Abstract
During cancer progression malignant cells undergo epithelial-mesenchymal transitions (EMTs) and mesenchymal-epithelial transitions (METs) as part of a broad invasion and metastasis program. We previously observed MET events among lung metastases in a preclinical model of prostate adenocarcinoma that suggested a relationship between epithelial plasticity and metastatic spread. We thus sought to translate these findings into clinical evidence by examining the existence of EMT in circulating tumor cells (CTCs) from patients with progressive metastatic solid tumors, with a focus on men with castration-resistant prostate cancer (CRPC) and women with metastatic breast cancer (BC). We show that the majority (>80%) of these CTCs in patients with metastatic CRPC co-express epithelial proteins such as EpCAM, CK, and E-cadherin, mesenchymal proteins, including vimentin, N-cadherin, and O-cadherin, and the stem cell marker CD133. Equally, we find that over 75% of CTCs from women with metastatic BC co-express cytokeratin, vimentin, and N-cadherin. The existence and high frequency of these CTCs co-expressing epithelial, mesenchymal, and stem-cell markers in patients with progressive metastases has important implications for the application and interpretation of approved methods to detect CTCs.
Keywords: metastatic castration resistant prostate cancer (mCRPC), epithelial mesenchymal transition (EMT), mesenchymal epithelial transition (MET), circulating tumor cells (CTCs), metastatic breast cancer, N-Cadherin, CD133, O-Cadherin, EpCAM
Introduction
Most metazoan cells can be classified as either epithelial or mesenchymal based on morphology, behavior and molecular signatures. In adult animals epithelial and mesenchymal cells usually remain in one phenotypic state; that is, epithelial cells do not change their properties and become mesenchymal. During development, however, epithelial cells of the early embryo give rise to all three embryonal layers (endoderm, mesoderm and ectoderm), which include mesenchymal cells (1). Therefore, these early embryonal cells have the ability to transition between epithelial and mesenchymal states, a property we define as epithelial plasticity (for a slightly different definition see (2)). Indeed, observations in embryos demonstrated epithelial-mesenchymal transitions (EMTs) as well as mesenchymal-epithelial transitions (METs) (3), which may be viewed, perhaps naively, as forward and reverse directions of the same reaction mechanism (for review, see (2, 4)).
While it is convenient to present EMT/MET as a reversible reaction between binary states, there are suggestions that intermediate states exist and that these may play important roles. The importance of EMT and MET in cancer progression is now widely, albeit not unanimously, accepted (see recent reviews (4–8)). EMT is observed in human cancer cells in vitro and in xenografts, and in the leading edge of invasive carcinomas in vivo (5, 6). In human prostate carcinoma, loss of E-cadherin expression and over-expression of N-cadherin, which indicates the presence of an EMT, independently correlates with high Gleason score and systemic and metastatic recurrence after surgery, linking EMT to more aggressive clinical behavior (9–12). In addition, recent studies have demonstrated the importance of N-cadherin expression during castration-resistant metastatic progression in preclinical models of prostate cancer and in human metastases These translational studies have suggested a link between loss of epithelial markers, gain of mesenchymal markers, and the induction of signaling pathways that promote survival and androgen-receptor independent growth (13). In breast cancer, a similar link has been established between EMT markers in primary and disseminated bone marrow tumor cells and aggressive clinical behavior (14–18). Likewise, evidence for MET was obtained from microscopic analysis of colorectal carcinoma metastases, which adopted epithelial characteristics of the non-invasive regions of the primary tumor (19). In prostate cancer, attachment of metastatic cells to bone cells correlates with expression of E-cadherin (20). These and many other studies describe the existence of these transitions during carcinogenesis and raise questions about their functional importance. There is strong evidence that EMT is important for metastatic behavior and chemoresistance (18, 21); however, the importance of MET has been more difficult to ascertain. Previously, we found that the preponderance of MET events among lung metastases in rats bearing AT3 rat prostate adenocarcinoma tumors suggested an important functional relationship between the capacity to revert to a more epithelial state and metastatic growth in the lung parenchyma (22, 23).
A strict view of epithelial plasticity in cancer posits that a mesenchymal-like state reached post-EMT is the driver of malignant fitness. Indeed there is strong evidence that the mesenchymal properties of invasiveness and motility are required for metastases (see above) and that EMT leads to expression of cancer stem cell markers, including CD44 (24). Nonetheless, observations above suggest that mesenchymal properties per se are not sufficient for optimal malignant behavior (19, 22, 23, 25). A broader interpretation suggests that the ability to easily transition between epithelial-like and mesenchymal-like states, which we define as phenotypic plasticity, may be linked to stem cell-like properties and is a more important determinant of aggressive metastatic behavior than the properties of the end states. In a preclinical model examining the importance of stem cells and cancer growth, subcutaneous injection of CD133-positive cells, which are predicted to be stem-cell like, into immunodeficient mice causes tumor growth, whereas injection of CD133-negative cells do not (26). A recent clinical study that measured mRNAs coding for stem-like markers in the bloodstream of patients with resected colorectal cancer found that the expression of CD133, CEA, and cytokeratin RNAs was associated with recurrent disease and an overall poor prognosis (27).
Therefore, we posit that the most plastic cells will be those that inhabit transitional or intermediate states with properties of both epithelium and mesenchyme, and that these transitional cells will be particularly malignant and stem-like. In order to test this proposal in human disease, we sought evidence for markers of both mesenchymal and stemness phenotypes in CTCs from patients with metastatic castration-resistant prostate cancer (CRPC) and metastatic breast cancer (BC).
