Abstract
Background
Both statins and ezetimibe lower LDL-C, but ezetimibe’s effect on atherosclerosis is controversial. We hypothesized that lowering LDL-C cholesterol by adding ezetimibe to statin therapy would regress atherosclerosis measured by magnetic resonance imaging (MRI) in the superficial femoral artery (SFA) in peripheral arterial disease (PAD).
Methods
– Atherosclerotic plaque volume was measured in the proximal 15–20 cm of the SFA in 67 PAD patients (age 63±10, ABI 0.69±0.14) at baseline and annually x 2. Statin-naïve patients (n=34) were randomized to simvastatin 40mg (S, n=16) or simvastatin 40mg + ezetimibe 10mg (S+E, n=18). Patients already on statins but with LDL-C>80 mg/dl had open-label ezetimibe 10mg added (E, n=33). Repeated measures models estimated changes in plaque parameters over time and between-group differences.
Results
– LDL-C was lower at year 1 in S+E (67±7mg/dl) than S (91±8mg/dl, p<0.05), but similar at year 2 (68±10 vs. 83±11mg/dl, respectively). Plaque volume did not change from baseline to year 2 in either S+E (11.5±1.4cm3 to 10.5±1.3cm3, p=NS) or S (11.0±1.5cm3 to 10.5±1.4cm3, p=NS). In E, plaque progressed from baseline to year 2 (10.0±0.8 cm3 to 10.8±0.9, p<0.01) despite a 22% decrease in LDL-C.
Conclusions
– Statin initiation with or without ezetimibe in statin-naïve patients halts progression of peripheral atherosclerosis. When ezetimibe is added to patients previously on statins, peripheral atherosclerosis progressed. Thus, ezetimibe’s effect on peripheral atherosclerosis may depend upon relative timing of statin therapy.
Clinical Trial Registration Information
- NCT00587678 http://clinicaltrials.gov/ct2/show/NCT00587678
Keywords: peripheral vascular disease, lipids, magnetic resonance imaging, plaque, atherosclerosis
INTRODUCTION
Imaging approaches for quantifying atherosclerotic plaque regression with statins include ultrasound carotid artery intimal medial thickness (CIMT)(1), magnetic resonance imaging (MRI)(2), and invasive intravascular ultrasound (IVUS)(3). Prior MRI atherosclerosis regression studies imaged the carotid or aorta. Lower extremity peripheral arterial disease (PAD) is highly prevalent(4), particularly in patients with co-existing atherosclerosis(5), and is associated with significant morbidity(6). Our group recently established a technique using 3mm thick multi-slice black blood turbo spin echo MRI of the proximal 15–20cm of the superficial femoral artery (SFA) which reliably and reproducibly quantifies atherosclerosis, allowing lower patient numbers for clinical trials of atherosclerosis progression/regression(7). The ability to study peripheral arterial atherosclerosis using the SFA is attractive given the length of the vessel, volume of atherosclerosis, and lack of motion compared to the carotid. Studying atherosclerotic plaque in PAD has important implications for patients suffering from lower extremity claudication as well as serving as a surrogate clinical marker for generalized atherosclerosis progression/regression given its high prevalence in other vascular beds in such patients(8).
The benefit of lipid lowering therapy with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), on overall mortality and cardiovascular events is well-established(9). Furthermore, studies suggest an incremental benefit in reducing cardiovascular events with the intensity of low-density lipoprotein (LDL-C) cholesterol lowering from statins(10). In addition to LDL-C lowering, evidence suggests that statins exert pleiotrophic effects including improving endothelial function, stabilizing atherosclerotic plaque, and decreasing vascular inflammation(11). Ezetimibe is a non-statin lipid lowering drug which is effective in lowering LDL-C cholesterol when added to statin therapy(12). However, controversy exists over the role of ezetimibe in atherosclerotic plaque regression and clinical events(13,14,15).
In this study we investigated the role of LDL-C lowering on SFA atherosclerosis in patients with PAD using two parallel studies with the same primary endpoint of change in SFA vessel wall volume over two years as measured by MRI. In the first study, statin-naïve patients were randomized to receive either simvastatin or a combination of simvastatin plus ezetimibe and were followed for degree of LDL-C lowering and effect on SFA atherosclerosis. The second part of the study evaluated patients with lower extremity PAD already on a statin with LDL-C > 80 mg/dl, who received open-label ezetimibe in addition to ongoing statin therapy to determine if this would regress SFA atherosclerosis.
Methods
Study Design
Our study was a single-center, prospective, double-blind, randomized trial of statin-naïve patients with PAD begun on either simvastatin or simvastatin plus ezetimibe, as well as a parallel prospective study of open-label ezetimibe in patients with PAD already on statin therapy with LDL-C > 80mg/dl. Patients provided written informed consent prior to study enrollment. The study protocol was approved by the Human Investigation Committee at the University of Virginia and complies with the Declaration of Helsinki.
