Abstract
Rationale
Quantitative real-time data on the stress experienced by drug misusers in their daily lives may provide additional insight into stress’s role in drug use.
Objective
The purpose of this study is to evaluate stress in relation to craving, mood, relapse-trigger exposure, and cocaine use in cocaine-dependent outpatients.
Methods
Methadone-maintained cocaine- and heroin-abusing outpatients (N=114) provided ecological momentary assessment data on handheld computers. Ratings of stress were compared to those of craving and mood and past-hour exposure to putative drug-use triggers in randomly prompted entries and in the 5 h prior to participant-initiated cocaine use reports.
Results
Stress had significant positive relationships with current ratings of craving for cocaine, heroin, and tobacco and with ratings of tiredness, boredom, and irritation, and had significant negative relationships with ratings of happiness and relaxation. Stress was significantly greater in entries in which participants also reported past-hour exposure to negative-mood triggers, most of the drug-exposure triggers, or any trigger involving thoughts about drugs (e.g., tempted out of the blue). The linear increase in stress during the 5-h preceding individual episodes of cocaine use was not significant (p=0.12), though there was a trend for such an increase before the use episodes that participants attributed to stressful states when they occurred (p=0.87).
Conclusions
The findings suggest a complex role of stress in addiction. Stress reported in real time in the natural environment showed strong cross-sectional momentary relationships with craving, mood, and exposure to drug-use trigger. However, the prospective association between stress ratings and cocaine-use episodes was, at best, weak.
Keywords: Stress, Cocaine, Craving, Addiction, Human, Ecological momentary assessment
As is apparent throughout this special issue of Psychopharmacology and other recent literature (Briand and Blendy 2010; Koob 2009; Uhart and Wand 2009), a converging body of evidence from laboratory studies is pointing to a key role for stress in drug addiction. But stress in daily life, like other transient mental events (Ariely 1998; Shiffman et al. 1997), may be recalled quite differently from the way it is experienced. Recall biases may stymie detection of—or give false evidence of—the relationship of stress with addictive behavior in drug misusers’ daily environments.
One way to circumvent this problem is to use electronic-diary methods such as ecological momentary assessment (EMA). In EMA, study participants provide real-time reports of their activities and moods1 throughout daily life, typically on handheld computers that time-stamp each report (Shiffman et al. 2008). In cigarette smokers who reported their lapses to smoking, the content of EMA entries was often drastically different from the content of retrospective interviews conducted several weeks later, and many of the differences seemed to reflect inaccurate or biased recall in the retrospective interviews (Shiffman et al. 1997).
Using EMA, we have been able to assess stress in real time among heroin and cocaine users in their daily environments over periods of up to 25 weeks. In the analyses presented here, we examined stress in terms of its relationship with mood, craving, and putative triggers of drug use/relapse. We also examined stress ratings throughout the day as predictors of episodes of cocaine use on a 5-h time scale, attempting to replicate our prior prospective analyses of other types of triggers (Epstein et al. 2009).
We collected our EMA data in a natural-history study in which we did not systematically manipulate stress, and thus, our data cannot resolve questions of causation. Rather, they address the possibility that correlations taken as truisms are merely artifacts of biased recall (Sutton 1993). They also set the stage for hypothesis testing in future EMA studies using randomized designs.
Methods and methods
Participants and setting
Participants were cocaine- and heroin-using individuals seeking outpatient treatment for opioid dependence. Inclusion criteria were: (1) age between 18 and 65 years, (2) evidence of physical dependence on opioids (by self-report and physical examination), and (3) evidence of cocaine and opiate use (by self-report and urine drug testing). Exclusion criteria were: (1) current psychotic disorder (by Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria), history of bipolar disorder, current major depressive disorder, (2) current dependence on alcohol or any sedative-hypnotic (by DSM-IV criteria), (3) cognitive impairment severe enough to preclude informed consent or valid self-report, and (4) medical illness that would compromise study participation.
