Abstract
Background
Human medullary thyroid cancer (MTC), is a neuroendocrine (NE) tumor, derived from thyroid C-cells. Besides surgery, there are no curative therapies for MTC. This emphasizes the need for the development of new therapies. In MTC, Notch1 signaling pathway is absent and Notch1 activation in MTC-TT cells has been shown to reduce growth and NE markers in vitro. While the in vitro studies will provide insight into the potential mechanisms by which Notch inhibits growth, only by in vivo model one can recreate the conditions found in patients with MTC and assess effects on metastatic potential and microscopic disease.
Materials and Methods
Doxycycline inducible TT-NOTCH1 cells were utilized in a murine subcutaneous xenograft model to study tumor development and growth. Doxycycline was used to induce the expression of Notch1 in these tumors.
Results
Measurements of tumor volume showed that doxycycline treated mice had slower tumor growth than control mice. Western blot analysis of tumor lysates demonstrated activation of Notch1 protein only in doxycycline treated mice suggesting that active Notch1 slowed tumor growth. Furthermore, this activation led to a significant reduction in the levels of achaete-scute complex-like1 and chromogranin A important NE markers.
Conclusion
Based on these data, activation of Notch signaling pathway could be a therapeutic strategy to treat patients with MTC.
Keywords: Notch1 pathway, tumor suppressor, medullary thyroid cancer (MTC), chromogranin A, achaete-scute complex-like 1, xenograft model
INTRODUCTION
Medullary thyroid carcinoma (MTC) is a sub-type of neuroendocrine (NE) tumor that originates from calcitonin producing parafollicular C-cells and accounts up to 5% of all thyroid cancers [1]. A wide variety of tumor markers including calcitonin (CT), chromogranin A (CgA), and achaete-scute complex-like1 (ASCL1) are produced at high levels by MTC [2–4]. We have shown that the active Notch1 intracellular domain is absent in human MTC tumor tissue samples and MTC-TT cells. Overexpression of active Notch1 in MTC-TT cells resulted in a significant reduction in hormone production and growth suppression indicating that Notch1 acts as a tumor suppressor in MTC [5]. Thus, activating Notch pathway may be a novel strategy to treat patients with MTC [4;6].
Notch1 is a transmembrane receptor; upon ligand binding it undergoes two proteolytic cleavages that release the intracellular domain. The active cleaved product, Notch1 intracellular domain (NICD1), translocates into the nucleus where it binds to the transcriptional repressor CBF1 (also named CSL, RBjκ, Su(H) or Lag-1) resulting in the activation of various genes including HES-1 [6]. Multiple studies have shown that HES-1 induction down-regulates the transcription of ASCL1 [7–11].
Utilizing doxycycline inducible NOTCH1 intracellular domain in MTC cells, we have previously shown that overexpression of active Notch1 (NICD1) down-regulates ASCL1 and CgA in an in vitro model. In the present study, we show that induction of Notch1 by doxycycline in murine xenograft model resulted in slowing down the tumor progression. Furthermore, we show by western analysis that Notch1 is expressed only in doxycycline treated tumor lysates. This is associated with reduction in NE markers CgA and ASCL1.
METHODS
Cell culture conditions
The Notch1-expressing MTC cell line, TT-NOTCH1, was established as previously described [5]. Conditional expression of Notch1 was achieved by using the TET-ON system. TT-NOTCH1 cells were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, California, USA) supplemented with 16% tetracycline - free fetal bovine serum (Clontech, Moutain View, California, USA), 100 IU/ml penicillin (Invitrogen), 0.6 μg/ml G418 (Invitrogen) and 0.4 μg/ml hygromycin (Invitrogen). Cells were cultured at 37° C in 5% humidified CO2.
Animals
Four-week –old male athymic nude mice bearing the nu/nu gene were obtained from Charles Rivers (Wilmington, Maryland, USA). They were allowed to acclimate one week in the animal facility to reduce stress after arrival. Mice were maintained under pathogen free conditions. At the end of the experiment, animals were euthanized by CO2 exposure. All experimental procedures were done in compliance with our animal care protocol approved by the University of Wisconsin-Madison Animal Care and Use Committee.
