Abstract
The present study demonstrates pDNA complexes of recombinant silk proteins containing poly(L-lysine) and tumor-homing peptides (THPs), which are globular and approximately 150–250 nm in diameter, show significant enhancement of target specificity to tumor cells by additions of F3 and CGKRK THPs. We report herein the preparation and study of novel nano-scale silk-based ionic complexes containing pDNA able to home specifically to tumor cells. Particular focus was on how the THP, F3 (KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK) and CGKRK, enhanced transfection specificity to tumor cells. Genetically engineered silk proteins containing both poly(L-lysine) domains to interact with pDNA and the THP to bind to specific tumor cells for target-specific pDNA delivery were prepared using Escherichia coli, followed by in-vitro and in-vivo transfection experiments into MDA-MB-435 melanoma cells and highly metastatic human breast tumor MDA-MB-231 cells. Non-tumorigenic MCF-10A breast epithelial cells were used as a control cell line for in-vitro tumor-specific delivery studies. These results demonstrate that combination of the bioengineered silk delivery systems and THP can serve as a versatile and useful new platform for non-viral gene delivery.
Keywords: Gene Delivery, Ionic Complex, Silk Protein, Tumor-Homing Peptide (THP)
INTRODUCTION
Gene delivery is an important technique to treat various diseases including cancers and inherited diseases. Recently, nonviral vectors, i.e. cationic polymer-based vectors, have attracted attention as safer gene vectors compared to viral vectors.1–3 Nonviral plasmid DNA (pDNA) delivery systems using cationic liposomes or chemically derived synthetic polymers such as polyethyleneimine have improved over the past decade in terms of transfection efficiency, cell-binding, and endosomal release.4,5 Nevertheless, cytotoxicity and target specificity remain key challenges with lipid- and synthetic polymer-based gene delivery systems. A suitable nonviral gene vector would be biocompatible, biodegradable, non-toxic, efficient, and able to be designed to target specific cell types. These are challenging design goals to meet with liposomes or chemical synthesized polymers, mainly due to the limits inherent with chemically synthesis.
Silk proteins have been used successfully in the biomedical field as sutures for decades, and also explored as biomaterials for cell culture and tissue engineering, achieving Food and Drug Administration approval for such expanded utility because of their excellent mechanical properties, versatility in processing and biocompatibility.6–8 Virgin silk with the associated contaminant sericin proteins was reported as a potential allergen causing a Type I allergic, due to upregulated IgEs in response to the sericins, however, once the sericins are properly removed, there is minimal response from the core fibroin structural proteins.9,10 Further, alpha-chymotrypsin degradation products of silk proteins are nontoxic.9–11 Silk-based gene delivery systems have recently been reported to provide biodegradability, biocompatibility, high transfection efficiency, and DNase resistance.12–14 The secondary structure of the silk sequences in these pDNA complexes can be used to regulate enzymatic degradation rates of the complexes, and hence control the release profile of carried genes from these complexes.13 Therefore, if target specificity can be added into these silk-based gene delivery systems via recombinant techniques, then a more efficient and useful non-viral gene delivery system can be realized, in which the chemistry can be precisely tailored for cell targeting. To our knowledge, there is no such non-viral tailored gene delivery system currently available.
In the present study, we report the preparation and study of novel nano-scale silk-based ionic complexes containing pDNA able to home specifically to tumor cells. Silk-based block copolymers were bioengineered with both poly(L-lysine) domains to interact with pDNA and the tumor-homing peptide (THP) to bind to specific tumor cells for target-specific pDNA delivery. Particular focus was on how the THP, F3 (KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK) and CGKRK, enhanced transfection specificity to tumor cells. F3 peptides have been reported to home to nucleolin, which is expressed at the surface of tumor angiogenic endothelial cells, and bind specifically to MDA-MB-435 cells.15,16 CGKRK peptides were reported to bind specifically to heparan sulfate in tumor vessels.17,18 Genetically engineered silk proteins containing poly(L-lysine) and the THPs were prepared using Escherichia coli, followed by in-vitro and in-vivo transfection experiments into MDA-MB-435 melanoma cells and highly metastatic human breast tumor MDA-MB-231 cells. Non-tumorigenic MCF-10A breast epithelial cells were used as a control cell line for in-vitro tumor-specific delivery studies.
