Abstract
The genetic engineering, host cell expression, purity, identity, and in vivo brain drug targeting properties are described for a new IgG-fusion protein, designated the cTfRMAb-AV fusion protein. Avidin (AV) is fused to the carboxyl terminus of the heavy chain of the genetically engineered chimeric monoclonal antibody (MAb) against the mouse transferrin receptor (TfR). The TfRMAb binds the endogenous TfR on the blood-brain barrier (BBB), which triggers transport into brain from blood. The cTfRMAb-AV fusion protein is produced in stably transfected Chinese hamster ovary cells, which are grown in serum free medium under conditions of biotin starvation. Following affinity purification, the purity and identity of the cTfRMAb-AV fusion protein was verified by electrophoresis and Western blotting. The affinity of the cTfRMAb for the murine TfR is high, KI = 4.6±0.5 nM, despite fusion of avidin to the antibody heavy chain. The model peptide radiopharmaceutical used in this study is the Aβ1-40 amyloid peptide of Alzheimer’s disease (AD), which in a brain-penetrating form could be used to image the amyloid plaque in brain in AD. The BBB transport and brain uptake of the [125I]-Aβ1-40 peptide was measured in mice injected intravenously (IV) with the peptide either free or conjugated to the cTfRMAb-AV fusion protein. The brain uptake of the free Aβ1-40 peptide was very low, 0.1 % of injected dose (ID)/gram brain following IV injection, and is comparable to the brain uptake of a brain blood volume marker. However, the brain uptake of the Aβ1-40 peptide was high, 2.1 ± 0.2 % ID/gram brain, following attachment of the biotinylated peptide to the cTfRMAb-AV fusion protein. Capillary depletion analysis showed the peptide penetrated the brain parenchyma from blood. The cTfRMAb-AV fusion protein is a new drug delivery system that can target to mouse brain mono-biotinylated peptide or antisense radiopharmaceuticals.
INTRODUCTION
Peptides and antisense agents are potential radiopharmaceuticals for imaging brain disorders. However, peptides and antisense agents do not cross the blood-brain barrier (BBB). Peptides and antisense agents can be re-formulated for brain penetration with BBB transport technology using molecular Trojan horses (MTH). A BBB MTH is an endogenous peptide or peptidomimetic monoclonal antibody (MAb) that binds an endogenous BBB receptor transporter, such as the insulin receptor or transferrin receptor (TfR), which triggers receptor-mediated transport into brain1. A peptide or protein drug, which is not normally transportable across the BBB, is genetically fused to the MTH, and the fusion protein is transported into brain via endogenous BBB transporters. However, it is often not feasible to engineer a fusion protein of the MTH and a peptide radiopharmaceutical. In addition, it is not possible to engineer a fusion protein of a MTH and an antisense radiopharmaceutical. An alternative strategy for linking the peptide or antisense imaging agent to the MTH is avidin-biotin technology. With this approach, a fusion protein is engineered that is comprised of avidin (AV) and the MTH. Prior work has described the genetic engineering of a fusion protein of avidin and a MAb to the human insulin receptor (HIR), and this fusion protein is designated HIRMAb-AV2. However, the HIRMAb-AV fusion protein cannot be validated in rodent models, because the HIRMAb only cross reacts with the insulin receptor of Old World primates3. There is no known MAb against the rat or mouse insulin receptor that can be used as a BBB MTH in rodents. Therefore, a surrogate MTH has been engineered for brain drug delivery in the mouse, which is a chimeric MAb against the mouse TfR, and designated the cTfRMAb4.
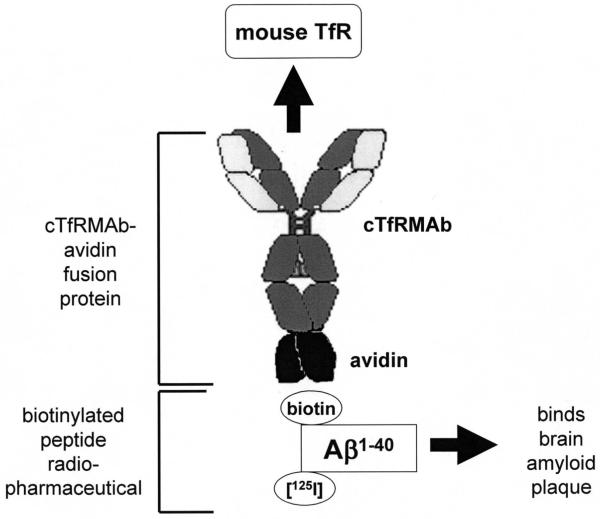
The present work describes the genetic engineering and eukaryotic host cell expression of a fusion protein of avidin and the cTfRMAb, designated the cTfRMAb-AV fusion protein. This IgG-avidin fusion protein is formed by fusion of the amino terminus of avidin to the carboxyl terminus of the heavy chain of the cTfRMAb (Figure 1). The model peptide radiopharmaceutical used in these studies is the Aβ1-40 amyloid peptide of Alzheimer’s disease (AD). The mono-biotinylated Aβ1-40 peptide is captured by the avidin moiety of the fusion protein (Figure 1). AD is caused by the formation of amyloid plaque in brain, and this plaque is comprised of the Aβ1-42/42 amyloid peptide5,6. Radiolabeled forms of the Aβ1-40 amyloid peptide are used to detect plaque in tissue sections in vitro of AD brain7. Owing to the self-assembly of amyloid plaque, the Aβ1-40 peptide radiopharmaceutical selectively associates with amyloid plaque in brain. This property makes the Aβ1-40 peptide a potential radiopharmaceutical for imaging the brain amyloid plaque burden in AD brain in vivo. However, the Aβ1-40 does not cross the BBB8. The present work describes the formulation of a brain penetrating peptide radiopharmaceutical, wherein the cTfRMAb-AV fusion protein is formulated in one vial, and the [125I, N-biotinyl]-Aβ1-40 peptide is formulated in a second vial. The vials are mixed just prior to intravenous administration in the mouse. The cTfRMAb-AV fusion protein is produced by stably transfected Chinese hamster ovary (CHO) cells under conditions of biotin depletion, and the N-biotinyl-Aβ1-40 is radio-labeled with the [125I]-Bolton-Hunter reagent.
Figure 1.
