Abstract
Background
Early studies indicate that prolongation of the interval between the peak and end of the T wave (Tpeak to Tend, TpTe) on the 12-lead ECG, is a marker of ventricular arrhythmogenesis. However, community-based studies have not been conducted.
Methods and Results
TpTe and other ECG predictors were evaluated in the ongoing Oregon Sudden Unexpected Death Study based in the Portland, Oregon metropolitan area using a case-control design. Cases had sudden cardiac death (SCD), and were compared to living controls with coronary artery disease (CAD) from the same region. Analysis of TpTe and selected ECG intervals was limited to sinus rhythm 12-lead ECGs; for cases, these were obtained prior to and unrelated to SCD. Independent-samples t tests and multiple logistic regression were used. Mean TpTe was significantly greater in cases [89.4 (87.7 – 91.2) ms, p <0.0001; n=353, 66.6 (65.1 – 68.1) yr, 67% male] vs. controls [76.1 (74.8 – 77.4) ms; n=342, 64.7 (63.4 – 66.0) yr, 69% male)]. The other ECG intervals [Corrected QT interval (QTc), QRS duration (QRSD), and TpTe/QT ratio) were also significantly prolonged among cases vs. controls (p≤0.01). TpTe remained a significant predictor of SCD after adjusting for age, gender, QTc, QRSD and left ventricular function. Odds of SCD increased more with a one standard deviation increase in TpTe (12 ms) among subjects with prolonged QRSD [OR 3.49, 95% CI (2.06-5.91)] than with a one SD increase in TpTe among subjects with normal QRSD [OR1.96, 95% CI (1.65-2.32)]. TpTe remained significantly associated with SCD in subjects with normal QTc.
Conclusions
Prolongation of the TpTe interval measured in lead V5 was independently associated with SCD, with particular utility when the QTc was normal or not measurable due to prolonged QRSD.
Keywords: death, sudden, coronary disease, population, risk factors, stratification, electrocardiogram
Introduction
Sudden cardiac death (SCD) claims 250,000 – 300,000 lives annually in the United States.1, 2 The majority of these SCDs are associated with significant coronary artery disease (CAD).3 Furthermore, SCD can be the first manifestation of coronary artery disease for many (up to 68% of younger subjects).4 Unfortunately, overall survival following out of hospital cardiac arrest remains below 5% even in countries with advanced first responder systems.5 Therefore innovations in interventional cardiology and device technology are ineffective for the majority of cases that are unable to reach the hospital alive. It is therefore critical to enhance risk stratification methodology for SCD and since the annual incidence is 53/100,000 population6 it becomes necessary to investigate SCD in the general community.7
We and others have previously highlighted the significant association between prolonged ventricular repolarization (measured as the corrected QT interval) and risk of SCD in the community.8, 9 However, these findings are not uniformly observed for all subjects that suffer SCD (some have normal QTc) and on occasion, the QTc is not measurable due to the presence of intraventricular conduction delay or block.
The interval from the peak of the T wave to the end of the T wave on the 12-lead ECG (Tpeak to Tend interval, TpTe) is a measure of transmural dispersion of repolarization in the left ventricle10-12 with prolongation of this interval representing a period of potential vulnerability to re-entrant ventricular arrhythmias.13, 14 Prolonged TpTe has been associated with increased risk of mortality in the congenital and acquired long QT syndromes,15 hypertrophic cardiomyopathy with troponin I mutations16 as well as patients undergoing primary PCI for myocardial infarction.17 However, there is no information available regarding the potential predictive ability of TpTe for SCD in the general population. We evaluated the association between TpTe and risk of SCD in the general population. We also hypothesized that TpTe is an effective risk marker among SCD subjects with normal QTc or un-measurable QTc.
Methods
Study participants
The Oregon Sudden Unexpected Death Study (Oregon SUDS) prospectively identifies cases of SCD from out of hospital cardiac arrests occurring among residents of the Portland, Oregon metropolitan region (population approximately 1,000,000).8, 18-22 SCD Cases were identified from the emergency medical response system, the medical examiner's office, and local emergency rooms. SCD was defined as a sudden unexpected, pulse-less condition of likely cardiac origin and survivors were also included. If un-witnessed, patients who were found dead within 24 hours of having last been seen alive and in a normal state of health, were also included. A diagnosis of SCD was based on a review of available medical records, circumstances of cardiac arrest, and autopsy records when available. Patients with terminal illness such as cancer and known non-cardiac causes of death such as pulmonary embolism, cerebrovascular accidents and drug overdose were excluded. Cases of SCD were compared to controls with coronary artery disease (CAD) from the same geographical area in the same time frame (2002 – 2009). We restricted the analysis to cases and controls ≥18 years of age, with availability of resting 12-lead ECGs in sinus rhythm.