EXPERIMENTAL PROCEDURES
Design and Cloning of Recombinant Silk Sequence
The spider silk repeat unit was selected based on the consensus repeat (SGRGGLGGQGAGAAAAAGGAGQGGYGGLGSQGT) derived from the native sequence of the dragline protein MaSp1 sequence from the spider Nephila clavipes (Accession P19837). The Silk6mer-30lys containing six contiguous copies of this repeat and 30 lysines was developed through the transfer of cloned inserts to pET-30a, according to our previously published procedures.12,15–18 The sequences of the synthetic oligonucleotides encoding F3 and CGKRK residues were as follows: F3-a: 5′-CTAGCAAAGATGAACCGCAGCGCCGCAGCGCGCGCCTGAGCGCGAAACCGGCGCCGCCGAAACCGGAACCGAAACCGAAAAAAGCGCCGGCGAAAAAAA-3′, F3-b: 5′-CTAGTTTTTTTCGCCGGCGCTTTTTTCGGTTTCGGTTCCGGTTTCGGCGGCGCCGGTTTCGCGCTCAGGCGCGCGCTGCGGCGCTGCGGTTCATCTTTG-3′, CGKRK-a: 5′-CTAGCTGCGGCAAACGCAAAA-3′, CGKRK-b: 5′-CTAGTTTTGCGTTTGCCGCAG-3′. The restriction sites for SpeI are italicized. F3-a and F3-b as well as CGKRK-a and CGKRK-b are complementary oligonucleotides which were annealed to form double stranded DNA. The double stranded DNAs of the sequences were ligated into pET30-Silk6mer-30lys to generate pET30-Silk6mer-30lys-monomeric F3 (mF3), pET30-Silk6mer-30lys-monomeric CGKRK (mCGKRK), pET30-Silk6mer-30lys-dimeric CGKRK (dCGKRK), as shown in Figure 1, by DNA ligase (New England Biolabs Inc, Ipswich, MA).
Figure 1.
Amino acid sequences of the recombinant proteins with 30 lysines and tumor-homing peptides (THP). Bold: THP sequences. Underline: representative 6mer of spider silk sequence.
Protein Expression and Purification
The constructs pET30-Silk6mer-30lys-mF3, pET30-Silk6mer-30lys-mCGKRK, and pET30-Silk6mer-30lys-dCGKRK were used to transform E. coli strain RY-3041, and the expression and purification of these proteins were carried out by methods reported previously.14,19–21 SDS-polyacrylamide gel electrophoresis (PAGE) was performed using 4–12% precast NuPage Bis-Tris gels (Invitrogen, Carlsbad, CA). The gel was stained with Colloidal blue (Invitrogen, Carlsbad, CA). Purified samples were extensively dialyzed against acetic buffer and then Milli-Q water. For dialysis, Spectra/Por Biotech Cellulose Ester Dialysis Membranes with MWCO of 100–500 Da (Spectrum Laboratories Inc, Rancho Dominguez, CA) were used.
Preparation and Characterization of pDNA Complexes
pDNA encoding Firefly Luciferase (Luc, 7041 bp) was amplified in competent DH5α E. coli (Invitrogen) and purified using EndoFree Plasmid Maxi Kits (Qiagen, Hilden, Germany). The DNA concentration was determined by absorbance at 260 nm. To prepare the complexes of the recombinant silk proteins with pDNA, a solution containing silk protein (0.1 mg/mL) was mixed with the pDNA solution (370 μg/mL) at various N/P ratios. Here, N/P ratio refers to the ratio of number of amines/ phosphates from pDNA. The mixture of recombinant silk and pDNA was incubated at room temperature (~20ºC) overnight prior to characterization. The pDNA complexes were characterized by zeta potential meter (Zetasizer Nano-ZS, Malvern Instruments Ltd, Worcestershire, UK) and Atomic Force Microscopy (AFM, Dimension V, Veeco Instruments Inc, Plainview, NY). The pDNA complex solution (around 70 μL) was added to ultra pure water (450 μL, Invitrogen) and then used as a sample for zeta potential and size measurement. Zeta potential and zeta deviation of samples were measured three times by a zeta potential meter, and the average data were obtained using Dispersion Technology Software version 5.03 (Malvern Instruments Ltd). The pDNA complex solution was cast on cleaved mica, and observed in air at room temperature using a 200–250 μm long silicon cantilever with a spring constant of 2.8 N/m in tapping mode AFM. Calibration of the cantilever tip-convolution effect was carried out to obtain the true dimensions of objects by previously reported methods.19,20
Cell Culture, in-vitro Transfection, and Cell Viability