Tumor model
TT-NOTCH1 cells (107 cells in 200 μl of Hanks Balanced Salt Solution, Mediatech, Inc, Manassas, Virginia, USA) were injected subcutaneously into the left flank of five weeks old male nude mice. When tumors have reached palpable size mice were divided into a control group (n=6) receiving only the vehicle and treated group (n=7) receiving 1 mg of doxycycline per mouse. Treatments were given every other day by intraperitoneal injection for 28 days. Tumor volumes were measured by external caliper every four days. Tumor volume based on caliper measurements were calculated by the modified ellipsoidal formula
At the end of the experiment, mice were sacrificed; the tumors were dissected free from the surrounding tissues, weighed and flash frozen in liquid nitrogen for storage in −80 °C. Postmortem examination of the lungs, liver, kidneys, and spleen were performed to be sure that there were no evidence of metastasis and tumor growth outside of the inoculation site.
Preparation of Tumor Extracts
Tumor tissue (2 mm3) was pulverized in liquid nitrogen and the tissue powder was used for both total RNA extraction (as described below in Quantitative Real-Time PCR/Methods) and protein lysates preparation. Total cell lysates were prepared as described previously [12]. Briefly, the tissue powder was dissolved in 500 μl of lysis buffer containing 50 mmol/l Tris, pH 7.5, 150 mmol/l NaCl, 1% Igepal CA-630, 0.1% sodium dodecyl sulfate, 0.1 μmol/l phenylmethylsulfonyl fluoride, 5 mmol/l ethylene diaminetetraacetic acid, 12 μl/ml Protease Inhibitor Cocktail (Sigma, St Louis, Missouri, USA); incubated on ice for 45 minutes; and centrifuged at 13,000 RPM for 30 minutes at 4°C. The supernatants were collected, and protein concentration was determined by the bicinchoninic acid protein assay kit (Pierce, Rockford, Illinois, USA).
Western Blotting
Equal amounts of protein (20 μg) were denatured by boiling and subjected to electrophoresis on 7.5% or 10% SDS – polyacrylamide gels (Bio-Rad Laboratories, Hercules, California, USA). Proteins were transferred onto nitrocellulose membrane (Bio-Rad), blocked for 1h in milk solution (1 X PBS, 5% nonfat dry milk, 0.1% Tween 20) and incubated at 4°C overnight with the appropriate primary antibody. Following primary antibody incubation, membranes were washed 3 × 5 min in 1x PBS-T (1x PBS with 0.1% Tween 20) and incubated with a horseradish peroxidase-conjugated secondary antibody for 1h and 30 min at room temperature. Then, the membranes were washed 3× 10 min in 1x PBS-T, and protein visualized by chemiluminescence. The Immun-star detection system (Bio-Rad) was used for CgA and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the Super Signal West Pico and SuperSignal West Femto kits (Pierce) were used for Notch1 and ASCL1 respectively. The antibodies Notch1 (Santa Cruz, California, USA), ASCL1 (BD Pharmingen, San Diego, California, USA), and CgA (ZYMED Laboratories, San Francisco, California, USA) were used in a dilution of 1:1000. GAPDH (Trevigen, Gaithersburg, Maryland, USA) was used in a dilution 1:10,000. The horseradish peroxidase linked goat secondary antibodies (Cell Signaling Biolabs, San Diego, California, USA) were used in a dilution of 1:3000.