Patients between the ages of 30 and 85 years with symptoms of intermittent claudication and an ankle-brachial index (ABI) between 0.4 and 0.9, based on vascular lab testing done during the screening period, were eligible. Exclusion criteria included rest pain, critical limb ischemia, contraindication to MRI, and pregnancy. Approximately 350 vascular lab patients were screened for participation over the 15 month recruitment period.
In the randomized study, statin-naïve patients (no statin therapy for at least the prior 6 months) regardless of baseline LDL-C cholesterol underwent a double-blind randomization to simvastatin 40mg (group S) or a combination of simvastatin 40mg plus ezetimibe 10mg (group S+E) daily using a block randomization scheme. For the parallel direct treatment study, patients were enrolled already on statin therapy but with LDL-C >80mg/dl and had open-label ezetimibe 10mg daily added (group E). The investigators were blinded to therapy until follow-up studies and data analysis were complete.
Study Protocol
Patients were imaged at baseline and annually for two years. The MRI protocol was completed on an Avanto 1.5T scanner (Siemens Medical Solutions, Erlangen, Germany). Lipids were quantified at study entry and annually for two years. Patients were screened at 8 weeks via study coordinator verbal questioning and annually thereafter for symptoms of muscle pain, weakness, or tenderness, and blood was drawn for liver function tests at the same time points.
Over the two year study period, patients were also followed for major adverse cardiovascular events (MACE = death, myocardial infarction, stroke, and transient ischemic attack).
SFA Wall Volume
Wall volume in the SFA was assessed as previously described using a custom-built surface coil array placed over the thigh of the subject’s most symptomatic leg and a fat-suppressed multi-slice turbo spin echo pulse sequence to obtain a series of interleaved 3mm slices in the first 15 to 20cm of the SFA(16). The bifurcation of the common and superficial femoral arteries served as a landmark to allow comparison of the same length of SFA in a given patient over time. Wall volume, a surrogate for plaque volume (PV), was defined as total vessel area minus lumen area multiplied by the slice thickness and was calculated for the entire length of the SFA studied. The plaque volume analysis was done primarily by two experienced investigators (JDC, AMW) blinded to study drug and time point with VesselMASS software (University of Leiden, the Netherlands). The blinded studies were all overseen and validated by one investigator (AMW). The total vessel volume (TVV) and lumen volume (LV) were also measured.
Statistical Analysis
Test-retest reliability data from our laboratory demonstrated a mean SFA PV of 6.26cm3, standard deviation of 0.27 cm3, and intraclass correlation coefficient of 0.996(7). Our primary outcomes were LDL-C cholesterol and plaque volume over time. All other lipid subsets and plaque parameters were secondary outcomes. From prior carotid studies(17), we anticipated an 18% reduction from baseline PV in the SFA over 2 years with simvastatin and a further 20% reduction with simvastatin plus ezetimibe. To detect a difference between strategies in statin-naïve patients (S vs. S+E), power calculation suggested a sample size of 24 patients per group to provide 80% power, α = 0.05. For the open-label ezetimibe arm, a sample size of 42 patients provided 80% power, α = 0.05 to detect a 4% reduction in vessel wall area. We estimated an attrition rate of 15%. A total of 87 patients were enrolled at baseline.
All patients’ baseline characteristics were presented as mean (SD) for continuous variables and n (%) for categorical variables. For lipid analysis and plaque parameters, all values are reported as mean (SE). A p value ≤0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, North Carolina). Comparisons of categorical data between groups were made with the Chi-square test. A linear repeated measures model, with time as the only predictor in the model, was used to estimate changes in plaque parameters over time within each group. To compare changes in plaque parameters between S and S+E, a repeated measures model used terms for time and group. All lipid and plaque parameters are reported from the repeated measures model. The results were adjusted for pairwise comparison using Bonferroni correction. Subjects with incomplete data were assumed to be missing at random. Given the relatively small sample size for each group, imputation analysis was not performed as it may distort the distribution of variables and introduce bias. Bivariate relationships between changes in lipid parameters and changes in vessel wall parameters were examined using either Pearson’s correlation coefficient or Spearman’s rank correlation coefficient.
Results
Patients
87 patients were initially enrolled, but two patients withdrew prior to completing baseline imaging due to claustrophobia (Figure 1). Thus, 85 patients were evaluated at baseline (S, n=22; S+E, n=22; E, n=41) and 72 returned for follow-up (11 lost to follow-up, 2 deceased). The baseline patient characteristics for the randomized study and the parallel study for E group are presented in Table 1. Two patients in S and 6 in S+E had been on statins previously, for a mean of 61±52 days. The mean time to year 1 visit was 383±64 days and 393±99 days later for year 2. Five of the 72 were excluded from final analysis due to occluded SFA at baseline (n=1), interval SFA intervention (n=3) and inadequate image quality (n=1), leaving 67 for plaque analysis (S, n=16; S+E, n=18; E, n=33).