The MDA-MB-435 melanoma cell line, MDA-MB-231 human breast tumor cell line, and MCF10A non-tumorigenic mammary breast epithelial cells were also used to investigate target specificity of the pDNA complexes. Cultures were grown to confluence using media consisting of Dulbecco’s Modified Eagle Medium (DMEM) and 10% FBS for MDA-MB-435 and MDA-MB-231 cells. The medium for MCF10A was composed of DMEM/F12, 5% horse serum, 20 ng/mL EGF, 0.5 μg/mL hydrocortisone, 0.1 μg/mL cholera toxin, and 10 μg/mL insulin. The cultures were detached from their substrates using 0.25% trypsin (Invitrogen), and then replated in the 24-multiwell plate at a density of 8000 cells/well. Before transfection experiments, the cells were washed with PBS to remove antibiotic and insulin. Media for the in-vitro transfection experiments was consisting DMEM and 10% FBS. pDNA (1.2 μg) and recombinant silk (appropriate amount) complexes were added into each well. After incubation of the cells for 6 h at 37ºC, the media was exchanged to the media without pDNA complexes. To evaluate luciferase gene expression quantitatively, a Luciferase assay (Promega, Madison, WI) was performed (n=4). The amount of protein in each well was determined using a BCA protein assay (Pierce Biotechnology, Rockford, IL), and then the Relative Light Units (output) / weight of protein (RLU/mg) was obtained. Lipofectamine 2000 (Invitrogen) was used as a positive control vector. For cell viability, MCF10A cells (8000 cells/well) were seeded into the 96-wells plates containing the pDNA complexes and cultured for 48 h in the media (100 μL) used in the transfection experiment. Cytotoxicity to the cells of the pDNA complexes was characterized by standard 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega, Madison, WI) according to the manufacturer’s instructions (n=8).
In-vivo Transfection
All experimental procedures involving animals were performed under an animal protocol approved by Tufts Institutional Animal Care and Use Committee. The MDA-MB-231 breast tumor was established by injecting a mixture of 5×105 MDA-MB-231 cells suspended in 13.3 μL of phosphate buffer solution (PBS, Invitrogen) and 6.7 μL of matrigel (BD Bioscience, San Jose, CA) into both the left and right mammary fat pads. Tumors were allowed to grow for 2 weeks to reach an average diameter of 5 mm. The pDNA complexes (pDNA: 1.2 μg, N/P: 2) in PBS were delivered via multiple tail vain injections to the tumor-bearing mice (n=4) twice a week. As a control experiment, the pDNA complexes were also injected to mice without tumor (n=2) twice a week. Three days, one week, and four weeks later, the mice were subjected to optical imaging on Xenogen IVIS200 (Xenogen Corp., Alameda, CA). Mice were anesthetized with 3% isoflurane and injected with D-luciferin (10 mg/kg intraperitoneal injection) approximately 10 min before imaging. Light emitted from the tumors was quantified using Living Image software (Xenogen). Photons produced by luciferase were normalized as photons per second per centimeter squared per steradian.
Statistical Analysis
The particle sizes on mica substrates were measured by AFM using a Research Nanoscope software version 7.30 (Veeco Instruments Inc). The average value of 20 measurements was used. Statistical differences in particle sizes by AFM, cell transfection efficiency, and cell viability were determined by unpaired t-test with a two-tailed distribution and differences were considered statistically significant at p<0.05. The data in the AFM, cell transfection efficiency, and cell viability experiments are expressed as means ± standard deviation.
RESULTS AND DISCUSSION
Biosynthesis of Recombinant Silk Proteins
The recombinant silk proteins containing poly(L-lysine) (30 aa) and THP, monomeric F3 (SL-mF3), monomeric CGKRK (SL-mCGKRK), or dimeric CGKRK (SL-dCGKRK), were expressed in E. coli and purified with Ni-NTA chromatography. The domain structure and amino acid sequences of the spider silk variants generated with poly(L-lysine) and THPs are shown in Figures 1 and 2. Yields of the recombinant silk proteins were approximately 0.8 mg/L after purification and dialysis. The proteins after purification by Ni-NTA chromatography and dialysis were analyzed by SDS-PAGE and stained with Colloidal blue to evaluate purity (Figure 3). SL-mF3, SL-mCGKRK, and SL-dCGKRK showed a major band corresponding to a molecular weight of approximately 24 kDa, respectively, almost identical to the theoretical molecular weights (monoisotopic mass) of 28737.77, 25897.10, and 26657.50 Da, respectively, by consideration of silk-based polymers generally do not run true to size on SDS-PAGE gels, due to the hydrophobic nature of the protein,14,17 confirming that the bioengineered proteins were the expected recombinant proteins.