The cTfRMAb-AV fusion protein is formed by fusion of avidin to the carboxyl terminus of the heavy chain of the chimeric monoclonal antibody against the mouse transferrin receptor (cTfRMAb). The Aβ1-40 peptide radiopharmaceutical is biotinylated on the amino terminus of the peptide, to enable attachment to the cTfRMAb-AV fusion protein, and is radio-iodinated on internal lysine residues with the [125I]-Bolton-Hunter reagent. The cTfRMAb-AV fusion protein is formulated in one vial, and the [125I, N-biotinyl]-Aβ1-40 peptide is formulated in a second vial, and the two vials are mixed prior to intravenous administration in mice. The cTfRMAb part of the fusion protein delivers the Aβ1-40 peptide across the BBB, where the peptide then attaches to Aβ amyloid plaque, which forms in AD brain behind the BBB.
EXPERIMENTAL PROCEDURES
Production of CHO line
A tandem vector (TV) containing within a single plasmid DNA the expression cassettes encoding the cTfRMAb heavy chain-AV fusion protein, the cTfRMAb light chain (LC), and the murine dihydrofolate reductase (DHFR), was engineered similar to a TV described previously9. The cDNA encoding the 128 amino acid (AA) avidin protein was amplified by PCR as described previously2, and subcloned at the 3′-end of the cTfRMAb HC to form the pcTfRMAb-AV tandem vector. The structure of the TV has been described previously9. The sequence of the TV was confirmed by bi-directional DNA sequencing performed at Eurofins MWG Operon (Huntsville, AL) using custom sequencing oligodeoxynucleotides synthesized at Midland Certified Reagent Co. (Midland, TX). The TV was linearized and CHO cells were electroporated, followed by selection in hypoxanthine-thymine deficient medium and amplification with graded increases in methotrexate (MTX) up to 80 nM in serum free medium (SFM). The CHO line was dilutionally cloned at 1 cell/well, and high producing clones were selected by measurement of medium mouse IgG concentrations by enzyme-linked immunosorbent assay (ELISA). The CHO line was stable through multiple generations, and produced medium IgG levels of 5-10 mg/L in shake flasks at a cell density of 1-2 million cells/mL in serum free medium (Hyclone, Logan, UT, HYQ SFM4CHO-Utility, catalogue number SH30516.02), which contains 800 nM biotin.
The IgG-avidin fusion protein must be expressed in biotin-free medium. Otherwise, the expressed fusion protein would be fully saturated with biotin. Owing to the very slow dissociation of biotin from avidin10, it is very difficult to strip biotin from avidin. Therefore, IgG-avidin fusion proteins are expressed in CHO cells cultured in biotin-depleted medium2. Following propagation in biotin-containing medium, the CHO cells were centrifuged and resuspended in custom, biotin-free medium from Hyclone (HYQ SFM4CHO-Utility without biotin, catalogue number RR10659-01), which was supplemented with 20 nM biotin. The cells were expanded to a volume of 0.8L in shake flasks in medium containing 20 nM biotin, followed by expansion to 2.4L with biotin-free medium. Over the course of 4 weeks, a total of 7.7 L of conditioned medium was harvested and replaced by biotin-free medium. The medium pool was concentrated to 2 L with tangential flow filtration, clarified by depth filtration, and applied to a protein G column.
Protein G affinity purification
The medium was ultra-filtered with a 0.2 um Sartopore-2 sterile-filter unit (Sartorius Stedim Biotech, Goettingen, Germany), and applied to a 25 mL protein G Sepharose 4 Fast Flow (GE Life Sciences, Chicago, IL) column equilibrated in 25 mM Tris/25 mM NaCl/5 mM EDTA/pH=7.1. The column was washed with 25 mM Tris/1 M NaCl/5 mM EDTA/pH=7.1, and the fusion protein was eluted with 0.1 M glycine/pH=2.8. The acid eluate was pooled and neutralized to pH=6.0 with 1M Tris base, aliquoted and stored at −70C. Protein content was determined with the bicinchoninic acid (BCA) assay from Pierce-Thermo (Rockford, IL).
SDS-PAGE and Western blotting
The homogeneity of the cTfRMAb-AV fusion protein was evaluated with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing and non reducing conditions using 4-15 % and 7.5% Tris-HCl gels (Biorad Life Science, Hercules, CA), respectively. Western blot analysis was performed with a goat anti-mouse IgG (H+L) antibody (Bethyl Labs, Montgomery, TX) for the mouse IgG Western, and with a rabbit anti-avidin antibody (Abcam, Cambridge, MA) for the avidin Western. The immunoreactivity of the cTfRMAb-AV fusion protein was compared with the cTfRMAb described previously4 and with avidin (Pierce-Thermo).
TfR radio-receptor assay
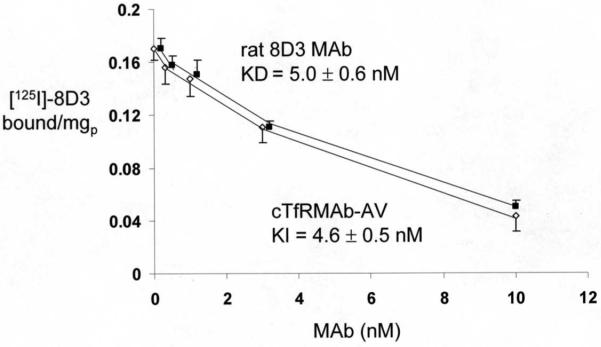
Binding of the cTfRMAb-AV fusion protein to the mouse TfR was measured with a radio-receptor assay (RRA) using the [125I]-labeled 8D3 rat MAb to the mouse TfR as the binding ligand, as described previously4. The 8D3 TfRMAb was purified from ascites with a protein G affinity column, and the antibody was radio-labeled with [125I]-iodine and chloramine T to a specific activity of 4.5 uCi/ug and a trichloroacetic acid (TCA) precipitability of 99%. Mouse 3T3 fibroblasts were plated on collagen coated BD Biocoat 24-well dishes (Becton-Dickinson, Franklin Lakes, NJ), and the binding assay was performed at 4C for 3 hours4. The [125I]-8D3 TfRMAb was added at a tracer concentration of 0.4 nM, and increasing concentrations of either unlabeled 8D3 TfRMAb, to measure the KD of binding, or the cTfRMAb-AV fusion protein, to measure the KI of binding, were co-mixed with the labeled 8D3 TfRMAb. The 8D3 self-inhibition study allowed for computation of the dissociation constant, KD, and the maximal binding, Bmax, and the cTfRMAb-AV fusion protein cross-inhibition study allowed for determination of the KI of binding to the mouse TfR9. Binding curves were fit by non-linear regression analysis using the BMDP2007e software (Statistical Solutions, Dublin, Ireland).