Methadone maintenance was initiated at the time of enrollment and continued for up to 28 weeks at a treatment research clinic in Baltimore, MD. Participants attended 7 days per week for oral methadone (target dose, 100 mg/day) administration; individual counseling was available once weekly, and drug use was monitored in urine drug screens conducted three times per week. In counseling sessions, reduction of substance use was the primary goal; sessions were devoted to discussion of cessation of all drugs. Counselors had access to urine drug screen results but did not have access to EMA reports of drug use. In weeks 7–18, abstinence reinforcement was in place to increase drug abstinence; participants could earn up to US $2,310 in vouchers in exchange for urine specimens negative for cocaine, opiates, or both.
The Institutional Review Board (IRB) of the NIDA Intramural Research Program approved this study. Participants gave written informed consent.
Study design
The study was designed to assess the natural history of craving and lapse in patients receiving treatment for cocaine and opioid dependence with methadone maintenance and abstinence reinforcement. At the end of the third week of the study, each participant was trained to use and was issued a personal digital assistant (PDA, i.e. Palm Zire or Palm Zire 21, Palm, Inc., Sunnyvale, CA) running our Transactional Electronic Diary software (Vahabzadeh et al. 2004). Participants were instructed to make two types of entries: event-contingent entries and randomly prompted. At each entry, participants reported where they were, whom they were with, and what they were doing and answered questions about their mood.
Participants were asked to initiate an event-contingent entry whenever they used cocaine, heroin, or both or craved without using. As part of the battery of questions, they were asked to indicate what they thought had precipitated the craving or use event, choosing from a list that included 18 putative triggers.
Random prompts were triggered two to five times per day for up to 25 weeks and were timed to occur only during each participant’s typical waking hours, which were programmed separately for each day of the week. At each random prompt, participants answered the following questions, among others, a series of questions beginning with, “In the past hour…,” which were designed to assess exposure to putative triggers of drug craving or drug use; these items were adapted from triggers reported by participants in prior studies (Heather et al. 1991; Marlatt and Gordon 1985) and were the focus of one of our previous papers (Epstein et al. 2009). Mood was assessed with six adjectives (happy, relaxed, tired, irritated, stressed, and bored) in six items worded “Right now, do you feel…” and rated on a four-point scale: “NO!! no?? yes?? YES!!.” These response anchors have been used in EMA studies by Shiffman and Paty (2006); the small number of adjectives was chosen to sample positive and negative mood without undue response burden on participants. Craving for cocaine, heroin, and tobacco smoking was assessed on the same scale and worded: “Right now, do you crave …?”
A total of 130 participants (84 men, 46 women) enrolled in the study; 114 (72 men, 42 women) attended clinic long enough to be issued a PDA and provided sufficient data for the main analyses reported here. These 114 participants did not differ significantly demographically from the other 16 in terms of demographic characteristics or drug-use history. Their mean age was 40.9 years (SD 8.1 years, range 20–58 years). Employment status was 38% unemployed, 34% employed full-time, and 27% employed part-time. Race/ethnicity was 61% African American, 37% white, and 2% Hispanic. All participants met DSM-IV criteria for cocaine dependence by structured interview (Robins et al. 1995) at study intake, though this was not an inclusion criterion; they reported having used cocaine a mean of 20.0 days out of the past 30 days (SD 9.1 days, range 4–30 days); primary routes of administration were smoking (48%), intravenous (42%), and intranasal (8%). Current use of tobacco was reported by 106 (93%) of participants. Additional demographic details for an almost identical subset of these participants are reported in previous publications of other aspects of their EMA data (Epstein et al. 2009, 2010; Preston et al. 2009).
Overall, participants made 26,734 random-prompt entries, 2,589 event-contingent entries (710 cocaine use, 663 cocaine craving, 66 heroin use, 288 heroin craving, 232 use of both drugs, and 630 craving for both drugs). During the ED portion of the study, the participants’ mean percentage of cocaine-negative urine specimens was 25% (SD 36%, median 4%, range 0% to 100%) and their mean percentage of opioid-negative urine specimens was 60% (SD 32%, median 67%, range 0% to 100%).