Figure 2.
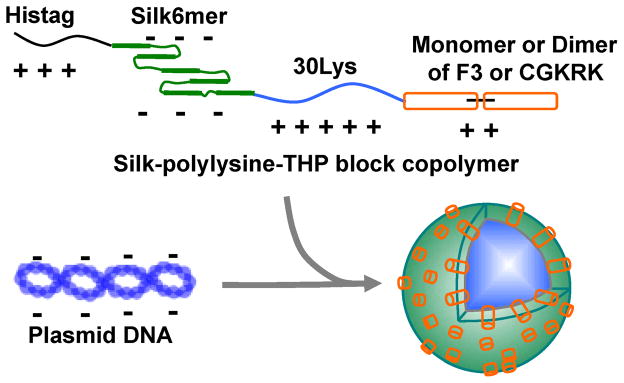
Schematic of formation of the pDNA complexes of the recombinant silk proteins, namely the silk-poly(L-lysine)-THP block copolymers.
Figure 3.
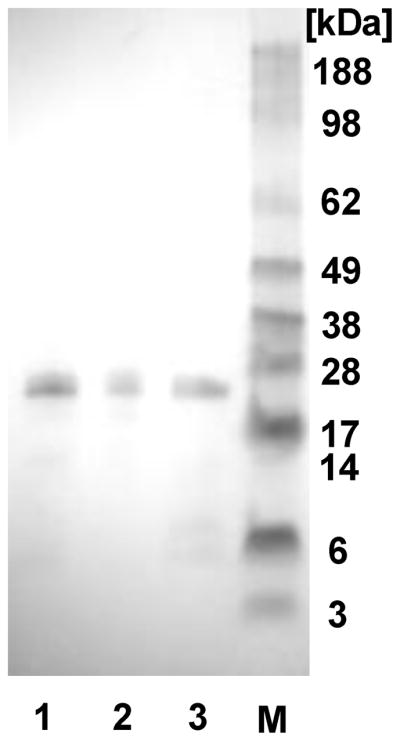
SDS-PAGE of the silk-poly(L-lysine)-THP block copolymers after the purification with Ni-NTA chromatography and dialysis. Lane 1: SL-mCGKRK, lane 2: SL-dCGKRK, lane 3: SL-mF3, and M: molecular weight marker.
Characterization of pDNA Complexes
Ionic complex formation with pDNA encoding a reporter gene and the recombinant silk proteins (SL-mF3, SL-mCGKRK and SL-dCGKRK) were characterized with different N/P ratios (the ratio of number of amines/ phosphates from pDNA) by zeta-nanosizer and AFM. The hydrodynamic diameter and zeta potential of the pDNA complexes of the recombinant silks are listed in Table 1. The average diameters of the SL-mF3, SL-mCGKRK and SL-dCGKRK at the concentration of 0.1 mg/mL without pDNA were 170, 363 and 204 nm, respectively, indicating that the recombinant silk proteins formed monodispersed micelles without pDNA via hydrophobic interaction of the silk sequences. The recombinant silk proteins with pDNA formed ionic complexes via ionic polylysine-pDNA interactions and played a role of core of the ionic complexes in consideration of the previous reports on ionic complexes of silk-like polymer and pDNA,14 which means THPs and silk sequences could exist at the surface of the complexes and also neutralized their electrical charges as shown in Figure 2. This micelle structure will help the complex to interact with the tumor cells via THPs. The average diameters of the complexes with pDNA decreased from over 500 nm to approximately 200 nm with an increase in N/P ratio, and the pDNA complexes prepared at an N/P 5 demonstrated almost the same values as the complexes at an N/P 2. The zeta potential of pDNA complexes increased slightly with an increase in N/P ratio. Also, the SL-mF3 showed positive zeta potentials. Based on these sizes and zeta potentials as well as previous studies,12,14 the most suitable pDNA complexes for in-vitro transfection were determined to be those prepared at an N/P 2, because of their smaller and less negative charge features of the complexes. The complexes (N/P 2) of SL-mF3, SL-mCGKRK and SL-dCGKRK were 143, 261 and 274 nm in diameter and 13.8 ± 3.6, 11.1 ± 6.6 and −14.5 ± 6.5 mV in zeta potential, respectively (Table 1).
Table 1.