Quantitative Real-Time PCR
Total RNA from pulverized tumors was extracted with RNeasy Mini Total RNA Purification Kit (Qiagen, Hilden, Germany) and reverse transcribed with the iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR was done by iCycler IQ detection system (Bio-Rad). A 25 μl volume reaction containing 2 μl cDNA sample (200 ng/μl), 200 nM forward primers for ASCL1 (5′-TCCCCCAACTACTCCAACGAC -3′) or GAPDH (5′-ACCTGCCAAATATGATGAC -3′) and 200 nM reverse primers for ASCL1 (5′-CCCTCCCAACGCCACTG-3′) or GAPDH (5′-ACCTGGTGCTCAGTGTAG-3′), and 12.5 μl SYBR Green Supermix (Bio-Rad) was used for the quantitative real-time PCR analysis. Primers were obtained from Integrated DNA Technologies (Coralville, Iowa, USA). The reaction was performed at conditions: 3 minutes at 95°C, 35 cycles of: 30 seconds at 95°C, 25 seconds at 60°C and 30 seconds at 72°C followed by 1 minute at 95°C and 1 minute at 55°C. Cycle numbers obtained at the log-linear phase of the reaction were plotted against a standard curve prepared with serially diluted control samples. Expression of ASCL1 was normalized by GAPDH mRNA levels measured concurrently. Expression was then plotted as average ± standard error of the mean (SEM).
Statistical analysis
One way ANOVA and contrasts of the treated group against control were obtained in R version 2.8.0 (R Development Core Team, 2008) [14] A p value of equal or <0.05 was considered as significant.
RESULTS AND DISCUSSION
Our long-term goal is to identify novel therapies for patients with NE tumors including MTC. Given that the surgical removal of neoplastic tissue is the only curative treatment for patients with MTC, identification of new targets for intervention is crucial for improving debilitating outcomes [1;4]. As we have shown previously [5], Notch signaling represents a potential target for antitumor therapy for patients with this type of thyroid cancer. One of the downstream targets of Notch1 pathway is ASCL1, a transcriptional factor important for development of C- cells which is expressed at high baseline level by MTC cells [3;13]. In addition, MTC cells secrete a high level of CgA, which has been proven to be a sensitive marker for the diagnosis of various types of NE tumors [14;15]. To investigate the Notch1 function on MTC cell growth and NE tumor markers production, we developed an in vitro model of doxycycline induced expression of NICD1 in TET-ON system, based on a tetracycline-controlled transactivator protein, tTA [16]. Our in vitro study showed that overexpression of NICD1 in MTC cells significantly reduced ASCL1 which is associated with a reduction in calcitonin and CgA.
To examine the influence of active NICD1 in vivo, we have created MTC xenograft mice to monitor the tumor growth and measure the expression of Notch1 and NE tumor markers. During the course of our in vivo study we observed no weight loss and significant pathological changes of the mice. In addition, postmortem examination of the lungs, liver, kidneys, and spleen showed no evidence of metastasis or tumor growth outside of the inoculation site.
Notch1 induction in TT-NOTCH1 cells reduces NE markers
We have shown that induction of Notch1 by doxycycline in TT-NOTCH1 cells resulted in growth suppression and reduction of NE markers ASCL1 and CgA [5]. To ensure that TT-NOTCH1 cells express functional Notch1 protein in response to doxycycline treatment, we carried out an experiment before proceed to murine xenograft study. To determine the induciblity and functional activity of Notch1 after doxycycline treatment, we measured the levels of ASCL1 and CgA from the doxycycline treated cell lysates. As shown in Fig. 1, TT-NOTCH1 cells treated with control showed no induction of Notch1 which is associated with endogenous levels of ASCL1 and CgA. However, doxycycline treatment showed significant induction of Notch1 that is associated with marked reduction of ASCL1 and CgA. This result is consistent with previous observation [5].
Fig. 1. Induction of active Notch1 caused reduction in NE markers.
