Abstract
Head and neck squamous cell carcinoma tumors (HNSCC) contain a dense fibrous stroma which is known to promote tumor growth, although the mechanism of stroma mediated growth remains unclear. As dysplastic mucosal epithelium progresses to cancer there is incremental overexpression of extracellular matrix metalloprotease inducer (EMMPRIN) which is associated with tumor growth and metastasis. Here we present evidence that gain of EMMPRIN expression allows tumor growth to be less dependent on fibroblasts by modulating fibroblast growth factor receptor-2 (FGFR2) signaling. We show that silencing EMMPRIN in FaDu and SCC-5 HNSCC cell lines inhibits cell growth, but when EMMPRIN-silenced tumor cells were co-cultured with fibroblasts or inoculated with fibroblasts into SCID mice, the growth inhibition by silencing EMMPRIN was blunted by the presence of fibroblasts. Co-culture experiments demonstrated fibroblast-dependent tumor cell growth occurred via a paracrine signaling. Analysis of tumor gene expression revealed expression of FGFR2 was inversely related to EMMPRIN expression. To determine the role of FGFR2 signaling in EMMPRIN silenced tumor cells, ligands and inhibitors of FGFR2 were assessed. Both FGF1 and FGF2 enhanced tumor growth in EMMPRIN silenced cells compared to control vector transfected cells, while inhibition of FGFR2 with blocking antibody or with a synthetic inhibitor (PD173074) inhibited tumor cell growth in fibroblast co-culture, suggesting the importance of FGFR2 signaling in fibroblast mediated tumor growth. Analysis of xenografted tumors revealed EMMPRIN silenced tumors had a larger stromal compartment compared to control. Taken together, these results suggest that EMMPRIN acquired during tumor progression promotes fibroblast independent tumor growth.
Keywords: Head and neck squamous cell carcinoma, stroma fibroblast, extracellular matrix metalloprotease inducer and fibroblast growth factor receptor
Introduction
Head and neck squamous cell carcinoma (HNSCC) develops from dysplastic mucosal epithelium and typically progresses to invasive islands of tumor cells. Transition to a highly invasive phenotype is thought to include a myriad of alterations including the upregulation of extracellular matrix metalloprotease inducer (EMMPRIN, also known as CD147) on the cell surface which promotes tumor-stromal signaling (1–3). EMMPRIN is a cell surface glycoprotein (4) that is overexpressed in malignant neoplasms with significant dyspastic reactions including bladder (5), lung (6), breast(7), and head and neck squamous cell carcinoma (HNSCC) (8, 9), but not in normal tissues (10). During the development of mucosal squamous cell carcinoma EMMPRIN expression gradually increases as cells develop from dysplastic lesions to carcinoma in situ to invasive cancer (8). These factors suggest that gain of EMMPRIN during carcinogenesis contributes to the malignant phenotype.
Elevated EMMPRIN expression levels correlate with tumor proliferation, angiogenesis, metastasis and invasion (11–13). Conversely, inhibition of EMMPRIN through genetic alterations or targeted inhibition in vivo results in inhibition of tumor growth (14–15). In fact, anti-EMMPRIN antibody has been studied as a potential therapeutic agent alone and in combination with conventional therapies in HNSCC (16, 17). Although the mechanism by which EMMPRIN promotes tumor growth is not fully understood, it has been demonstrated that EMMPRIN expression on the tumor cell surface stimulates surrounding fibroblasts and endothelial cells to secrete matrix metalloproteinases (MMPs) (18, 19) and vascular endothelial growth factor (VEGF) (20, 21). The release of these cytokines within the tumor microenvironment favors tumor metastasis, invasion and angiogenesis.
Fibroblast growth factors (FGFs) signal through FGF receptors (FGFRs) and were originally discovered regulating fundamental developmental pathways of multiple organ systems (22, 23). Consistent with other embryologically critical ligand-receptor pathways that are resurrected in malignant transformation, FGF signaling has been found to promote angiogenesis and mediate tumor and stroma communication during tumor progression via a paracrine feedback pathway (24–27). Although little is reported about FGFR in head and neck cancer, increased expression of the FGF2 receptor has been associated with disease progression (28–30).
Head and neck squamous cell carcinoma tumors contain tumor cells along with dense fibroblasts which are known to promote tumor growth (31). However, the mechanism of fibroblast mediated growth remains unclear. Although EMMPRIN is known to induce fibroblast expression of MMPs and various angiogenic stimuli (18–21), the effect of EMMPRIN on fibroblast enhanced tumor growth has not been characterized. In this study, we found that downregulation of tumor cell derived EMMPRIN inhibits cell proliferation as well as promotes fibroblast-dependent tumor growth. Our results suggest that EMMPRIN plays a role in fibroblast-dependent tumor growth by modulating FGF-FGFR signaling. Gain of EMMPRIN expression during tumor progression not only corresponds to promotion of tumor growth but also allows the tumor growth to be less dependent on fibroblasts.
Materials and Methods
Cell Culture and Reagents
FaDu (ATCC) and SCC-5 (University of Michigan) were grown in DMEM supplemented with 10% fetal bovine serum (FBS) and penicillin (100 U/mL), streptomycin (100 μg/mL). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. To obtain human normal dermal fibroblasts (NDFs), normal skin specimens were minced, washed in 70% ethanol followed by PBS, and then dried on six-well culture plates in triplicate for 30 minutes before the addition of culture media. Specimens were incubated for 21 to 28 days in DMEM supplemented with 20% fetal calf serum, penicillin (100 U/mL), streptomycin (100 μg/mL), and amphotericin B (2.5μg/L). Cells were then transferred by brief trypsinization (0.25% for 30 s with gentle agitation at room temperature) to six-well dishes and grown to confluence. Mouse normal fibroblasts were kindly provided by Dr. Tim Townes and cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and penicillin (100 U/mL), streptomycin (100 μg/mL). Mouse monoclonal anti-human EMMPRIN antibody and mouse IgG1 were purchased from biolegend (San Diego, CA). FITC conjugated mouse anti-human EMMPRIN along with its FITC isotype control (Mouse IgG1k-FITC), hamster anti- mouse H2K[d]-PE as well as its isotype IgG2ak-PE were obtained from BD Bioscience (San Jose, CA). Rabbit and mouse monoclonal anti-human FGFR2 antibodies and rabbit anti-human FGFR1 polyclonal antibodies were purchased from abcam (Cambridge, MA). Horseradish peroxidase-conjugated mouse monoclonal anti-human β-actin, Mouse monoclonal anti-human FGFR3 (B-9) antibodies were obtained from Santa Cruz biotechnology (Santa Cruz, CA). Mouse monoclonal agianst vimentin (V-9) antibody was purchased from Millipore (Billerica, MA).