Materials and Methods
Analysis of human circulating tumor cells
Patients eligible for the CTC biomarker protocols included 1) men with mCRPC, with metastatic progression by PSA (two consecutive rises over nadir separated by >1 week) or radiologic criteria (RECIST or new bone scan lesions), a PSA ≥5, age ≥18 years; or 2) women with mBC with disease progression or with initiation of a new systemic therapy, who were >18 years of age, and who were at least 7 days from treatment with an anthracycline-containing regimen. All subjects provided informed consent as part of an IRB-approved prospective clinical protocol. Blood (15ml) was collected from patients and processed within 48 hours at the Duke University Department of Molecular Pathology and clinical pathology laboratory using the Cell Search System (Veridex, Raritan, NJ). Veridex profile kits, which isolate EpCAM positive cells using a ferromagnetic immunoabsorbtion assay without additional staining, were used to collect CTCs. An additional tube was collected and processed in parallel for CTC enumeration using the Veridex Cellsearch method using the standard test kit. Following profile kit processing, the isolated cells were either processed immediately or stored overnight in 4% paraformaldehyde (PFA) and processed the next day. An initial wash using a bench top magnet to enrich the EpCAM-bound cells was performed to further isolate CTCs, with resuspension of the cell pellet after magnet release into 100 μL PBS. Immunostaining was done on teflon coated slides. Briefly, cells were pipetted into the wells of the slides and left to settle for ~ 30 minutes followed by standard immunostaining procedures with careful aspiration to minimize cell loss at room temperature. Following 4% PFA fixation, permeabilization with PBT (PBS with 0.2% v/v Triton), and blocking with 10% goat serum for 30 minutes, triple immunostaining was performed using CD45 antibody labeled with Alexa 647, cytokeratin labeled with Alexa 555 and either Vimentin (BD Biosciences, San Jose CA, 550513), N-cadherin (BD Biosciences), O-cadherin (Invitrogen, Carlsbad CA), or CD133 (Novus Biologics, Littleton CO) labeled with Alexa 488. Supplementary Table 1 provides specifics on the control cells, dilutions, and products used. Nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI) was then performed. A CTC was defined as an intact cell, containing a nucleus and expressing cytokeratin but lacking CD45 expression. Control cells were evaluated in parallel with each patient sample for immunofluorescent staining intensity and scoring (see Supplementary Table 1 for controls used and Supplementary Figure 1 for images of these real time controls). Human peripheral blood mononuclear cells (PBMCs), obtained by Ficoll purification of buffy coats from normal American Red Cross donors, were kindly provided by Micah Luftig (Duke) and used as positive control cells for CD45 expression and negative controls for CK and cadherin proteins. In addition, the co-expression of E-cadherin (BD Biosciences) with N-cadherin on EpCAM-captured, CD45-negative DAPI positive cells, irrespective of their cytokeratin expression, was examined. In this situation, all CD45-negative cells were manually enumerated and the proportion of CD45-negative nucleated cells that expressed E- or N-cadherin were scored. In order to ensure that the antibodies against E- and N-cadherin (Supplementary Table 1) did not cross-react with their respective antigens, a series of control cells with known E- and N-cadherin expression levels were examined by western blot analysis against E- and N-cadherin as well as actin as a loading control.
The slides were mounted with gel/mount media (Biomeda, Foster City, CA). The slides were analyzed with an Olympus (Melville, NY) IX 71 epifluorescence microscope, and images were acquired using an Olympus DP70 digital camera. Image processing was done with DP Controller software (Olympus). All fields on each slide were analyzed sequentially, with each cytokeratin-positive nucleated cell that was CD45-negative being counted as a CTC. Standardized exposure times optimized for each antibody were used consistently throughout the analysis for case and control cells.
Immunohistochemical (IHC) analysis of metastases
Under the same informed consent protocol, men undergoing CTC collection additionally consented to have a radiologic-guided metastatic biopsy for analysis of biomarker expression by IHC. Samples were obtained through core needle biopsies during light sedation, and immediately formalin-fixed and paraffin embedded. For analysis, slides were deparaffinized, rehydrated, and endogenous peroxidase was inactivated for 30 min. in 0.3% H2O2 (hydrogen-peroxide) in methanol. Specific antigen retrieval steps were performed for individual antigens. Three markers were evaluated by IHC: vimentin (M7020, Dako, 1:150; antigen retrieval with pepsin treatment at 37ºC for 15 minutes), cytokeratin cocktail (18–0132, Invitrogen, 1:50 and 349205, BD Biosciences 1:50, antigen retrieval with pepsin treatment at 37ºC for 15 minutes) and CD45 (M0701, Dako, 1:200; antigen retrieval with sodium citrate 10 mM, pH 6.0 at 100ºC for 30 minutes). Primary antibody was incubated for 60 minutes at room temperature. Dako Envision horseradish peroxidase secondary antibody was used for 30 minutes at room temperature and the signal was detected with DAB reagent (Vector kit SK 4100). Slides were counter stained with hematoxylin and eosin and assessed by a trained pathologist for expression using appropriate positive (localized prostate tissue microarray sections) and negative controls (mock antibody) for each marker.
Statistical analyses
We used simple descriptive statistics to estimate the prevalence of mesenchymal and CD133 antigen co-expression on EpCAM-captured CTCs, summarizing these findings on both an intra-individual level and within group (CRPC and BC) level. In order to compare CTC count (standard Cellsearch® method) against the proportion of CTCs that co-express vimentin, N/O-cadherin, or CD133, linear regression analysis was performed. Goodness of fit was tested by analysis of variance.