Data analysis
For statistical analyses, stress, mood, and craving ratings were converted from NO!!, no??, yes??, and YES!! to values 0, 1, 2, and 3, respectively. Event-contingent reports of use or craving were coded as stress-related if participants attributed the event (“I think it happened because…”) to any of the following nonmutually exclusive choices: feeling “worried, anxious, or tense,” feeling “angry or frustrated” (either “with myself or because things were not going my way” or “because of my relationship with someone else”), or feeling that “others were being critical of me.”
To evaluate the relationships between stress ratings and concurrent ratings of mood or severity of ongoing “background” craving, we used data from all random-prompt entries. Each mood or craving item (happy, relaxed, tired, bored, irritated, crave cocaine, crave heroin, crave tobacco) was used as the independent variable in a repeated-measures linear regression (SAS Proc Mixed). Each model included control terms for sex, race (dichotomized as white and nonwhite), age, and the number of records that each participant contributed to the dataset. A first-order autoregressive error structure provided the best fit to the data.
To evaluate the relationships between stress ratings and past-hour exposure to putative triggers, we again used data from all random-prompt entries. Each of the 18 possible triggers was used as the independent variable in a repeated-measures linear regression (SAS Proc Mixed). Each model included control terms for sex, race, age, and the number of records that each participant contributed to the dataset. A first-order autoregressive error structure provided the best fit to the data.
To evaluate the time course of stress ratings prior to discrete episodes of cocaine craving and cocaine use, we examined the random-prompt entries in each of the 5 h preceding each event-contingent entry (with craving or use treated separately) using a method devised by Shiffman and Waters (2004). Thus, all stress ratings analyzed were from random-prompt entries, but the selection of random-prompt entries was determined by their proximity to a subsequent event-contingent entry. To determine whether stress ratings increased over the 5-h period, we used repeated-measures logistic regressions (SAS GLIMMIX macro), with hours before event (1, 2, 3, 4, or 5) as the within-subject independent variable. The pre-craving and pre-use datasets were analyzed in parallel. Each GLIMMIX model included control terms for sex, race, age, and the number of records that each participant contributed to the dataset. A first-order autoregressive error structure provided the best fit to the data. Contrast coefficients were used to test for a linear trend in hours before event. In an additional analysis, we examined random-prompt entries preceding the subset of cocaine-use reports that participants specifically attributed to presumably stressful states (having felt worried/anxious/tense, angry/frustrated, or having felt criticized by others).
For all analyses, the criterion for significance was p<.05, two-tailed, with trends noted at p<.10. Because of the large number of analyses of putative triggers, we applied a Bonferroni correction (.05÷18) to maintain a nominal alpha of .05 in that family of tests.
Results
The data used in these analyses included 26,734 random-prompt entries collected from 114 participants. The demographic variables age, sex, and race were included as controls in each of the analyses. In most of the analyses, age had a significant negative relationship with stress, but the relationship was very small—generally a decrease of .07 points (on a four-point scale) for every 10-year increase in age (data not shown). Sex and race generally did not have consistent relationships with stress ratings.