Generation of EMMPRIN-silenced Fadu and SCC-5 cell lines
shRNA lentiviral particle delivery system was used to generate EMMPRIN-silenced tumor cell lines according to the manufacturer’s instructions (Clontech, Mountain View, CA). Briefly, two pairs of DNA oligo nucleotides (listed below) which specifically targeted EMMPRIN were separately cloned into pLVX-shRNA1 vector. The vectors with EMMPRIN shRNA were subsequently packaged into lentiviral particles. The lentiviral particles were then transduced into Fadu or SCC-5 cells. After selection under puromycin (1μg/ml), the drug resistant cells were assessed by flow cytometer for EMMPRIN expression and further confirmed by western blot.
DNA sequences used for targeting EMMPRIN:
SiE1: GCTGTGAAGTCGTCAGAACATTCAAGAGATGTTCTGACGACTTCACAGTTTTTTG
SiE2: GCAGCACCAGAATGACAAATTCAAGAGATTTGTCATTCTGGTGCTGCTTTTTTG
Tumor cell proliferation assay
Tumor Cell Proliferation
FaDu, SCC-5 or the cells with EMMPRIN silenced were seeded into 24 well plated at density of 104 cells/per well, after 72 hr culture, the cells were trypsonized. The cell numbers were counted by accuri flow cytometer.
Tumor cell proliferation in co-culture with fibroblast cells
Normal dermal fibroblast cells were first labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) following the manufacture’s instruction (Invitrogen, Carlsbad, CA). Briefly, normal dermal fibroblast cells were cultured in 100 mm cell culture dish until the cells reached confluence. Culture medium was replaced with prewarmed PBS containing 10 uM CFSE. After incubation at 37°C for 15 mins, the cells were washed with medium three times and cultured for additional 24 hrs. For tumor and fibroblast cell co-culture, 6.6×103 tumor cells were mixed with 3.3×103 CFSE-labeled fibroblast cells and then seeded into 24 well plates. After 96 hrs culture, the cells were trypsinized and analyzed using flow cytometer.
Tumor cell proliferation in transwell plate with fibroblast cells
24 well plates with 0.4 μm pore size transwell were used for the experiment. 6.6×103 tumor cells were placed on bottom compartment of well while 3.3 × 103 fibroblast cells were seeded in transwell. The cells were cultured for 96 hrs and then subjected to cell number analysis. All in vitro assays were performed a minimum of three times to confirm findings, and representative data is shown.
Western blot analysis
Cells were grown to 70%–80% confluence, washed twice with cold PBS, and lysed in lysis buffer [50 mM Tris-HCl(pH7.5), 150 mM NaCl, 1%(v/v) NP40, 0.5 % (w/v) sodium deoxycholate, 1 mM EDTA, 0.1%SDS], and a protease inhibitor cocktail tablet ( Roche Applied Science, Indianapolis, IN) was added. The cleared lysates were collected by centrifugation at 12000×g for 20 mins at 4°C. The protein concentrations were measured by BCA protein assay (Thermo Scientific, Rockford, IL). Lysates with 10 μg of total protein were resolved by SDS-PAGE and transferred to PVDF membranes. The membranes were incubated with the primary antibody. After washing and incubating with horseradish peroxidase-conjugated secondary antibodies, the membranes were washed again and detected by the Amersham ECL Western blotting detection system (GE healthcare, Buckinghamshire, UK). The membranes were reprobed with horseradish peroxidase-conjugated mouse monoclonal anti-human β-actin to ensure equal protein loading.
Assessing effect of FGFR2 inhibitors on fibroblast mediated tumor cell growth
Tumor cells were cultured with or without CFSE labeled normal dermal fibroblast cells as described above. Anti-FGFR2 antibody (20 μg/ml, Abcam, MA, clone MM0278-6L19), its isotype mouse IgG1 (Biolegend, CA) or 100 nM PD173074 (Sigma-Aldrich, MO) were used at in tumor cell culture with or without NDF. After 96 hrs culture, the cells were trypsinized and analyzed using flow cytometer.
Assessment of effect of FGF1 and FGF2 on tumor proliferation
2×103 tumor cells were seeded into 48 well tissue culture plate, 5 ng/ml FGF1 (Sigma-Aldrich, MO) or 5 ng/ml FGF2 (Sigma-Aldrich, MO) along with 10 μg/ml heparin (Sigma-Aldrich, MO) were used in cell culture. The cells were cultured for 96 hrs and then subjected to cell number analysis.
Examination of FGF1 and FGF2 expression in NDF and tumor cells
2×105 cells were cultured in 6-well plate for overnight. Then, the cells were cultured in serum free medium for 48 hrs. FGF1 or FGF2 concentration in the medium was measured using human FGF acidic and human FGF basic kits (R&D System, MN).
Assessment of tumor cell growth in vivo
Mice with severe combined immune deficiency (SCID, 8 week old females) were obtained from National Cancer Institute-Frederick (Frederick, MD) and maintained in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines of University of Alabama at Birmingham. Animals were inoculated s.c. on the right flank with 2×106 tumor cells or 1.5×106 tumor cells plus 0.5×106 normal dermal fibroblast cells in 0.2 ml serum free DMEM. Animals were followed for 4 weeks after the tumor cell inoculation. Tumor measurement were converted to a calculated tumor weight (in milligram) using the formula (width (mm)2 × length (mm))/2 (32).