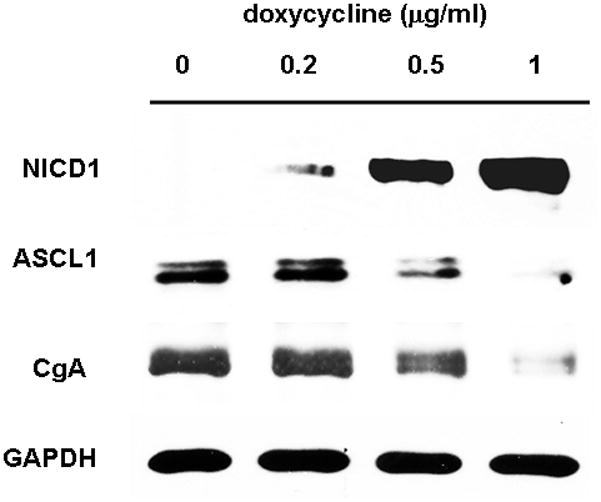
Lysates from TT-NOTCH1 cells treated with or without doxycycline for 4 days showed marked induction of Notch1 only after doxycycline treatment. This is associated with a significant reduction in CgA and ASCL1. GAPDH was used as loading control.
Up-Regulation of Notch1 reduces MTC tumor volume
Doxycycline or PBS treatment started when xenograft reached a mean tumor volume of 250 mm3. Day 0 was assigned for the first intraperitoneal injection followed by the tumor volume measurement every 4 days for 28 days. The tumor growth in doxycycline treated animals slowed down after 8 days of treatment, whereas tumors of the PBS-treated mice continued to grow as evidenced by caliper measurement. After four weeks of treatment, the mean tumor volumes of doxycycline injected group were significantly smaller (650 mm3) than the vehicle (PBS) treated group (1500 mm3), resulting in an average reduction of tumor volume of 57% (p<0.05). In the control group, the fold increase in the tumor volume was 6 whereas the doxycycline treated group’s fold increase was only 2.6 (Fig 2A). The average weight of the tumors from mice injected with doxycycline were significantly lower than that of mice injected with the vehicle (58% reduction, p<0.05) (Fig 2B).
Fig. 2. Overexpression of Notch1 suppresses MTC tumor growth in nude mice.

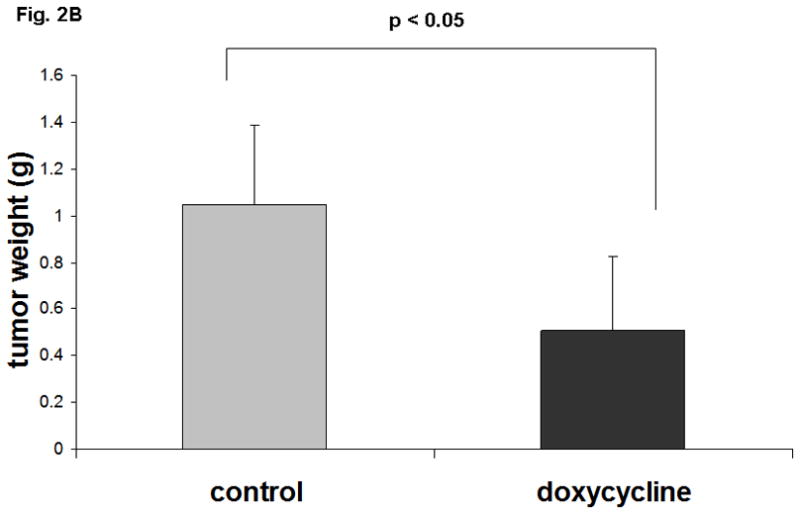
A. Doxycycline induced expression of Notch1 resulted in an average 57% reduction of tumor volume (p<0.05) compared to the control group after 28 days treatment. Standard deviation (SD) was calculated for the control and treated group. B. Tumor weights at necropsy have showed 58% reduction in the group treated with doxycycline.
Expression of Notch1 and its effects on NE markers in vivo
Measurements of tumor volume showed that doxycycline treated mice have slower tumor growth than control mice. However, it is not known that the tumor regression is associated with Notch1 expression. To determine whether Notch1 is actually induced in the doxycycline treated groups, western analysis was carried out using the tumor lysates from both control and doxycycline treated mice. As shown in Fig 3, there is a marked induction of active Notch1 in doxycycline treated mice compared to the PBS injected control group. The minimal level of Notch1 cleaved protein in control samples may be originated from blood vessels and mural cells present in the tumor tissue rather than in MTC cells [17]. Thus doxycycline treatment was able to induce expression of NICD1 in mice with TT-NOTCH1 tumors.