Figure 1.
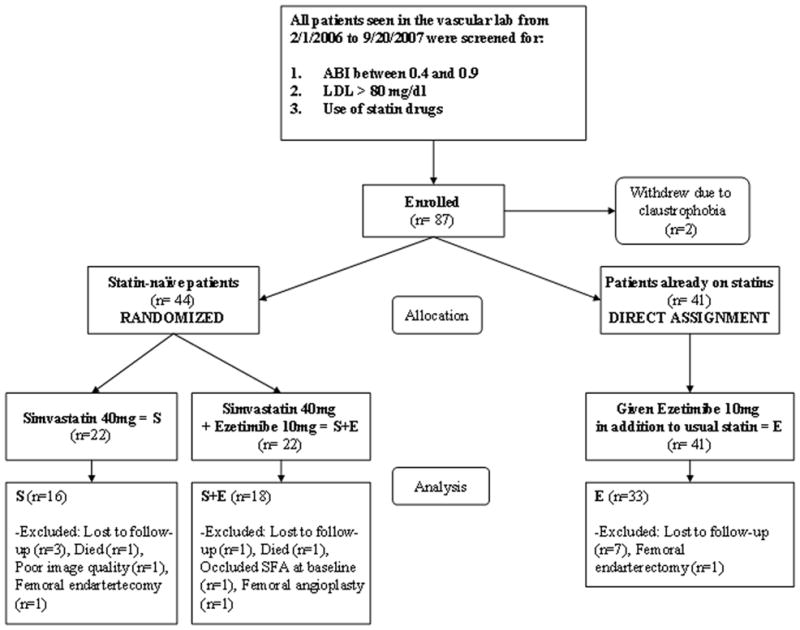
Patient flow diagram through the study.
Table 1.
Patient characteristics at study enrollment according to treatment group. The S and S+E groups were randomized and the E group represents a parallel study.
| Characteristic | All patients (n=67) | S (n=16) | S+E (n=18) | E (n=33) |
|---|---|---|---|---|
| Age - yr | 62±10 | 59±10 | 62±8 | 65±10 |
| Ankle Brachial Index (ABI) | 0.69±0.14 | 0.72±0.14 | 0.62±0.15 | 0.72±0.12 |
| Body Mass Index, kg/m2 | 29±6 | 30±7 | 28±6 | 30±5 |
| Male sex – (%) | 37 (55) | 11 (69) | 10 (56) | 16 (48) |
| Prior lower extremity revascularization – (%) | 13 (19) | 2 (13) | 4 (22) | 7 (21) |
| Leg of interest – (%) | 8 (12) | 2 (13) | 3 (17) | 3 (9) |
| Race – (%) | ||||
| White | 51 (76) | 13 (81) | 14 (78) | 24 (73) |
| Black | 11 (16) | 2 (13) | 3 (17) | 6 (18) |
| American Indian | 5 (7) | 1 (6) | 1 (5) | 3 (9) |
| Risk Factors – (%) | ||||
| Tobacco Use | 33 (50) | 8 (50) | 13 (72) | 12 (36) |
| Diabetes mellitus | 20 (30) | 5 (31) | 5 (28) | 10 (30) |
| Hypertension | 56 (84) | 13 (81) | 14 (78) | 29 (88) |
| History of CAD | 38 (57) | 8 (50) | 10 (56) | 20 (61) |
| Hypercholesterolemia | 51 (76) | 9 (56) | 11 (61) | 31 (94) |
| Medications – (%) | ||||
| Aspirin/Clopidogrel | 49(73) | 11 (69) | 13 (72) | 25 (76) |
| ACE-Inhibitor | 33 (49) | 8 (50) | 5 (28) | 20 (61) |
| ARB | 8 (12) | 2 (13) | 4 (22) | 2 (6) |
| β-Blocker | 25 (37) | 6 (38) | 6 (33) | 13 (39) |
| Cilastazol | 8 (12) | 2 (13) | 2 (11) | 4 (12) |
S - simvastatin only, S+E - simvastatin+ezetimibe, E - open label ezetimibe added to ongoing statin. Plus-minus values are means ±SD. ACE - angiotensin converting enzyme, ARB - angiotensin receptor blocker. Body-mass index - weight in kilograms divided by the square of the height in meters. There were no differences in any demographics parameters between S and S+E.