Biotin binding
The quantitative binding of biotin to the cTfRMAb-AV fusion protein was determined with an ultrafiltration assay, as described previously2. The assay used [3H]-biotin (Perkin-Elmer, Boston, MA), which had a specific activity of 50 uCi/nmol, and an Ultrafree-MC filter unit (Millipore, Billerica, MA), which has a low protein binding regenerated cellulose filter with a 10 kDa molecular weight cutoff. A 100 uL volume of PBS (0.01 M Na2HPO4/0.15 M NaCl/pH=7.4) was added to a microfuge tube (MFT), which contained 0.1 uCi/ml of 3H-biotin (2 nM), various concentrations of unlabeled biotin (25-1000 nM), and a fixed concentration of either avidin (5 ug/ml, 80 nM) or the cTfRMAb-AV fusion protein (15 ug/mL, 80 nM). After a 30 minute incubation at room temperature, the solution was transferred to the Ultrafree-MC ultrafiltration unit, which was placed in a 1.5 mL MFT, and the unit was centrifuged at 10,000 g for 5 minutes at room temperature. The total biotin binding was determined from the total biotin concentration and the percent binding of the [3H]-biotin, which was determined by sampling the ultrafiltrate for 3H radioactivity using a Perkin Elmer liquid scintillation counter. The number of biotin binding sites per protein was determined from the ratio of the bound biotin concentration relative to the concentration of the avidin or the cTfRMAb-AV fusion protein.
Cell biotin uptake assay
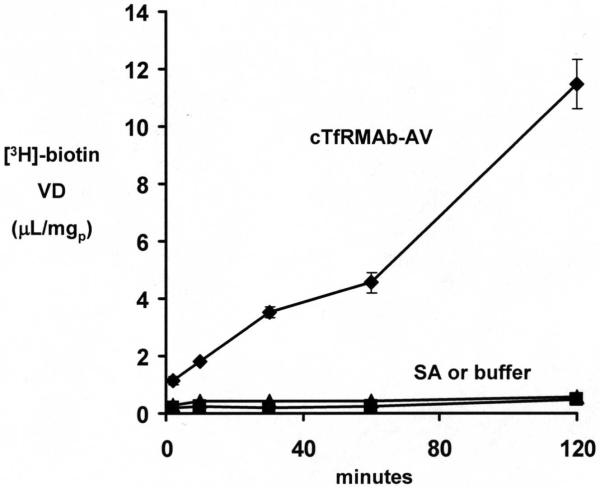
Mouse 3T3 fibroblasts (400,000 cells/well) were plated in 24 well dishes as described above, and cultured in DMEM medium with 10% fetal bovine serum. The incubation was initiated by aspiration of medium, washing with Tris-buffered saline (TBS), and addition of 400 uL/well of TBS (pH=7.4) containing 0.5 uCi/mL of [3H]-biotin, 0 or 1.0 ug/mL recombinant streptavidin (Sigma Chemical Co, St. Louis, MO), or 0 or 5.0 ug/mL cTfRMAb-AV fusion protein. The incubation at 37C was terminated at 2, 10, 30, 60, or 120 min by aspiration followed by washing of the monolayer 3 times with cold TBS. The monolayer was solubilized with 0.5 mL/well of 1 N NaOH, followed by heating at 60C for 30 min. The [3H] radioactivity was determined with a Perkin-Elmer liquid scintillation counter using Ultima-Gold (Downers Grove, IL), and the monolayer protein was determined with the BCA assay. The cell uptake was expressed as a volume of distribution (VD), which is equal to the (DPM/mg protein)/(DPM/uL medium).
Radio-iodination of N-biotinyl-Aβ1-40
The N-biotinyl-LC-Aβ1-40 amyloid peptide was purchased from AnaSpec, Fremont, CA (catalogue no. 24645-01), where LC=6 carbon long chain linker between the biotin and the peptide amino terminus. The N-biotinyl-Aβ1-40 peptide was radiolabeled with [125I]-Bolton-Hunter reagent from American Radiolabeled Chemicals (St. Louis, MO). The peptide was solubilized in 60% dimethylsulfoxide in water and 50 ug of peptide was reacted with 2.0 mCi of [125I]-Bolton-Hunter reagent. The labeled peptide was purified with a 1×28 cm Sephadex G-25 gel filtration column, with an elution buffer of 0.01 M Na2HPO4/0.15 M NaCl/pH=7.4/0.1% Tween-20. The N-biotinyl-Aβ1-40 peptide was labeled to a specific activity of 31 uCi/ug and a trichloroacetic acid (TCA) precipitability of >99%.
Pharmacokinetics and brain uptake in the mouse
Adult male C57BL/6J mice, weighing 24 grams, were obtained from Jackson Labs (Bar Harbor, ME). All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health. Mice in groups of 3 were anesthetized with intra-peritoneal (IP) ketamine (100 mg/kg) and xylazine (10 mg/kg), and injected intravenously (IV) in the tail vein with 60 uL (0.11 nmol/mouse, 15 uCi/mouse) of [125I, N-biotinyl]-Aβ1-40 either alone or co-mixed with the cTfRMAb-AV fusion protein. The injection dose (ID) of the cTfRMAb-AV fusion protein was 0.14 nmol/mouse, which is equivalent to 1 mg/kg of fusion protein. An aliquot (50 uL) of venous blood was collected at 0.25, 2, 5, 15, 30, and 60 min after injection by sampling the orbital vein. The blood was centrifuged for collection of plasma, which was analyzed for radioactivity. At 60 min after injection, the mice were euthanized and major organs (liver, spleen, kidney, heart, lung), and the cerebral hemispheres were removed and weighed for each mouse. One hemisphere was homogenized for capillary depletion analysis, as described below. The radioactivity of plasma and tissue samples was determined with a Wizard gamma counter (Perkin-Elmer, Downers Grove, IL). Organ uptake at 60 min after injection was expressed as % of injected dose (ID)/gram tissue. The plasma radioactivity that was precipitable with cold 10% TCA was determined at each time point.