Subjects were categorized as CAD if they had a ≥50% stenosis of a major coronary artery or history of myocardial infarction (MI), coronary artery bypass grafting, or percutaneous coronary intervention. Since ≥80% of subjects with SCD have been demonstrated to have significant CAD by autopsy,3 the control group was required to have significant CAD but no history of SCD. In order to include controls over the broad spectrum of CAD, these were identified from patients undergoing coronary angiography at one of the region's major participating health systems, or patients transported by the region's EMS system with complaints suggestive of coronary ischemia. Medical records for each potential control were reviewed to identify those with CAD. Left ventricular function was categorized as severely reduced (≤35% ejection fraction by echocardiogram, angiogram, or MUGA) or not severely reduced; for case subjects these tests were also required to be performed prior to and unrelated to the SCD event.
Measurement of TpTe, QT and QRS Intervals from the 12-Lead ECG
For SCD cases, the most recent ECG available in medical records, before and unrelated to the cardiac arrest was used. For the controls, an ECG prior to ascertainment was selected if available; if unavailable, an ECG post-ascertainment or peri-ascertainment was analyzed. We used a standard 12-lead ECG tracing at 25-mm/s paper speed and 10-mm/mV amplitude. QRSD was as reported on the ECG recording. Intraventricular conduction delay (IVCD) was defined as QRSD≥120 ms. Measurements of other ECG intervals were conducted manually using digital on-screen software (Datinf measure, Datainf Gmbh, Tübingen). TpTe was measured from the peak of the T wave to the end of the T wave. TpTe and QT intervals were measured in lead V5.23 If V5 was not suitable, lead V4 and V6 in that order were measured.17 The end of the T wave was defined as the intersection of the tangent to the down slope of the T–wave and the isoelectric line24 when not followed by a U wave or if distinct from the following U wave. If a U wave followed the T wave, the T-wave offset was measured as the nadir between the T and U waves. If the T wave amplitude was less than 1.5 mm in a particular lead, that lead was excluded from analysis. The QT interval was measured from the earliest onset of the QRS complex to the end of the T wave. After measurements in all precordial and limb leads, the longest QT interval was recorded. The TpTe/QT ratio was calculated as the ratio of TpTe in that lead to the corresponding QT interval.16 The QT interval was corrected for heart rate using Bazett's formula.25 ECG intervals were analyzed as continuous variables, and by use of gender-specific QTc categories (for men, normal QTc ≤430 ms; for women, normal QTc ≤450 ms).9 ECG readers were blinded to case status. The study was approved by the Institutional Review Boards of Cedars-Sinai Medical Center, Oregon Health and Science University and all participating hospitals and health systems.
Statistical Analysis
SPSS and SAS 9.1 statistical software were utilized. Independent-samples t tests and Pearson's chi-square tests were used for univariate case-control comparisons of continuous and categorical variables for all cases vs. controls, respectively. Additional, exploratory univariate analyses were conducted for two clinically relevant subgroups a) those with normal QTc according to the Rotterdam criteria (males with QTc ≤ 430 ms and females with QTc ≤ 450 ms) and b) those with prolonged QRSD due to IVCD. The rationale for these additional analyses is that the QTc value is normal in one group and not measurable in the other (since QRSD forms part of the QTc); we hypothesized that TpTe could be helpful for these two subgroups. Unadjusted odds ratio estimates were determined for the ECG intervals as well as LV systolic function. Multiple logistic regression models were used to estimate odds ratios (ORs) for SCD associated with TpTe interval, adjusted for variables that were significant in the univariate analyses. Odds ratios presented for TpTe are the estimated increase in odds of SCD associated with a one-standard-deviation (1-SD) increase in the interval, using the standard deviation among controls. In order to assess the predictive value of TpTe among subjects with normal vs. abnormal QTc and among subjects with or without IVCD, we evaluated possible interactions between TpTe and binary variables of QTc and QRSD. Since LV function values were available for approximately half of the subjects, this variable was categorized as: normal, severe LV systolic dysfunction (EF ≤ 35%) and LV function unknown. In addition, an ROC curve was produced for each ECG parameter for the entire group to compare each ECG parameter's ability to predict SCD. Models adjusted for co-morbidities (diabetes, obesity, hypertension, and sleep apnea) were also run. Finally, we also modeled the ECG parameters as categorical variables (prolonged TpTe >85ms, QRSD≥ 120ms and QTc >430ms for males and >450ms for females) adjusting for LVSD and co-morbidities. For all analyses, p value<0.05 was considered significant.