Results
In order to examine the co-expression of mesenchymal and/or stemness antigens on CTCs from patients with metastatic disease, we took advantage of the existing FDA-approved capture method to initially capture and isolate cells from whole blood. CTCs have both independent prognostic and predictive significance in multiple epithelial malignancies, including mCRPC and mBC (28, 29), and can be collected, isolated, and analyzed for a variety of biomarkers relevant to cancer biology (30–32). The approved technology for CTC capture relies on the expression of EpCAM (Epithelial Cell Adhesion Molecule) on the surface of epithelial cells, and thus currently measured CTCs must be epithelial-like. Yet these cells have escaped from the primary tumor, possibly as a result of an EMT/invasiveness program, and may have mesenchymal properties.
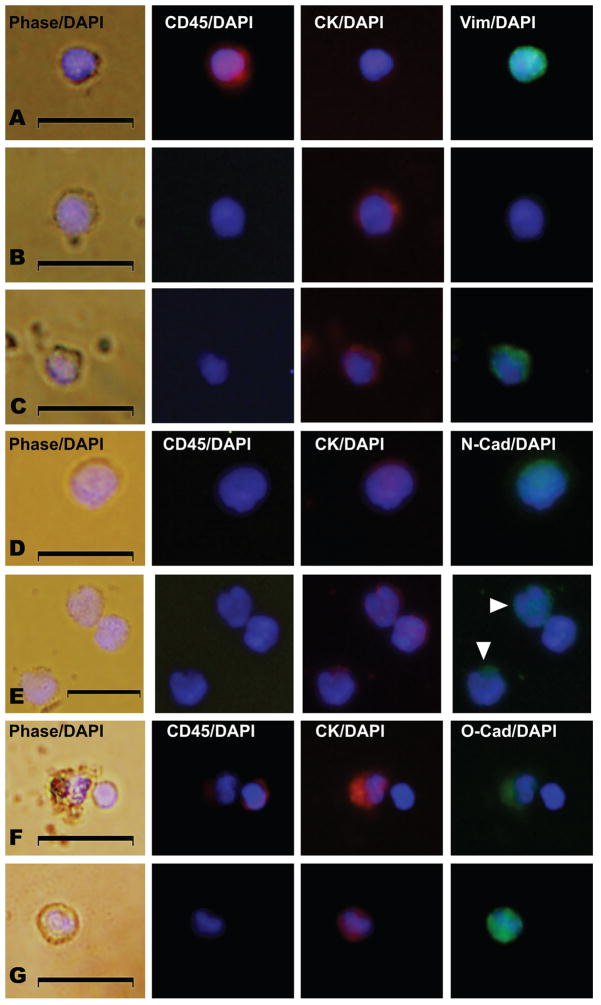
To test for the existence of mesenchymal-like CTCs, blood was collected from 41 men with mCRPC and 16 women with mBC (see baseline characteristics for the patients in Table 1 and Supplementary Table 2) and CTCs were processed using the CellSearch® EpCAM-based immunocapture method and profiled for expression of CD45 (PTPRC) (a leukocyte marker), cytokeratins (CK) and E-cadherin (CDH1) (epithelial markers), vimentin (VIM) , N-cadherin (CDH2), and O-cadherin (CDH11) (mesenchymal markers), and CD133 (a stem cell marker) by immunofluorescence (IF) (10, 33) (Supplementary Table 1). Leukocytes were defined as nucleated (DAPI positive), CD45-positive and CK-negative cells (Figure 1A), whereas CTCs were defined as nucleated (DAPI positive), CD45-negative and CK-positive cells (Figure. 1B–G). Among CTCs we identified subsets of cells that additionally expressed vimentin (Fig. 1C) or N-cadherin (Figure. 1D & E) or O-cadherin (Figure 1F & G). Real-time positive and negative control cells were used at the time of individual patient CTC analysis to determine positive or negative IF expression using identical exposure, antibody concentration, and camera settings without alteration. Real-time control cell images for each corresponding antigen and subject depicted in Figures 1, 3 and 4 are provided in Supplementary Figure 1.
Table 1.
Baseline demographic and clinical characteristics of the men with metastatic CPRC in this study (n=41).
| DEMOGRAPHICS | n = 41 | |
|---|---|---|
| Median Age, years (range) | 71 (50–89) | |
| Race, Ethnicity | ||
| White, non-Hispanic | 73 % | |
| Black, non-Hispanic | 27 % | |
|
| ||
|
BASELINE DISEASE HISTORY
| ||
| Median Gleason Score (range) | 8 (5–10) | |
| Median Baseline PSA1 (ng/dl, range) | 248.9 (14.0–13,419.5) | |
| Median Baseline Pain (range)2 | 0 (0–7) | |
| Median Karnofsky Performance Status (range) | 90 (60–100) | |
| Median Number of Prior Hormonal Therapies (range) | 2.5 (0–5) | |
| Prior Chemotherapy | 68 % | |
| Prior Bisphosphonates | 73 % | |
|
| ||
|
SITES OF METASTATIC DISEASE
|
||
| Visceral (lung + liver) | 54% | |
| Lymph Node Only | 0% | |
| Metastatic to Bone: | ||
| Metastatic to Bone With Lymph Nodes (no visceral metastases) | 24% | |
| Metastatic to Bone Without Lymph Nodes (no visceral metastases) | 22% | |
PSA: prostate specific antigen.
