Abstract
Objectives
To determine the pharmacokinetics of pentobarbital in neonates, infants and young children with congenital heart disease post-operative from open heart surgery.
Study design
35 subjects, (3 days – 4.4 years), following open heart surgery who received pentobarbital as standard of care were enrolled. Serial pharmacokinetic blood samples were obtained. A population-based, nonlinear mixed–effects modeling approach was used to characterize pentobarbital pharmacokinetics.
Results
A two-compartment model with weight as a covariate allometrically expressed on clearance (CL), inter-compartmental clearance (Q), central (V1) and peripheral volume of distributions (V2), bypass time as a covariate on CL and V1, and age and ventricular physiology as covariates on CL best described the pharmacokinetics.. A typical infant (two-ventricle physiology, 6.9 kg, 5.2 months, and bypass time of 60 minutes) had a CL of 0.12 L/hr/kg, V1 of 0.45 L/kg and V2 of 0.98 L/kg. The bypass effect was poorly estimated. For subjects less than 12 months age, an age effect on CL remained after accounting for weight, and was precisely estimated.
Conclusions
Pentobarbital pharmacokinetics is influenced by age and weight. Subjects with single-ventricle physiology demonstrated a 15% decrease in clearance when compared with subjects with two-ventricle physiology.
The barbiturate, pentobarbital, is the oxygen analogue of thiopental. Pentobarbital has a potent effect on GABA-sensitive chloride channels, and is a potent central nervous system depressant. Pentobarbital enters the brain more rapidly than phenobarbital or diazepam, and is a very potent antiepileptic drug [1]. Pentobarbital is an ideal drug when sedation is the primary goal. It has been recommended as a sedative agent for diagnostic imaging studies [2–7]. It has respiratory depressant and hypotensive side effects in some patients. Pediatric populations who may benefit from the favorable pharmacodynamic effects of pentobarbital are neonates, infants, and children with congenital heart disease who are status post corrective surgery and require sedation to tolerate the conditions of the immediate post-operative period. Tracheal intubation and mechanical ventilation are frequently required in the post-operative period for some of these patients. Fresh cardiac suture lines mandate that patients remain adequately sedated to decrease the risk of post-operative bleeding. These patients frequently have sufficient residual narcotic from intra-operative administration and are pain free but require additional sedation to remain still to accept a chest tube, trans-thoracic lines, a foley catheter and a strange environment.
Although pentobarbital pharmacology has been well studied in adults, limited pediatric pharmacokinetic (PK) and pharmacodynamic (PD) data are available to guide therapy, particularly in neonates, infants and children with congenital heart disease, who exhibit a wide range of anatomy and physiology, from ventricular septal defects to single ventricle physiology. The impact of immature drug-metabolizing enzyme systems, altered physiology, and intra-operative procedures such as cardiopulmonary bypass and hypothermic circulatory arrest on pentobarbital drug disposition has not been studied. Understanding the clinical pharmacology of pentobarbital is necessary to allow for rational drug administration in this critically ill pediatric subpopulation. The primary objective of this study was therefore to describe the pharmacokinetics of pentobarbital in neonates, infants and children following open heart surgery.
Methods
After IRB approval and written informed parental consent, neonates, infants and children with congenital heart disease undergoing open heart surgery, with adequate hepatic function and who received pentobarbital for sedation as standard of care in the post operative period were eligible for enrollment. Doses of pentobarbital were administered at the discretion of the clinical team. Pharmacokinetic samples, consisting of one milliliter of blood, were drawn immediately before and after the bolus dose, and at 30 min, 60 min, 2 hours, 4–6 hours, 12–18 hours, 18–24 hours, 36–48 hours and 56–72 hours after the bolus. For patients who received two consecutive boluses, the sampling scheme was identical except PK samples were obtained after each bolus and the 56–72 hour PK sample was not obtained. For subjects who received three or more consecutive boluses, PK samples were obtained after the first three bolus doses, and then 15 minutes, 30 minutes, 2 hours, 4–6 hours, 12–18 hours and 36–48 hours after the last bolus.. Plasma was separated by centrifugation and stored at −80°C. Pentobarbital plasma concentrations were determined using a validated high-performance liquid chromatography-tandem mass spectrometry assay with a lower limit of quantitation of 0.05 mcg/mL. The intra-day accuracy and precision (coefficient of variation (%CV)) ranged from 97.6 to 113.2% and 0.8 to 6.0%, respectively. The inter-day accuracy ranged from 104.2 and 105.7%, and the precision (coefficient of variation (%CV)) was ≤ 7.3%.
