Abstract
Objective
Cerebral edema is a devastating complication of pediatric diabetic ketoacidosis (DKA). We examined measures describing potential causes of whole brain and regional brain edema [mean transit time (MTT), apparent diffusion coefficient (ADC) and relative cerebral blood volume (relCBV)] during treatment of DKA in children.
Design
Prospective observational study
Setting
Regional Children’s Hospital
Interventions
None
Measurements and Main Results
After IRB approval, children admitted with DKA (pH < 7.3, HCO3 < 15 meq/L, Glucose > 300 mg/dL, and ketosis) underwent two serial paired contrast-enhanced (gadolinium) and diffusion magnetic resonance imaging (MRI) scans. Change in whole brain and regional (frontal lobe, occipital lobe and basal ganglia) MTT, ADC and relCBV between the two time periods (12–24 hours) and (36–72 hours) after start of insulin treatment (Time 0) were determined.
Thirteen children (median age 10.3 ± 1.1 years; 7 female) with DKA were examined. Overall, whole brain and regional MTT decreased from Time 1 (first MRI after Time 0) to Time 2 (second MRI after Time 0) by 51 ± 59% (p=0.01), without differences between the brain regions examined. Whole brain ADC increased by 4.7% ± 3.4% (p=0.001), without differences between the brain regions examined. There was no change in relCBV for the whole brain and for the 3 brain regions examined.
Conclusions
In this study, whole brain MTT decreased and ADC increased, suggesting a vasogenic process between the two study periods during DKA treatment.
Keywords: mean transit time, apparent diffusion coefficient, cerebral blood volume, diabetic ketoacidosis
INTRODUCTION
Diabetic ketoacidosis (DKA) is a serious complication of Type I diabetes where cerebral injury, including cerebral infarcts and cerebral edema may occur. (1–6) Despite these central nervous system complications in pediatric DKA, changes in cerebral physiology in DKA and during DKA treatment have not been well characterized.
The genesis of cerebral injury in DKA may involve both cytotoxic and vasogenic pathways and imaging technology and image based calculations such as apparent diffusion coefficient (ADC), cerebral blood volume (CBV) and mean transit time (MTT) have begun to shed light on these changes during DKA. (6–8) Briefly, ADC reflects diffusion of water molecules within cerebral tissue, where disease states associated with dominant acute vasogenic edema formation or chronic tissue destruction usually show elevations of the ADC (Gass et al, 2001). (9) Cerebral blood volume measures the amount of blood in the vessels examined and MTT reflects the nominal time required for the blood to travel from a feeding artery to tissue voxel. An increase in MTT indicates either a relative increase in CBV or relative decrease in CBF. In one recent study of MRI examinations in 26 children acutely ill with DKA, there was a decrease in ADC from initial examination early during DKA treatment to after recovery in most brain areas, suggesting resolution of brain edema over time. (10) A separate study by the same group showed that 14 children demonstrated lower MTT early (between 2–12 hours) during treatment compared to late (post recovery > 72 hours. (8) However, there are no data characterizing changes in these variables from intermediate time intervals (between 12 and 72 hours) when DKA treatment occurs and cerebral injury may continue to occur. Our previous work documented increases in whole blood brain barrier permeability and a greater increase in the occipital lobe during DKA treatment between 12–24 hours and 36–48 hours. (11) The aim of this study was to examine the associations between whole brain and regional changes in MTT, ADC, and relCBV that may contribute to cerebral injury in DKA.
MATERIALS AND METHODS
After institutional approval from the Seattle Childrens (SCH) hospital’s Human Subjects Review Committee, we conducted a prospective cohort study in children admitted with DKA. Consent was obtained from parents or legal guardians and assent was obtained from patients per institutional protocol. This study is part of a larger project designed to examine mechanisms of cerebral injury and edema in DKA; a convenience sample of patients underwent MRI scanning as part of the study. Data from these 13 of the 43 subjects in the larger study who contributed MTT, ADC and relCBV data are reported here. Four of these 13 subjects contributed data to our previous work on changes in blood brain permeability in DKA. (11)
Study Participants
Following approach by the clinical team, consent/assent, patients to be admitted to SCH with the diagnosis of DKA were consented and prospectively enrolled in the emergency department, pediatric intensive care unit or hospital medical unit. Eligibility criteria included: age ≤ 17 years, pH < 7.3, glucose > 300 mg/dL, HCO3 < 15 meq/l and urinary ketosis. Since patients had to be transported out of the PICU for MRI imaging, we excluded children who were deemed to have hemodynamic instability by the treating PICU attending (IRB requirement) and patients with no available parents/guardians at the time of enrollment. We reviewed medical records for eligibility, relevant medical history, and physiologic data.