There was a low rate of study drug discontinuation. In S, one patient discontinued study drug at year 1and an additional patient reported study drug non-compliance at year 2. In S+E, one patient discontinued study drug between year 1 and 2. In E, atorvastatin was the most commonly used statin (n=17, mean dose 33mg), followed by simvastatin (n=11, mean 45mg) and one patient each taking lovastatin 40mg and rosuvastatin 5mg. Three patients in E were unable to tolerate continuing statin usage due to nonspecific complaints, one additional patient self-discontinued their statin at year 1, as did another at year 2. All data was analyzed as intention-to-treat. Other lipid lowering agents were used by few study subjects and included niacin (n=1 for S, n=2 for E), fibrates (n=2 for S+E, n=1 for E) and fish oil (n=1 for S, S+E and E).
Laboratory Results
All groups had significant decreases in LDL-C and total cholesterol from baseline to year 1, which were sustained at year 2 (Table 2). The baseline LDL-C was similar between S+E and S (118±41mg/dl, 118±34mg/dl, respectively). Although the year one LDL-C was less for S+E than S (67±31mg/dl vs. 91±32mg/dl, p<0.05), the difference was less pronounced at year two (68±49mg/dl vs. 85±29mg/dl, p=NS). There was no change in HDL or triglycerides over the study period for any group.
Table 2.
Changes in lipid parameters over time between the randomized groups S and S+E as well as in the parallel study group E.
| Group | Baseline | Year 1 | Year 2 |
|---|---|---|---|
| Total Cholesterol (mg/dl) | |||
|
| |||
| S | 194±11 | 162±10* | 152±12* |
| S+E | 189±10 | 135±9*† | 136±12* |
| E | 170±6 | 144±7* | 144±6* |
|
| |||
| LDL-C (mg/dl) | |||
|
| |||
| S | 118±10 | 91±8* | 83±11* |
| S+E | 118±9 | 67±7*† | 68±10* |
| E | 100±4 | 80±6* | 77±5* |
|
| |||
| HDL (mg/dl) | |||
|
| |||
| S | 45±4 | 46±3 | 44±4 |
| S+E | 48±4 | 42±3 | 46±3 |
| E | 42±2 | 42±2 | 43±3 |
|
| |||
| Triglycerides (mg/dl) | |||
|
| |||
| S | 227±47 | 173±27 | 171±30 |
| S+E | 130±21 | 126±18 | 119±20 |
| E | 157±20 | 140±12 | 152±15 |
Values are listed as mean±S.E.
p≤0.05 vs. baseline,
p<0.05 vs. S.
In E, LDL-C decreased from baseline to year 1 (100±25mg/dl to 79±33mg/dl, p=0.001) and was stable at year 2 (78±27mg/dl, p<0.001 vs. baseline). Similar findings were seen with total cholesterol from baseline to year 1 (170±36mg/dl to 144±41mg/dl, p<0.01), without a further decrease at year 2 (145±32mg/dl).
MRI Plaque Parameters
Change in plaque volume measured in the SFA over time (Figures 2 and 3, Table 3) was the primary outcome measure. No significant difference between S and S+E was found in any of the plaque parameters (PV, TVV or LV). Given the number of randomized patients who completed analysis (S, n=16 and S+E, n=18), there was 72% power for detecting a difference between S and S+E. There was no difference between S and S+E for plaque volume (mean±SE) at baseline (11.0±1.5 cm3 and 11.5±1.4 cm3, p=NS), year one (10.3±1.5 cm3 and 11.1±1.4 cm3, p=NS) or year two (10.5±1.4 cm3 and 10.5±1.3 cm3, p=NS). The change in plaque volume from baseline to year one was similar in S and S+E (−0.7±0.2 cm3 and −0.4±0.2 cm3, respectively, p=NS), as was the change from baseline to year two (−0.4±0.3 cm3 and −1.0±0.4 cm3, respectively, p=NS).
Figure 2.