The TCA-precipitable plasma radioactivity, DPM/mL, was converted to % injected dose (ID)/mL, and the %ID/mL was fit to a bi-exponential equation,
The intercepts (A1, A2) and the slopes (k1, k2) were used to compute the pharmacokinetic (PK) parameters, including the median residence time (MRT), the central volume of distribution (Vc), the steady state volume of distribution (Vss), the area under the plasma concentration curve (AUC) at 60 minutes, the steady state AUC (AUCss), and the systemic clearance (CL). Non-linear regression analysis used the AR subroutine of the BMDP Statistical Software. Data were weighted by 1/(%ID/mL)2.
The BBB permeability-surface area (PS) product is computed from the terminal brain uptake (%ID/g) and the 60 min plasma AUC (%IDmin/mL) as follows:
Capillary depletion method
One cerebral hemisphere from each mouse was processed for the capillary depletion method as described previously11, which separates the brain homogenate into the capillary pellet and the post-vascular supernatant. The volume of distribution (VD) was determined for each of the 3 fractions from the ratio of total 125I radioactivity in the fraction, DPM/gram brain, divided by the 125I radioactivity in the 60 min terminal plasma, DPM/uL. A high VD in the post-vascular supernatant, as compared to the VD in the capillary pellet, is evidence for transport of the protein through the BBB into brain parenchyma.
RESULTS
DNA sequencing of the cTfRMAb-AV expression plasmid encompassed 6,475 nucleotides (nt), which spanned 3 separate and tandem expression cassettes encoding the fusion protein of the cTfRMAb heavy chain (HC) and avidin, the cTfRMAb light chain (LC), and the murine DHFR selection gene. The LC was formed by 234 AA, including a 20 AA signal peptide, and had a predicted molecular weight of 23,554 Da and an isoelectric point (pI) of 5.73. The HC fusion protein was formed by 592 AA, including a 19 AA signal peptide, a 118 AA cTfRMAb HC variable region, a 324 AA mouse IgG1 constant (C) region, a 3 AA linker (Ser-Ser-Ser), and the 128 AA avidin, and had a predicted molecular weight of 63,375 Da and a pI of 7.64. The fusion HC contained 2 predicted N-linked glycosylation sites, with one site in the mouse IgG1 C-region and one site within the AV domain. The predicted molecular weight of the cTfRMAb-AV hetero-tetramer, without glycosylation, is 173,858 Da.
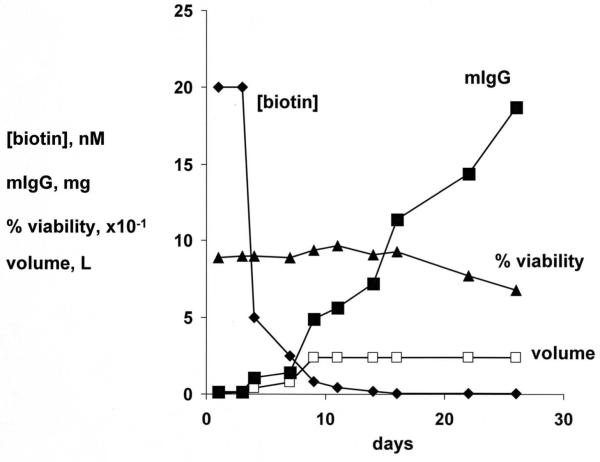
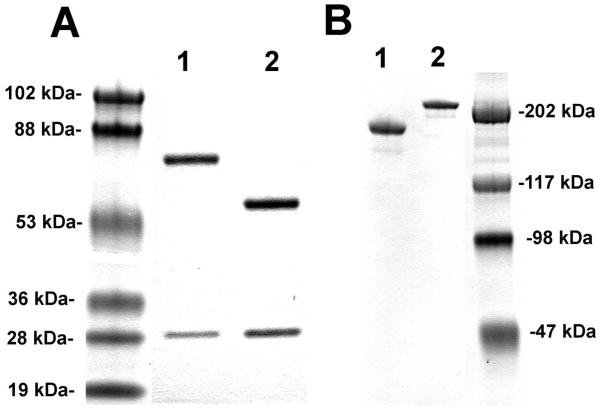
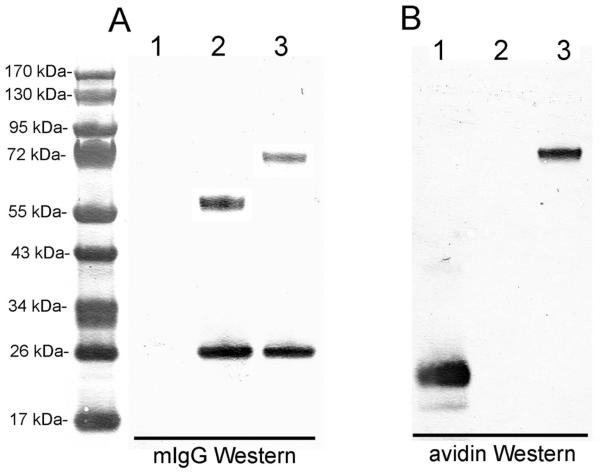
The CHO cells, stably transfected with the cTfRMAb-AV tandem vector, were propagated in serum free medium in 1 L flasks on an orbital shaker with a medium biotin concentration of 20 nM. Over the next 7 days, the medium was replaced with biotin-free medium until the medium biotin was <0.05 nM (Figure 2). The CHO cells were propagated for an additional 3 weeks in medium with <0.05 nM biotin, and during this time, the production of the cTfRMAb-AV fusion protein increased linearly (Figure 2). The cell viability was >90% for 3 weeks and then the viability decreased to 70% during the fourth week of biotin starvation (Figure 2). The cTfRMAb-AV fusion protein was affinity purified from the terminal, serum free conditioned medium by protein G affinity chromatography to homogeneity on reducing (Figure 3A) and non-reducing (Figure 3B) SDS-PAGE. The molecular weight (MW) of the cTfRMAb and the cTfRMAb-AV heavy and light chains was estimated by linear regression analysis based on the migration of the MW standards in Figure 3. The light chain of both proteins migrated at 28 kDa, while the heavy chain of the cTfRMAb and the cTfRMAb-AV fusion protein migrated at 59 kDa and 75 kDa, respectively, on the reducing SDS-PAGE (Figure 3A). The cTfRMAb and the cTfRMAb-AV fusion protein migrated at a MW of 181 kDa and 214 kDa, respectively, on the non-reducing SDS-PAGE gel (Figure 3B). The identity of the cTfRMAb-AV fusion protein was verified by mouse IgG and avidin Western blotting (Figure 4). An anti-mouse IgG primary antibody reacted equally well with the light chain of either the cTfRMAb or the cTfRMAb-AV fusion protein, but did not react with avidin (Figure 4A). An anti-avidin primary antibody reacted equally well with avidin or the heavy chain of the cTfRMAb-AV fusion protein, but did not react with the heavy chain of the cTfRMAb (Figure 4B).