The population pharmacokinetic analysis was conducted using NONMEM (ICON Development Solutions, Ellicott City, MD) version VI, Level 1.1 All models were run with the first order conditional estimation with interaction (FOCE-I) method. S-Plus Version 6.2 (Insightful, Inc., Data Analysis Products Division, Seattle, WA) was used for goodness-of-fit diagnostics and graphical displays. The goodness-of-fit from each NONMEM run was assessed by the examination of the following criteria: visual inspection of diagnostic scatter plots (observed vs. predicted concentration, observed and predicted concentration vs. time, and weighted residual vs. predicted concentration or time), the precision of the parameter estimates as measured by asymptotic standard errors derived from the covariance matrix of the estimates, successful minimization with at least 3 significant digits in parameter estimates, changes in the Akaike Information Criterion (minimum value of the objective function plus two times the total number of parameters), and changes in the estimates of inter-individual and residual variability for the specified model. No formal sample size estimation was performed. However, prior work [8] has demonstrated that a population of 30 subjects is sufficient for the estimation of a clearance in a typical population when the inter-individual variability is less than 75%. Given this information and clinical feasibility, we deemed a sample size of 35 subjects as adequate.
One and two-compartment models were investigated. A two-compartment disposition model was deemed optimal to define the pentobarbital plasma concentration profile based on results from the model building process and previously published data. Models were parameterized by clearance (CL, liters (L)/hour), inter-compartmental clearance (Q, L/hour), volume of central compartment (V1, L), and volume of peripheral compartment (V2, L). The impact of weight on all pharmacokinetic variables was investigated using an allometric model: TVP = θTVP* (WTi/WTref)θallo where TVP is the typical value of a model parameter, described as a function of individual body weight, θTVP is an estimated parameter describing the typical PK parameter value for an individual with weight equal to the reference weight, WTi is an individual subject’s body weight, WTref is the reference metric (6.9 kg for this analysis), and θallo is an allometric power parameter fixed at a value of 0.75 for clearances, and a value of 1 for volumes based on physiologic consideration of size impact on metabolic processes [9,10]
An exponential variance model was used to describe the variability of pharmacokinetic variables across individuals in the form: Pi = θk exp(ηki) where Pi is the estimated parameter value for the individual subject i, θk is the typical population value of parameter k, ηki are the inter-individual random effects for individual i and parameter k. Models were explored using various inter-individual random effect covariance structures. Inter-individual variability was initially estimated for clearance, and then for the additional variables.
Additive, proportional and combined (additive and proportional) residual error models were considered during the model building process. Ultimately, a proportional error model was used to model random residual variability according to: Cij = (COij* (1+εijP)) where Cij is the observed concentration j in individual i, COij is the individual predicted concentration, and εijP is the proportional residual random error.
Once the final base model was selected, a full covariate modeling method, as implemented in Ravva et al [11] was constructed to estimate effects of covariates on pentobarbital disposition. Covariate effects were pre-defined based on clinical interest, prior knowledge and physiologic plausibility. Contrary to step-wise hypothesis testing, the full model approach is advocated when the goal of the analysis is effect estimation and avoids the problem of selection bias, which is particularly problematic in small data sets (9, 10). The full model included effects of age, weight, total cardiopulmonary bypass time and ventricular physiology (single or two-ventricle). Age was considered as an additional covariate on clearance. Total bypass time was evaluated as a covariate on clearance, based on the hypothesis that a longer bypass time would impair clearance immediately post-operatively. The effect of total bypass time on central volume of distribution was also of clinical interest and was included in the full model. Finally, ventricular physiology was evaluated as a covariate on clearance to assess the impact of altered blood flow.