Assessing tumor cells and host stromal cells in tumor tissue by flow cytometry and H&E stain
Fresh tumors were cut into a small piece and incubated with PBS with 2 mg/ml collagenase (Fisher, Scientific, Waltham, MA) at 37°C for 2 hrs to disaggregate cells. Dead cells and debris were removed by density centrifugation (800×g, 15 min) using Ficoll-Paque Plus. Interface cells were resuspended in Hanks’ buffer (containing 3% FBS) then incubated with antibodies to mouse CD16/CD32 (eliminating nonspecific binding), mouse H2k[d]-PE and human EMMPRIN-FITC, PI negative cells were electronically gated and then analyzed with respect to mouse H2k[d] and human EMMPRIN surface staining. The rest of tumor was fixed with formalin, subsequently embedded in paraffin and stained with hematoxylin and eosin as previously described (9). Tumors from each animal (n=7) underwent flow analysis and multiple sections were analyzed with similar results and representative data shown.
Assessment of effect of tumor cells with altered EMMPRIN on fibroblast migration
Cloning cylinders were put in 12-well tissue culture plate with 100 μl of type I collagen (4 mg/ml) in each well. The plate was then left in tissue culture hood with cover opened until collagen solution was dried and sealed the cylinder to bottom of the plate. 4 × 104 tumor cells with different level of EMMPRIN were placed inside of cylinders and 1 × 105 fibroblasts were cultured outside. After 24 hrs culture, both tumor cells and fibroblast grow to confluence and cylinders were then removed. A consistent gap appeared between tumor cells and fibroblasts. The cells were kept culture for additional 24 hrs. Alteration of gap represents the relative migration rate of cells. Tumor cell migration was also assessed as control.
Results
EMMPRIN regulates fibroblast-dependent tumor cell growth
To examine effect of EMMPRIN on fibroblast-dependent tumor cell growth, tumor cell EMMPRIN was genetically manipulated by lentiviral particle delivery system. Because HNSCC express high level of EMMPRIN, further exogenous overexpression was not performed and only the impact of EMMPRIN downregulation was assessed. HNSCC cells stably silenced for EMMPRIN expression (SiE1 and SiE2), as well as cells with vector as control (VC) transfected cells were generated from SCC-5 and FaDu cell lines. The EMMPRIN expression of these cells was verified by flow cytometry and Western blot (Fig. 1A and B). Loss of EMMPRIN expression resulted in decreased tumor cell proliferation (Fig. 1C). It is well established that tumor cells obtain a significant growth advantage from interaction with fibroblasts (33). To determine the role of EMMPRIN in fibroblast-dependent tumor cell growth, we performed a co-culture assay in which HNSCC cells silenced for EMMPRIN expression were mixed with carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled fibroblasts. As labeled fibroblasts emitted fluorescence, tumor cells and fibroblasts were able to be separated by flow cytometry and counted (Fig. 2A). Although either EMMPRIN silenced or control vector transfected tumor cells demonstrated an increased growth in response to fibroblasts, proliferation of SiE1 or SiE2 cells was more dramatic in the presence of fibroblasts (Fig. 2B). Co-culture of tumor cells with normal human dermal fibroblasts significantly accelerated growth of tumor cells with less EMMPRIN in vitro. In order to assess this phenomenon in vivo, EMMPRIN silenced tumor cells with and without fibroblasts were inoculated into the flank SCID mice. Normal dermal fibroblasts (tumor cell: fibroblast ratio, 3:1) accelerated growth in the EMMPRIN silenced cell lines (SiE1) to a far greater extent than control vector transfected cells (Fig. 2C). Similar results were also obtained from cells with SiE2 (data not shown). These findings suggest that silencing EMMPRIN promotes tumor growth sensitive to the presence of fibroblasts; gain of EMMPRIN accelerates tumor growth and allows the growth to be less dependent on stromal fibroblasts.
Figure 1.
Effects of EMMPRIN expression on Head and neck squamous cell carcinoma (HNSCC) proliferation. A. Confirmation of EMMPRIN silencing by flow cytometry. Two HNSCC cell lines, FaDu and SCC-5 were individually transduced by lentiviral particles carrying vectors with EMMPRIN shRNA SiE1, SiE2 or vector as control (VC). The cells were stained with FITC conjugated anti-human EMMPRIN antibodies. EMMPRIN expression was evaluated by flow cytometry. *, significant difference from control (VC). B. Verification of EMMPRIN expression by western blotting assay. Cell lysates from control cells as well as from EMMPRIN silenced cells were subjected to western blotting using antibody against EMMPRIN. β-actin was used as loading control. C. Effects of silencing EMMPRIN on HNSCC cell proliferation. The tumor cells with SiE1, SiE2 or VC were cultured for 72 hrs and then measured by Acuuri flow cytometry. Columns, mean for triplicate; bars, SD; p, SiE versus VC. The results presented are representative of three independent experiments.
Figure 2.
Effects of EMMPRIN expression on fibroblast mediated HNSCC cell growth. A. Assessment feasibility of a technique for investigating tumor cell growth in co-culture with fibroblasts. NHSCC and CFSE-labeled fibroblast cells were cultured separately as well as co-cultured together for 96 hr and then subject for flow cytometry analysis. The tumor cells or fibroblasts from co-culture were able to be separated by their different fluorescence intensity and counted individually by Acuuri flow cytometry. B. Assessment of influence of silencing EMMPRIN on fibroblast mediated tumor cell proliferation. HNSCC cells with EMMPRIN silenced or control vector were cultured alone or with human normal dermal fibroblasts (NDF). After 96 hr culture, the tumor cell numbers were measured by Acuuri flow cytometry. Columns, mean for triplicate; bars, SD; *, p<0.05 (+NDF versus −NDF); p, fold difference (+NDF/−NDF) of SiE cells versus fold difference (+NDF/−NDF) of VC cells. The data presented are representative of three independent experiments. C. Assessment of effect of silencing EMMPRIN on fibroblast mediated tumor cell growth in vivo. HNSCC cells with vector control as well as EMMPRIN silenced were inoculated into SCID mice alone or along with fibroblasts. Tumor sizes were measured. Average tumor size represents mean for tumors from 7 mice in each group. p, fold difference (+NDF/−NDF) of SiE1 tumors versus fold difference (+NDF/−NDF) of VC tumors.