Figure 2.
Medium biotin concentration, nM (◆); medium mouse IgG (mIgG) by ELISA, mg produced (■); medium volume, liters (□); and CHO cell viability, % (▲); over the course of 28 days in culture in serum free, biotin depleted medium.
Figure 3.
(A) Reducing SDS-PAGE of the cTfRMAb-AV fusion protein (lane 1) and the cTfRMAb (lane 2). (B) Non-reducing SDS-PAGE of the cTfRMAb (lane 1) and the cTfRMAb-AV fusion protein (lane 2).
Figure 4.
Western blot with primary antibody against mouse IgG (mIgG) (A) or avidin (B) of avidin (lane 1), cTfRMAb (lane 2), or cTfRMAb-AV fusion protein (lane 3).
The cTfRMAb-AV fusion protein retained high affinity binding to the mouse TfR, as the KI of fusion protein binding to the mouse TfR, 4.6 ± 0.5 nM, was no different from the KD of binding of the 8D3 rat MAb to the mouse TfR, 5.0 ± 0.6 nM (Figure 5). The biotin binding assay demonstrated that there was 1 and 4 free biotin binding sites per cTfRMAb-AV fusion protein and avidin molecule, respectively. The cellular uptake of [3H] biotin by mouse 3T3 fibroblasts was low in the presence of either buffer or recombinant streptavidin (SA), but was increased following co-mixing with the cTfRMAb-AV fusion protein (Figure 6).
Figure 5.
Binding of [125I]-labeled rat 8D3 MAb against the mouse TfR is either self-competed by unlabeled 8D3 MAb (■) or cross competed by unlabeled cTfRMAb-AV fusion protein (◇). Data are mean ± SE (n=4 per point).
Figure 6.
The cell/medium ratio, or volume of distribution (VD), of [3H]-biotin during a 120 min incubation in the presence of buffer, streptavidin (SA), or the cTfRMAb-AV fusion protein. Data are mean ± SE (n=4 per point).
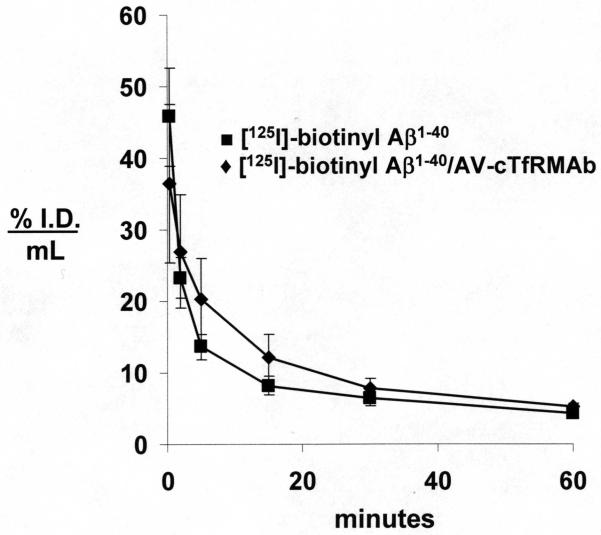
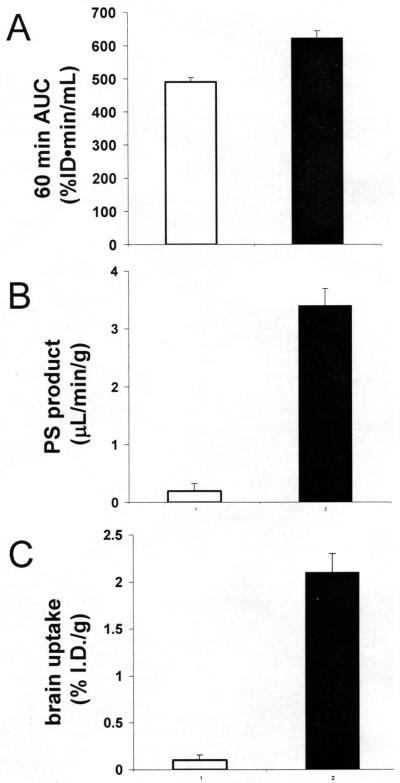
The rate of clearance of [125I, N-biotinyl]-Aβ1-40 from plasma in the mouse was comparable following IV injection of the peptide either with or without pre-mixing with the cTfRMAb-AV fusion protein (Figure 7). The PK analysis showed there was no difference in the systemic clearance (CL) of the labeled peptide with or without attachment to the cTfRMAb-AV fusion protein (Table 1). However, the organ uptake of the [125I, N-biotinyl]-Aβ1-40 was altered following co-injection with the fusion protein. Organ uptake by the kidney was suppressed, while organ uptake by brain, liver, spleen, and lung was increased following conjugation of the [125I, N-biotinyl]-Aβ1-40 to the cTfRMAb-AV fusion protein (Table 2). The brain uptake of the free [125I, N-biotinyl]-Aβ1-40 was nil, while there was a >20-fold increase in the brain uptake of the peptide following co-injection with the cTfRMAb-AV fusion protein (Figure 8C). The 60 min plasma AUC was comparable in mice injected with either the free [125I, N-biotinyl]-Aβ1-40 or with the conjugate of [125I, N-biotinyl]-Aβ1-40 bound to the cTfRMAb-AV fusion protein (Figure 8A). The marked increase in brain uptake of the peptide following attachment to the cTfRMAb-AV fusion protein was caused by an increase in the BBB permeability coefficient (Figure 8B).
Figure 7.