Pain is scored as a linear analog scale (0–10 range).
Figure 1. Co-expression of epithelial and mesenchymal proteins in CTCs from men with metastatic castration resistant prostate cancer (mCRPC).
All panels represent merged images derived from phase/DAPI, CD45/DAPI, CK/DAPI, and either vimentin(Vim)/DAPI, N-cadherin(N-cad)/DAPI expression, or O-cadherin(O-cad)/DAPI as indicated. Shown are examples of (A) a leukocyte with CD45 expression, (B) a CTC with no vimentin expression, (C) a CTC with vimentin expression, (D) a CTC with N-cadherin expression, (E) three CTCs, two with N-cadherin expression (arrowheads), (F) a CTC with O-cadherin expression and a nearby leukocyte, and (G) an additional CTC with O-cadherin expression. Scale bars represent 20 μm and were added from an image taken at identical magnification and resolution. Control cells were assayed in parallel at the same time of CTC collection and analysis with each set of patient samples and are shown in Supplementary Fig. 1.
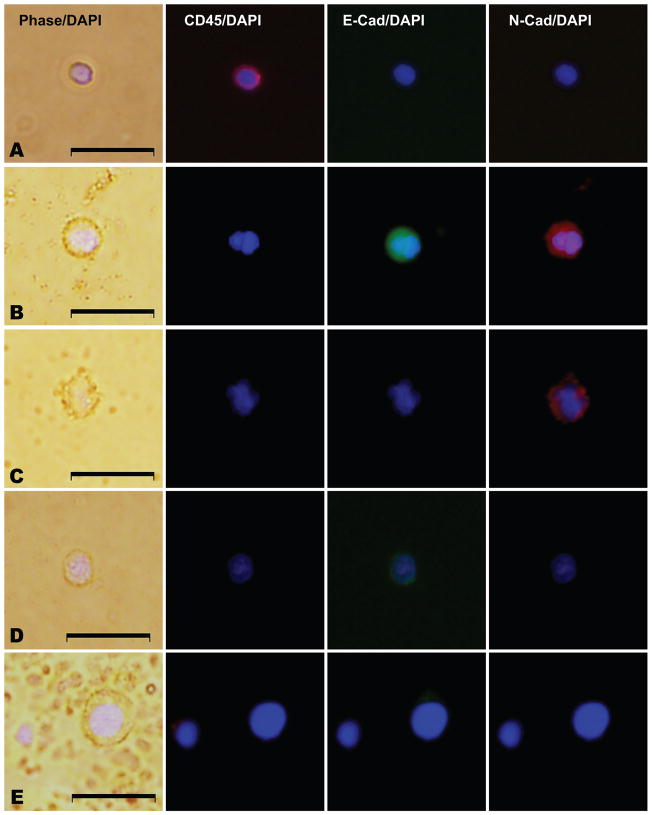
Figure 3. E-cadherin and N-cadherin co-expression among CD45 negative nucleated cells from men with metastatic CRPC.
All panels represent merged images derived from phase/DAPI, CD45/DAPI, E-cadherin(E-cad)/DAPI, and N-cadherin(N-cad)/DAPI expression as indicated. (A) a leukocyte with CD45 expression, (B) a CD45 negative nucleated cell, with E-cadherin and N-cadherin co-expression, (C) a CD45 negative nucleated cell with N-cadherin expression and no E-cadherin expression, (D) a CD45 negative nucleated cell with E-cadherin expression and no N-cadherin expression, and (E) a nucleated cell lacking expression of CD45, E-cadherin, and N-cadherin. Scale bars represent 20 μm and were added from an image taken at identical magnification and resolution. Control cells were assayed in parallel at the same time of CTC collection and analysis with each set of patient samples and are shown in Supplementary Figure 1.
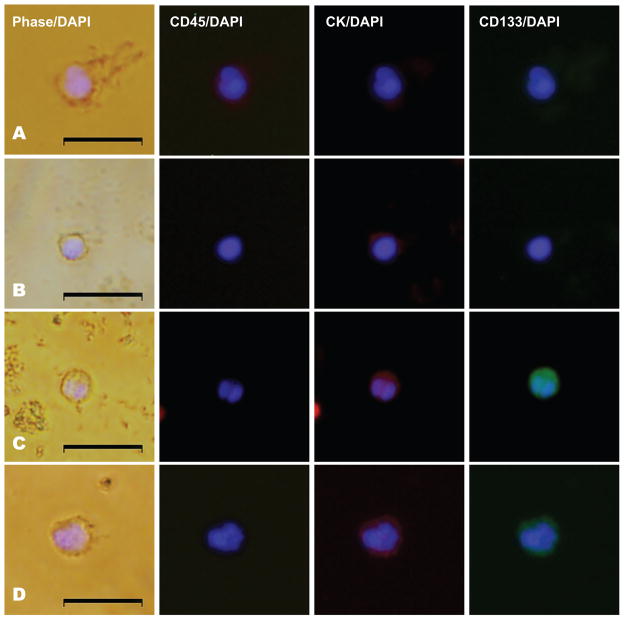
Figure 4. Expression of a stem-like cell marker CD133 in CTCs from men with mCRPC.
All panels represent merged images derived from phase/DAPI, CD45/DAPI, CK/DAPI, and CD133/DAPI expression as indicated. Shown are examples of (A) a leukocyte with CD45 expression, (B) a CD133 negative CTC, (C) a CD133 positive CTC, and (D) an additional example of a CD133 positive CTC. Scale bars represent 20 μm and were added from an image taken at identical magnification and resolution. Control cells were assayed in parallel at the same time of CTC collection and analysis with each set of patient samples and are shown in Supplementary Figure 1.