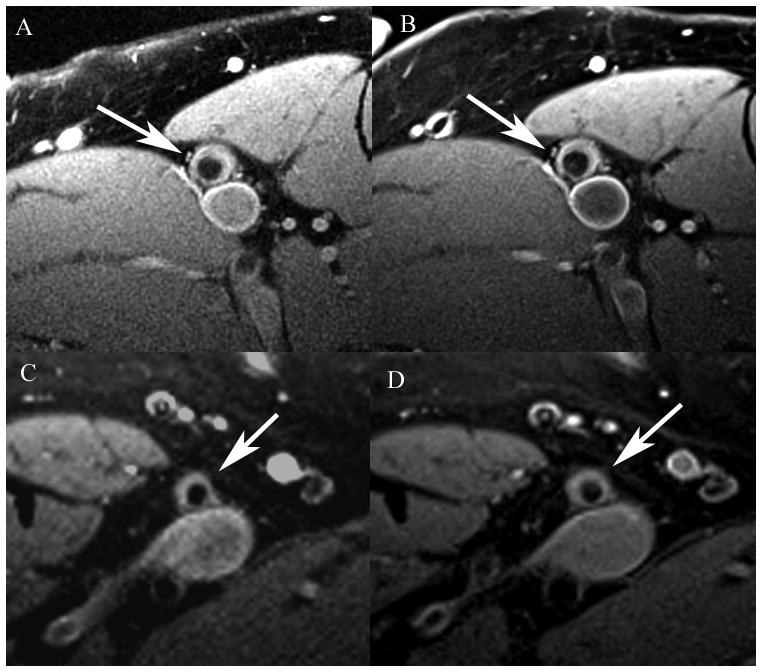
Representative black blood turbo spin echo MRI of one 3mm slice of the superficial femoral artery (arrow) in a S+E patient (top) at baseline (A) and year two (B) demonstrating lack of change over time and an E patient (bottom) at baseline (C) and year two (D) demonstrating subtle plaque progression. Note the excellent match between imaging locations at the different time points.
Figure 3.
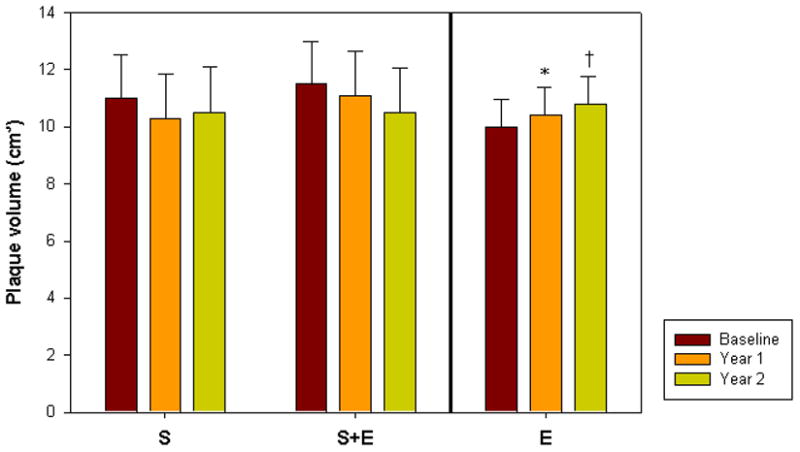
Graph comparing change in plaque volume over time for each of the study groups (S= simvastatin only, S+E = simvastatin plus ezetimibe and E = open label ezetimibe added to ongoing statin use at baseline). *p<0.05 vs. baseline, †p<0.01 vs. baseline
Table 3.
Changes in plaque parameters over time between the randomized groups S and S+E as well as in the parallel study group E.
| Baseline | Year 1 | Year 2 | |
|---|---|---|---|
|
Plaque Volume (cm3)
| |||
| S | 11.0±1.5 | 10.3±1.5 | 10.5±1.4 |
| S+E | 11.5±1.4 | 11.1±1.4 | 10.5±1.3 |
| E | 10.0±0.8 | 10.4±0.9* | 10.8±0.9† |
|
| |||
|
Total Vessel Volume (cm3)
| |||
| S | 17.7±2.5 | 16.9±2.5 | 17.2±2.6 |
| S+E | 17.5±2.4 | 16.9±2.4 | 16.3±2.4 |
| E | 15.7±1.2 | 16.0±1.2 | 16.6±1.3† |
|
| |||
|
Lumen Volume (cm3)
| |||
| S | 6.7±1.2 | 6.5±1.2 | 6.7±1.3 |
| S+E | 5.9±1.1 | 5.8±1.1 | 5.9±1.3 |
| E | 5.7±0.5 | 5.6±0.5 | 5.8±0.6 |
Values are listed as mean±S.E.
p<0.05 vs. baseline,
p<0.01 vs. baseline.
In E, atherosclerotic plaque volume progressed from baseline to year 1 (10.0±0.8 cm3 to10.4±0.9 cm3, p<0.02) and tended to increase further at year 2 (10.8±0.9 cm3, p<0.01 vs. baseline and p=0.09 compared to year 1). (Figure 2, Table 3) An increase in total vessel volume was seen between baseline and year 2 (15.7±1.2 cm3 to 16.6±1.3 cm3, p<0.01).
There was no correlation found between changes in plaque and lipid parameters for either S or S+E. In E, there was a significant negative correlation between % change in total cholesterol and plaque volume over two years (r=−0.45, p<0.02). A similar negative correlation was seen between % change in LDL-C with plaque volume over two years (r=−0.38, p=0.05).
Adverse Events
One S patient discontinued their study drug after year 1 due to muscle aches. Over the two year study period, occurrence of MACE was similar in S and S+E (2 and 4, respectively) and was 3 in E.