Sizes and zeta potentials of the pDNA complexes prepared in this study.
| Sizes and PDI [nm]a | Zeta potential and deviation [mV] | |||||
|---|---|---|---|---|---|---|
| SL-mF3 | SL-mCGKRK | SL-dCGKRK | SL-mF3 | SL-mCGKRK | SL-dCGKRK | |
| N/P 0.1 | 631 (0.384) | 826 (0.608) | 218 (0.778) | 8.17 (4.5) | −32.5 (5.9) | −21.2 (11) |
| N/P 1 | 331 (0.645) | 323 (0.558) | 239 (0.842) | 11.0 (5.5) | −20.5 (5.9) | −12.5 (7.6) |
| N/P 2 | 143 (0.176) | 261 (0.157) | 274 (0.687) | 13.8 (3.6) | −11.1 (6.6) | −14.5 (6.5) |
| N/P 5 | 167 (0.460) | 244 (0.139) | 258 (0.622) | 14.6 (4.6) | −10.6 (4.4) | −10.5 (5.9) |
| Silk polymer only without pDNA | 170 (0.790) | 363 (0.813) | 204 (0.882) | 10.9 (4.3) | −7.17 (4.4) | −9.76 (4.9) |
Determined by zeta-nanosizer.
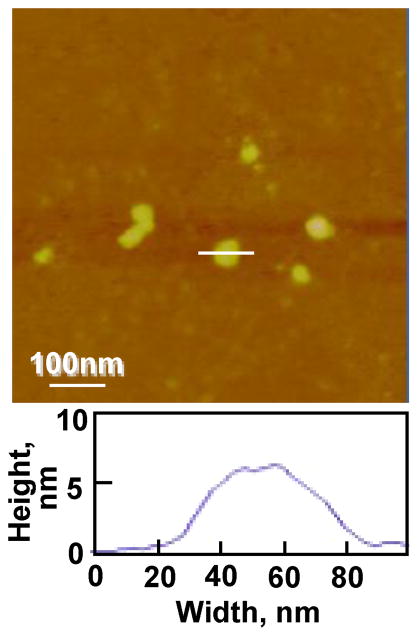
The pDNA complexes of SL-mF3 prepared at an N/P 2 were cast on mica and imaged by AFM (Figure 4). Based on these AFM observations, all of the complexes prepared at an N/P 2 formed homogeneous globular complexes, and the pDNA complexes of SL-mF3 at an N/P 2 demonstrated an average diameter and height of 95 ± 46 nm and 6.5 ± 2.3 nm, respectively (n=20). The calibration of the cantilever tip-convolution effect was performed to obtain the true dimensions of the pDNA complexes using the methods reported previously.19,20 On the basis of the volume of the pDNA complexes, the dimensions determined by zeta-nanosizer (dynamic light scattering) were slightly larger than those by AFM, since the pDNA complexes on mica were shrunk due to drying in air.20
Figure 4.
AFM height images of the pDNA complexes with silk-poly(L-lysine)-monomeric F3 (SL- mF3) casted on a mica substrate in air.
The cytotoxicity of the pDNA complexes with an N/P ratio of 2 for SL-mF3, SL-mCGKRK and SL-dCGKRK was determined using the standard MTS assay with MCF10A mammary breast epithelial cells, which represent healthy cells in the present study. The pDNA complexes of SL-mF3, SL-mCGKRK and SL-dCGKRK showed 106 ± 17, 111 ± 3 and 105 ± 8% of cell viability, respectively, indicating that the present silk-based systems have reasonably low cytotoxicity against non-tumorigenic cells; suitable to be used in drug and gene deliveries.