Fibroblast enhanced tumor growth is mediated by paracrine signaling
To characterize the interaction between tumor cells and surrounding fibroblasts, tumor cell proliferation was measured in transwell chambers (Fig. 3). Tumor cell proliferation was similar regardless of whether fibroblasts were in direct contact with tumor cells (NDF(c)) or whether cells were separated within the chambers of the transwell system (NDF(s)), indicating that a soluble factor secreted from fibroblasts plays a role in enhancing tumor growth; the event was fulfilled by paracrine rather than by juxtacrine communication.
Figure 3. Characterization of fibroblast mediated tumor cell growth.
HNSCC cells with vector or silenced EMMPRIN were cultured alone (−NDF), in contact with fibroblasts (+NDF(c)) or with fibroblasts in different chambers (+NDF(s)). Cells were cultured for 96 hr, and cell numbers were acquired by Acuuri flow cytometry. Columns, mean for three triplicate; bars, SD. *, p<0.05 (+NDF(c) versus −NDF or +NDF (s) versus −NDF ). The data presented are representative of results in three independent experiments.
Down-regulation of EMMPRIN augments FGFR2 expression; and FGFR2 participates in fibroblast mediated tumor growth
To determine the possible molecular mechanisms by which fibroblasts mediate tumor cell growth in this context, gene expression profile of EMMPRIN silenced cells was compared with control vector transfected cells by gene microarray analysis (Supplementary Figure 1A). FGFR2 gene expression was elevated in EMMPRIN silenced cells. To confirm this, FGFR2 expression was assessed by western blot assay (Fig. 4A) and demonstrated that silencing EMMPRIN enhances FGFR2 expression. Importantly, FGF signaling through FGFRs has been reported to mediate tumor and stroma communication during tumor progression via a paracrine loop (34). To determine the significance of FGFR2 expression in fibroblast mediated HNSCC tumor growth, ligands and inhibitors of FGFR2 were assessed. Supplement of FGF1 (5 ng/ml) or FGF2 (5 ng/ml), a ligand of FGFR2 enhanced tumor growth in HNSCC cells (Fig. 4C); however, EMMPRIN silenced cells display more aggressive acceleration of growth in response to both FGF1 and FGF2. Importantly, both FGF1 and FGF2 were detected in conditioned media of NDF culture (Fig. 4D). Addition of FGFR2 blocking antibody to co-cultures of tumor cells and fibroblasts abrogated the increase seen in tumor cell proliferation (Fig. 4B). Although anti-FGFR2 antibody can block NDF-enhanced tumor cell growth, the incomplete abrogation of fibroblast-dependent growth suggests FGFR2 independent signaling pathways contribute or the blocking antibody did not completely saturate FGFR2 in the SiE2 cell line. Furthermore, PD173074 (35), a specific inhibitor for FGF-FGFR signaling (FGFR1, 2, and 3), similarly blocked the fibroblast mediated proliferation in the co-culture assay (Fig. 4E). Gene microarray data and Western Blot results demonstrated that FGFR1 and FGFR3 expression levels are much lower than FGFR2 in HNSCC and EMMPRIN stimulation does not affect these receptor expression of FGFR1 and 3 (Supplementary Figure 1). This suggests that PD 173074 suppresses the fibroblast mediated tumor cell proliferation primarily through blocking FGFR2 function. These results suggest that FGFR2 mediates fibroblast-dependent cell proliferation and as EMMPRIN expression is silenced, tumor cell growth is more sensitive to fibroblast promotion via up-regulated FGFR2 signaling.
Figure 4.
Effects of FGFR2 mediated signaling on fibroblast-dependent tumor cell proliferation. A. Effects of EMMPRIN on FGFR2 expression. FGFR2 expressions in EMMPRIN silenced cells and control cells were examined by western blot; β-actin was used as loading control. B. Effect of anti-FGFR2 antibody on fibroblast mediated tumor cell growth. Anti-FGFR2 antibody 20 μg/ml) or its isotype mouse IgG1 were used in tumor cell culture with or without NDF. *, p<0.05 (+NDF versus −NDF); **, p>0.05 (+NDF versus −NDF). C. Effect of FGF1 or FGF2 on tumor proliferation. FGF1 (5 ng/ml) or FGF2 (5 ng/ml) along with heparin (10 μg/ml) was used in cell culture. *, p<0.05 (+FGF versus −FGF); D. Examination of FGF1 and FGF2 expression in NDF and tumor cells. The cells were cultured in serum free medium for 48 hrs. FGF1 as well as FGF2 concentrations in the medium were then measured by ELISA. E. Confirmation of essential of FGF-FGFR signaling in fibroblast mediated tumor cell growth. PD173074 (100 nM), a specific inhibitor for FGF-FGFR signaling was added into SCC-5 cell culture or SCC-5 and NDF co-culture. The cells were cultures for 96 hrs and cell proliferation was checked by Acuuri flow cytometry. *, p<0.05 (+NDF versus −NDF); **, p>0.05 (+NDF versus −NDF). Columns, mean for triplicate; bars, SD. The data presented are representative of three independent experiments.