Fig. 3. Doxycycline dependant expression of Notch1 in MTC xenografts suppresses NE markers.
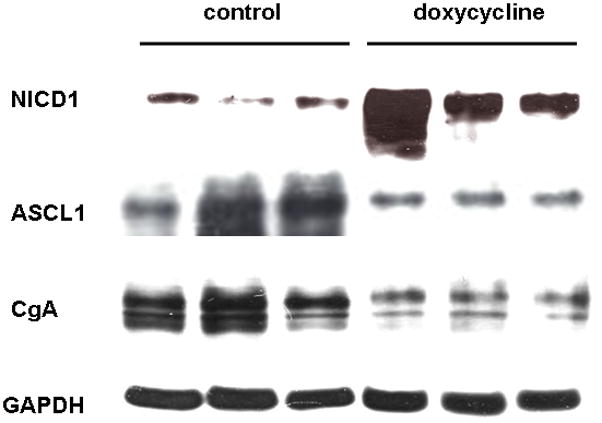
Western blot analysis showed doxycycline induction of the Notch1 intracellular domain (NICD1) in MTC cells (TT-NOTCH1) implanted into nude mice and protein expression of NE tumor markers, ASCL1 and CgA, down regulated by Notch1. GAPDH was used as a protein loading control.
We have demonstrated that Notch1 induction by doxycycline is associated with inhibition of tumor growth. We then analyzed the effect of Notch induction on the NE markers ASCL1 and CgA. CgA plays a crucial role in the diagnosis of NE tumors, including carcinoids, neuroblastoma, pheochromocytoma, pancreatic islet cell tumors, SCLC and MTC [15;18]. ASCL1 and CgA were reduced with the expression of Notch1 in vivo (Fig 3).
Once the Notch1 pathway is activated, active Notch1 binds to CBF-1 complex and upregulates HES-1 expression, which in turn binds to the promoter of ASCL1 and downregulates the ASCL1 mRNA [7]. Furthermore, Notch1 can induce rapid degradation of ASCL1 independent of HES-1 by directly binding to ASCL1 protein [19]. Therefore, to determine if the reduction in ASCL1 protein is due to a reduction in mRNA level, we performed quantitative Real-Time PCR analysis. We have observed a significant suppression of ASCL1 mRNA (two fold decrease; p<0.05) in Notch1 expressing tumors (Fig 4), suggesting that the regulation of ASCL1 is at the transcriptional level and may be mediated by HES-1.
Fig. 4. Notch1 expression suppresses ASCL1 mRNA.
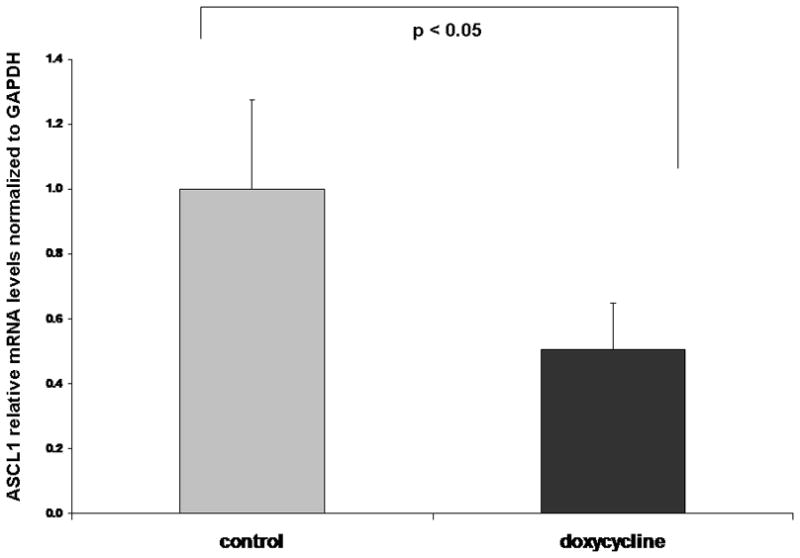
Quantitative real-time PCR has shown a 2 fold reduction in ASCL1 mRNA in mice treated with doxycycline compared to the control group.