Results
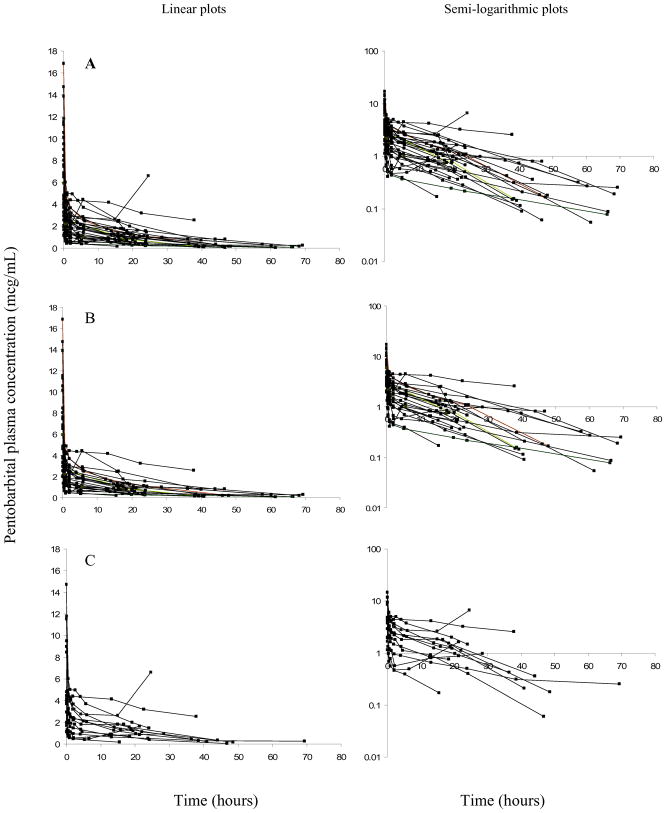
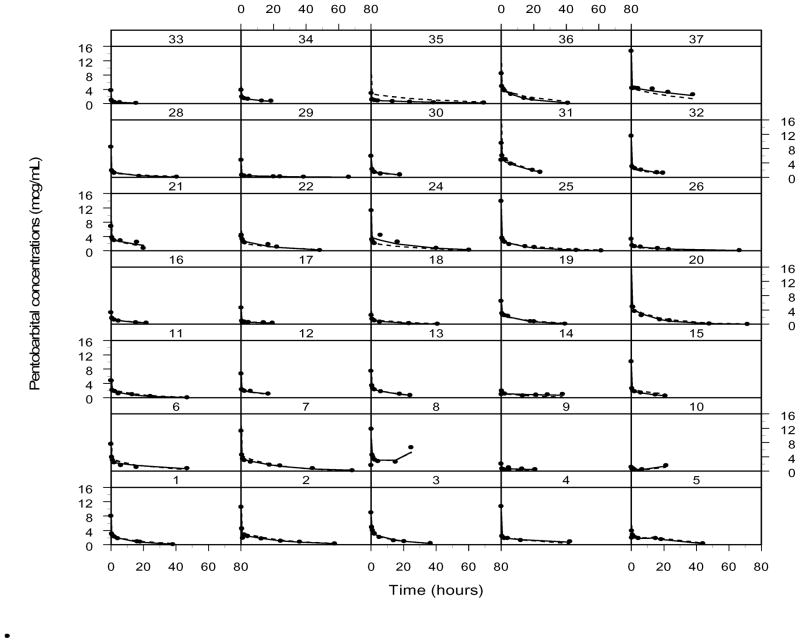
Thirty-seven subjects were enrolled in this study to achieve a total of thirty-five evaluable subjects. Demographics of evaluable subjects are listed in Table I. The median (range) age, weight and total bypass time of the subjects were 6.3 months (0.1 – 42.6), 6.9 kg (2.8 – 15.1), and 60 min (17–91) respectively. The administered total dose ranged from 0.8 to 14 mg/kg. Concentration-time profiles are represented in Figure 1.
Table 1.
Patient demographics.
| Age (months) | Weight (kg) | Bypass (min) | Amount (mg) | Amount (mg/kg) | Surgery |
|---|---|---|---|---|---|
| 0.1 | 2.8 | 86 | 11 | 4 | VSD repair/Arch augmentation |
| 0.2 | 3.2 | 67 | 20 | 6.3 | VSD repair |
| 0.6 | 3 | 73 | 15 | 5 | Coarctation of the aorta repair |
| 2.9 | 5.7 | 59 | 30 (3) | 5.3 | VSD repair |
| 3.2 | 5.7 | 91 | 20 | 3.5 | Tetralogy of Fallot repair |
| 4 | 7.6 | 40 | 50 | 6.6 | Tetralogy of Fallot repair |
| 4.1 | 6.5 | 34 | 15 (2) | 2.3 | Tetralogy of Fallot repair |
| 4.5 | 6.8 | 55 | 25 | 3.7 | Tetralogy of Fallot repair |
| 4.6 | 5.4 | 19 | 20 | 3.7 | Tetralogy of Fallot repair |
| 4.8 | 7 | 84 | 15 | 2.1 | Hemi Fontan PDA ligation |
| 4.9 | 6 | 58 | 50 (2) | 8.3 | VSD repair |
| 5 | 6.9 | 73 | 25 | 3.6 | Tetralogy of Fallot repair |
| 5.3 | 5.5 | 71 | 10 (2) | 1.8 | Hemi Fontan |
| 5.5 | 4.2 | 71 | 15 (2) | 3.6 | VSD closure |
| 5.8 | 5.7 | 47 | 75 (3) | 13.2 | Tetralogy of Fallot repair |
| 6.1 | 7.5 | 60 | 15 | 2 | Bidirectional Glenn |
| 6.1 | 6.6 | 18 | 40 | 6.1 | Bidirectional Glenn |
| 6.3 | 6.1 | 62 | 30 | 4.9 | Hemi-Fontan |
| 6.5 | 6.8 | 34 | 35 | 5.2 | Bidirectional Glenn |
| 6.7 | 6.6 | 27 | 5 | 0.8 | Bidirectional Glenn |
| 7.2 | 7.5 | 73 | 50 | 6.7 | Tetralogy of Fallot repair |