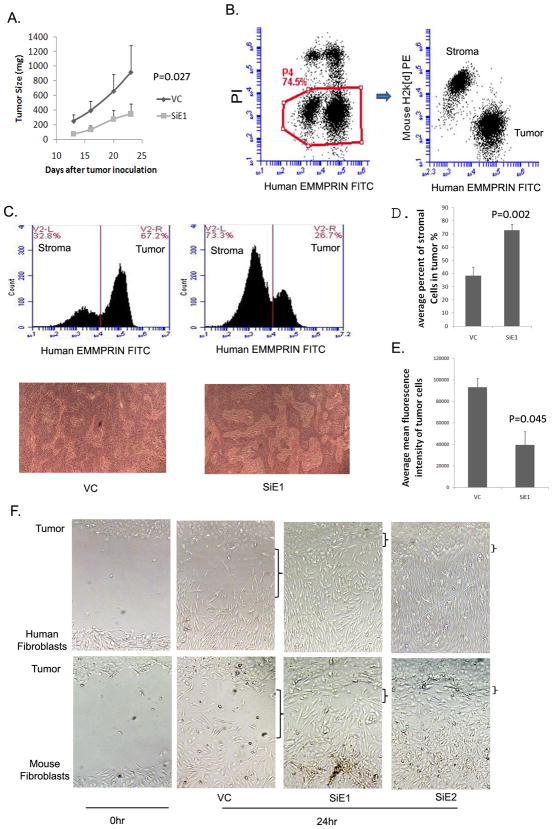
EMMPRIN silenced tumors are associated with a larger stromal compartment
If EMMPRIN expression compensates for fibroblast modulated enhancement of tumor proliferation, one would hypothesize that this stromal-independent growth would result in a smaller stromal component in cells with high levels of EMMPRIN expression. To this end, SCC-5 tumor cells were inoculated s.c. on a flank of SCID mice, and tumor growth was monitored. The EMMPRIN silenced cells grew at a slower rate (Fig. 5A). Xenografted tumors were harvested after 4 weeks of growth and assessed for mouse stromal elements as well as human tumor. Disaggregated tumor cells were stained with mouse H2k[d]-PE and human EMMPRIN-FITC antibodies. Tumor and stromal cell populations were separated by flow cytometry (Fig 5B). Although the EMMPRIN silenced tumors (SIE1) grew more slowly when measuring the entire tumor mass, they were associated with a significantly larger stromal compartment as measured by flow cytometry and histological analysis (Fig. 5C&D). Similar results were also obtained from cells with SiE2 (data not shown). Low EMMPRIN expression of silenced cells in vivo was confirmed (Fig. 5E). To determine if enlarged stromal compartments in EMMPRIN silenced tumors are the result stimulation of fibroblast migration, we evaluated the effects of EMMPRIN silenced tumor cells on migration of human and mouse fibroblasts. Tumor cells and fibroblasts were cultured atop type I collagen in different compartments separated by a cloning cylinder. When the cells grow to confluence, the cylinder was removed and a consistent gap appeared between tumor cells and fibroblasts. After a 24 hr culture, cell migration was examined (Fig. 5F). There was no apparent different migration between EMMPRIN silenced cells and control vector transfected cells during experiment period (data not shown), alteration of gap reflects the relative migration of fibroblasts. Migration of either human fibroblast cells or mouse fibroblast cells across the collagen matrix gap was enhanced by EMMPRIN silenced cells compared to control. The data suggest that EMMPRIN silenced tumor cells accelerates fibroblast migration, which may accounts for construction of larger stromal compartments inside the tumor.
Figure 5.
Effects of EMMPRIN on tumor stroma compartment. A. Silencing EMMPRIN reduced tumor growth in vivo. SCC-5 cells with vector or SiE1 were inoculated into flank of SCID mice. Tumor sizes were measured at indicated times. The data represent average sizes of tumors from 7 mice in each group. P, SiE1 versus VC. B. Analysis of both host stromal and tumor components by flow cytometry. Xenografted SCC-5 tumors isolated after 4 weeks of growth were disaggregated by collagenase degradation of extracellular matrix. The cells were stained with mouse H2k[d]-PE and human EMMPRIN-FITC. Live cells (PI negative) are either stained by Human EMMPRIN or mouse H2k[d] antibodies, clearly demonstrating tumor and host populations. C. Silencing EMMPRIN affects stromal compartment. In the upper panel, cells isolated from xenografted tumor were analyzed by flow cytometry. V2-L and V2-R display mouse stroma and human SCC-5 population respectively. Bottom panel displays H&E stain of xenografted tumor slides. The islands with spindle cells represent stroma compartment. The data presented are representative of 7 samples from each group. D. Statistic analysis of data from flow cytometry. Average percent of stromal cells were calculated from 7 mice of control or EMMPRIN silenced xenografted tumors. Bars, SD; p, SiE1 versus control. E. Confirmation EMMPRIN expression status in vivo. EMMPRIN expression was quantified by fluorescence intensity of tumor cells stained with anti-EMMPRIN FITC. Average mean fluorescence intensity represents data from 7 mice of control or EMMPRIN silenced xenografted tumors. EMMPRIN silenced SCC-5 cells still kept lower EMMPRIN level than control in vivo. Bars, SD; p, SiE1 versus control. F. EMMPRIN silenced tumor cells accelerated fibroblast migration. Tumor cells and fibroblasts were implanted in different chambers separated by a cloning cylinder. Consistent gap appeared between tumor cells and fibroblasts following removal of cylinders (left panel). Alteration of gap after 24 hour culture reflected fibroblast migration. The results presented are representative of three independent experiments.