Animal models have served as a surrogate for patients in the evaluation of compounds for in vivo anticancer activity. Importantly, the subcutaneous xenograft model is more strenuous as the test compound must cross physiologic barriers to reach the target tumor. However, the drawback of the model is the animals are immunosuppressed either by genetic alteration athymic mice (nu/nu) or as severe combined immunodeficient. Therefore, these mice are required to be kept at microbe-free environment which is not the same as normal environment. Most importantly, the tumor sites are in different than the normal human tumor origin and so it may have different tumor histology. Though results from the animal studies sometimes may not be fully applicable to humans, animal studies can be of great value and such studies are required before human administration of new medicines. Since there is no syngeneic model available for MTC, we have utilized the subcutaneous xenograft, a well established model to study the drug efficacy.
In summary, we report, for the first time that an active NICD1 is not only able to slow down MTC tumor growth but also reduces NE markers in an experimental mouse model. Based on our previous in vitro and present in vivo study, Notch1 specific activators may be novel therapeutic agents to treat MTC. It has been shown that valproic acid and suberoyl bishydroxamic acid activates Notch in neuroendocrine tumors[20–24]. Recently, we have identified resveratrol as a Notch activator based on our cell based assay using a high throughput screen. Treatment of carcinoid cells or MTC-TT cells with resveratrol suppresses growth and NE markers which is associated with Notch expression [25;26].
Acknowledgments
This research was supported in part by National Institutes of Health grants CA109053, CA121115, (HC), and P30 CA014520 from the National Cancer Institute.
References
- 1.Chen H, Roberts JR, Ball DW, Eisele DW, Baylin SB, Udelsman R, Bulkley GB. Effective long-term palliation of symptomatic, incurable metastatic medullary thyroid cancer by operative resection. Ann Surg. 1998;227:887–895. doi: 10.1097/00000658-199806000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Udelsman R, Zeiger MA, Ball DA. Human achaete-scute homolog-1 is highly expressed in a subset of neuroendocrine tumors. Oncology Reports. 1997;4:775–778. doi: 10.3892/or.4.4.775. [DOI] [PubMed] [Google Scholar]
- 3.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Chen H. The role of human achaete-scute homolog-1 in medullary thyroid cancer cells. Surgery. 2003;134:866–871. doi: 10.1016/s0039-6060(03)00418-5. [DOI] [PubMed] [Google Scholar]
- 4.Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13:539–547. doi: 10.1634/theoncologist.2007-0239. [DOI] [PubMed] [Google Scholar]
- 5.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281:39819–39830. doi: 10.1074/jbc.M603578200. [DOI] [PubMed] [Google Scholar]
- 6.Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12:535–542. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD, Baylin SB, Ball DW. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc Natl Acad Sci U S A. 1997;94:5355–5360. doi: 10.1073/pnas.94.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, Conlon RA. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- 9.Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- 10.Kunnimalaiyaan M, Yan S, Wong F, Zhang YW, Chen H. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery. 2005;138:1137–1142. doi: 10.1016/j.surg.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Soderholm H, Ortoft E, Johansson I, Ljungberg J, Larsson C, Axelson H, Pahlman S. Human achaete-scute homologue 1 (HASH-1) is downregulated in differentiating neuroblastoma cells. Biochem Biophys Res Commun. 1999;256:557–563. doi: 10.1006/bbrc.1999.0314. [DOI] [PubMed] [Google Scholar]