Results
Univariate analysis
All subjects
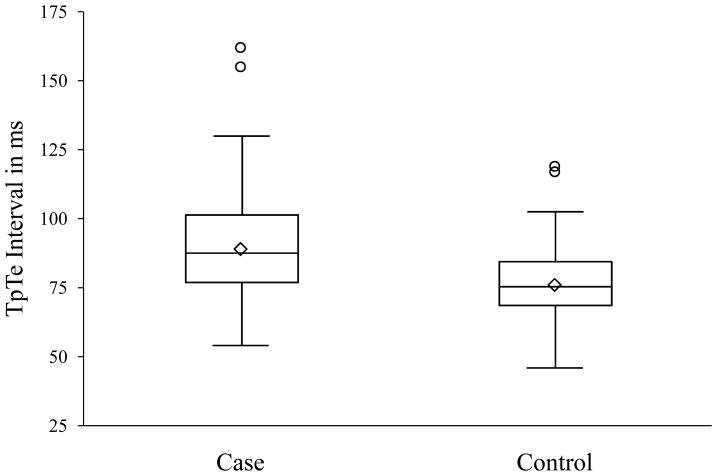
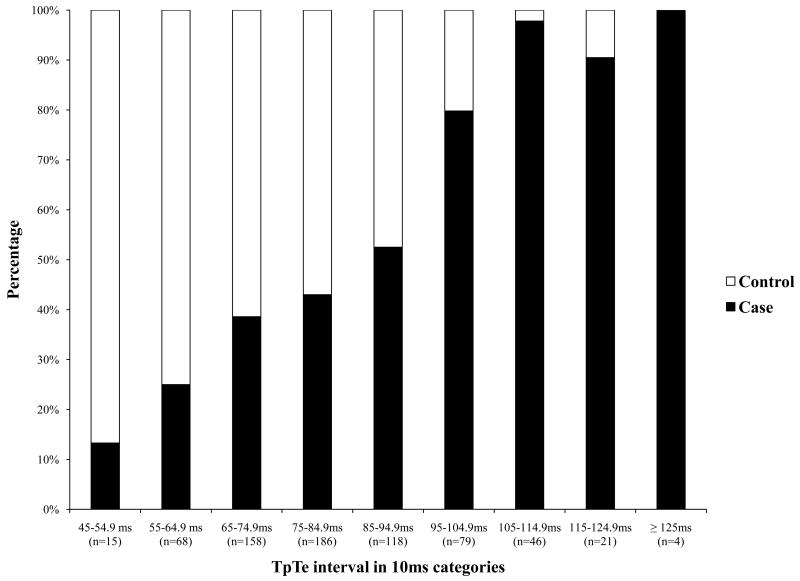
Among the total 353 cases and 342 controls that met criteria for analysis, age, gender, and history of co-morbidities were not significantly different (Table 1). TpTe was significantly associated with SCD with mean TpTe significantly prolonged in cases compared to controls (p< 0.0001; Table 1; Figure 1A). Evaluation of the distribution of TpTe in cases versus controls (Figure 1B) demonstrates that beyond TpTe >85 ms, 72% of subjects were cases; and >95 ms, ≥80% were cases. QTc, QRSD and TpTe/QT ratio were also significantly prolonged in cases compared to controls (Table 1). Unadjusted odds ratio estimates of significant factors are shown in Table 2.
Table 1. Patient characteristics and ECG measurements among subjects with SCD (cases) and without SCD (controls).
| Total subjects (n = 695) |
|||
|---|---|---|---|
| Categories | Cases (n = 353) |
Controls (n = 342) |
P value* |
| Age (yr) | 66.6 (65.1 – 68.1) | 64.7 (63.4 – 66.0) | 0.06 |
| Male | 237 (67.1%) | 236 (69.0%) | 0.60 |
| TpTe (ms) | 89.4 (87.7 – 91.2) | 76.1 (74.8 – 77.4) | < 0.0001 |
| Prolonged TpTe† | 193 (54.7%) | 75 (21.9%) | < 0.0001 |
| QTc (ms) | 447.4 (443.0 – 451.8) | 422.1 (418.3 – 425.8) | < 0.0001 |
| Prolonged QTc† | 208 (58.9%) | 106 (31.0%) | < 0.0001 |
| TpTe/QT | 0.22 (0.22 – 0.23) | 0.19 (0.19 – 0.19) | < 0.0001 |
| QRSD (ms)‡ | 100.5 (97.9 – 103.1) | 96.5 (94.5 – 98.4) | 0.01 |
| Prolonged QRSD† | 61 (17.3%) | 44 (12.9%) | 0.10 |
| Diabetes | 139 (39.4%) | 115 (33.6%) | 0.12 |
| Severe LVSD Missing§ |
52 (28.9%) 173 (49.0%) |
16 (11.6%) 204 (59.6%) |
0.0002 |
| Obesity Missing§ |
89 (32.0%) 75 (21.2%) |
133 (40.4%) 13 (3.8%) |
0.03 |
| Hypertension | 260 (73.6%) | 237 (69.3%) | 0.20 |
| Sleep apnea | 39 (11.0%) | 30 (8.8%) | 0.32 |
Results presented as n (%) or mean (95% CI).