MRI acquisition during Illness and Recovery
Study participants underwent MRI exams if they were considered hemodynamically stable by the treating physician, and if they were deemed cooperative for MRI scanning without sedation. No sedation was used to obtain MRI scans because of the confounding effects of sedative agents on cerebrovascular physiology.
Children admitted with DKA underwent two serial paired contrast enhanced and diffusion MRI scans: during illness and recovery. Imaging during illness was operationalized and defined in relation to start of insulin therapy (Time 0). The illness period was defined as the first 12–24 hours from Time 0 and we aimed to obtain the first MRI during this period of illness (Time 1). The recovery period was defined as between 36–72 hours from Time 0 and we aimed to obtain the second MRI during this recovery time period (Time 2). Additionally, at-least 12 hours must have lapsed between the first and second MRI scans for each patient. Change in whole brain and regional rel MTT, ADC and relCBV between the 2 MRI time intervals (Times 1 and 2) were determined.
Contrast-enhanced MRI scans were performed on a Siemens 1.5T Symphony (software version syngo MR 2004A 4VA25A or Avanto (version syngo B-13 or 15) scanner (Siemens, Erlangen, Germany). Dynamic contrast-enhanced imaging was performed with intravenous injection of 10 mL of Magnevist Gadolinium (Gd-DTPA) using a power injector at 3 ml/second followed by 2 mL flush of saline and the following acquisition parameters: axial spin-echo echo-planar 2D pulse sequence, TR/TE 2400/47 msec, 20 slices, 50 to 60 time volumes, phase-encoded steps 112, reconstructed matrix 128X128, slice thickness/gap 5/2mm, flip angle 90 degrees, field of view 230 mm, pixel spacing 1.875X1.875 mm.
Relative Cerebral Blood Volume (relCBV)
Relative cerebral blood volume (relCBV) was calculated from the time series perfusion MR signal on a voxel by voxel basis using the semi-automated tool available on the Siemens Leonardo workstation. This software reports relative values for CBV. Conversion to absolute values would require additional assumptions about the arterial input functions. Verification of these assumptions would require additional scan time that was not available for this study. The perfusion data were processed according to the equations described by Zaharchuk (12). Using tracer kinetic theory, the input and the output of a tracer from a voxel can be used to determine the volume of distribution (i.e., cerebral blood volume CBV). Ignoring recirculation, the plasma volume (vp = CBV * [1 − hematocrit (Hct)]) was calculated as the integral of the tissue concentration time curve:
vp is the plasma volume, Ctissue is the tissue concentration of the tracer (Gadolinium).
Calculation of Mean Transit Time (MTT)
Maps of MTT were generated using the automated tool available on the Siemens Leonardo workstation. The arterial input function was defined by manual selection of two or three voxels free of apparent partial volume (tissue/artery) effects and located within the middle cerebral artery. The Leonardo software estimates MTT using the techniques described by Østergaard et al. (13)
Apparent Diffusion Coefficient (ADC)
Apparent diffusion co-efficient (ADC) was measured by scanning with the following parameters: 2-D diffusion-weighted echo planar spin-echo pulse sequence, TR/TE 4800/88 milliseconds, slice thickness/gap 5/2 mm; 20 slices, phase encoding steps 110, reconstructed matrix 128 × 128, field of view 240 × 240 mm. Diffusion-weighted images are characterized by paired magnetic field gradients that attenuate the signal from randomly diffusing water. The degree of attenuation depends on the magnitude and timing of the gradients and can be assessed using the b value, which is defined as:
where: γ is the gyromagnetic ratio, G is the magnitude of the paired gradients, δ is the duration of the gradients, and Δ is the delay time between the gradients. In this study, 3 different b values (1000, 500, 0) were used and ADC was calculated by fitting the MR intensity values from these 3 different b values to the following equation:
where S0 is the signal intensity without the diffusion weighting, S is the signal with the gradient, and D is the diffusion-coefficient.
Statistical Analysis
The clinical characteristics of all patients enrolled in this study are presented using descriptive statistics. MTT, ADC, and relCBV data are presented for the whole brain and also for the brain regions (frontal, occipital and basal ganglia).