Stress and ratings of craving and mood
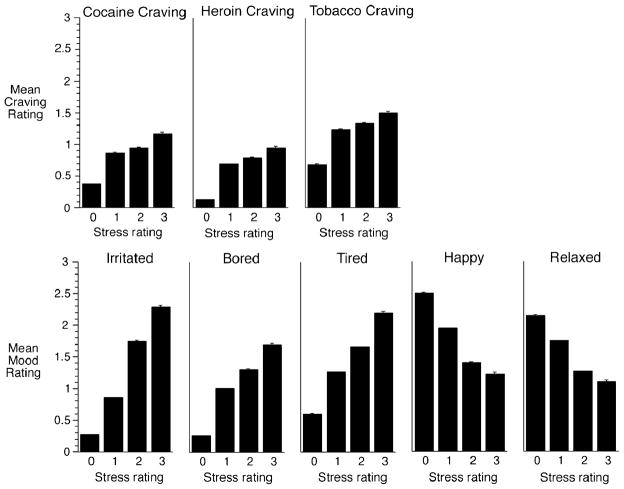
Mean ratings of craving and mood measures are shown in Fig. 1. as a function of ratings of stress. Ratings of stress were positively associated with craving for cocaine, heroin, and tobacco: cocaine [linear trend F(1,27000)=752.43, p<.0001]; heroin [linear trend F(1,26000)=1,060.48, p<.0001]; tobacco [linear trend F(1,27000)=547.56, p<.0001].2 Ratings of stress were also positively associated with ratings of the three negative-mood measures: tired [linear trend F(1,27000)= 2,552.17, p<.0001]; bored [linear trend F(1,27000)= 3,591.60, p<.0001]; irritated [linear trend F(1,27000)= 11,941.1, p<.0001]. Complementing this finding was the finding that stress was negatively associated with ratings of the two positive-mood measures: happy [linear trend F(1,27000)=3024.08, p<.0001] and relaxed [linear trend F(1,27000)=1172.64, p<.0001].
Fig. 1.
Relationship between ratings of stress and craving and mood. Mean ratings of craving and mood on a four-point (0 to 3) scale are shown as a function of stress ratings on the same scale. Brackets indicate SEM. The association between stress and each variable was tested with repeated-measures linear regressions (SAS Proc Mixed) in which each variable was treated as continuous showed significant relationships between stress and craving and mood measures
Stress and putative drug-use triggers
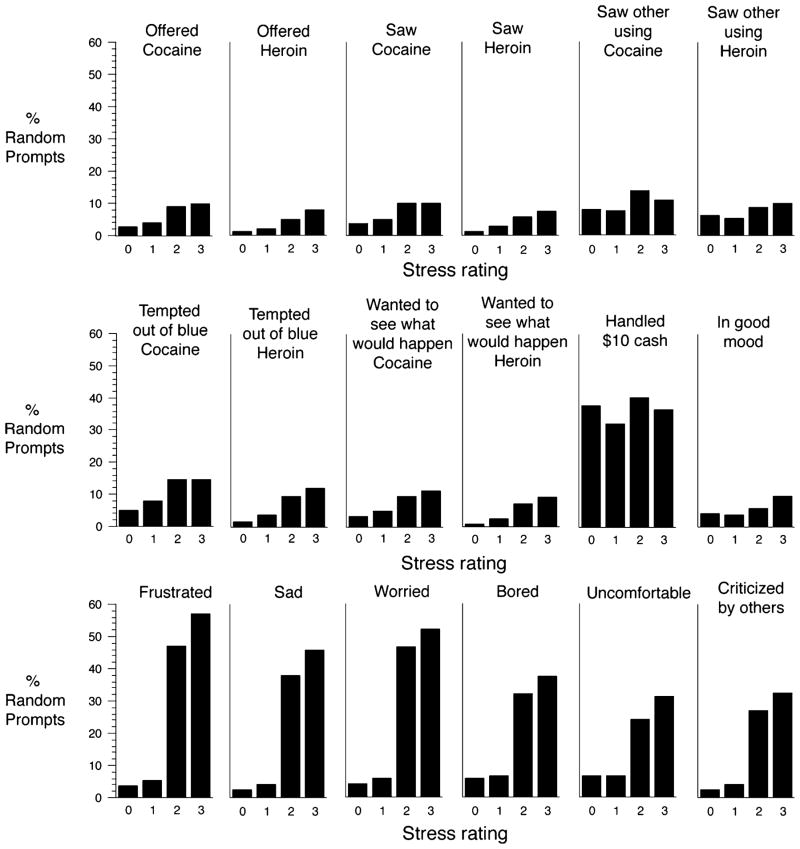
These data are shown in two ways. Table 1 (reflecting the strategy used for data analysis) shows mean current stress ratings in random prompts with and without endorsement of exposure to the triggers. Figure 2 shows current stress ratings histogramatically for each of the triggers. The overall pattern was for reports of exposure to triggers to be significantly greater as stress ratings grew higher. This relationship was especially robust for negative-mood-related triggers (having felt frustrated, worried, bored, uncomfortable, sad, or criticized by others) but was also present for most of the drug-exposure triggers (saw cocaine, saw heroin, saw someone using cocaine, was offered cocaine, and was offered heroin) and all the triggers involving thoughts about drugs (wanted to see what would happen if I tried just a little cocaine, wanted to see what would happen if I tried just a little heroin, was tempted out of the blue to use cocaine, was tempted out of the blue to use heroin). In contrast, current stress ratings were not related to reports of having been in a good mood or having handled at least US $10 in cash (the latter were uniformly high).