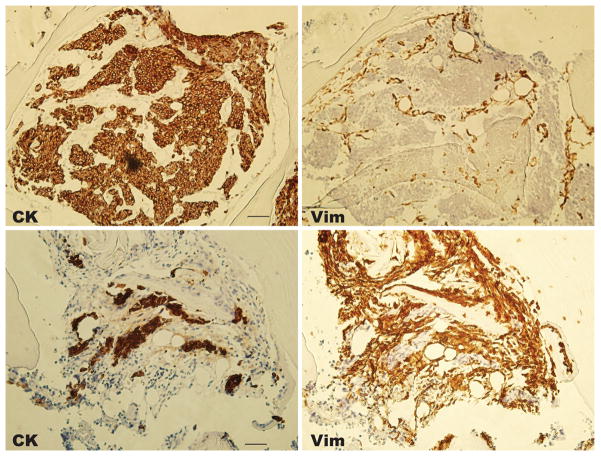
Among men with mCRPC, we found that CTCs co-expressed vimentin and CK in 10/10 (100%) patients, and by this criterion 108/126 (86%) of enumerated CTCs were transitional (Figure 1B–C, Table 2 and Supplementary Table 3, and also see Supplementary Figure 1 for control cells and Supplementary Figure 2 for additional examples). Biopsies of bony metastases performed within one week of initial CTC collection in two of these patients (patients 6 and 7 in table 1) revealed no vimentin expression in the CK positive tumor foci, but strong vimentin expression in the surrounding bone stroma, which lacks CK expression (Figure 2). These same patients had CTCs taken at the same time as the CT-guided tumor biopsy that commonly expressed co-expressed CK and vimentin. These findings are consistent with invasion and metastasis by CTCs that subsequently are undergo EMT/MET or exist in a transitional state; an alternative explanation may be that vimentin co-expression may be heterogeneous in metastases, similar to CTC expression.
Table 2.
Prevalence of EMT and stemness antigen marker expression by immunofluorescence in circulating tumor cells (intact, nucleated CK+, CD45-, DAPI + cells) from the circulation of men with metastatic castration-resistant prostate cancer (CRPC) and women with metastatic breast cancer (BC). Columns on the right indicate the number of manually scored CTCs scoring positive for each marker and the number of patients in each group who have at least one CTC that stains positive for a given marker.
| Antigen | n | Marker-Positive CTCs (%) | Patients with Marker-Positive CTCs (%) |
|---|---|---|---|
|
| |||
| Vimentin (CRPC) | 10 | 108/126 (86%) | 10/10 (100%) |
| Vimentin (BC) | 10 | 65/97 (65%) | 7/10 (70%) |
| N-Cadherin (CRPC) | 11 | 205/244 (84%) | 11/11 (100%) |
| N-Cadherin (BC) | 6 | 78/95 (82%) | 4/6 (67%) |
| O-Cadherin (CRPC) | 6 | 107/120 (89%) | 6/6 (100%) |
| CD133 (CRPC) | 11 | 127/153 (83%) | 9/11 (82%) |
Figure 2. Expression of vimentin and cytokeratin in prostate cancer metastases.
Images are taken from a CT-guided targeted bone metastasis biopsy at the same time as circulating tumor cells were collected and evaluated for vimentin co-expression by immunofluorescence as described. Images are from patient 6 (top) and patient 7 (bottom), with CK (left) and vimentin (right) expression assayed by immunohistochemistry (20X magnification). Scale bars in the CK panels represent 50 μm and were added from an image taken at identical magnification and resolution.
Among the next cohort of eleven men with mCRPC, we found CTCs co-expressing N-cadherin and CK in 11/11 (100%) patients, and by this criterion 205/244 (84%) of CTCs were identified as co-expressing these markers (Figure 1D & E, Table 2 and also see Supplementary Table 3, Supplementary Figure 1 for real-time control cells and Supplementary Figure 3 for additional examples). The expression of N-cadherin among CTCs varied from undetectable, determined by the real-time negative controls described in Supplementary Table 1, to very strong (Figure 1D). This variation was observed among CTCs from individual patients as can be seen by examination of three CTCs from patient 11 (Figure 1E). While we noted heterogenous vimentin expression among patient and control leukocytes, we did not observe N-cadherin expression among patient or control leukocytes.
Among ten women with mBC, nine had detectable CTCs and of these, we found evidence of vimentin co-expression in seven (78%) patients, and 55/88 CTCs overall (63%) co-expressed vimentin (Supplementary Figure 2, Table 2 and Supplementary Table 4, and controls in Supplementary Figure 1). Among another six women with detectable CTCs and mBC, four had evidence of CK and N-cadherin co-expression, and overall 78/95 CTCs (82%) had N-cadherin expression, with significant heterogeneity in expression in a given individual (Table 2, Supplementary Figure 3 and controls in Supplementary Figure 1). These data indicate that the majority of CTCs in patients with mBC and mCRPC co-express epithelial (EpCAM and cytokeratin) and mesenchymal (vimentin, N-cadherin) markers, and thus exist in a transitional phenotypic state, similar to that observed in our preclinical models.