Treatment start was defined by start of insulin administration (Time 0), regardless of transfer status. We operationalized the times of first (Time 1) and second MRI (Time 2) scan times from Time 0 (time post insulin) and by range since it is not possible to ensure that in each and every case, subjects can receive MRI scanning at the exact same time due to clinical stability status, MRI availability, and in some cases, transport to SCH from an outside facility.
MTT, ADC and relCBV and were calculated for the whole brain and for the 3 select regions of interest: frontal cortex (anterior cerebral circulation), occipital cortex (posterior circulation), and basal ganglia (deep structure) for Time 1 and Time 2. For whole brain and for each brain region, we first calculated the percent increase for each region of interest and for the whole brain by comparing the mean for the group at time 1 vs. time 2. Paired Student’s T test was then used to examine whole brain and regional differences in MTT, ADC and relCBV between Time 1 and Time 2 because this approach adjusts for those subjects who may have contributed low baseline values and skewed the data. Data are presented as mean ± SEM if normally distributed (Kolmogorov-Smirnov Goodness-of-Fit Test), median ± SEM for nonparametric data, and p < 0.05 reflects significance.
RESULTS
Demographic and Clinical Characteristics (Table 1)
Table 1. Admission Characteristics of Thirteen Children with Diabetic Ketoacidosis.
1 Pretreatment blood gas showed mean values of pH 7.05 ± 0.15 mmHg, pvCO2 17.9 ± 7.1 mmHg, glucose 444 ± 71 g/dL, HCO3 4.8 ± 3.7 mEq/L, calculated serum osmolarity 336 ± 47 mosm/L, with improvement in these variables following 24 hours of therapy to pH 7.31 ± 0.08, pvCO2 33 ± 2.1 mmHg, HCO3 16.0 ± 1.1 mEq/L.
| Subject number | Age (years)/Gender | New Dx | Weight (kg) | Glucose (g/dL) | Blood gas (pH/pVCO2/HCO3) | Calculated serum osmolarity (mosm/L) | BUN (mg/dL) | Clinical CE |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 F | Y | 33 | 453 | 6.81/15/1 | 453 | 13 | M, MV, ICP |
| 2 | 9 M | Y | 27 | 459 | 7.14/24/8 | 284 | 29 | |
| 3 | 10 F | Y | 43 | 503 | 6.95/11/2 | 310 | 13 | |
| 4 | 12 F | N | 52 | 250 | 7.28/33/14 | 296 | 19 | |
| 5 | 10 M | N | 32 | 533 | 7.11/16/5 | 310 | 30 | |
| 6 | 17 F | N | 68 | 419 | 7.05/19/5 | 302 | 9 | M |
| 7 | 12 M | Y | 65 | 398 | 6.98/11/4 | 345 | 17 | |
| 8 | 11 F | N | 51 | 438 | 7.12/18/3 | 346 | 34 | |
| 9 | 9 M | N | 47 | 508 | 7.01/19/2 | 363 | 21 | |
| 10 | 10 M | N | 61 | 421 | 7.22/29/9 | 401 | 39 | |
| 11 | 8 F | N | 45 | 457 | 7.03/12/4 | 330 | 22 | |
| 12 | 6 M | Y | 39 | 429 | 6.81/10/1 | 329 | 19 | M |
| 13 | 7 M | Y | 41 | 501 | 7.20/16/5 | 302 | 25 |
MV-mechanical ventilation, ICP-intracranial pressure monitoring, and M= mannitol. Subject #1 received received ICP monitoring and treatment for clinical cerebral edema (CE) and subjects 1,6,12 received hypoerosmolar therapy for suspected CE.
The median admission pH was higher among screened and not enrolled vs. enrolled subjects (pH 7.40 ± 0.14 vs. pH 7.05 ± 0.04mmHg; p< 0.001) but there was no difference in mean age between the two groups (9 ± 1.7 vs. 10 ± 2.7 years; p = 0.26). All 13 children who were enrolled completed both sets of MRIs; there were no protocol violations.