p-value from Pearson chi-square test for categorical variables and t-test for continuous variables.
Prolonged TpTe defined as > 85 ms; prolonged QTc defined as > 430 ms for males and > 450 ms for females [16]; IVCD defined as QRSD≥ 120 ms; severe LVSD defined as ejection fraction ≤ 35%.
QRSD as reported on the ECG recording. TpTe and QT intervals were manually measured.
For variables with missing values, proportions and p-values are calculated using the non-missing data as the denominator.
LVSD, Left ventricular systolic dysfunction; QRSD, QRS duration; QTc, corrected QT interval; SCD, sudden cardiac death; TpTe, Tpeak to Tend interval.
Figure 1.
1A. Box plot of distribution of TpTe interval among cases and controls (TpTe, Tpeak to Tend interval) 1B. Distribution of TpTe interval among cases and controls Bar diagram showing relative clustering of cases towards the higher values of TpTe. Above the interval range 95-104.9ms of TpTe, ≥80% are cases.
(TpTe, Tpeak to Tend interval)
Table 2.
Unadjusted odds ratio estimates of SCD among entire study population.
| Categories | OR (95% CI) n = 695 |
|---|---|
| TpTe (1 SD increase) | 2.18 (1.87 – 2.53) |
|
| |
| QTc (1 SD increase) | 1.82 (1.57 – 2.12) |
|
| |
| QRSD (1 SD increase) | 1.17 (1.03 – 1.33) |
|
| |
| Normal LV function | Ref. |
| Severe LVSD | 3.10 (1.68 – 5.72) |
| LV function unknown | 0.81 (0.59 – 1.11) |
CI, Confidence Interval; LV, Left ventricle; LVSD, Left Ventricular Systolic Dysfunction; OR, Odds Ratio; QRSD, QRS duration; QTc, corrected QT interval; SD, Standard Deviation; SCD, Sudden Cardiac Death; TpTe, Tpeak to Tend interval.
Patients with normal QTc interval
Among patients with normal QTc interval by Rotterdam study criteria9 (n=145 cases and 236 controls), age, gender and presence of co-morbidities were not significantly different between cases and controls (p ≥ 0.12). In this sub-group, TpTe was significantly greater in cases versus controls [83.8 (81.4-86.1) ms vs. 74.5 (72.9-76.0) ms; p<0.0001]. TpTe/QT ratio was also significantly greater in cases versus controls (p<0.0001). Though all individuals in this group had a normal QTc, mean QTc remained significantly greater in cases vs. controls, with a smaller absolute case-control difference [411.3 (407.4-415.1) ms vs. 404.0 (401.1-406.8) ms; p=0.002]. QRSD was not associated with SCD risk in this subgroup (p=0.99).
Patients with intraventricular conduction delay/block
Among subjects with IVCD (61 cases and 44 controls), all ECG parameters (TpTe, QTc, QRSD, and TpTe/QT ratio) were significantly prolonged in cases vs. controls (p≤0.0005). TpTe was significantly greater in cases versus controls [98.6 (94.3-102.9) ms vs. 79.0 (75.5-82.6) ms; p<0.0001]. Age, gender and prevalence of co-morbidities were not significantly different (p≥0.13).
Multivariable odds ratio estimates
In a multiple logistic regression model for all cases and controls (n = 695), TpTe, QTc, QRSD and LVSD were included. There was no significant interaction between normal vs. prolonged QTc and TpTe (p=0.71). The interaction between normal vs. prolonged QRSD and TpTe was statistically significant (p=0.04) and the respective odds ratios of 1 SD increase in TpTe among these subgroups are presented in Table 3. Lack of significant interaction between TpTe and binary QTc shows that TpTe remains significantly associated with SCD in subjects with normal as well as prolonged QTc. With one standard deviation increase in the TpTe interval the increase in odds of SCD among subjects with IVCD [3.49 (95% CI 2.06-5.91)] was greater compared to the increase in odds associated with one standard deviation increase in TpTe among subjects with normal QRSD [OR 1.96 (95% CI 1.65-2.32)] (Table 3). Prolonged QTc [OR 2.08 (95%CI 1.45-2.96)] and severe LV dysfunction [OR 3.83 (95%CI 1.88-7.78)] were strongly associated with SCD in this group.