In-vitro Transfection
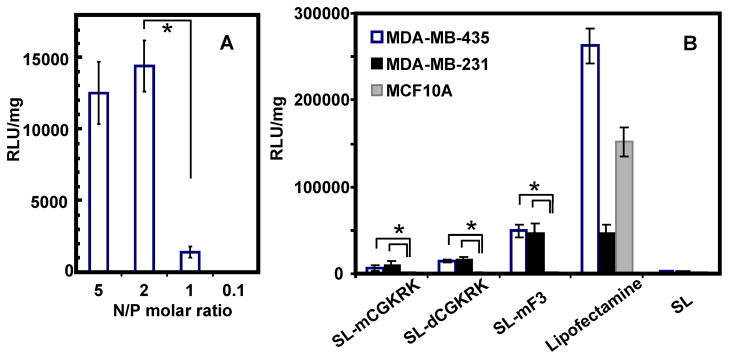
In-vitro transfection experiments were performed with MDA-MB-435, MDA-MB-231, and MCF10A cells in order to evaluate the effects of the THPs, F3 and CGKRK, on target specificity of the silk-based gene delivery. MDA-MB-435 cells had been considered to be breast cancer cells but were recently reported to show melanoma-like properties.21 Nevertheless, F3 peptides are reported to bind MDA-MB-435 and MDA-MB-231 via nucleolin or nucleolin-related proteins, whereas CGKRK peptides may bind to MDA-MB-435 by recognizing heparan sulfates in tumor vessels.22–26 MDA-MB-231 human breast tumor cells were used as a representative model for breast cancer cells. On the other hand, MCF10A non-tumorigenic mammary breast epithelial cells were used as a negative control to evaluate target specificity. To determine the most efficient N/P ratio of the pDNA complexes, MDA-MB-435 cells were transfected via the SL-dCGKRK with luciferase pDNA as a reporter gene. Figure 5A show the transfection efficiencies to MDA-MB-435 cells for pDNA complexes of SL-dCGKRK with N/P ratios ranging from 0.1 to 5 based on the luciferase assay (n=4). pDNA complexes prepared at an N/P 2 demonstrated the highest transfection efficiency among the different N/P ratios, followed by a steep decrease in efficacy, presumably due to excess recombinant silk interacting with the cells and pDNA. The transfection efficiency of the pDNA complexes prepared at an N/P 5 was nearly identical to that at an N/P 2. Therefore, the complexes at N/P 2, which contained less recombinant silk proteins, were used for the transfection experiments. Figure 5B shows the transfection efficiencies to MDA-MB-435, MDA-MB-231 and MCF10A cells for pDNA complexes of the recombinant silk proteins (N/P 2), as well as for the transfection regent Lipofectamine 2000 (Invitrogen), as a positive control. The silk block copolymers containing only silk6mer and poly(L-lysine) (30aa) sequences did not show useful transfection to these cell lines (Figure 5B). The pDNA complexes of SL-dCGKRK showed higher transfection efficiency in comparison with SL-mCGKRK. This may be because of fewer negative surface charges on the dimeric CGKRK sequences of SL-dCGKRK. The pDNA complexes of all three types of silk polymers exhibited transfection to only MDA-MB-435 and MDA-MB-231 cells, whereas they did not demonstrate useful transfection efficiency to MCF10A non-tumorigenic mammary breast epithelial cells, indicating that the target specificity of the present silk-based gene delivery systems was significantly improved by the additions of THP into the silk and poly(L-lysine) sequences. This also suggests that parts of THPs were present at the surface of the complexes with pDNA to specifically interact with the tumorigenic cells. The pDNA complexes were digested by enzymes in lysosomes inside cells, resulting in pDNA release, as in our prior study.12 DNase I showed no significant degradation of the pDNA complexed with the silk block copolymers.13
Figure 5.
In-vitro transfection results in loading pDNA complexes of the recombinant silk proteins. (A) In-vitro transfection results of silk-poly(L-lysine)-dimeric CGKRK (SL-dCGKRK) with different N/P ratios in MDA-MB-435 cells. (B) Transfection results of Silk-poly(L-lysine)-monomeric CGKRK (SL-mCGKRK), silk-poly(L-lysine)-dimeric CGKRK (SL-dCGKRK), silk-poly(L-lysine)-monomeric F3 (SL-mF3) and silk-poly(L-lysine) (SL: negative control) prepared at N/P 2 in MDA-MB-435, MDA-MB-231, and MCF10A cells. Lipofectamine 2000 was used as a positive control sample. Data are shown as means ± standard deviation (n=4). *Significant difference between two groups at p< 0.05. The transfection results of SL-mCGKRK, SL-dCGKRK, and SL-mF3 in MCF10A are significantly different from in MDA-MB-435 and MDA-MB-231 cells.
In-vivo Transfection
In-vivo transfection experiments were also carried out using SL-mF3 (N/P 2), which showed the highest transfection efficiency to MDA-MB-231 tumor cells (Figure 5B). Bioluminescent images of live tumor-bearing mice at 3 days, 1 week, and 4 weeks after injections of the pDNA complexes were captured to evaluate the luciferase expression (Figure 6). Three days were insufficient for expression of the pDNA in tumor cells in the mice (Figure 6a, d). Each tumor-bearing mouse significantly expressed luciferase in the tumor at day 7, one week after the first injection, whereas the mice without tumor cells did not show significant luciferase expression (Figure 6b, e). The expression level slightly increased for 4 weeks, while the control mice did not show significant expression (Figure 6c, f). More importantly, no significant toxicity was observed with the mice. Based on these limited in-vivo results, the pDNA complexes containing F3 THP have potential to treat tumors specifically, but more importantly, without side effects associated with toxicity. A more comprehensive study will be required to clarify the longevity of transfection as well as to fully evaluate the potential of these new systems.