Thirteen children, mean age of 10.1 ± 2.7 years (range 6 years–17 years), completed the study protocol without adverse events. Seven subjects were female and 6 subjects were newly diagnosed. Five children were transferred to SCH from an outside facility and 9 were admitted to the pediatric intensive care unit (PICU) for treatment. All children were treated with an intravenous insulin infusion (0.1u/kg/hr), and hydration with intravenous fluids (10–20 mL/kg) bolus followed by a maintenance infusion of a balanced salt solution (D51/4NS) for DKA prior to the first MRI scan. The rate of osmolality and glucose change was 2.2 mOsm/kg/hr and 76 mg/dL/hr. Subject #1 received ICP monitoring and treatment for clinical cerebral edema, and subjects 1,6, and 12 received hyperosmolar therapy for suspected cerebral edema (Table 1). All 13 children were discharged home with Glasgow Coma Scale 15 and without gross neurological deficits. For transfers, all DKA treatment data were available and Time 0 reflects time of insulin start at outside hospital.
MRI Acquisition
The timing of MRI scans following initiation of DKA treatment (Time 0) varied due to consent, patient care and/or logistic issues: the first scan was obtained between 14 and 24 hours (mean 20 ± 1 hours) and the second MRI scan ranged from 40–58 hours (44 ± 2 hours), with at least 12 hours elapsing between first and second MRI scans (mean 23 ± 2; range 14–37 hours between scans).
The median time from serum osmolality (calculated) and acid base data to the first MRI scan was shorter (3 ± 3 hours [range 1–6] than for the time between these tests and the second MRI scan (7 ± 2 hours [range 4–8]), reflecting recovery and less frequent laboratory testing at the time of the second MRI scan. Median calculated serum osmolality was higher closer to time of the first MRI scan (329 ± 14 vs. 301 ± 5 mosm/mL; p < 0.01).
Whole Brain and Regional Mean Transit Time (Figure 1)
Figure 1.
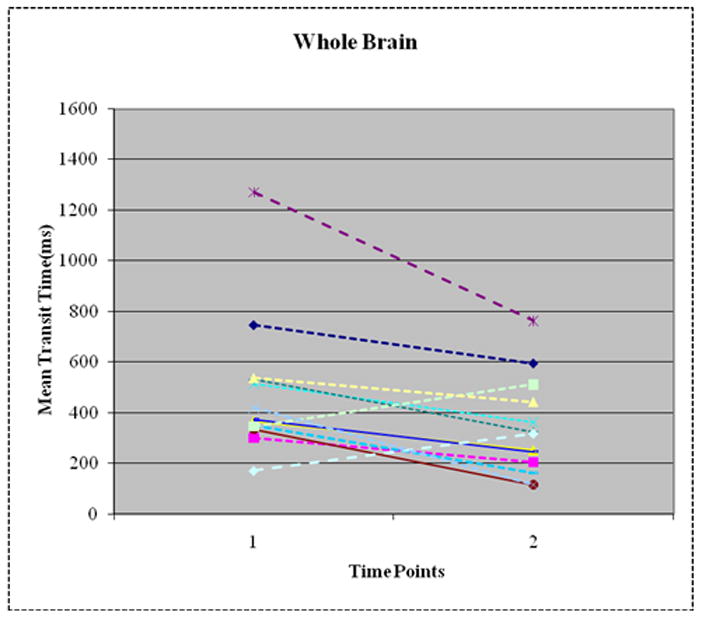
Decrease in Whole Brain Mean Transit Time (MTT) between Time 1 (14–24 hours after insulin start) and Time 2 (40–58 hours after insulin start) in 11/13 patients. Overall, whole brain MTT reduced by 50% (p=0.01). Frontal lobe, occipital lobe and basal ganglia show similar changes.
Eleven of the 13 subjects had lower whole brain MTT at Time 2 compared to Time 1, whereas 2 subjects had higher whole brain MTT. Mean decrease in MTT for the whole brain was 51± 59% (p=0.01). In general, MTT also decreased by approximately 50% from Time 1 to Time 2 in all 3 brain regions in the same 11 patients examined: frontal lobe (−51± 59%), occipital lobe (−51± 59%) and basal ganglia (−50± 59%; Figures 2A–D). There was no difference in the magnitude of change in MTT between the three brain regions (p = 0.98). The two subjects who had increases in MTT demonstrated a 32% and 46% increase, respectively.
Figure 2.