- 12.Vaccaro A, Chen H, Kunnimalaiyaan M. In-vivo activation of Raf-1 inhibits tumor growth and development in a xenograft model of human medullary thyroid cancer. Anticancer Drugs. 2006;17:849–853. doi: 10.1097/01.cad.0000217424.36961.47. [DOI] [PubMed] [Google Scholar]
- 13.Borges M, Linnoila RI, van d V, Chen H, Nelkin BD, Mabry M, Baylin SB, Ball DW. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997;386:852–855. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- 14.Massironi S, Conte D, Sciola V, Spampatti MP, Ciafardini C, Valenti L, Rossi RE, Peracchi M. Plasma chromogranin A response to octreotide test: prognostic value for clinical outcome in endocrine digestive tumors. Am J Gastroenterol. 2010;105:2072–2078. doi: 10.1038/ajg.2010.154. [DOI] [PubMed] [Google Scholar]
- 15.Peracchi M, Gebbia C, Basilisco G, Quatrini M, Tarantino C, Vescarelli C, Massironi S, Conte D. Plasma chromogranin A in patients with autoimmune chronic atrophic gastritis, enterochromaffin-like cell lesions and gastric carcinoids. Eur J Endocrinol. 2005;152:443–448. doi: 10.1530/eje.1.01862. [DOI] [PubMed] [Google Scholar]
- 16.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 17.ZhuGe Q, Zhong M, Zheng W, Yang GY, Mao X, Xie L, Chen G, Chen Y, Lawton MT, Young WL, Greenberg DA, Jin K. Notch-1 signalling is activated in brain arteriovenous malformations in humans. Brain. 2009;132:3231–3241. doi: 10.1093/brain/awp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouland AJ, Bevan S, White JH, Hendy GN. Human chromogranin A gene. Molecular cloning, structural analysis, and neuroendocrine cell-specific expression. J Biol Chem. 1994;269:6918–6926. [PubMed] [Google Scholar]
- 19.Sriuranpong V, Borges MW, Strock CL, Nakakura EK, Watkins DN, Blaumueller CM, Nelkin BD, Ball DW. Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol Cell Biol. 2002;22:3129–3139. doi: 10.1128/MCB.22.9.3129-3139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenblatt DY, Cayo M, Ning L, Jaskula-Sztul R, Haymart M, Kunnimalaiyaan M, Chen H. Suberoyl bishydroxamic acid inhibits cellular proliferation by inducing cell cycle arrest in carcinoid cancer cells. J Gastrointest Surg. 2007;11:1515–1520. doi: 10.1007/s11605-007-0249-1. [DOI] [PubMed] [Google Scholar]
- 21.Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, Ning L, Haymart M, Kunnimalaiyaan M, Chen H. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007;12:942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 22.Greenblatt DY, Cayo MA, Adler JT, Ning L, Haymart MR, Kunnimalaiyaan M, Chen H. Valproic acid activates Notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Ann Surg. 2008;247:1036–1040. doi: 10.1097/SLA.0b013e3181758d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ning L, Greenblatt DY, Kunnimalaiyaan M, Chen H. Suberoyl bis-hydroxamic acid activates Notch-1 signaling and induces apoptosis in medullary thyroid carcinoma cells. Oncologist. 2008;13:98–104. doi: 10.1634/theoncologist.2007-0190. [DOI] [PubMed] [Google Scholar]
- 24.Ning L, Jaskula-Sztul R, Kunnimalaiyaan M, Chen H. Suberoyl bishydroxamic acid activates notch1 signaling and suppresses tumor progression in an animal model of medullary thyroid carcinoma. Ann Surg Oncol. 2008;15:2600–2605. doi: 10.1245/s10434-008-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinchot SN, Jaskula-Sztul R, Ning L, Peters NR, Cook MR, Kunnimalaiyaan M, Chen H. Identification and validation of Notch pathway activating compounds through a novel high-throughput screening method. Cancer. 2010 doi: 10.1002/cncr.25652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truong M, Cook MR, Pinchot SN, Kunnimalaiyaan M, Chen H. Resveratrol Induces Notch2-Mediated Apoptosis and Suppression of Neuroendocrine Markers in Medullary Thyroid Cancer. Ann Surg Oncol. 2010 doi: 10.1245/s10434-010-1488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