| 7.5 | 6.6 | 17 | 5 | 0.8 | Bidirectional Glenn |
| 10.3 | 10.3 | 71 | 20 | 1.9 | Total anomalous pulmonary venous return repair |
| 11.1 | 9.6 | 24 | 25 | 2.6 | Arterio-venous canal defect repair |
| 20.4 | 8.7 | 81 | 122 (6) | 14 | VSD repair |
| 28.8 | 13.6 | 62 | 25 | 1.8 | Extracardiac fenestrated Fontan |
| 29.4 | 13.6 | 55 | 50 | 3.7 | Lateral tunnel fenestrated Fontan procedure |
| 30.5 | 14.6 | 65 | 50 | 3.4 | Extracardiac fenestrated Fontan |
| 32.7 | 11.5 | 75 | 10 | 0.9 | Extracardiac fenestrated Fontan |
| 36.2 | 14.9 | 62 | 50 | 3.4 | RV to PA shunt |
| 36.5 | 12.1 | 66 | 15 | 1.2 | Extracardiac fenestrated Fontan |
| 36.7 | 13.8 | 56 | 45 (2) | 3.3 | Lateral tunnel fenestrated Fontan |
| 42.6 | 15.1 | 23 | 45 | 3 | Patch closure of atrial septal defect |
| 43.5 | 13.5 | 44 | 50 | 3.7 | Replacement of right ventricular to pulmonary artery conduit |
| 52.7 | 18.1 | 50 | 30 | 1.7 | Repair atrial septal defect |
Amount indicates total dose administered. The number in parenthesis in amount column indicates total number of boluses if greater than one.
Figure 1.
Concentration-time plots for pentobarbital. Time is from the first bolus. Linear plots are represented on the left, with semi-logarithmic plots represented on the right. “A” panels represents the entire dosing cohort; “B” panels represent subjects who received one bolus; “C” panels subjects who received more than one bolus.
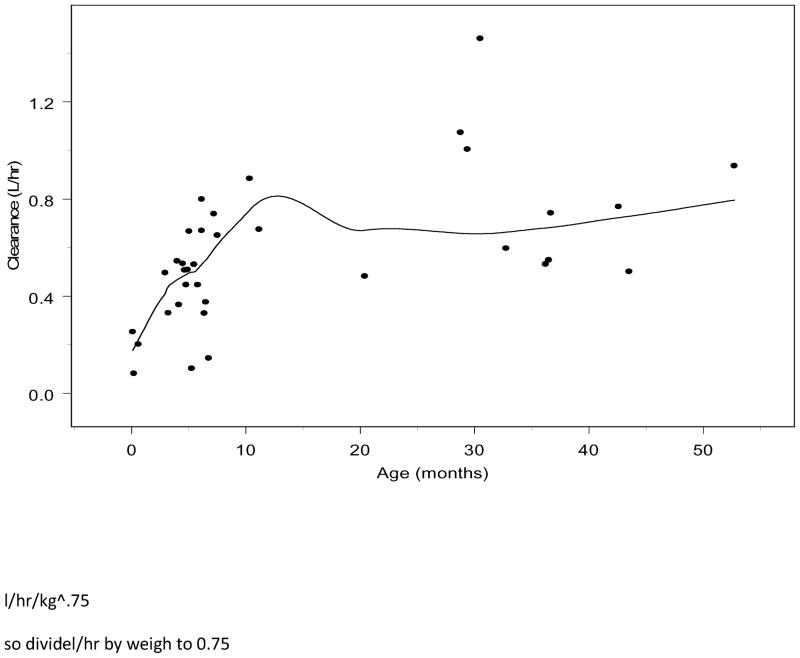
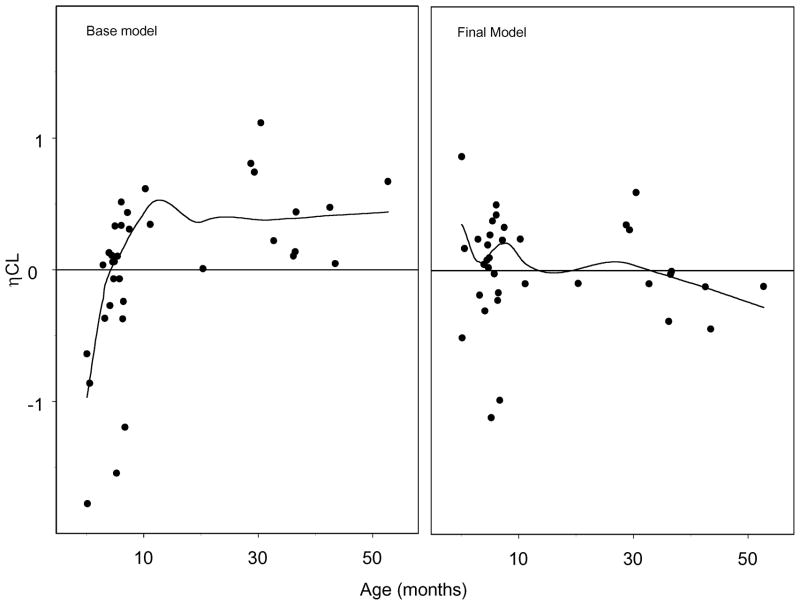
The final structural model was a two-compartment disposition model with inter-individual random effects estimated on CL, V1 and V2. Using FOCE-I estimation, the base model minimized with successful execution of the covariance step. Review of the relationship between CL and age from the base model revealed a direct