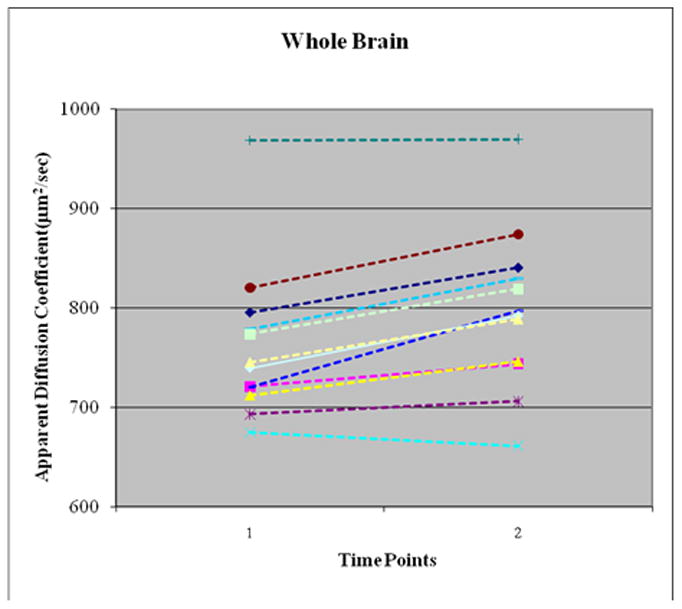
Increase in Whole Brain Apparent Diffusion Coefficient (ADC) between Time 1 (14–24 hours) vs. Time 2 (40–58 hours) after Insulin Start in 10/12 Subjects. Overall, whole brain ADC increased by 4.7% (p=0.001). Frontal lobe, occipital lobe and basal ganglia show similar changes.
Apparent Diffusion Coefficient (μm2/sec; Figures 2)
Data from one patient were excluded for technical reasons, leaving 12 subjects who contributed ADC data. Overall, whole brain ADC increased by 4.7 ± 3.4% (p=0.001) between Times 1 and 2. Eleven of 12 subjects experienced an overall increase in whole brain ADC and one patient showed a 2% lower ADC at Time 2. Regional increases in ADC ranged from 3.7 ± 3.0% (occipital lobe), 3.6 ± 4.6% (basal ganglia) and 2.8 ±3.3% (frontal lobe 2.7 ± 3.3) but these differences between the 3 brain regions were not significant (p = 0.35).
Whole Brain and Regional Relative Cerebral Blood Volume (mL/g tissue; Figure 3))
Figure 3.
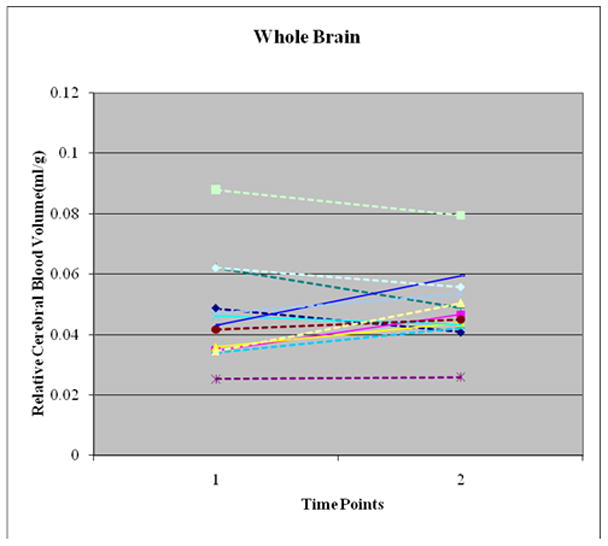
No Overall Change in Relative Cerebral Blood Volume (relCBV) between Time 1 (12–24 Hours after Insulin start) and Time 2 (36–72 hours after insulin start) in 13 Subjects. Seven patients had increase in whole brain relCBV while 6 patients had decrease in whole brain relCBV. Overall, the 9.7% increase in relCBV was not significant (p = 0.37). Frontal lobe, occipital lobe and basal ganglia show similar changes.
There was no overall significant difference in relCBV between Time 1 and Time 2 for the whole brain or for any of the 3 brain regions examined. The overall whole brain change in relCBV was 9.7 + 12% (p=0.37); 7/13 subjects had higher whole brain relCBV while 6 subjects had lower relCBV at Time 2 than Time 1. The regional changes in relCBV were 9.7 ± 22% (whole brain), 8.1 ± 20.2% (frontal lobe), 9 ± 22% (occipital lobe) and 8 ± 20% (basal ganglia).
DISCUSSION
The aim of this study was to characterize whole brain and regional cerebral changes in DKA using MTT, ADC, and relCBV to reflect the underlying pathophysiology that may play a role in cerebral injury during DKA treatment. The main findings of this study are that of the 13 children with DKA who were examined first between 14–24 hours and then between 40–58 hours during DKA treatment: 1) Most study participants had consistent and large decrease in whole brain (51%) and regional (50–59%) MTT, and 2) Most study participants had a small but consistent (2–5%) increase in whole brain and regional ADC. However, there was no change in whole brain or regional relCBV. Clinical features did not distinguish between the few study participants whose changes in changes in relCBV, MTT or ADC differed from the majority of participants or between subjects with new vs. an established diagnosis of Type I diabetes.