Discussion
EMMPRIN has been shown to mediate communication between malignant cells and the surrounding stromal compartment. Either cell surface or soluble EMMPRIN is able to stimulate production of MMP1, MMP2, MMP3 and MT1-MMP (19, 36, 37) as well as cytokines VEGF, IL-1β, IL-6 and IL8 (16, 20, 21)from adjacent tumor cells as well as from surrounding fibroblast or endothelial cells. EMMPRIN mediated secretion of these factors contributes to a microenvironment suitable for tumor cell growth. However, there is a limited understanding of how fibroblasts promote growth in head and neck squamous cell carcinoma tumors. To this end, we evaluated the growth promoting effects of fibroblasts on tumor cells with variable EMMPRIN expression. In the absence of high EMMPRIN expression, tumor cell proliferation was very sensitive to fibroblasts, which we identified as mediated by FGFR2 signaling.
We and others have demonstrated the importance of EMMPRIN in tumor cell proliferation in vitro and in vivo (12–15). The mechanism for EMMPRIN-promoted tumor cell proliferation remains to be elucidated. It is reported that EMMPRIN associated with hyaluronan and CD44 enhances lactate efflux, resulting in promoting cell proliferation (38). It is also likely that autocrine signaling is involved or alternatively, that there are fibroblasts mixed within tumor cells. Although tumor cell lines can occasionally be contaminated with fibroblasts, the FaDu and SCC-5 cell lines did not demonstrate morphologically and immunohistochemical staining for vimentin suggest that these cell lines did not include fibroblast cell types (Supplementary Figure 2).
In the current study, we demonstrate that EMMPRIN expression promotes tumor cell growth in a fibroblast independent manner. Our data illustrate the role of EMMPRIN in tumor progression. It is well known that EMMPRIN expression increases as epithelial cells transition from normal to dysplastic to cancer (8). We hypothesize that at early stage of tumor progression, the EMMPRIN expression is low and the cells need support from surrounding fibroblasts for growth. The tumor cells display sensitivity to fibroblasts via upregulating FGFR2. As tumor progression occurs, higher levels of EMMPRIN allow tumor cells to be less dependent on stroma for their proliferation. However, tumor progression needs angiogenesis and metastasis. it is possible that increased EMMPRIN stimulates stromal fibroblasts to produce VEGF and MMP which create a favorable microenvironment for this to occur. As EMMPRIN expression increases along with tumor progress, elevated EMMPRIN enhances tumor growth and allows the growth to be less dependent on stroma; and meanwhile it stimulates fibroblast to produce MMP1, MMP2, MMP3 and MT1-MMPto facilitate tumor cell migration and invasion, allowing focus of crosstalk between tumor and stroma shifts from tumor growth to tumor metastasis.
Previous immunohistochemical studies of EMMPRIN expression in human tumors have demonstrated a mosaic pattern of expression throughout the tumor (9). It is likely that tumor growth is both FGFR2-dependent and FGFR2-independent within the tumor mass depending on the expression of EMMPRIN within individual islands of tumor cells. EMMPRIN likely continues to stimulate surrounding tumor cells to generate the appropriate microenvonment. Taken together, the results suggest that FGF2-FGFR2 signaling mediates fibroblast enhanced tumor cell proliferation; and up-regulation of EMMPRIN suppresses FGFR2 expression, leading to fibroblast independent growth. Although we and others have demonstrated that EMMPRIN promotes tumor growth, here we demonstrate in vivo and in vitro that EMMPRIN expression reduces the dependence of tumor cells on fibroblasts by modulating FGFR2 expression. In vivo experiments using knockdown EMMPRIN cells confirmed in vitro findings. Although it is possible that murine EMMPRIN could compensate in the in vivo experiments, because EMMPRIN is a membrane anchored protein with very low level of expression in fibroblasts, it is unlikely that murine EMMPRIN could compensate silenced human EMMPRIN.
It is well established that FGF-FGFR signaling is critical for tumor growth via working on tumor endothelial cells to promote tumor angiogenesis (24–27). The direct association of FGFR with tumor growth was also documented. Aberrant FGFR2 signaling, through overexpression of FGFR2 and/or its ligands, mutations, and receptor amplification, has been found in endometrial, ovarian, breast, lung, gastric, as well as head and neck cancers (39–44). Although fibroblast growth factor expression is known to be important in head and neck cancer (28–30, 44), the mechanism by which it influences tumor growth has not been characterized. Consistent with our findings, FGFR2 expression in advanced stage squamous cell carcinoma revealed that higher level of FGFR2 expression associated with tumor dysplasia and early invasion, compared to later stages of oral carcinogenesis (28). This suggests that FGFR2 plays an important role in the initial stages of oral cancer development where EMMPRIN expression is also low since our data demonstrate that EMMPRIN level is inversely related with FGFR2 expression. FGFR2 has also been demonstrated to be important in fibroblast modulation of the tumor microenvironment for esophageal carcinoma progression (45).
EMMPRIN has been identified as a therapeutic target in head and neck because it is highly expressed in malignant but not normal tissues and expression of EMMPRIN correlates with poor patient prognosis. Furthermore, preclinical data has shown that silencing EMMPRIN expression leads to reduced cell proliferation and tumor size. The current study reveals that prior to upregulation of EMMPRIN, tumor cells are more dependent on fibroblast initiated FGF2 - FGFR2 signaling and enlarged stroma compartment. Hence, potential head and neck therapies involving EMMPRIN silencing must involve combination treatments to overcome FGFR2 signaling or tumor associated fibroblast function.
Supplementary Material
Acknowledgments
Grant sponsors: This work was supported by the National Institutes of Health/National Cancer Institute grants 5R01CA142637, K08CA102154 (Rosenthal) and T32 CA091078 (Bland).