relationship with CL and age until approximately 12 months of age, when CL reached a plateau (Figure 2; available at www.jpeds.com). In addition, exploratory graphics of the random effects for CL demonstrated a strong correlation between age and CL until approximately 12 months of age (Figure 3; available at www.jpeds.com). When individual clearance is normalized by allometrically scaled weight (individual CL/individual wt 0.75) there are no obvious trends with respect to age or weight. However, there may potential confounding by the differential effects of bypass time.
Figure 2.
Plot of CL versus age, demonstrating direct relationship until approximately twelve months of age.
Figure 3.
Random effects (η) for CL versus age from the base and final models
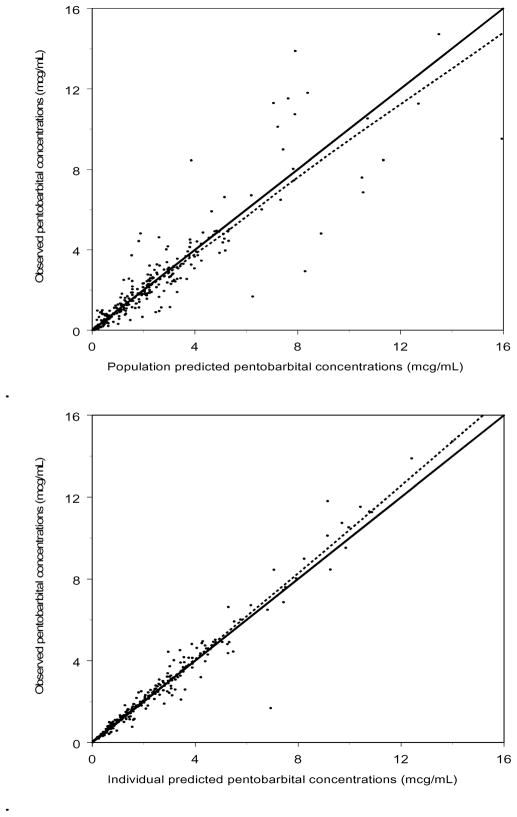
The full model was implemented under FOCE-I estimation and minimized with successful execution of the covariance step. The addition of age as a covariate on CL until 12 months of age resulted in an improvement in the random effects for CL versus age (Figure 3). Observed versus population and individual predicted values revealed no systematic bias in the prediction of plasma concentrations for the entire study (Figure 4). This is also represented in Figure 5 (available at www.jpeds.com), a plot of the observed, population predicted, and individual predicted concentrations versus time.
Figure 4.
Observed versus population (top) and individual (bottom) predicted concentrations for the final pentobarbital pharmacokinetic model.. A loess smoother is represented by the dashed line. R2 is 0.81 and 0.96 for population and individual predicted, respectively.
Figure 5.
Observed (solid circles), population (dashed line) and individual predicted (solid line) pentobarbital concentrations versus time.