Table 1.
Mean stress ratings and past-hour exposure to putative relapse triggers in random prompts
| Trigger | No Exposure
|
Yes Exposure
|
Analysisa | ||
|---|---|---|---|---|---|
| Mean stress | SEM | Mean stress | SEM | ||
| Saw cocaine | 0.87 | 0.011 | 1.02 | 0.022 | F(1,85)=56.39, p<.0001b |
| Saw heroin | 0.88 | 0.011 | 1.03 | 0.028 | F(1,77)=32.12, p<.0001b |
| Saw other using | |||||
| Cocaine | 0.88 | 0.011 | 1.03 | 0.042 | F(1,47)=12.41, p=.001b |
| Heroin | 0.88 | 0.011 | 1.00 | 0.048 | F(1,47)=5.95, p=.0185 |
| Was offered | |||||
| Cocaine | 0.87 | 0.011 | 1.05 | 0.023 | F(1,87)=67.79, p<.0001b |
| Heroin | 0.88 | 0.011 | 1.01 | 0.030 | F(1,70)=22.47, p<.0001b |
| Wanted to see what would happen | |||||
| Cocaine | 0.87 | 0.011 | 1.10 | 0.022 | F(1,82)=129.0, p<.0001b |
| Heroin | 0.87 | 0.011 | 1.13 | 0.029 | F(1,67)=87.04, p<.0001b |
| Tempted out of blue to use | |||||
| Cocaine | 0.86 | 0.011 | 1.07 | 0.019 | F(1,94)=150.27, p <.0001b |
| Heroin | 0.87 | 0.011 | 1.10 | 0.025 | F(1,79)=93.15, p<.0001b |
| Handled US $10 or more | 0.88 | 0.012 | 0.88 | 0.013 | F(1,109)=0.53, n.s. |
| Good mood | 0.88 | 0.011 | 0.90 | 0.023 | F(1,93)=0.63, n.s. |
| Frustrated | 0.78 | 0.010 | 1.51 | 0.015 | F(1,103)=2,723.32, p<.0001b |
| Uncomfortable | 0.84 | 0.011 | 1.20 | 0.019 | F(1,96)=434.61, p<.0001b |
| Bored | 0.84 | 0.011 | 1.19 | 0.018 | F(1,100)=500.58, p<.0001b |
| Worried | 0.79 | 0.010 | 1.46 | 0.016 | F(1,101)=2,154.53, p<.0001b |
| Sad | 0.80 | 0.010 | 1.53 | 0.018 | F(1,101)=1,954.9, p<.0001b |
| Felt others critical | 0.84 | 0.011 | 1.39 | 0.019 | F(1,93)=964.98, p<.0001b |
Each analysis used data from 114 participants. However, due to the use of the between-within method for denominator degrees of freedom (df), the denominator df varies as a function of how many participants endorsed both “yes” and “no” responses in a given analysis
Significant after Bonferroni correction
Fig. 2.