Abbreviation
- HNSCC
head and neck squamous cell carcinoma
- EMMPRIN
extracellular matrix metalloprotease inducer
- FGFR
fibroblast growth factor receptor
- FGF
fibroblast growth factor
- NDF
normal dermal fibroblast
References
- 1.Bordador LC, Li X, Toole B, Chen B, Regezi J, Zardi L, Hu Y, Ramos DM. Expression of EMMPRIN by oral squamous cell carcinoma. Int J Cancer. 2000;85:347–52. [PubMed] [Google Scholar]
- 2.Rosenthal EL, Zhang W, Talbert M, Raisch KP, Peters GE. Extracellular matrix metalloprotease inducer-expressing head and neck squamous cell carcinoma cells promote fibroblast-mediated type I collagen degradation in vitro. Mol Cancer Res. 2005;3:195–202. doi: 10.1158/1541-7786.MCR-04-0203. [DOI] [PubMed] [Google Scholar]
- 3.Cao Z, Xiang J, Li C. Expression of extracellular matrix metalloproteinase inducer and enhancement of the production of matrix metalloproteinase-1 in tongue squamous cell carcinoma. Int J Oral Maxillofac Surg. 2009;38:880–5. doi: 10.1016/j.ijom.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–9. [PubMed] [Google Scholar]
- 5.Als AB, Dyrskjøt L, von der Maase H, Koed K, Mansilla F, Toldbod HE, Jensen JL, Ulhøi BP, Sengeløv L, Jensen KM, Orntoft TF. EMMPRIN and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13:4407–14. doi: 10.1158/1078-0432.CCR-07-0109. [DOI] [PubMed] [Google Scholar]
- 6.Sidhu SS, Nawroth R, Retz M, Lemjabbar-Alaoui H, Dasari V, Basbaum C. EMMPRIN regulates the canonical Wnt/beta-catenin signaling pathway, a potential role in accelerating lung tumorigenesis. Oncogene. 2010;29:4145–56. doi: 10.1038/onc.2010.166. [DOI] [PubMed] [Google Scholar]
- 7.Yang JM, O’Neill P, Jin W, Foty R, Medina DJ, Xu Z, Lomas M, Arndt GM, Tang Y, Nakada M, Yan L, Hait WN. Extracellular matrix metalloproteinase inducer (CD147) confers resistance of breast cancer cells to Anoikis through inhibition of Bim. J Biol Chem. 2006;281:9719–27. doi: 10.1074/jbc.M508421200. [DOI] [PubMed] [Google Scholar]
- 8.Vigneswaran N, Beckers S, Waigel S, Mensah J, Wu J, Mo J, Fleisher KE, Bouquot J, Sacks PG, Zacharias W. Increased EMMPRIN (CD 147) expression during oral carcinogenesis. Exp Mol Pathol. 2006;80:147–59. doi: 10.1016/j.yexmp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal EL, Shreenivas S, Peters GE, Grizzle WE, Desmond R, Gladson CL. Expression of extracellular matrix metalloprotease inducer in laryngeal squamous cell carcinoma. Laryngoscope. 2003;113:1406–10. doi: 10.1097/00005537-200308000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Riethdorf S, Reimers N, Assmann V, Kornfeld JW, Terracciano L, Sauter G, Pantel K. High incidence of EMMPRIN expression in human tumors. Int J Cancer. 2006;119:1800–10. doi: 10.1002/ijc.22062. [DOI] [PubMed] [Google Scholar]
- 11.Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193–9. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- 12.Newman JR, Bohannon IA, Zhang W, Skipper JB, Grizzle WE, Rosenthal EL. Modulation of tumor cell growth in vivo by extracellular matrix metalloprotease inducer. Arch Otolaryngol Head Neck Surg. 2008;134:1218–24. doi: 10.1001/archotol.134.11.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voigt H, Vetter-Kauczok CS, Schrama D, Hofmann UB, Becker JC, Houben R. CD147 impacts angiogenesis and metastasis formation. Cancer Invest. 2009;27:329–33. doi: 10.1080/07357900802392675. [DOI] [PubMed] [Google Scholar]
- 14.Baba M, Inoue M, Itoh K, Nishizawa Y. Blocking CD147 induces cell death in cancer cells through impairment of glycolytic energy metabolism. Biochem Biophys Res Commun. 2008;374:111–6. doi: 10.1016/j.bbrc.2008.06.122. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Lin J, Kanekura T, Su J, Lin W, Xie H, Wu Y, Li J, Chen M, Chang J. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res. 2006;66:11323–30. doi: 10.1158/0008-5472.CAN-06-1536. [DOI] [PubMed] [Google Scholar]
- 16.Dean NR, Knowles JA, Helman EE, Aldridge JC, Carroll WR, Magnuson JS, Clemons L, Ziober B, Rosenthal EL. Anti-EMMPRIN antibody treatment of head and neck squamous cell carcinoma in an ex-vivo model. Anticancer Drugs. 2010 Oct;21(9):861–7. doi: 10.1097/CAD.0b013e32833d1a11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean NR, Newman JR, Helman EE, Zhang W, Safavy S, Weeks DM, Cunningham M, Snyder LA, Tang Y, Yan L, McNally LR, Buchsbaum DJ, Rosenthal EL. Anti-EMMPRIN monoclonal antibody as a novel agent for therapy of head and neck cancer. Clin Cancer Res. 2009;15:4058–65. doi: 10.1158/1078-0432.CCR-09-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642–50. doi: 10.1038/sj.onc.1204097. [DOI] [PubMed] [Google Scholar]
- 19.Caudroy S, Polette M, Nawrocki-Raby B, Cao J, Toole BP, Zucker S, Birembaut P. EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin Exp Metastasis. 2002;19:697–702. doi: 10.1023/a:1021350718226. [DOI] [PubMed] [Google Scholar]
- 20.Bougatef F, Quemener C, Kellouche S, Naïmi B, Podgorniak MP, Millot G, Gabison EE, Calvo F, Dosquet C, Lebbé C, Menashi S, Mourah S. EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2alpha-mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood. 2009;114:5547–56. doi: 10.1182/blood-2009-04-217380. [DOI] [PubMed] [Google Scholar]