Final parameter estimates, inter-individual variability and residual variability are represented in Table II, with the respective standard errors of the point estimates. We estimate a typical value of CL of 0.52 L/hr for a patient who was the median weight of 6.9 kg, median age of 5.5 months, and experienced the median bypass time of 60 minutes (0.12 L/hr/kg0.75). Using allometric scaling this would translate to a CL of 2.96 L/hr for a 70 kg adult. The median age for subjects less than 12 months was 5.2 months. Calculated estimates of CL and V1 for different weights are represented in Table III (available at www.jpeds.com). The percent effect of age relative to the typical value of CL for a range of ages independent of weight and bypass time are represented in Table IV (available at www.jpeds.com).
Table 2.
Variable estimates from the final pentobarbital population pharmacokinetic model.
| Variable | Estimate | SE% |
|---|---|---|
| CL (L/hr) | 0.52 | 8.4 |
| V1(L) | 3.10 | 7.6 |
| Q (L/hr) | 9.64 | 5.2 |
| V2 (L) | 6.75 | 4.8 |
| Age effect CL | 0.23 | 50.0 |
| TBYP effect CL | 0.0039 | 4641.0 |
| TBYP effect V1 | −0.04 | 404.5 |
| Single-Ventricle effect CL | 0.85 | 8.1 |
|
| ||
| Variable | Inter-Individual Variability | SE% |
|
| ||
| ω2Cl | 42.3 | 41.7 |
| ω2V1 | 41.5 | 31.5 |
| ω2V2 | 32.7 | 46.1 |
|
| ||
| Variable | Covariance | SE% |
|
| ||
| CovCL, V1 | 0.01 | 367.6 |
|
| ||
| CovCL, V2 | 0.04 | 98.6 |
|
| ||
| CovV1, V2 | 0.01 | 363.4 |
|
| ||
| Residual Variability | Variance | SE% |
|
| ||
| σ2proportional | 0.01 | 100 |
SE% = (standard error/parameter estimate) *100
Inter-individual variability = (square-root of variance) *100
For age less than 12 months:
CL = θCL* (WT/6.9)0.75*(AGE/5.2)0.23* (TBYP/60)0.0039* [0.85(for single-ventricle only)]
For age greater than 12 months:
CL = θCL* (WT/6.9)0.75* (TBYP/60)0.0039* [0.85(for single-ventricle only)]
For all ages:
V1 = θV1 * (WT/6.9)1* (TBYP/60)−0.04
Q = θQ* (WT/6.9)0.75
V2 = θV2* (WT/6.9)1
Table 3.
Impact of weight alone on clearance and central volume of distribution, estimated using the final model with a median age of 5.2 months and a median total bypass time of 60 minutes.
| Clearance | V1 | |||
|---|---|---|---|---|
| Wt (kg) | L/hr | L/hr/kg | L | L/kg |
| 1 | 0.12 | 0.12 | 0.45 | 0.45 |
| 5 | 0.41 | 0.08 | 2.25 | 0.45 |
| 7 | 0.53 | 0.08 | 3.14 | 0.45 |
| 10 | 0.69 | 0.07 | 4.49 | 0.45 |
| 12 | 0.79 | 0.07 | 5.39 | 0.45 |
| 15 | 0.93 | 0.06 | 6.74 | 0.45 |
Table 4.
Percent effect of age (independent of weight) on CL relative to typical value of clearance for a 12 month old child
| Age (months) | Percent Effect on Typical CL |
|---|---|
| 0.1 | 33% |
| 3 | 73% |
| 6 | 85% |
| 9 | 94% |
| 12 | 100% |
Discussion
A typical infant (two-ventricle physiology, median weight of 6.9 kg, median age of 5.2 months, and median bypass time of 60 minutes) post-operative from cardiac surgery had a total systemic CL of 0.12 L/hr/kg, Q of 2.26 L/hr/kg, V1 of 0.45 L/kg and V2 of 0.98 L/kg. The bypass effect on CL and V1 was poorly estimated. There is a trend towards a direct relationship between bypass time and CL, and an inverse relationship between bypass time and V1. An age effect on CL remained after accounting for weight for subjects less than 12 months age, and was poorly estimated as demonstrated by the percent standard error. Single versus two-ventricle physiology did not appear to affect CL in a clinically relevant way. Subjects with single-ventricle physiology experienced only a 15% decrease in clearance, which was precisely estimated as demonstrated by the 8.1 percent standard error.