Plasma concentration, expressed as % I.D./mL, of [125I]-biotinyl Aβ1-40 following injection of labeled peptide either alone (■) or co-injected with 1:1 molar ratio of the cTfRMAb-AV fusion protein (◆). Data are mean ± SE (n=3 mice per point).
Table 1.
Pharmacokinetic parameters in the mouse
| parameter | units | [125I]-biotinyl Aβ1-40 | [125I]-biotinyl Aβ1-40/ cTfRMAb-AV |
|---|---|---|---|
| A1 | %ID/mL | 38.6 ± 3.5 | 23.2 ± 2.7 |
| A2 | %ID/mL | 10.1 ± 0.8 | 12.8 ± 1.9 |
| k1 | min-1 | 0.477 ± 0.059 | 0.195 ± 0.050 |
| k2 | min-1 | 0.014 ± 0.002 | 0.015 ± 0.003 |
| MRT | min | 64 ± 9 | 58 ± 11 |
| Vc | mL/kg | 81 ± 4 | 120 ± 8 |
| Vss | mL/kg | 308 ± 23 | 263 ± 29 |
| AUCss | %IDmin/mL | 797 ± 62 | 958 ± 91 |
| CL | mL/min/kg | 4.8 ± 0.4 | 4.5 ± 0.4 |
Parameters estimated from the plasma clearance data in Figure 7. The parameters are defined in Methods.
Table 2.
Organ uptake in the mouse of biotinyl-Aβ1-40 with and without conjugation to the cTfRMAb-AV fusion protein
| organ | [125I]-biotinyl Aβ1-40 | [125I]-biotinyl Aβ1-40/ AV-cTfRMAb |
|---|---|---|
| brain | 0.10 ± 0.06 | 2.1 ± 0.2 |
| heart | 1.6 ± 0.2 | 1.8 ± 0.3 |
| lung | 3.3 ± 0.5 | 9.9 ± 2.5 |
| kidney | 32.3 ± 3.8 | 15.8 ± 4.8 |
| spleen | 2.3 ± 0.5 | 29.7 ± 2.4 |
| liver | 18.1 ± 4.1 | 26.1 ± 4.3 |
Data are % ID/gram; mean ± SE (n=3 mice/group).
Figure 8.
Plasma AUC at 60 minutes after injection (A), BBB PS product (B), or brain uptake (C) of [125I]-biotinyl Aβ1-40 following injection of labeled peptide either alone (open bars) or co-injected with 1:1 molar ratio of the cTfRMAb-AV fusion protein (closed bars). Data are mean ± SE (n=3 mice per point).
The increase in brain uptake of the biotinyl-Aβ1-40 peptide following conjugation to the cTfRMAb-AV fusion protein represented distribution into brain parenchyma, as demonstrated by the capillary depletion method (Table 3). The VD in whole brain homogenate of the free biotinyl-Aβ1-40 peptide, 12 ± 2 uL/g, is no different from the brain plasma volume. Conversely, the VD in whole brain homogenate of the biotinyl-Aβ1-40 peptide conjugated to the cTfRMAb-AV fusion protein, 308 ± 2 uL/g, is 26-fold greater than the homogenate VD for the free biotinyl-Aβ1-40 peptide (Table 3). The VD of the conjugate in the post-vascular supernatant is 12-fold greater than the VD in the vascular pellet (Table 3), indicating >90% of the conjugate taken up by brain has distributed into brain parenchyma beyond the BBB. The TCA precipitability of the radioactivity in the post-vascular supernatant is 19 ± 2 % and 93 ± 1 % for the unconguagted biotinyl-Aβ1-40 peptide and for the biotinyl Aβ1-40 peptide conjugated to the cTfRMAb-AV fusion protein, respectively (Table 3).
Table 3.
Capillary depletion analysis of biotinyl-Aβ1-40 distribution in brain with and without conjugation to the cTfRMAb-AV fusion protein
| Parameter | [125I]-biotinyl Aβ1-40 | [125I]-biotinyl Aβ1-40/ AV-cTfRMAb |
|---|---|---|
| Homogenate VD | 12 ± 2 | 308 ± 9 |
| Post-vascular supernatant VD | 12 ± 2 | 141 ± 12 |
| Brain capillary pellet VD | 2.5 ± 0.5 | 12 ± 1 |
| TCA precipitation (%), 60 min post-vascular supernatant |
19 ± 2 | 93 ± 1 |
| TCA precipitation (%), 60 min plasma |
65 ± 3 | 76 ± 1 |
VD=volume of distribution (uL/g); TCA=trichloroacetic acid. Mean ± SE (n=3 mice/group).
The brain uptake of the conjugate of biotinyl-Aβ1-40 bound to the cTfRMAb-AV fusion protein declined with time after IV administration. The brain uptake at 6 hours after IV injection was 0.63 ± 0.08 % ID/gram (mean ± SE, n=4 mice), which is reduced 70% compared to the brain uptake of the targeted peptide at 1 hour after IV injection, 2.1 ± 0.2 % ID/gram (Figure 8C).
DISCUSSION
The results of this study are consistent with the following conclusions. First, the cTfRMAb-AV fusion protein is expressed well by stably transfected CHO cells cultured in serum free medium, and the cells can be maintained with high viability under conditions of biotin starvation for at least 4 weeks (Figure 2). Second, the affinity purified cTfRMAb-AV fusion protein is structurally intact with no degradation products or aggregation (Figures 3-4), and is a bi-functional protein, which both binds biotin (Figure 6) and the murine TfR with high affinity (Figure 5). Third, the cTfRMAb-AV fusion protein mediates the brain uptake of a model peptide radiopharmaceutical, biotinyl-Aβ1-40, and the brain uptake of the conjugate is on the order of 2% ID/gram brain (Figure 8).
Avidin, and avidin fusion proteins, have been produced in bacterial and baculovirus expression systems12,13, as well as mammalian host cells, such as myeloma cells14. However, cell culture medium contains very high concentrations of biotin (Methods), and any avidin fusion protein expressed in mammalian host cells cultured in high biotin medium will be secreted already complexed with biotin. Owing to the extremely high affinity of avidin binding of biotin, which is characterized by a KD of 10−15 M, and a dissociation half-time of 89 days15, it is difficult to separate biotin from avidin. Therefore, prior work on the expression of an IgG-AV fusion protein in CHO cells developed a 1-month biotin starvation protocol for production of biotin-depleted IgG-AV fusion protein by CHO cells2, and this method was used in the present work to produce the cTfRMAb-AV fusion protein. The biotin concentration was reduced from the maintenance level of 800 nM (Methods) to <0.05 nM (Figure 2). The failure to produce the IgG-AV fusion protein under conditions of biotin starvation will generate a biotin-loaded fusion protein with minimal capacity for binding biotinylated therapeutics.