Discussion
This study used MRI to quantify atherosclerotic plaque volume in the first 15–20 cm of the superficial femoral artery in patients with symptomatic PAD treated with lipid-lowering therapy for 2 years. Statin-naïve patients randomized to simvastatin or simvastatin + ezetimibe demonstrated no change in plaque volume over time or difference between groups despite greater initial LDL-C lowering in the simvastatin + ezetimibe group. In the second part of the study, patients previously on a statin with ezetimibe added at study enrollment demonstrated plaque progression despite a significant reduction in LDL-C cholesterol. In fact, the extent of LDL-C lowering correlated with plaque progression, raising questions regarding the utility of ezetimibe added to patients previously on long-standing statin therapy.
Ezetimibe effectively lowers LDL-C cholesterol. Older studies have found an improvement in cardiovascular outcomes with LDL-C lowering from non-statin mechanisms, such as binding resins and ileal-bypass(18,19). Despite the evidence for effective LDL-C lowering, the impact of ezetimibe on atherosclerotic plaque regression and cardiovascular events has been debated(20). Similar to the present study, The SANDS trial demonstrated equivalent effects of aggressive LDL lowering of statins and statins with ezetimibe added on CIMT regression (15). In the ENHANCE (Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression) trial(13), Kastelein et al randomized patients with familial hypercholesterolemia to receive simvastatin 80mg with either ezetimibe 10 mg or placebo and compared change in CIMT measurements. After 2 years, despite a significantly greater reduction in LDL-C in the patients on ezetimibe, there was no difference in CIMT and both groups demonstrated plaque progression. When comparing ENHANCE to this study, 81% of the study participants from ENHANCE were already on statin therapy prior to study enrollment and as a result, the impact of statins on the lipid content of plaque may have already occurred (similar to the E group in the present study). In addition, the ENHANCE trial was performed in younger patients with familial hypercholesterolemia, in contrast to the patients in our study who are older with extensive peripheral atherosclerosis and thus, the rates of atherosclerotic progression may be different. Despite these differences, the results of our open-label ezetimibe study are concordant with the negative results of the ENHANCE trial.
A recent trial, ARBITER 6-HALTS, evaluated patients with coronary heart disease or equivalent already on long-term statin therapy who were randomized to receive niacin or ezetimibe with CIMT measurements after 14 months(14). Ezetimibe therapy decreased LDL-C significantly (-19.2% to 66mg/dl) without a change in CIMT, whereas niacin lowered CIMT. The patients in the ezetimibe group with greater reductions in LDL-C paradoxically had increases in CIMT (R=-0.31, p<0.001), remarkably similar to the findings of the present study. The ARBITER-6 investigators suggested that this paradox could be related to alterations in cholesterol transport by ezetimibe(14). Ezetimibe may be associated with proatherogenic mechanisms(21), such as inhibition of scavenger receptor B1 (SRB1) and ATP-binding cassette transporter A1 (ABCA1) as well as increases in HMG-CoA reductase activity(22).
Corti et al studied the effect of initiation of simvastatin on carotid and aortic atherosclerosis using high-resolution MRI. In 18 patients studied over one year, there was a decrease in LDL-C and total cholesterol as well as a significant reduction in vessel wall area, without a change in lumen area(2). In a follow-up study, Corti et al (16) reported two year data on aortic and carotid artery plaques in 21 patients begun on simvastatin and found a further decrease in vessel wall area at 24 months as well as a very slight increase in lumen area. The data from this study supported the idea that statin-induced plaque regression and vascular remodeling occur before any changes in lumen size. The present study showed similar findings with no significant change in lumen volume over time in any of the groups studied.
From studies using intravascular ultrasound (IVUS), there is substantial evidence of a beneficial effect of statins on coronary plaque volume, especially upon statin initiation and with more aggressive LDL-C lowering. In general, the studies of IVUS and statin use have demonstrated plaque volume stabilization. Nissen et al (3) found that in patients with known coronary artery disease (CAD) (30% of whom had used statins previously) treated with 18 months of 80mg of atorvastatin had significantly reduced progression of coronary atherosclerosis compared with patients treated with 40 mg of pravastatin. In the atorvastatin group, 97% achieved an LDL-C less than 100mg/dl compared to only 65% in the pravastatin group. In a pooled analysis of 4 prospective randomized trials of IVUS and statin therapy in patients with coronary atherosclerosis(23), there was a mean decrease in LDL-C from 124±38 to 88±29mg/dl and average increase in percent coronary atheroma volume of 0.5±3.9%. However, the subgroup of patients with regression of greater than 5% atheroma volume occurred in those with LDL-C cholesterol during treatment less than 88mg/dl. The final LDL-C after 2 years of lipid lowering therapy in all 3 groups in the present study was less than this value.