Table 3. Multivariable odds ratio estimates of SCD *.
| Categories | OR (95% CI) n = 695 |
|---|---|
| TpTe (1 SD increase) | |
| Among subjects with normal QRSD | 1.96 (1.65 – 2.32) |
| Among subjects with IVCD | 3.49 (2.06 – 5.91) |
|
| |
| Prolonged QTc vs. normal | 2.08 (1.45 – 2.96) |
|
| |
| Normal LV function | Ref. |
| Severe LVSD | 3.83 (1.88 – 7.78) |
| LV function unknown | 0.93 (0.65 – 1.33) |
Normal QTc defined as ≤ 430 ms for males and ≤ 450 ms for females [14]; IVCD defined as QRSD ≥120 ms; severe LVSD defined as ejection fraction ≤ 35%.
CI, Confidence Interval; LV, Left ventricle; LVSD, Left Ventricular Systolic Dysfunction; OR, Odds Ratio; QRSD, QRS duration; QTc, corrected QT interval; SD, Standard Deviation; SCD, Sudden Cardiac Death; TpTe, Tpeak to Tend interval.
Sensitivity analyses
While diabetes, obesity, hypertension, and sleep apnea were not strongly associated with SCD risk in univariate comparisons, we conducted additional analyses to determine whether they were potential confounders of the association between ECG parameters and SCD risk. TpTe remained a significant predictor of SCD after adjustment for all co-morbidities mentioned in Table 1 [OR 2.03 (1.70 – 2.42)]. In addition, we evaluated the predictive ability of TpTe using a cut-point of 85 ms (prolonged TpTe). In this separate model using the ECG intervals as categorical variables, a prolonged TpTe increased the risk of SCD [OR 3.53 (95% CI 2.41 – 5.18)] in all subjects.
Receiver operating characteristic (ROC)curves
We examined the predictive ability of each of the ECG parameters separately by plotting ROC curves (Fig. 2). The area under the ROC curve for TpTe was 0.73 (95% CI 0.69-0.77), which was significantly better (p=0.042) than the area under the curve for QTc (0.68 (95% CI 0.64-0.72) although these differences were observed at moderate sensitivity and specificity. The ROC curve for QRSD was not significantly different than 0.5, indicating that QRSD did not improve prediction of SCD beyond chance alone (0.53, 95% CI 0.49-0.57).
Figure 2.
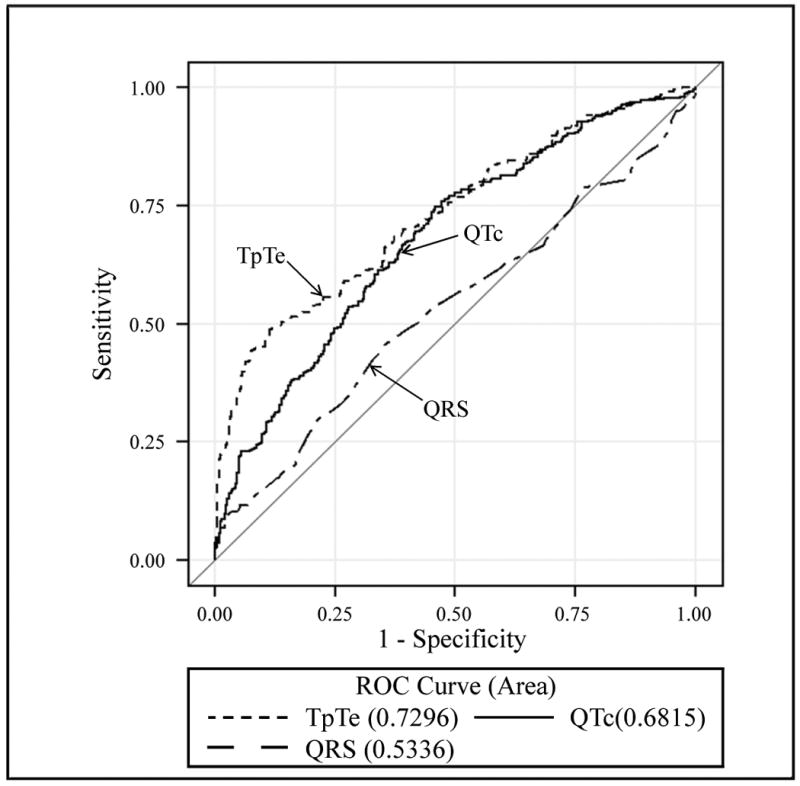
Receiver operating characteristic (ROC) curve for comparing TpTe, QTc and QRSD. The area of the ROC curves were 0.73 for TpTe, 0.68 for QTc and 0.53 for QRSD.