Given these findings, we next sought to better characterize the dual expression of the epithelial E-cadherin with the more mesenchymal N-cadherin on circulating CD45-negative nucleated cells. Western blot analysis of control cells (T47D, BT549, PC-3, and PBMC cells) indicated a lack of cross-reactivity of our E, N, or O-cadherin antibodies against their respective antigens (data not shown), providing assurance that these antibodies are able to measure the intended target antigen. In an additional cohort of 6 men with progressive metastatic CRPC, we characterized all CD45 negative cells that were initially captured using the standard Cellsearch® method. Given the limited number of proteins that may be evaluated using IF in an individual cell, we examined the presence of both E and N-cadherin expression on these CD45 negative cells but are not able to additionally measure CK expression and therefore cannot define a CTC as described above. Interestingly, as shown in Table 3, Figure 3, and Supplementary Figure 1, we found clear evidence for four distinct subtypes of CD45-negative cells, including those cells that co-expressed both E and N-cadherin (20–71% of cells examined in a given subject), cells that expressed E but not N-cadherin (2–22% of cells), cells that expressed N but not E-cadherin (0–45% of cells), and cells that lacked both cadherin family members (25–48% of cells). The proportion of CD45 negative cells that expressed N-cadherin was similar to the proportion that expressed E-cadherin (61% vs. 53%), although this varied greatly in individual men (48–72% vs. 23–75%, respectively). Finally, we noted that the subtype of E-cadherin negative/ N-cadherin positive cells was relatively rare among these EpCAM-captured cells except in two men (subjects 39 and 40) who had progressive disease on their most recent treatment with the mTOR inhibitor temsirolimus as part of an experimental protocol. While these analyses are limited to those cells captured based on EpCAM expression, the results indicate that CTCs co-express epithelial and mesenchymal proteins, and suggest the existence of currently undetected CTCs with reduced or absent epithelial markers. Methods that evaluate whole blood for non-leukocyte nucleated cellular populations without initial EpCAM-based capture may thus be able to identify these additional cells.
Table 3.
Prevalence of E-cadherin and N-cadherin expression on CD45 negative nucleated cells isolated from the circulation using an EpCAM-based ferrofluid (Cellsearch® method) among men with progressive metastatic CRPC. Clinical CTC= enumeration using the FDA approved method. Each column represents the number and percent of CD45 nucleated cells that were identified based on dual marker expression. *indicates two patients with metastatic CRPC who were progressing on therapy with the mTOR inhibitor temsirolimus.
| Subject Number | Clinical CTC | CD45 Negative Cells | E + N+ Cells (%) | E−N− Cells (%) | E+ N− Cells (%) | E− N+ Cells (%) |
|---|---|---|---|---|---|---|
| 35 | 7 | 24 | 17 (71%) | 6 (25%) | 1 (4%) | 0 (0%) |
| 36 | 55 | 23 | 11 (48%) | 8 (35%) | 4 (17%) | 0 (0%) |
| 37 | 112 | 122 | 82 (67%) | 30 (25%) | 4 (3%) | 6 (5%) |
| 38 | 1000 | 65 | 27 (42%) | 22 (34%) | 14 (22%) | 2 (3%) |
| 39* | 45 | 44 | 9 (30%) | 21 (48%) | 1 (2%) | 13 (30%) |
| 40* | 16 | 83 | 18 (22%) | 26 (31%) | 2 (22%) | 37 (45%) |
| Summary | 1235 | 361 | 164 (45%) | 113 (31%) | 26 (7%) | 58 (16%) |
Given that prostate cancer has a tendency to metastasize to bone, we next hypothesized that adhesion molecules that favor an osteoblastic tumor microenvironment may be visualized on CTCs from men with CRPC. O-cadherin has been recently linked to metastasis to bone in preclinical models of prostate cancer (34, 35), and we thus wanted to examine its co-expression with CK in CTCs. Indeed we found co-expression in six out of six men detectable CTCs by EpCAM–based ferromagnetic capture, and O-cadherin was expressed in 64–96% of CTCs, and any O-cadherin expression in 100% of all men with CRPC (examples shown in Figure 1F & G, summarized in Table 2 and Supplementary Table 3 with controls in Supplementary Figure 1). These findings suggest that prostate cancer CTCs co-express adhesion molecules that may promote homing to bone and homotypic binding to osteoblastic cells.
Given the expression of the stem cellassociated antigen CD133 in putative prostate cancer stem cells and other cancer stem cell populations (27, 33, 36), we investigated CD133 expression in CTCs from men with mCRPC. We found CD133 to be expressed in 11/11 (100%) men with CTCs, and in 127/153 (83%) of CTCs from these men (Figure 4, Table 2, Supplementary Figure 4 and controls in Supplementary Figure 1). These data suggest that CTCs from patients with common epithelial malignancies co-express both epithelial and mesenchymal markers, suggesting that EMT/MET transitions may be contributing to metastatic progression. In addition, in men with metastatic CRPC, the co-expression of the stemness antigen CD133 in the majority of CTCs suggests that these cells may have acquired properties of stemness during their migration into the bloodstream (21, 24).
Discussion
In these studies, we have identified biomarkers suggestive of epithelial plasticity and stemness in CTCs from patients with common metastatic epithelial malignancies, including breast and prostate cancer. The identification of both epithelial and mesenchymal phenotypes among CTCs in a significant subset of patient samples offers several important clinical opportunities. These data suggest that CTCs may undergo phenotypic changes from epithelial to more mesenchymal transitional states during metastatic transit, while metastases themselves may be more epithelial in phenotype and marker expression. Our findings also suggest that, in addition to cells expressing both epithelial and mesenchymal markers, there may be an unknown number of CTCs that are more mesenchymal-like and thus are EpCAM-negative. These cells will be missed by the FDA approved CellSearch® method, the Adna Test (AdnaGen AG) system, and current microfluidic technologies, which enrich for CTCs by immunoabsorbtion of cells expressing MUC1 or EpCAM (37). Indeed, recent studies in breast cancer have suggested that “normal” type breast cancer cell lines that overexpress EMT and stem cell antigens (CD44+, CD24-) may lack EpCAM and are thus not detectable by currently approved CTC detection systems (38). Therefore, it is possible that the number of CTCs in patients with metastatic cancer is much higher than currently appreciated.