The baseline plaque volume in the E group was lower than that of S or S+E which may have been because E patients were already on statins and had achieved much of their benefits by that time. The mean LDL-C for all groups in this study at one year was substantially less than 100mg/dl; however, despite achieving a lower LDL-C with the addition of ezetimibe in patients on ongoing statin there was plaque progression. In addition, the statin-naïve patients randomized to simvastatin plus ezetimibe had a significantly lower LDL-C at one year compared to those on simvastatin only, however there was no difference in plaque volume over time between these groups. Thus, the intensity of LDL-C lowering may not be the most important factor in determining atherosclerotic plaque progression/regression. Instead, the mechanism underlying LDL-C reduction, as well as the timing of statin initiation, appears to have an important impact.
An advantage to using MRI for atherosclerotic plaque regression studies is the high reproducibility and reliability allowing smaller patient numbers to detect differences between groups. In a multi-center trial of carotid artery wall volume(24), the interclass correlation coefficients for wall volume was 0.99 (95% CI: 0.98, 0.99), and the intraclass correlation coefficient was 0.98 (95% CI: 0.97, 0.99). In the validation studies done at our institution for superficial femoral artery plaque wall volume using MRI(7), the intra-observer intraclass correlation was R=0.997, inter-observer was R=0.987, and test-retest reproducibility was R=0.996. In studies using CIMT(1) or IVUS(3), the number of patients needed to detect differences in plaque volume is in the 200–500 patient range, which is much larger than is necessary with SFA MRI, as seen in this study. Further evidence to the small patient numbers needed to detect changes in atherosclerotic plaque volume is found in the study by Lima et al. of 27 patients treated with simvastatin for 6 months with significant LDL-C reduction who demonstrated a decrease in thoracic aorta plaque volume using MRI(25).
Limitations
The study was slightly underpowered (72%) to detect a difference in change in atherosclerotic plaque volume between S and S+E, due in part to the difficulty finding PAD patients who were statin-naïve, as well as the attrition rate. Twenty three % (20/87) of the patients initially enrolled in the study were not included in the final analysis for a variety of reasons. PAD patients are among the sickest of cardiovascular disease patients and thus the attrition rate in our study is not entirely surprising. In addition, a few of the patients underwent revascularization procedures in the leg of interest, precluding data analysis. Given the relatively small size of the study, we did not perform a multi-variable analysis.
No arm in this study was placebo-controlled. There was no placebo group in the statin-naïve group due to the known cardiovascular benefit of statins in PAD patients(26). The study in E patients was not placebo-controlled, but the annualized rate of plaque progression despite statin therapy and further LDL-C lowering was 4%. A recent multi-center study of MRI of the carotid artery in patients with stenosis > 50% showed an annualized rate of plaque progression in patients on ongoing statin therapy of just over 1%(24). However, without a placebo comparison for the E group, it is unclear whether the plaque progression is related to the natural history of lipid-depleted atherosclerosis or if it is due to an effect of ezetimibe. In addition, patients in the E group did not have their dose of statin increased as part of the study design and as a result, their LDL-C did not reach contemporary levels of less than 70mg/dl. Although the additive benefit of nicotinic acid on HDL levels and atherosclerotic plaque regression in patients already treated with statins has been shown in both MRI(27) and CIMT(14) studies, this study was designed only to look at changes in LDL-C and total cholesterol.
Only one MRI tissue weighting of all of the slices imaged in each patient at each time point was performed. Thus, characterization of plaque components could not be performed as part of this study. MRI provides a non-invasive assessment of the degree of atherosclerosis in the SFA which may or may not be reflective of changes in other vascular territories, such as the coronary arteries, carotids and aorta. Further studies are needed to correlate changes in SFA atherosclerosis with other vascular beds. This study was not powered to measure the effect of different lipid lowering strategies on clinical outcomes in patients with PAD. Future, large multi-center trials will address the efficacy of ezetimibe in combination with statins on clinical outcomes in patients with cardiovascular disease, such as IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial)(28) with results expected in 2013 and the SHARP (Study of Heart and Renal Protection) trial(29).
Conclusions
In patients with peripheral arterial atherosclerosis, initiation of a statin with or without ezetimibe leads to a halting of plaque progression. In contrast, patients already on a statin who were treated with ezetimibe demonstrated plaque progression despite pronounced further LDL-C lowering. Thus, both the timing of statin initiation and mechanism of LDL-C lowering may be important in the progression of peripheral atherosclerosis.
Acknowledgments
We would like to acknowledge the contributions of David Isbell MD, Jayne Missel RN, Edward Van Opstal, and the University of Virginia Vascular Lab.