Significance of describing changes between 2 and 72 hours in DKA
In 2004, Glaser and colleagues examined ADC and MTT between DKA and recovery from DKA in 14 children (8). In this study, illness was defined as 2–12 hours and the recovery time period was defined as > 72 hours. This broad time interval for evaluation of cerebral changes allowed for identification of a larger longitudinal change with treatment and recovery and also for detection of effect. In 2008, Glaser et al (10), reported elevated ADC during illness compared with recovery and that the degree of edema formation during illness in children was correlated with the degree of dehydration and hyperventilation at presentation, but not with factors related to initial osmolality or osmotic changes during treatment. However, exclusion of middle time periods of evaluation in previous studies may have led to missing identification of important changes in cerebral pathophysiology, and missed opportunities to prevent and treat cerebral injury. Thus, we chose time periods to detail the natural history of cerebral changes in DKA and during DKA treatment in this middle (between 14 and 58 hours) time period, not examined in previous work. Thus, our findings cannot be directly compared Glaser’s data set but rather should add to their work and to our understanding of the progressive changes during DKA treatment (10) Since we too do not have data from multiple time points (for example, hourly) during DKA treatment, we cannot fully describe detailed changes in MTT, ADC and relCBV. However, based on previous work and the present study, we speculate that changes in MTT, ADC and relCBV may not be linear, and that very early in DKA, some degree of cerebral injury occurs which may increase and peak during DKA treatment (middle time period) before recovering beyond the 72 hour period. Therefore, it appears that relCBF (relCBV/MTT) is higher early in DKA compared to recovery but that it increases between the early and middle periods during DKA treatment before returning to normal, suggesting a vasogenic component (reperfusion injury) to cerebral injury and edema during the treatment phase of DKA in children. (10)
Significance of observed changes in MTT and ADC
The decrease in MTT and increase in ADC at Time 2 suggests two potential pathways involved in cerebral injury: decrease in CBV or increase in CBF. Since relCBV did not change, increases in CBF may account for the observed decrease in MTT during the study period. Moreover, the increase in ADC suggests an increase in brain water content between these two time periods. In children 8 to 13 years, ADC has been determined between 7.0 and 8.0 × 10−4 mm2/s (14) and in another study, ADC values of 8–9 × 10−4 mm2/s are reported for children between 12 and 60 months of life (15, 16) These values are generally comparable to what we presently report in children with DKA at both time points. However, there does appear to be an increase in ADC during pediatric DKA treatment and our findings reflect relative changes in MTT, CBV and ADC between the two time points examined.
Consequently, changes in CBF during DKA may account for the majority of changes in MTT and ADC observed during DKA treatment.. Our previous study suggests that increases in blood brain barrier permeability may also play a role in the development of cerebral edema but that magnitude of this change was smaller than the changes observed with MTT, suggesting that changes in CBF may be more contributory than changes in blood brain barrier permeability. (11) Dehydration decreased over time, as expected. We could not find published literature on this subject but if we consider cerebral autoregulation to be intact and the blood pressure were within the range of normal (aka not below the lower limit of autoregulation), then dehydration would not be expected to decrease MTT. We have reported a high prevalence of hypertension in children admitted with DKA that persists beyond the first 24 hours after start of insulin infusion (17) and although neurological deterioration during an episode of DKA is usually assumed to be caused by cerebral edema and cerebral vascular accidents, occult infarct from intracerebral hemorrhage has been described in a pediatric patient during DKA treatment. (18) The role of hypertension during DKA is unclear as it may be protective during intracranial hypertension to maintain cerebral perfusion. Conversely, if autoregulation is impaired during DKA, as has been suggested (7), hypertension can theoretically result in increased CBF. Our findings do not necessarily refute the classic teaching that idiogenic osmoles cause cytotoxic edema as serum osmolarity drops during treatment as both processes may coexist; rather it may be an issue as to which is the predominant mechanism during which time interval, which we are not able to answer. Although the precise mechanism remains elusive, collectively, the present findings suggest a possible vasogenic/reperfusion process that might lead to cerebral injury during this period of pediatric DKA treatment.