It is well appreciated that cells induced to undergo EMT activate stem cell pathways (24). Indeed, a recent study demonstrated a striking relationship between prostate cancer-associated fibroblast (CAF) chemokine expression of interleukin-6 or TGF-beta and the acquisition of tumor invasiveness, metastatic propensity, EMT antigen expression, and stemness characteristics (39). Our findings suggest that CTCs captured using an epithelial-based capture assay, the current FDA standard method, express the stemness marker CD133. These findings are consistent with the results of Mani et al. (24) demonstrating a relationship between EMT and stemness states in breast cancer models. It is tempting to speculate that these CTCs may represent transitional cells with both epithelial and mesenchymal phenotypes, and this heterogeneity or plasticity may also extend to stem cell markers as well as to treatment-induced effects. For example, we observed an increased predominance of N-cadherin expression in CTCs from two men who were progressing on their most recent treatment with an mTOR inhibitor, suggesting that certain systemic agents may alter CTC phenotype. An alternative explanation to this transitional state theory is that tumor heterogeneity for these markers exist, without the need to transition phenotypically. Further preclinical and clinical studies of metastatic tumors in which the EMT/MET process is experimentally disrupted or induced may shed further light on these observations.
Our working model of plasticity also predicts that cells with maximal stem-cell character, which by definition will be highly malignant, should display both epithelial and mesenchymal traits, because they inhabit intermediate states in the epithelial-mesenchymal axis. Here we show that patients with metastatic breast and prostate carcinomas have CTCs that exist in phenotypic states that may be intermediate to epithelial and mesenchymal states. While the enumeration of epithelial antigen (EpCAM)-captured CTCs or CD133 positive CTCs correlates with disease progression, there is great heterogeneity to the number of CTCs isolated from individual patients with metastatic carcinomas, and thus a further refinement in the methods of CTC detection using EMT antigen-based capture methods may result in improved prognostication CTC identification (27–29). Aktas and colleagues recently showed that a population of cells enriched in CTCs expressed RNAs encoding mesenchymal markers; however, the study could not prove co-expression of epithelial and mesenchymal markers in the same cell (40). Based on the results presented here, further studies to explore methods to capture cells based on these markers in addition to EpCAM are warranted to investigate their relevance to metastatic progression and chemoresistance.
There are several inherent limitations to this work. First, we have not demonstrated true stemness among CTCs, which would require further experimental evidence of serial clonal passage and transplantation, or prolonged culture of CTCs from patients. This is not technically feasible with current methods. Our markers of stemness are correlative in nature only, and may be associated with properties other than stemness. We also acknowledge that co-expression of epithelial and mesenchymal markers does not, in itself, represent plasticity, as we are unable to observe this dynamic in vivo in patients. Our clinical observations suggest plasticity based on the co-expression in real time on CTCs during the process of metastasis, as well as the lack of expression of vimentin in paired metastases from the same patients. The importance of this plasticity to highly aggressive metastatic behavior can only be tested through experimental manipulation of preclinical systems in which either EMT/MET is prevented; future experiments will need to address this issue. Finally, these studies have not correlated co-expression of EMT factors on CTCs with clinical outcomes; these prognostic studies require large appropriately powered studies and patients with long-term follow-up, such as has been recently reported with CD133 positive colorectal CTCs and post-operative outcomes (27). Our findings, however, suggest that the measurement of CTCs collected through both EpCAM-enriched and EMT antigen-enriched methods may complement each other in providing prognostic or predictive information during systemic therapy that should be prospectively evaluated.
Finally, CTCs expressing mesenchymal or stem-like markers expression, which comprise the majority of cells isolated in this study, and additional cells that may go undetected due to EpCAM loss, represent a therapeutic problem. It has been well documented that EMT alters drug sensitivity (21, 41, 42) and it has been challenging to direct therapy to cancer cells with stem cell-like properties, perhaps because of their recalcitrance to undergo apoptosis (43). While recent studies suggest both a screening method and actual compounds (e.g., salinomycin) that can selectively target cancer stem cells (21), these aggressive cells still represent a formidable challenge. Our findings suggest that these cell types may be highly prevalent among patients with metastatic epithelial tumors, and suggest methods for the improved detection of these cells in vivo to assist in developing novel therapeutic strategies.
Supplementary Material
Acknowledgments
Grant Support
NIGMS grant R01 GM63090 (M.A. Garcia-Blanco); NCI grant R01 CA127727, (M.A. Garcia-Blanco). A.J. Armstrong was supported by Prostate Cancer Foundation Young Investigator Award Program, the Duke Cancer Institute K12 program (5K12-CA-100639-05, PI H.K. Lyerly), a Department of Defense Physician Research Training Award (W81XWH-10-1-0483), the American Cancer Society Pilot Grant program, and the H.L. Kirkpatrick Foundation. M.S. Marengo was supported by National Research Service Awards T32-CA059365 and F32 CA142095.
We thank Drs. M. Dewhirst, P. Febbo, J Somarelli and J. Pearson for important discussions. We also thank Dr. M. Luftig for providing buffy coats from normal donors.