Funding
This work was supported by the National Heart Lung and Blood Institute at the National Institutes of Health, grant number: R01 HL075792 (CMK) and the National Center for Research Resources, grant number: M01RR000847 and the National Institute of Biomedical Imaging and Bioengineering, grant number: T32 EB003841 (JDA, AMW). Study drugs were supplied by Merck Schering Plough.
Footnotes
COMPETING INTEREST
Drs. Epstein, Meyer, Hagspiel, and Kramer receive research support from Siemens Medical Solutions. All other authors have no declared conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smilde TJ, van Wissen S, Wollersheim H, et al. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolemia (ASAP): a prospective, randomised, double-blind trial. Lancet. 2001;357:577–81. doi: 10.1016/s0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 2.Corti R, Fayad ZA, Fuster V, et al. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation. 2001;104:249–52. doi: 10.1161/01.cir.104.3.249. [DOI] [PubMed] [Google Scholar]
- 3.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–80. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Denenberg JO, Langer RD, et al. The epidemiology of peripheral arterial disease: importance of identifying the population at risk. Vasc Med. 1997;2:221–6. doi: 10.1177/1358863X9700200310. [DOI] [PubMed] [Google Scholar]
- 6.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–99. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 7.Isbell DC, Meyer CH, Rogers WJ, et al. Reproducibility and reliability of atherosclerotic plaque volume measurements in peripheral arterial disease with magnetic resonance imaging. J Cardiovasc Magn Reson. 2007;9:71–6. doi: 10.1080/10976640600843330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Kuijk JP, Flu WJ, Welten GMJM, et al. Long-term prognosis of patients with peripheral arterial disease with or without polyvascular atherosclerotic disease. Eur Heart J. 2010;31:992–9. doi: 10.1093/eurheartj/ehp553. [DOI] [PubMed] [Google Scholar]
- 9.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 10.de Lemos JA, Blazing M, Wiviott S, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–16. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 11.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–9. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 12.Gagne C, Bays HE, Weiss SR, et al. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1084–91. doi: 10.1016/s0002-9149(02)02774-1. [DOI] [PubMed] [Google Scholar]
- 13.Kastelein JJP, Akdim F, Stroes ESG, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–43. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 14.Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361:2113–22. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 15.Fleg JL, Mete M, Howard BV, Umans JG, Roman MJ, Ratner RE, Silverman A, Galloway JM, Henderson JA, Weir MR, Wilson C, Stylianou M, Howard WJ. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes. J Am Coll Cardiol. 2008;52:2198–2205. doi: 10.1016/j.jacc.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson JA, Epstein FH, Meyer CH, et al. Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol. 2009;54:628–34. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corti R, Fuster V, Fayad ZA, et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions. Circulation. 2002;106:2884–7. doi: 10.1161/01.cir.0000041255.88750.f0. [DOI] [PubMed] [Google Scholar]
- 18.Lipid Research Clinics Program. The lipid research clinics coronary primary prevention trial results. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–64. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 19.Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the program on the surgical control of hyperlipidemias. N Engl J Med. 1990;323:946–55. doi: 10.1056/NEJM199010043231404. [DOI] [PubMed] [Google Scholar]
- 20.Al Badarin FJ, Kullo IJ, Kopecky SL, et al. Impact of ezetimibe on atherosclerosis: is the jury still out? Mayo Clin Proc. 2009;84:353–61. doi: 10.1016/S0025-6196(11)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005;135:2305–12. doi: 10.1093/jn/135.10.2305. [DOI] [PubMed] [Google Scholar]
- 22.Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL-C receptor protein expression and on LDL-C receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis. 2008;198:198–207. doi: 10.1016/j.atherosclerosis.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 23.Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007;297:499–508. doi: 10.1001/jama.297.5.499. [DOI] [PubMed] [Google Scholar]
- 24.Boussel L, Arora S, Rapp J, et al. Atherosclerotic plaque progression in carotid arteries: monitoring with high-spatial-resolution MR imaging - multicenter Trial. Radiology. 2009;252:789–96. doi: 10.1148/radiol.2523081798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lima JA, Desai MY, Steen H, et al. Statin-induced cholesterol lowering and plaque regression after 6 months of magnetic resonance imaging-monitored therapy. Circulation. 2004;110:2336–41. doi: 10.1161/01.CIR.0000145170.22652.51. [DOI] [PubMed] [Google Scholar]
- 26.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvistatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(11)61125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JMS, Robson MD, Yu LM, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol. 2009;54:1787–94. doi: 10.1016/j.jacc.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 28.ClinicalTrials.gov. IMPROVE-IT: Examining outcomes in subjects with acute coronary syndrome: vytorin (ezetimibe/simvastatin) vs simvastatin. Study P04103AM3
- 29.ClinicalTrials.gov. SHARP:Study of heart and renal protection. Study NCT00125593