Figure 6.
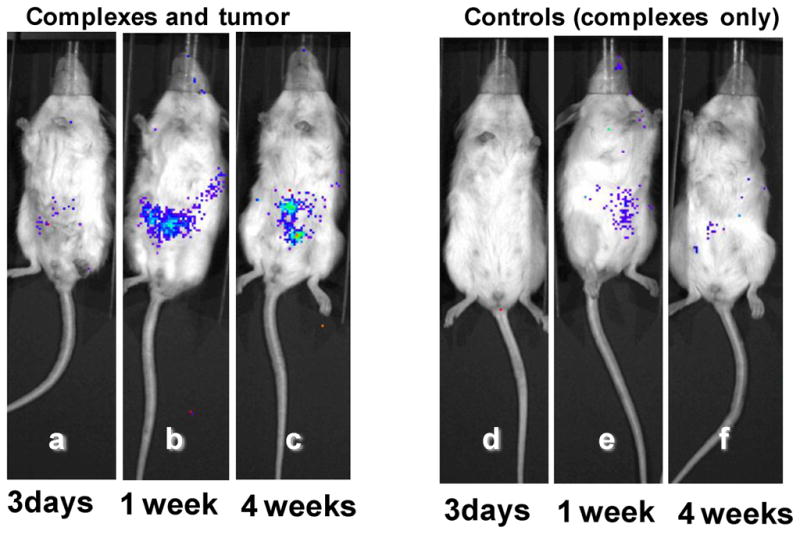
In-vivo transfection results in loading pDNA complexes of SL-mF3 prepared at N/P 2 in MDA-MB-231 tumor cells in mice (a,b,c) and their control experiments without tumor cells (d,e,f). Typical bioluminescent images of 3 days (a,d), 1 week (b,e), and 4 weeks (c,f) luciferase expression in mice.
In conclusion, the present study demonstrated that pDNA complexes of recombinant silk proteins containing poly(L-lysine) and THP, which are globular and approximately 150–250 nm in diameter, show significant enhancement of target specificity to tumor cells by additions of F3 and CGKRK THPs. The low cytotoxicity of the silk-based pDNA complexes is significant based on MTS assays, in-vitro and in-vivo transfection experiments, which is an important advantage for biomedical applications. The present study also suggests that the silk-based delivery systems containing functional peptides, such as THP, can be tailor-made delivery systems by virtue of those peptides as well as have shown potentials as a versatile and useful new platform polymer for non-viral gene delivery.
Acknowledgments
This work has been supported by grants from the NIH (P41 Tissue Engineering Resource Center, EB002520) and the NSF (DK). KN was supported by a JSPS Postdoctoral Fellowship for Research Abroad.