The cTfRMAb-AV fusion protein is a hetero-tetrameric protein comprised of 2 heavy and 2 light chains (Figure 1). The fusion of avidin to the carboxyl terminus of each heavy chain of the cTfRMAb has no effect on the affinity of the cTfRMAb part of the fusion protein for the murine TfR, as shown by the cell-based radio-receptor assay in vitro (Figure 4). The retention of the high affinity of the cTfRMAb part of the fusion protein for the TfR predicts high brain uptake of the cTfRMAb-AV fusion protein, and this is observed (Figure 8C). The brain uptake (%ID/gram) of the conjugate of the [125I, N-biotinyl]-Aβ1-40 and the cTfRMAb-AV fusion protein, 2.1 ± 0.2 % ID/gram (Figure 8C), is equal to the brain uptake of the cTfRMAb4. The enhanced uptake in liver and spleen (Table 2) is due to the highly fenestrated capillary wall in these organs, which enables rapid distribution of the conjugate to the TfR on the parenchymal cells in liver and spleen. The suppression of uptake by kidney (Table 2) is due to the greater molecular size of the [125I, N-biotinyl]-Aβ1-40 following conjugation to the cTfRMAb-AV fusion protein.
The use of the cTfRMAb-AV fusion protein as a BBB drug delivery system has the potential to deliver to brain virtually any mono-biotinylated therapeutic, and this is demonstrated in the present study for the biotinylated Aβ1-40 amyloid peptide of AD. The cTfRMAb-AV fusion protein is formulated in one vial, and the [125I, N-biotinyl]-Aβ1-40 peptide radiopharmaceutical is formulated in a second vial. The two vials are mixed just prior to intravenous administration to form the conjugate shown in Figure 1. In the absence of the drug delivery system, the N-biotinyl-Aβ1-40 peptide does not penetrate the brain, owing to the lack of transport of the peptide across the BBB (Figure 8). The brain volume of distribution (VD) of the N-biotinyl-Aβ1-40, 12 ± 2 uL/gram (Table 3), or the brain uptake of the N-biotinyl-Aβ1-40, 0.10 ± 0.06 %ID/gram (Figure 8C), is no different than the brain VD, or brain %ID/gram, of a brain blood volume marker. The OX26 murine MAb against the rat TfR does not recognize the mouse TfR, and is a blood volume marker in the mouse brain16. The brain VD of the OX26 MAb in the mouse is 11 ± 1 uL/gram, and the brain uptake of the OX26 MAb in the mouse is 0.1 % ID/gram, and these parameters are equal to the brain uptake parameters of the N-biotinyl-Aβ1-40 amyloid peptide, which shows that the biotinylated peptide alone does not cross the BBB. However, following conjugation to the cTfRMAb-AV fusion protein, the brain uptake of the N-biotinyl-Aβ1-40 amyloid peptide increases to 2.1 ± 0.2 % ID/gram (Figure 8C). This level of brain uptake in the mouse is comparable to the brain uptake of lipid soluble small molecules. For example, the brain uptake in the mouse of lipid soluble, small molecule brain amyloid imaging agents is 2-3% ID/gram at 60 min after IV injection17, which is comparable to the brain uptake of the Aβ peptide radiopharmaceutical conjugated to the cTfRMAb-AV fusion protein (Figure 8C). The transport of the cTfRMAb-AV fusion protein across the BBB in the mouse in vivo results in distribution into brain parenchyma, as demonstrated by the capillary depletion method. The brain VD of the cTfRMAb-AV fusion protein in the post-vascular supernatant is 12-fold greater than the VD in the brain vasculature (Table 3), which indicates that 90% of the fusion protein bound by the vasculature in brain has transcytosed into brain parenchyma. The measurement of activity of brain capillary specific enzymes in the fractions produced with the capillary depletion method shows this technique results in a 95% removal of the microvasculature from the post-vascular supernatant18. The distribution of the conjugate radioactivity into the post-vascular supernatant was not an artifact caused by the brain uptake of TCA soluble metabolites, as the TCA precipitability of the radioactivity in the post-vascular supernatant of mice injected with the conjugate, 93 ± 1% (Table 3), was high compared to the TCA precipitability in the post-vascular supernatant of mice injected with the unconjugated [125I, N-biotinyl]-Aβ1-40 peptide, 19 ± 2% (Table 3).
The N-biotinyl-Aβ1-40 peptide used in these studies is mono-biotinylated. The biotin is incorporated at the amino terminus of the 40-amino acid amyloid peptide. It is important that the targeted therapeutic contain no more than 1 biotin moiety per molecule. Higher degrees of biotinylation could lead to aggregation of the complex, owing to the multi-valency of avidin binding of biotin. In the case of peptide or antisense radiopharmaceuticals, such as peptide nucleic acids19, a single biotin moiety can be incorporated at the level of solid phase synthesis.
The cTfRMAb-AV fusion protein is specific for the mouse, and is the homologue of the HIRMAb-AV fusion protein described previously for use in humans2. With regard to potential immune reactions in humans, the primary immunogenic component of the IgG-avidin delivery system is avidin. However, there are several factors that may reduce the potential immunogenicity of avidin in humans. The delivery agent is an IgG-avidin fusion protein, and the constant region of IgG contains amino acid sequences called Tregitopes, which induce T-cell immune tolerance20. Humans may be immune tolerant to avidin. Avidin is abundant in Western diets, as avidin is a major protein of egg whites, and oral antigen feeding induces immune tolerance21. Avidin has been administered to humans intravenously at high doses without immune reactions22,23. Finally, diagnostic agents are not intended for chronic use.