(TpTe, TpTe interval; QTc, QTc interval; QRSD, QRS duration).
Discussion
In this population-based study of SCD, TpTe interval on the resting 12-lead ECG was prolonged in SCD cases compared to control subjects and was significantly associated with SCD independently of age, gender, QTc, QRSD and LV dysfunction. Furthermore, TpTe was significantly associated with risk of SCD when QTc could not be employed for risk assessment, i.e. among SCD cases with normal QTc or prolonged QRSD due to intraventricular conduction delay/block. It is important to recognize that significant differences in the TpTe interval were identified between two groups of subjects that had significant heart disease (controls were also required to have significant CAD).
There are findings from the bench that link TpTe to mechanisms of ventricular arrhythmia. Using a canine myocardial wedge preparation model, Antzelevitch and co-workers have explored the genesis of TpTe as well as the potential mechanisms that link TpTe prolongation to increased risk of ventricular arrhythmogenesis. There are three electrophysiologically distinct cell types identifiable in the ventricular myocardium: the endocardial, epicardial and subendocardial M cells (Masonic mid-myocardial Moe cells).26, 27 During bradycardia or due to a repolarization-prolonging insult, the action potential of the M cells is more vulnerable to prolongation compared to the other two cell types,12 likely due to larger late sodium and sodium/calcium exchange currents and a weaker slowly activating delayed rectifier current (IKs).28 TpTe corresponds with transmural dispersion of repolarization in the ventricular myocardium, a period during which the epicardium has repolarized and is fully excitable, but the M cells are still in the process of repolarization and vulnerable to the occurrence of early after-depolarizations (EADs).29, 30 If conditions are favorable, these EADs can lead to reentry and its perpetuation, resulting in polymorphic VT or VF. Hence, a prolonged TpTe likely corresponds to an extended vulnerable period and given the right conditions, could increase risk of ventricular arrhthmogenesis.13, 31-33 Others have suggested that abnormal transmural dispersion of repolarization underlies ventricular arrhythmogenesis in Brugada syndrome,34 patients undergoing primary percutaneous intervention for acute myocardial infarction,17 hypertrophic cardiomyopathy with Troponin I mutations,16 short QT and long QT syndromes.15, 35 Lubinski et al. observed that patients with coronary artery disease and inducible VT had a higher TpTe (74 ± 14ms vs. 63 ± 16 ms; p < 0.004) and TpTe/QT ratio expressed as percentage (21 ± 4% vs. 17 ± 3%; p = 0.02) than subjects who were survivors of myocardial infarction without inducible VT.36 To our knowledge, the association between prolonged TpTe and SCD among subjects in the community is a novel finding.
What absolute value of TpTe should be considered abnormally prolonged? TpTe values greater than 100ms during acute myocardial infarction17 and >113ms in the setting of acquired bradyarrhythmias15 have been previously reported as “high risk” in the literature. In our study, the vast majority of subjects with a TpTe value >100ms (90.4%), were cases. Our findings are comparable with a smaller study of patients with acute myocardial infarction that evaluated overall mortality (not SCD) in which 10 out of 11 patients who died had TpTe >100ms.17 However, mean TpTe in our cases was lower, at 89.4 ± 16.9 ms (95% CI 87.7 – 91.2). In this study, TpTe value of ≥ 100ms had a specificity of 97%, but sensitivity was only 27%. Hence, the cut off for TpTe for SCD risk prediction in the community may be lower than values published earlier. This fact is supported from data by Lubinski et al36 who reported that mean TpTe interval observed in subjects with CAD and inducible VT was relatively low (74 ± 14 ms). However, further studies are needed for establishing cut offs for patients at risk of SCD in the community.
A disproportionate increase in transmural dispersion of repolarization relative to the total duration of repolarization (the TpTe/QT ratio) has also been found to have proarrhythmic effects.29 TpTe/QT stays relatively constant, in a narrow range of 0.17-0.23 across several species from guinea pig to the cow, may have a role in electrical stability of the myocardium29 and could be especially useful in eliminating effects of heart rate as well as inter-individual variations in QT interval.29 In normal healthy adults average TpTe/QT is 0.21 in the precordial ECG leads29 and has also been proposed as a marker of arrhythmogenesis for long QT syndrome35 and HCM with K183del mutation in the cTnI gene.16 In the present study, mean TpTe/QT was significantly greater among cases than controls in the entire study population and the subgroups analyzed.