Significance of examining regional differences in MTT, ADC and relCBV
We previously reported significant regional differences in permeability ratio during a similar time interval, suggesting a greater and particular increase in blood brain permeability in the occipital lobe during DKA treatment. (11) We, therefore, reasoned that some brain areas may be more susceptible to DKA and its treatment and that similar regional differences might exist in relCBV, MTT and ADC during the middle time period. However, unlike blood brain barrier permeability, this study suggested that changes in both MTT and ADC were more consistent across the 3 brain regions we examined and reflected the magnitude of changes observed in the whole brain. While our findings regarding regional consistency in direction of changes in MTT are generally similar to that of Glaser and colleagues (8), unlike our study, Glaser’s work reported that the occipital lobe exhibited a decrease in ADC between 2–12 hours compared to > 72 hours after DKA start when compared to the frontal lobe, thalamus, basal ganglia and periaqueductal gray matter, where ADC significantly increased. The reasons for these contrasting ADC findings in the occipital lobe are unclear yet differences in study design (different time intervals), treatment strategies (administration of sedation, patient factors, and imaging factors) or other site-specific factors may be contributory. Finally, long term intellectual performance may be affected by early childhood onset of type 1 diabetes and history of DKA (18, 19) Mild central brain atrophy, larger ventricular volumes and significant differences in intellectual performance re reported in adults with early onset diabetes, implying that neurodevelopment may be adversely affected by early onset diabetes and that differences observed in brain structure may play a role in these observed outcomes. (19, 20) These data and our present study suggest that research is needed to examine how DKA and DKA treatment impact regional brain structure and function and long term outcomes.
Limitations of this study
The sample size is small, the age range is wide, and of the magnitude of changes in MTT, ADC and relCBV during DKA treatment may be overestimated or underestimated. While the timing of observations differs from earlier reports, we too only have two study times primarily because it was not feasible to obtain more than 2 MRI scans in each patient. We did not obtain these data from times earlier than 12 hours after insulin start and may have missed very early and pretreatment changes. Our time estimates may not be completely accurate given that some patients were transferred from outside facilities. We abstracted documented times from transfer record, suggesting that our estimates may approximate insulin treatment time. In the absence of baseline pre-morbid data, we acknowledge that we cannot comment on whether increase in ADC during treatment represents return of ADC to baseline. We cannot from this study, exclude the presence of cerebral ischemia prior to initiation of insulin treatment or during times periods earlier than examined. Three patients received mannitol prior to MRI scanning, which could have affected CBF and CBV. The reliability of our perfusion measurements may be questioned but is likely to be similar ot previously published studies and high (16, 21) MTT can be defined as the average time required for an individual particle of contrast agent to pass through the measurement volume. In general, it is inversely related to perfusion. In our measurements, MTT tended to decrease from time 1 to time 2 and this suggests that perfusion may have increased following treatment. However, some caution should be used in making this interpretation as the MTT can also be affected by other hemodynamic parameters. Therefore, our results should be considered preliminary, pending additional, more direct measurements of perfusion which are difficult to obtain and more prone to error. Pertaining to ADC, limitations of ADC diffusion are caused by artifacts in MR signal which can result from motion, metal, or susceptibility. Limitations for MR perfusion techniques to measure cerebral blood volume are caused by possible instability/drift of the time series MR signal that is used to calculate CBV.
Lastly, these data are observational. Despite these limitations, our data are new, important and increase knowledge of the changes in cerebral physiology during DKA treatment in children.
CONCLUSIONS
In summary, this study documents significant whole brain and regional changes in MTT and ADC, consistent with a vasogenic process beyond the early phase of DKA and during DKA treatment. We speculate that the “middle” (between 14 and 58 hours) time after insulin start may represent an interval where reperfusion and cerebral injury could occur in pediatric DKA.
Footnotes
Reprints: No reprints will be ordered.
Disclosure/Conflict of interest: This research was supported in part by NIHR21-HD044632 (MSV). None of the authors have financial interest in this research.