In summary, the present study describes the genetic engineering, CHO cell expression, purification, and validation of a novel IgG-avidin fusion protein that selectively targets the TfR in the mouse. The cTfRMAb-AV fusion protein can potentially deliver a wide variety of peptide radiopharmaceuticals to the brain of the mouse for in vivo imaging applications. Following re-formulation of peptide radiopharmaceuticals with the IgG-avidin fusion protein, the brain uptake of the large molecule brain imaging agents is comparable to the brain uptake of small molecule, lipid soluble brain imaging agents.
ACKNOWLEDGEMENT
Winnie Tai and Phuong Tram provided expert technical assistance. This work was supported by NIH grant R43-NS-062458.
LITERATURE CITED
- (1).Pardridge WM. Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug. Chem. 2008;19:1327–1338. doi: 10.1021/bc800148t. [DOI] [PubMed] [Google Scholar]
- (2).Boado RJ, Zhang Y, Xia CF, Wang Y, Pardridge WM. Genetic engineering, expression, and activity of a chimeric monoclonal antibody-avidin fusion protein for receptor-mediated delivery of biotinylated drugs in humans. Bioconjug. Chem. 2008;19:731–739. doi: 10.1021/bc7004076. [DOI] [PubMed] [Google Scholar]
- (3).Pardridge WM, Kang YS, Buciak JL, Yang J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm. Res. 1995;12:807–816. doi: 10.1023/a:1016244500596. [DOI] [PubMed] [Google Scholar]
- (4).Boado RJ, Zhang Y, Wang Y, Pardridge WM. Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol. Bioeng. 2009;102:1251–1258. doi: 10.1002/bit.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- (6).Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U. S. A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Maggio JE, Stimson ER, Ghilardi JR, Allen CJ, Dahl CE, Whitcomb DC, Vigna SR, Vinters HV, Labenski ME, Mantyh PW. Reversible in vitro growth of Alzheimer disease beta-amyloid plaques by deposition of labeled amyloid peptide. Proc. Natl. Acad. Sci. U. S. A. 1992;89:5462–5466. doi: 10.1073/pnas.89.12.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Saito Y, Buciak J, Yang J, Pardridge WM. Vector-mediated delivery of 125I-labeled beta-amyloid peptide A beta 1-40 through the blood-brain barrier and binding to Alzheimer disease amyloid of the A beta 1-40/vector complex. Proc. Natl. Acad. Sci. U. S. A. 1995;92:10227–10231. doi: 10.1073/pnas.92.22.10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Boado RJ, Zhou QH, Lu JZ, Hui EK, Pardridge WM. Pharmacokinetics and brain uptake of a genetically engineered bifunctional fusion antibody targeting the mouse transferrin receptor. Mol. Pharm. 2010;7:237–244. doi: 10.1021/mp900235k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wilchek M, Bayer EA. Applications of avidin-biotin technology: literature survey. Methods Enzymol. 1990;184:14–45. doi: 10.1016/0076-6879(90)84257-h. [DOI] [PubMed] [Google Scholar]
- (11).Zhou QH, Boado RJ, Lu JZ, Hui EK, Pardridge WM. Monoclonal antibody-glial-derived neurotrophic factor fusion protein penetrates the blood-brain barrier in the mouse. Drug Metab. Dispos. 2010;38:566–572. doi: 10.1124/dmd.109.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hytonen VP, Laitinen OH, Airenne TT, Kidron H, Meltola NJ, Porkka EJ, Horha J, Paldanius T, Maatta JA, Nordlund HR, Johnson MS, Salminen TA, Airenne KJ, Yla Herttuala S, Kulomaa MS. Efficient production of active chicken avidin using a bacterial signal peptide in Escherichia coli. Biochem. J. 2004;384:385–390. doi: 10.1042/BJ20041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Airenne KJ, Oker-Blom C, Marjomaki VS, Bayer EA, Wilchek M, Kulomaa MS. Production of biologically active recombinant avidin in baculovirus-infected insect cells. Protein Expr. Purif. 1997;9:100–108. doi: 10.1006/prep.1996.0660. [DOI] [PubMed] [Google Scholar]
- (14).Penichet ML, Kang YS, Pardridge WM, Morrison SL, Shin SU. An antibody-avidin fusion protein specific for the transferrin receptor serves as a delivery vehicle for effective brain targeting: initial applications in anti-HIV antisense drug delivery to the brain. J. Immunol. 1999;163:4421–4426. [PubMed] [Google Scholar]
- (15).Green NM. Avidin. Adv. Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- (16).Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J. Pharmacol. Exp. Ther. 2000;292:1048–1052. [PubMed] [Google Scholar]
- (17).Ono S, Cheng Y, Kimura H, Cui M, Kagawa S, Nishii R, Saji H. Novel 18F-labeled benzofuran derivatives with improved properties for positron emission tomography (PET) imaging of β-amyloid plaques in Alzheimer’s brains. J. Med. Chem. 2011;54:2971–2979. doi: 10.1021/jm200057u. [DOI] [PubMed] [Google Scholar]
- (18).Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J. Neurochem. 1990;54:1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
- (19).Suzuki T, Wu D, Schlachetzki F, Li JY, Boado RJ, Pardridge WM. Imaging endogenous gene expression in brain cancer in vivo with 111In-peptide nucleic acid antisense radiopharmaceuticals and brain drug-targeting technology. J. Nucl. Med. 2004;45:1766–1775. [PubMed] [Google Scholar]
- (20).De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, Scott DW, Martin W. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood. 2008;112:3303–11. doi: 10.1182/blood-2008-02-138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Weiner HL. Oral tolerance. Proc Natl Acad Sci U S A. 1994;91:10762–10765. doi: 10.1073/pnas.91.23.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Paganelli G, Magnani P, Zito F, Lucignani G, Sudati F, Truci G, Motti E, Terreni M, Pollo B, Giovanelli M, Canal N, Scotti G, Comi G, Koch P, Maecke HR, Fazio F. Pre-targeted immunodetection in glioma patients: tumour localization and single-photon emission tomography imaging of [99mTc]PnAO-biotin. Eur. J. Nucl. Med. 1994;21:314–321. doi: 10.1007/BF00947966. [DOI] [PubMed] [Google Scholar]
- (23).Samuel A, Paganelli G, Chiesa R, Sudati F, Calvitto M, Melissano G, Grossi A, Fazio F. Detection of prosthetic vascular graft infection using avidin/indium-111-biotin scintigraphy. J. Nucl. Med. 1996;37:55–61. [PubMed] [Google Scholar]