Pentobarbital is metabolized primarily by the hepatic microsomal enzyme system, and the metabolic products are excreted in the urine, and less commonly, in the feces [12]. In a study of 7 healthy adult men and women, 100 mg of pentobarbital was administered as an intravenous bolus. A 2 compartment model determined the central Vd to be 27.8 L, with a peripheral Vd of 35.6 L. Using the reported mean weight of 64 kg, this translates to 0.43 L/kg and 0.56 L/kg. The elimination half life (t 1/2) was 22.3 hours [13].
High-dose pentobarbital infusions have been advocated as an effective adjunct in controlling persistent intracranial hypertension after severe head trauma in patients refractory to conventional therapy. Pentobarbital disposition was assessed in 10 adults with severe nonpenetrating head injury. An intravenous loading dose of pentobarbital (10 mg/kg) was infused over 1 hour, followed by a continuous infusion at 0.5 to 3.0 mg/kg/hr. Pharmacokinetic parameters of Vd, CL, and t 1/2 were determined. The mean t 1/2, Vd and CL were 15.6 hours, 44L, and 2 L/hr were reported [14]. In another study, six adult patients with severe head injury received a 25–34 mg/kg intravenous loading dose followed by a 1–3 mg/kg/hr continuous infusion of pentobarbital for 61–190 hours. Dosing rates were adjusted based on the patient’s clinical status. The mean weight was 73 kg. The mean CL was 3 L/hr with a volume of distribution of 75 L (1.03 L/kg) and a t 1/2 of 19.1 hr [15].. Our model would predict a CL of 3.02 L/hr (0.04 L/hr/kg) using a weight of 73 kg, with no age or bypass effects.
The pharmacokinetics of pentobarbital was examined in 11 children (5 months – 15 years) with Reye syndrome, hypoxic encephalopathy, or acute head injury. Nine of these patients were hypothermic (less than 32 degrees C). The total CL was reported to be 0.7 L/hr. The CL and Vd of pentobarbital were significantly reduced in these pediatric patients when compared with previous data in normothermic healthy adult volunteers. It was concluded that the “diminished” systemic clearance of pentobarbital in children (0.7 L/hr) as compared with that in adults (3 L/hr) may result from decreases in intrinsic enzyme activity that accompany hypothermia, as well as hepatic dysfunction in patients with Reye syndrome [16].. However, the authors compared the clearances in terms of L/hr, when a weight-normalized CL (L/hr/kg) is a more appropriate comparison. For example, using their weight of 24.5 kg, our current allometrically scaled model would predict a CL of 1.35 L/hr or 0.05 L/hr/kg, assuming no age or bypass effects. Although the predicted CL in L/hr in the pediatric (1.35) population is less than that in the adult population (3.02 L/hr), the predicted weight-normalized CL in the pediatric population (0.055 L/hr/kg) is actually greater than that in the adult population (0.04 L/hr/kg). This finding contributes to the growing empirical evidence in support of allometrically-based body size scaling of pharmacokinetic variables, an observation which is well established and based on a theoretical derivation [9,10]. It is because of the allometric nature of physiologic processes, such as systemic clearance, that CL estimates that are not weight normalized (L/hr) appear to result in greater clearance in adults as compared with smaller children. This is demonstrated in Table III by the 2-fold change in L/hr when moving from a 7 kg child to a 15 kg child. The factors pointed out by the authors (enzyme activity and hepatic dysfunction) may impact CL, but it is impossible to understand the influence of these factors without an appropriate weight based adjustment. The model presented here allows us to describe change in CL across a range of weights using a single variable. Once differences in body weight were accounted for, the influences of age on CL could be further explored. For pentobarbital, the relationship between age and CL remained for children less than one year of age, even after the body size contribution to the variability in CL was accounted for. From a practical point of view and based on pharmacokinetics alone because the per kg CL is not constant, the dosing requirements on a per kg basis may not be constant, and younger infants may require more drug when dosed on a per kg basis. Of course this CL weight relationship must be viewed in the context of safety and efficacy. A link to pharancodynamic endpoints would be required to construct a formal dosing rule.
Previously reported CL values in hypothermic children with hepatic dysfunction are slightly lower than our model predicts. Ongoing trials may be warranted to further explore the impact of cardiopulmonary bypass on pharmacokinetic parameters. Additional pharmacodynamic studies will be essential to derive meaningful dosing guidance in the complex pediatric population, with focus on measures of sedation, the use of adjunct sedatives and cardio-respiratory adverse events.