References
- 1.Rewers A, Chase HP, Mackenzie T, et al. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002;287:2511–2518. doi: 10.1001/jama.287.19.2511. [DOI] [PubMed] [Google Scholar]
- 2.Rewers A, Klingensmith G, Davis C, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. Pediatrics. 2008;121:e1258–66. doi: 10.1542/peds.2007-1105. [DOI] [PubMed] [Google Scholar]
- 3.Hanas R, Lindblad B, Lindgren F. Diabetic ketoacidosis and cerebral edema in Sweden, a 2-year population study (Abstract) Diabetes. 2004;53 (Suppl 2):A421. doi: 10.1111/j.1464-5491.2007.02200.x. [DOI] [PubMed] [Google Scholar]
- 4.Roche EF, Menon A, Gill D, et al. Clinical presentation of type 1 diabetes. Pediatr Diabetes. 2005;6:75–78. doi: 10.1111/j.1399-543X.2005.00110.x. [DOI] [PubMed] [Google Scholar]
- 5.Edge JA. Cerebral oedema during treatment of diabetic ketoacidosis: are we any nearer finding a cause? Diabetes Metab Res Rev. 2000;16:316–324. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr143>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Glaser NS, Barnett P, McCaslin I, et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. N Engl J Med. 2001;344:264–269. doi: 10.1056/NEJM200101253440404. [DOI] [PubMed] [Google Scholar]
- 7.Roberts JS, Vavilala MS, Schenkman KA, et al. Cerebral hyperemia and impaired cerebral autoregulation associated with diabetic ketoacidosis in critically ill children. Crit Care Med. 2006;34:2258–2259. doi: 10.1097/01.CCM.0000227182.51591.21. [DOI] [PubMed] [Google Scholar]
- 8.Glaser NS, Wootton-Gorges SL, Marcin JP, et al. Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr. 2004;145:164–171. doi: 10.1016/j.jpeds.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 9.Gass A, Niendorf T, Hirsch JG. Acute and chronic changes of the apparent diffusion coefficient in neurological disorders--biophysical mechanisms and possible underlying histopathology. J Neurol Sci. 2001;186(Suppl 1):S15–23. doi: 10.1016/s0022-510x(01)00487-7. [DOI] [PubMed] [Google Scholar]
- 10.Glaser NS, Marcin JP, Wootton-Gorges SL, et al. Correlation of clinical and biochemical findings with diabetic ketoacidosis-related cerebral edema in children using magnetic resonance diffusion-weighted imaging. J Pediatr. 2008;153:541–6. doi: 10.1016/j.jpeds.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Vavilala MS, Richards TL, Roberts JS, et al. Change in blood-brain barrier permeability during pediatric diabetic ketoacidosis treatment. Pediatr Crit Care Med. 2010;11:332–8. doi: 10.1097/PCC.0b013e3181c013f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaharchuk G. Theoretical basis of hemodynamic MR imaging techniques to measure cerebral blood volume, cerebral blood flow, and permeability. AJNR. 2007;28(10):1850–8. doi: 10.3174/ajnr.A0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostergaard L, Weisskoff RM, Chesler DA, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–25. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 14.Snook L, Paulson LA, Roy D, et al. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005 Jul 15;26(4):1164–73. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Hermoye L, Saint-Martin C, Cosnard G, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006 Jan 15;29(2):493–504. doi: 10.1016/j.neuroimage.2005.08.017. Epub 2005 Sep 27. [DOI] [PubMed] [Google Scholar]
- 16.Bonekamp D, Nagae LM, Degaonkar M, et al. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007 Jan 15;34(2):733–42. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeter K, Roberts JS, Pihoker C, et al. Hypertension Despite Dehydration in Pediatric Diabetic Ketoacidosis. Pediatric Diabetes. doi: 10.1111/j.1399-5448.2010.00695.x. (in-press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin JJ, Lin KL, Wang HS, et al. Occult infarct with acute hemorrhagic stroke in juvenile diabetic ketoacidosis. Brain Dev. 2008;30:91–3. doi: 10.1016/j.braindev.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson SC, Blane A, Wardlaw J, Frier BM, Perros P, McCrimmon RJ, Deary IJ. Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care. 2005;28:1431–7. doi: 10.2337/diacare.28.6.1431. [DOI] [PubMed] [Google Scholar]
- 20.Shehata G, Eltayeb A. Cognitive function and event-related potentials in children with type 1 diabetes mellitus. J Child Neurol. 2010;25:469–74. doi: 10.1177/0883073809341667. [DOI] [PubMed] [Google Scholar]
- 21.Luby M, Bykowski JL, Schellinger JG, et al. Intra- and Interrater Reliability of Ischemic Lesion Volume Measurements on Diffusion-Weighted, Mean Transit Time and Fluid- Attenuated Inversion, Recovery MRI. Stroke. 2006;37:2951–2956. doi: 10.1161/01.STR.0000249416.77132.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]