Footnotes
Disclosure of Potential Conflicts of Interest: Drs. Armstrong, Oltean, George, and Garcia-Blanco are listed as inventors in a related patent application (Application Number PCT/US10/50233) filed on September 24, 2010.
Author Contributions
A.J. Armstrong, S. Oltean, D. George, and M.A. Garcia-Blanco conceived of the original study. A.J. Armstrong and M.A. Garcia-Blanco directed the research. G. Kemeny, S. Oltean, and M.S. Marengo carried out laboratory experiments. A.J. Armstrong, J. Turnbull, C.I. Herold, P.K. Marcom, and D. George carried out clinical procedures and recruitment. While all co-authors contributed to the writing of the manuscript, A.J. Armstrong, M.S. Marengo and M.A. Garcia-Blanco did the majority of writing and editing.
References
- 1.Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis. 1995;26:678–90. doi: 10.1016/0272-6386(95)90610-x. [DOI] [PubMed] [Google Scholar]
- 2.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–49. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blick T, Widodo E, Hugo H, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–42. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 6.Hugo H, Ackland ML, Blick T, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 7.Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am J Pathol. 2009;174:1588–93. doi: 10.2353/ajpath.2009.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberg RA. Twisted epithelial-mesenchymal transition blocks senescence. Nat Cell Biol. 2008;10:1021–3. doi: 10.1038/ncb0908-1021. [DOI] [PubMed] [Google Scholar]
- 9.Contreras HR, Ledezma RA, Vergara J, et al. The expression of syndecan-1 and -2 is associated with Gleason score and epithelial-mesenchymal transition markers, E-cadherin and beta-catenin, in prostate cancer. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–11. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 11.Tomita K, van Bokhoven A, van Leenders GJ, et al. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60:3650–4. [PubMed] [Google Scholar]
- 12.Zhau HE, Odero-Marah V, Lue HW, et al. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin Exp Metastasis. 2008;25:601–10. doi: 10.1007/s10585-008-9183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka H, Kono E, Tran CP, et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010;16:1414–20. doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco MJ, Moreno-Bueno G, Sarrio D, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–6. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 15.Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc Natl Acad Sci U S A. 2008;105:14867–72. doi: 10.1073/pnas.0807146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–97. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 17.Willipinski-Stapelfeldt B, Riethdorf S, Assmann V, et al. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;11:8006–14. doi: 10.1158/1078-0432.CCR-05-0632. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Brabletz T, Jung A, Reu S, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–61. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha B, Arase A, Imam SS, et al. Overexpression of E-cadherin and beta-catenin proteins in metastatic prostate cancer cells in bone. Prostate. 2008;68:78–84. doi: 10.1002/pros.20670. [DOI] [PubMed] [Google Scholar]
- 21.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–59. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oltean S, Febbo PG, Garcia-Blanco MA. Dunning rat prostate adenocarcinomas and alternative splicing reporters: powerful tools to study epithelial plasticity in prostate tumors in vivo. Clin Exp Metastasis. 2008;25:611–9. doi: 10.1007/s10585-008-9186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oltean S, Sorg BS, Albrecht T, et al. Alternative inclusion of fibroblast growth factor receptor 2 exon IIIc in Dunning prostate tumors reveals unexpected epithelial mesenchymal plasticity. Proc Natl Acad Sci U S A. 2006;103:14116–21. doi: 10.1073/pnas.0603090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 26.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 27.Iinuma H, Watanabe T, Mimori K, et al. Clinical Significance of Circulating Tumor Cells, Including Cancer Stem-Like Cells, in Peripheral Blood for Recurrence and Prognosis in Patients With Dukes’ Stage B and C Colorectal Cancer. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.30.5151. [DOI] [PubMed] [Google Scholar]
- 28.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 29.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 30.Attard G, Swennenhuis JF, Olmos D, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–8. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 31.Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–9. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wulfing P, Borchard J, Buerger H, et al. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res. 2006;12:1715–20. doi: 10.1158/1078-0432.CCR-05-2087. [DOI] [PubMed] [Google Scholar]
- 33.Zhu L, Gibson P, Currle DS, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–7. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu K, Cheng CJ, Ye X, et al. Cadherin-11 promotes the metastasis of prostate cancer cells to bone. Mol Cancer Res. 2008;6:1259–67. doi: 10.1158/1541-7786.MCR-08-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang CF, Lira C, Chu K, et al. Cadherin-11 increases migration and invasion of prostate cancer cells and enhances their interaction with osteoblasts. Cancer Res. 2010;70:4580–9. doi: 10.1158/0008-5472.CAN-09-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, Isaacs JT. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008;68:9703–11. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–9. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sieuwerts AM, Kraan J, Bolt-de Vries J, et al. Molecular characterization of circulating tumor cells in large quantities of contaminating leukocytes by a multiplex real-time PCR. Breast Cancer Res Treat. 2009;118:455–68. doi: 10.1007/s10549-008-0290-0. [DOI] [PubMed] [Google Scholar]
- 39.Giannoni E, Bianchini F, Masieri L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–56. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 40.Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11:R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomson S, Buck E, Petti F, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–62. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 42.Yauch RL, Januario T, Eberhard DA, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005;11:8686–98. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- 43.Li HZ, Yi TB, Wu ZY. Suspension culture combined with chemotherapeutic agents for sorting of breast cancer stem cells. BMC Cancer. 2008;8:135. doi: 10.1186/1471-2407-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.