Limitations
As is the case for QTc, the difficulty in locating T end when the T wave morphology is flat or multiphasic may affect TpTe measurements. However readers were blinded, any such errors would be unrelated to case status. Therefore, any bias introduced by this error would be unlikely to affect the validity of the findings. LV function assessment was available only in a subset. This is not unexpected for a community-based study, since 40-50% of patients suffer SCD as an unfortunate first manifestation of their illness, and are unlikely to have undergone cardiac investigations. Finally, because we conducted subgroup analyses, it is possible that our family-wise Type I error was somewhat inflated. However, application of a Bonferroni-corrected p-value of 0.01 (p=0.05 / 5 groups total) as the cut-off for statistical significance did not change any conclusions based on univariate or multivariable analyses.
Conclusions
TpTe was significantly and independently associated with increased odds of SCD among subjects with coronary artery disease. TpTe measurement may extend the value of repolarization beyond the QTc, particularly in situations where QTc is either normal or not valid due to prolongation of QRS duration. Prolonged TpTe has potential for enhancement of SCD risk stratification and warrants evaluation in additional, larger populations.
Acknowledgments
We would like to acknowledge the significant contribution of American Medical Response, Portland/Gresham fire departments and the Oregon State Medical Examiner's office
Funding Sources: Funded by National Heart Lung and Blood Institute R01HL088416, R01 HL088416-03S1 and R01 HL105170 to Dr Chugh. Dr Chugh is the Pauline and Harold Price Professor of Cardiac Electrophysiology at the Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA. Dr Panikkath is the recipient of the Asher Kimchi and Chun Hwang post doctoral fellowship award.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K, Jui J. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O'Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng ZJ. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adabag AS, Peterson G, Apple FS, Titus J, King R, Luepker RV. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159:33–39. doi: 10.1016/j.ahj.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corrado D, Thiene G, Pennelli N. Sudden death as the first manifestation of coronary artery disease in young people (less than or equal to 35 years) Eur Heart J. 1988;9:139–144. doi: 10.1093/eurheartj/9.suppl_n.139. [DOI] [PubMed] [Google Scholar]
- 5.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 7.Chugh SS. Early identification of risk factors for sudden cardiac death. Nat Rev Cardiol. 2010;7:318–326. doi: 10.1038/nrcardio.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Jui J. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–670. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 10.Taggart P, Sutton PM, Opthof T, Coronel R, Trimlett R, Pugsley W, Kallis P. Transmural repolarisation in the left ventricle in humans during normoxia and ischaemia. Cardiovasc Res. 2001;50:454–462. doi: 10.1016/s0008-6363(01)00223-1. [DOI] [PubMed] [Google Scholar]
- 11.Opthof T, Coronel R, Janse MJ. Is there a significant transmural gradient in repolarization time in the intact heart?: Repolarization Gradients in the Intact Heart. Circ Arrhythm Electrophysiol. 2009;2:89–96. doi: 10.1161/CIRCEP.108.825356. [DOI] [PubMed] [Google Scholar]
- 12.Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu DW. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991;69:1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- 13.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 14.Antzelevitch C, Shimizu W, Yan GX, Sicouri S. Cellular basis for QT dispersion. J Electrocardiol. 1998;30:168–175. doi: 10.1016/s0022-0736(98)80070-8. [DOI] [PubMed] [Google Scholar]
- 15.Topilski I, Rogowski O, Rosso R, Justo D, Copperman Y, Glikson M, Belhassen B, Hochenberg M, Viskin S. The morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmias. J Am Coll Cardiol. 2007;49:320–328. doi: 10.1016/j.jacc.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu M, Ino H, Okeie K, Yamaguchi M, Nagata M, Hayashi K, Itoh H, Iwaki T, Oe K, Konno T, Mabuchi H. T-peak to T-end interval may be a better predictor of high-risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol. 2002;25:335–339. doi: 10.1002/clc.4950250706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haarmark C, Hansen PR, Vedel-Larsen E, Pedersen SH, Graff C, Andersen MP, Toft E, Wang F, Struijk JJ, Kanters JK. The prognostic value of the Tpeak-Tend interval in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. J Electrocardiol. 