Acknowledgments
This research would not be possible without the support of the Cardiac Center and Cardiac ICU staff, Clinical and Translational Research Center nursing, and the Division of Critical Care Medicine study coordinator, Mary Ann DiLiberto, and research assistant, David Kang.
Supported by NIH, CTRC UL1 RR024134 and NICHD, K23 HD048817.
List of abbreviations
- kg
kilogram
- L
liter
- mcg
microgram
- mL
milliliter
Footnotes
Reprints: Reprints will not be available.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Pharmacologic Approach to the Critically Ill Patient. Baltimore: Williams and Wilkins; 1994. [Google Scholar]
- 2.Hubbard AM, Markowitz RI, Kimmel B, Kroger M, Bartko MB. Sedation for pediatric patients undergoing CT and MRI. J Comput Assist Tomogr. 1992;16:3–6. doi: 10.1097/00004728-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Strain JD, Campbell JB, Harvey LA, Foley LC. IV Nembutal: safe sedation for children undergoing CT. AJR Am J Roentgenol. 1988;151:975–9. doi: 10.2214/ajr.151.5.975. [DOI] [PubMed] [Google Scholar]
- 4.Strain JD, Harvey LA, Foley LC, Campbell JB. Intravenously administered pentobarbital sodium for sedation in pediatric CT. Radiology. 1986;161:105–8. doi: 10.1148/radiology.161.1.3763851. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg SB, Adams RC, Aspinall CL. Initial experience with intravenous pentobarbital sedation for children undergoing MRI at a tertiary care pediatric hospital: the learning curve. Pediatr Radiol. 2000;30:689–91. doi: 10.1007/s002470000304. [DOI] [PubMed] [Google Scholar]
- 6.Kienstra AJ, Ward MA, Sasan F, Hunter J, Morriss MC, Macias CG. Etomidate versus pentobarbital for sedation of children for head and neck CT imaging. Pediatr Emerg Care. 2004;20:499–506. doi: 10.1097/01.pec.0000136065.22328.df. [DOI] [PubMed] [Google Scholar]
- 7.Rooks VJ, Chung T, Connor L, Zurakowski D, Hoffer FA, Mason KP, Burrows PE. Comparison of oral pentobarbital sodium (nembutal) and oral chloral hydrate for sedation of infants during radiologic imaging: preliminary results. AJR Am J Roentgenol. 2003;180:1125–8. doi: 10.2214/ajr.180.4.1801125. [DOI] [PubMed] [Google Scholar]
- 8.Ette EI, Sun H, Ludden TM. Balanced designs in longitudinal population pharmacokinetic studies. J Clin Pharmacol. 1998;38:417–23. doi: 10.1002/j.1552-4604.1998.tb04446.x. [DOI] [PubMed] [Google Scholar]
- 9.Anderson BJ, McKee AD, Holford NH. Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin Pharmacokinet. 1997;33:313–27. doi: 10.2165/00003088-199733050-00001. [DOI] [PubMed] [Google Scholar]
- 10.West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284:1677–9. doi: 10.1126/science.284.5420.1677. [DOI] [PubMed] [Google Scholar]
- 11.Ravva P, Gastonguay MR, Tensfeldt TG, Faessel HM. Population pharmacokinetic analysis of varenicline in adult smokers. Br J Clin Pharmacol. 2009;68(5):669–81. doi: 10.1111/j.1365-2125.2009.03520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nembutal (pentobarbital Sodium) Physician’s Desk Reference. 2006. [Google Scholar]
- 13.Ehrnebo M. Pharmacokinetics and distribution properties of pentobarbital in humans following oral and intravenous administration. J Pharm Sci. 1974;63:1114–8. doi: 10.1002/jps.2600630717. [DOI] [PubMed] [Google Scholar]
- 14.Bayliff CD, Schwartz ML, Hardy BG. Pharmacokinetics of high-dose pentobarbital in severe head trauma. Clin Pharmacol Ther. 1985;38:457–61. doi: 10.1038/clpt.1985.204. [DOI] [PubMed] [Google Scholar]
- 15.Wermeling DP, Blouin RA, Porter WH, Rapp RP, Tibbs PA. Pentobarbital pharmacokinetics in patients with severe head injury. Drug Intell Clin Pharm. 1987;21:459–63. doi: 10.1177/106002808702100517. [DOI] [PubMed] [Google Scholar]
- 16.Schaible DH, Cupit GC, Swedlow DB, Rocci ML., Jr High-dose pentobarbital pharmacokinetics in hypothermic brain-injured children. J Pediatr. 1982;100:655–60. doi: 10.1016/s0022-3476(82)80780-4. [DOI] [PubMed] [Google Scholar]