- 21.Tang Y, Nakada MT, Rafferty P, Laraio J, McCabe FL, Millar H, Cunningham M, Snyder LA, Bugelski P, Yan L. Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res. 2006;4:371–7. doi: 10.1158/1541-7786.MCR-06-0042. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Weinstein M, Li C, Deng C. Fibroblast growth factor receptors (FGFRs) and their roles in limb development. Cell Tissue Res. 1999;296:33–43. doi: 10.1007/s004410051264. [DOI] [PubMed] [Google Scholar]
- 23.Szebenyi G, Fallon JF. Fibroblast growth factors as multifunctional signaling factors. Int Rev Cytol. 1999;185:45–106. doi: 10.1016/s0074-7696(08)60149-7. [DOI] [PubMed] [Google Scholar]
- 24.Knights V, Cook SJ. De-regulated FGF receptors as therapeutic targets in cancer. Pharmacol Ther. 2010;125:105–17. doi: 10.1016/j.pharmthera.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009;8:580–8. doi: 10.4161/cc.8.4.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffers M, LaRochelle WJ, Lichenstein HS. Fibroblast growth factors in cancer: therapeutic possibilities. Expert Opin Ther Targets. 2002;6:469–82. doi: 10.1517/14728222.6.4.469. [DOI] [PubMed] [Google Scholar]
- 27.Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009;9:639–51. doi: 10.2174/156800909789057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vairaktaris E, Ragos V, Yapijakis C, Derka S, Vassiliou S, Nkenke E, Yannopoulos A, Spyridonidou S, Vylliotis A, Papakosta V, Loukeri S, Lazaris A, et al. FGFR-2 and −3 play an important role in initial stages of oral oncogenesis. Anticancer Res. 2006;26:4217–21. [PubMed] [Google Scholar]
- 29.Raimondi AR, Molinolo AA, Itoiz ME. Fibroblast growth factor-2 expression during experimental oral carcinogenesis. Its possible role in the induction of pre-malignant fibrosis. J Oral Pathol Med. 2006;35:212–7. doi: 10.1111/j.1600-0714.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 30.Hase T, Kawashiri S, Tanaka A, Nozaki S, Noguchi N, Kato K, Nakaya H, Nakagawa K. Correlation of basic fibroblast growth factor expression with the invasion and the prognosis of oral squamous cell carcinoma. J Oral Pathol Med. 2006;35:136–9. doi: 10.1111/j.1600-0714.2006.00397.x. [DOI] [PubMed] [Google Scholar]
- 31.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Eltoum IE, Guo B, Beck BH, Cloud GA, Lopez RD. Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J Immunol. 2008;180:6044–53. doi: 10.4049/jimmunol.180.9.6044. [DOI] [PubMed] [Google Scholar]
- 33.Bhowmick NA, Moses HL. Tumor-stroma interactions. Curr Opin Genet Dev. 2005;15:97–101. doi: 10.1016/j.gde.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Root LL, Shipley GD. Human dermal fibroblasts express multiple bFGF and aFGF proteins. In Vitro Cell Dev Biol. 1991;27A:815–22. doi: 10.1007/BF02631248. [DOI] [PubMed] [Google Scholar]
- 35.Pardo OE, Latigo J, Jeffery RE, Nye E, Poulsom R, Spencer-Dene B, Lemoine NR, Stamp GW, Aboagye EO, Seckl MJ. The fibroblast growth factor receptor inhibitor PD173074 blocks small cell lung cancer growth in vitro and in vivo. Cancer Res. 2009;69:8645–51. doi: 10.1158/0008-5472.CAN-09-1576. [DOI] [PubMed] [Google Scholar]
- 36.Taylor PM, Woodfield RJ, Hodgkin MN, Pettitt TR, Martin A, Kerr DJ, Wakelam MJ. Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2 release through a phospholipase A(2) and 5-lipoxygenase catalyzed pathway. Oncogene. 2002;21:5765–72. doi: 10.1038/sj.onc.1205702. [DOI] [PubMed] [Google Scholar]
- 37.Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer. 2002;99:520–8. doi: 10.1002/ijc.10390. [DOI] [PubMed] [Google Scholar]
- 38.Slomiany MG, Grass GD, Robertson AD, Yang XY, Maria BL, Beeson C, Toole BP. Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res. 2009;69:1293–01. doi: 10.1158/0008-5472.CAN-08-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byron SA, Pollock PM. FGFR2 as a molecular target in endometrial cancer. Future Oncol. 2009;5:27–32. doi: 10.2217/14796694.5.1.27. [DOI] [PubMed] [Google Scholar]
- 40.Byron SA, Gartside MG, Wellens CL, Goodfellow PJ, Birrer MJ, Campbell IG, Pollock PM. FGFR2 mutations are rare across histologic subtypes of ovarian cancer. Gynecol Oncol. 2010;117:125–9. doi: 10.1016/j.ygyno.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Qiu LX, Wang ZH, Leaw SJ, Wang BY, Wang JL, Cao ZG, Gao JL, Hu XC. Current evidence on the relationship between three polymorphisms in the FGFR2 gene and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2010;124:419–24. doi: 10.1007/s10549-010-0846-7. [DOI] [PubMed] [Google Scholar]
- 42.Jayson GC, Vives C, Paraskeva C, Schofield K, Coutts J, Fleetwood A, Gallagher JT. Coordinated modulation of the fibroblast growth factor dual receptor mechanism during transformation from human colon adenoma to carcinoma. Int J Cancer. 1999;82:298–304. doi: 10.1002/(sici)1097-0215(19990719)82:2<298::aid-ijc23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 43.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–8. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 44.Dellacono FR, Spiro J, Eisma R, Kreutzer D. Expression of basic fibroblast growth factor and its receptors by head and neck squamous carcinoma tumor and vascular endothelial cells. Am J Surg. 1997;174:540–4. doi: 10.1016/s0002-9610(97)00169-4. [DOI] [PubMed] [Google Scholar]
- 45.Zhang C, Fu L, Fu J, Hu L, Yang H, Rong TH, Li Y, Liu H, Fu SB, Zeng YX, Guan XY. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clin Cancer Res. 2009;15:4017–27. doi: 10.1158/1078-0432.CCR-08-2824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.