2009;42:555–560. doi: 10.1016/j.jelectrocard.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Chugh SS, Uy-Evanado A, Teodorescu C, Reinier K, Mariani R, Gunson K, Jui J. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The Ore-SUDS (Oregon Sudden Unexpected Death Study) J Am Coll Cardiol. 2009;54:2006–2011. doi: 10.1016/j.jacc.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teodorescu C, Reinier K, Dervan C, Uy-Evanado A, Samara M, Mariani R, Gunson K, Jui J, Chugh SS. Factors associated with pulseless electric activity versus ventricular fibrillation: the Oregon sudden unexpected death study. Circulation. 2010;122:2116–2122. doi: 10.1161/CIRCULATIONAHA.110.966333. [DOI] [PubMed] [Google Scholar]
- 20.Reddy PR, Reinier K, Singh T, Mariani R, Gunson K, Jui J, Chugh SS. Physical activity as a trigger of sudden cardiac arrest: the Oregon Sudden Unexpected Death Study. Int J Cardiol. 2009;131:345–349. doi: 10.1016/j.ijcard.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinier K, Stecker EC, Vickers C, Gunson K, Jui J, Chugh SS. Incidence of sudden cardiac arrest is higher in areas of low socioeconomic status: a prospective two year study in a large United States community. Resuscitation. 2006;70:186–192. doi: 10.1016/j.resuscitation.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 23.Antzelevitch C, Sicouri S, Di Diego JM, Burashnikov A, Viskin S, Shimizu W, Yan GX, Kowey P, Zhang L. Does Tpeak-Tend provide an index of transmural dispersion of repolarization? Heart Rhythm. 2007;4:1114–1116. doi: 10.1016/j.hrthm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkiomaki JS, Koistinen MJ, Yli-Mayry S, Huikuri HV. Dispersion of QT interval in patients with and without susceptibility to ventricular tachyarrhythmias after previous myocardial infarction. J Am Coll Cardiol. 1995;26:174–179. doi: 10.1016/0735-1097(95)00122-g. [DOI] [PubMed] [Google Scholar]
- 25.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–357. [Google Scholar]
- 26.Antzelevitch C, S W, Yan GX. Electrical heterogeneity and the development of arrhythmias. In: Olsson SB, Yuan S, Amlie JP, editors. Dispersion of ventricular repolarization:state of the art. Armonk (NY): Futura Publishing Company; 2000. p. 3. [Google Scholar]
- 27.Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. The M cell. Circ Res. 1991;68:1729–1741. doi: 10.1161/01.res.68.6.1729. [DOI] [PubMed] [Google Scholar]
- 28.Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol. 2007;293:H2024–2038. doi: 10.1152/ajpheart.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, Yan GX. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567–574. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Brown BS, Wu Y, Antzelevitch C, Kowey PR, Yan GX. Blinded validation of the isolated arterially perfused rabbit ventricular wedge in preclinical assessment of drug-induced proarrhythmias. Heart Rhythm. 2006;3:948–956. doi: 10.1016/j.hrthm.2006.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antzelevitch C, Shimizu W, Yan GX, Sicouri S, Weissenburger J, Nesterenko VV, Burashnikov A, Di Diego J, Saffitz J, Thomas GP. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 32.Emori T, Antzelevitch C. Cellular basis for complex T waves and arrhythmic activity following combined I(Kr) and I(Ks) block. J Cardiovasc Electrophysiol. 2001;12:1369–1378. doi: 10.1046/j.1540-8167.2001.01369.x. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe N, Kobayashi Y, Tanno K, Miyoshi F, Asano T, Kawamura M, Mikami Y, Adachi T, Ryu S, Miyata A, Katagiri T. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol. 2004;37:191–200. doi: 10.1016/j.jelectrocard.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Castro Hevia J, Antzelevitch C, Tornes Barzaga F, Dorantes Sanchez M, Dorticos Balea F, Zayas Molina R, Quinones Perez MA, Fayad Rodriguez Y. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828–1834. doi: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi M, Shimizu M, Ino H, Terai H, Uchiyama K, Oe K, Mabuchi T, Konno T, Kaneda T, Mabuchi H. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin Sci (Lond) 2003;105:671–676. doi: 10.1042/CS20030010. [DOI] [PubMed] [Google Scholar]
- 36.Lubinski A, Kornacewicz-Jach Z, Wnuk-Wojnar AM, Adamus J, Kempa M, Krolak T, Lewicka-Nowak E, Radomski M, Swiatecka G. The terminal portion of the T wave: a new electrocardiographic marker of risk of ventricular arrhythmias. Pacing Clin Electrophysiol. 2000;23:1957–1959. doi: 10.1111/j.1540-8159.2000.tb07061.x. [DOI] [PubMed] [Google Scholar]