LITERATURE CITED
- 1.Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007--an update. J Gene Med. 2007;9:833–842. doi: 10.1002/jgm.1100. [DOI] [PubMed] [Google Scholar]
- 2.Edelstein ML, Abedi MR, Wixon J, Edelstein RM. Gene therapy clinical trials worldwide 1989–2004-an overview. J Gene Med. 2004;6:597–602. doi: 10.1002/jgm.619. [DOI] [PubMed] [Google Scholar]
- 3.Lundstrom K. Latest development in viral vectors for gene therapy. Trends Biotechnol. 2003;21:117–122. doi: 10.1016/S0167-7799(02)00042-2. [DOI] [PubMed] [Google Scholar]
- 4.Fischer D, Bieber T, Li YX, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: Effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–1279. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 5.Ross PC, Hui SW. Lipoplex size is a major determinant of in vitro lipofection efficiency. Gene Ther. 1999;6:651–659. doi: 10.1038/sj.gt.3300863. [DOI] [PubMed] [Google Scholar]
- 6.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 7.Numata K, Kaplan DL. Silk-based delivery systems of bioactive molecules. Adv Drug Deliv Rev. 2010;62:1497–1508. doi: 10.1016/j.addr.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Dewair M, Baur X, Ziegler K. Use of Immunoblot Technique for Detection of Human Ige and Igg Antibodies to Individual Silk Proteins. J Allergy Clin Immun. 1985;76:537–542. doi: 10.1016/0091-6749(85)90772-9. [DOI] [PubMed] [Google Scholar]
- 10.Zaoming W, Codina R, FernandezCaldas E, Lockey F, Bukanz SC. Partial characterization of the silk allergens in mulberry silk extract. J Allergy Clin Immun. 1996;97:109–109. [PubMed] [Google Scholar]
- 11.Numata K, Cebe P, Kaplan DL. Mechanism of enzymatic degradation of beta-sheet crystals. Biomaterials. 2010;31:2926–2933. doi: 10.1016/j.biomaterials.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Numata K, Hamasaki J, Subramanian B, Kaplan DL. Gene delivery mediated by recombinant silk proteins containing cationic and cell binding motifs. J Control Release. 2010;146:136–143. doi: 10.1016/j.jconrel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Numata K, Kaplan DL. Silk-Based Gene Carriers with Cell Membrane Destabilizing Peptides. Biomacromolecules. 2010;11:3189–3195. doi: 10.1021/bm101055m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Numata K, Subramanian B, Currie HA, Kaplan DL. Bioengineered silk protein-based gene delivery systems. Biomaterials. 2009;30:5775–5784. doi: 10.1016/j.biomaterials.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bini E, Foo CW, Huang J, Karageorgiou V, Kitchel B, Kaplan DL. RGD-functionalized bioengineered spider dragline silk biomaterial. Biomacromolecules. 2006;7:3139–3145. doi: 10.1021/bm0607877. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Valluzzi R, Bini E, Vernaglia B, Kaplan DL. Cloning, expression, and assembly of sericin-like protein. J Biol Chem. 2003;278:46117–46123. doi: 10.1074/jbc.M307792200. [DOI] [PubMed] [Google Scholar]
- 17.Prince JT, McGrath KP, DiGirolamo CM, Kaplan DL. Construction, cloning, and expression of synthetic genes encoding spider dragline silk. Biochemistry. 1995;34:10879–10885. doi: 10.1021/bi00034a022. [DOI] [PubMed] [Google Scholar]
- 18.Yan SZ, Beeler JA, Chen YB, Shelton RK, Tang WJ. The regulation of type 7 adenylyl cyclase by its C1b region and Escherichia coli peptidylprolyl isomerase, SlyD. J Biol Chem. 2001;276:8500–8506. doi: 10.1074/jbc.M010361200. [DOI] [PubMed] [Google Scholar]
- 19.Numata K, Hirota T, Kikkawa Y, Tsuge T, Iwata T, Abe H, Doi Y. Enzymatic degradation processes of lamellar crystals in thin films for poly[(R)-3-hydroxybutyric acid] and its copolymers revealed by real-time atomic force microscopy. Biomacromolecules. 2004;5:2186–2194. doi: 10.1021/bm0497670. [DOI] [PubMed] [Google Scholar]
- 20.Numata K, Kikkawa Y, Tsuge T, Iwata T, Doi Y, Abe H. Adsorption of biopolyester depolymerase on silicon wafer and poly[(R)-3-hydroxybutyric acid] single crystal revealed by real-time AFM. Macromolecular Bioscience. 2006;6:41–50. doi: 10.1002/mabi.200500160. [DOI] [PubMed] [Google Scholar]
- 21.Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 22.Porkka K, Laakkonen P, Hoffman JA, Bernasconi M, Ruoslahti E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc Natl Acad Sci U S A. 2002;99:7444–7449. doi: 10.1073/pnas.062189599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman JA, Giraudo E, Singh M, Zhang L, Inoue M, Porkka K, Hanahan D, Ruoslahti E. Progressive vascular changes in a transgenic mouse model of squamous cell carcinoma. Cancer Cell. 2003;4:383–391. doi: 10.1016/s1535-6108(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 25.Makela AR, Matilainen H, White DJ, Ruoslahti E, Oker-Blom C. Enhanced baculovirus-mediated transduction of human cancer cells by tumor-homing peptides. J Virol. 2006;80:6603–6611. doi: 10.1128/JVI.00528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soundararajan S, Chen WW, Spicer EK, Courtenay-Luck N, Fernandes DJ. The nucleolin targeting aptamer AS1411 destabilizes bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008;68:2358–2365. doi: 10.1158/0008-5472.CAN-07-5723. [DOI] [PubMed] [Google Scholar]