Relationship between ratings of stress and exposure of putative relapse triggers in the past hour. The percentage of random prompt entries in which exposure to a putative trigger was endorsed is shown for each of the ratings of stress on a four-point (0 to 3, low to high) scale
Stress across the hours preceding discrete episodes of cocaine use and craving
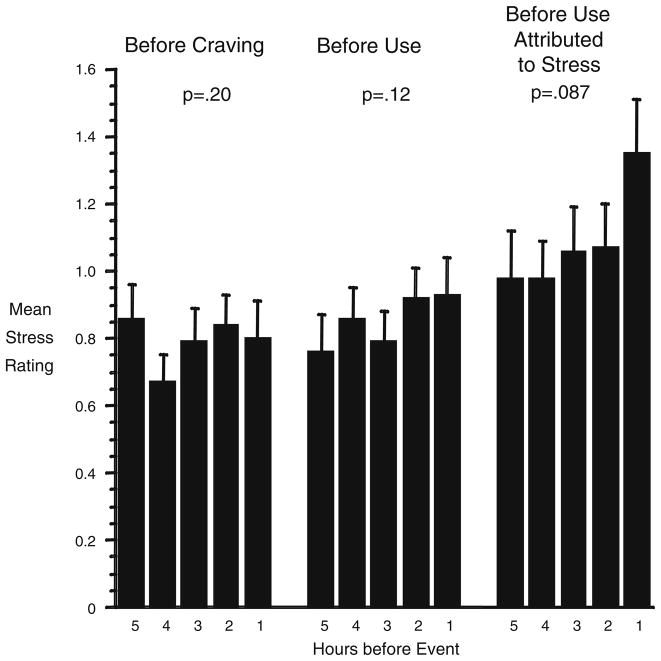
As shown in Fig. 3, stress ratings did not increase in the 5 h preceding reports of discrete episodes of cocaine craving [linear trend F(1,119)=1.66, p=.20] or cocaine use [linear trend F(1,125)=2.43, p=.12]. The relationship was stronger, but significant only at trend level, in the 45% of cocaine-use reports that participants specifically attributed to presumably stressful states such as frustration, worry, or having felt criticized by others [linear trend F(1,102)=2.98, p=.087].
Fig. 3.
Real-time participant ratings of stress in the 5 h of random-prompt entries preceding each episode (participant-initiated report) of cocaine craving, cocaine use, and cocaine uses attributed to presumably stressful triggers (such as having felt criticized by others). Brackets indicate SEM. The median number of datapoints per bar is 80 (range 52 to 102). P values are for tests of linear trends. The “before use” data shown in the middle third of the figure also appeared in one of our previous publications (Preston et al. 2009) but were not discussed in detail there
Our strategy of testing for linear trends had been chosen a priori, but the data on the right-hand side of Fig. 3 suggested that craving spiked during the hour before use rather than increasing linearly. In an exploratory test, we changed the contrast coefficients to compare hour 1 with the 4 h preceding it. The result of this post hoc contrast was not significant [F(1,102)=2.09, p=.15]. Thus, a spike in craving at the 1-h time point was not as strongly supported as a linear increase in craving across 5 h.
Discussion
In this study, we examined electronic-diary data collected prospectively in randomly prompted and participant-initiated entries as participants went about their daily lives, in order to investigate stress and its relationship to craving and drug use. We found a positive cross-sectional momentary association between ratings of stress and ratings of craving for cocaine, heroin, and tobacco. All of the participants in our study met DSM-IV criteria for cocaine and opioid dependence, and a very large proportion (106 of 114) of them were smokers (Epstein et al. 2010). Laboratory studies have shown that stress increases craving in stimulant, opiate, and tobacco users (Buchmann et al. 2010; Colamussi et al. 2007; Elman et al. 2003; Hamidovic et al. 2010; Hyman et al. 2007; Sinha et al. 1999, 2000). In our EMA data, the relationship between stress and craving may have reflected reciprocal causation: craving itself may induce stress among outpatients who are trying to avoid use. It should be noted, however, that the relationship we found between stress and craving for cocaine (drug participants were trying to stop using) was about as strong as the relationship we found between stress and craving for nicotine/tobacco (drug participants were not necessarily trying to stop using).
In our random-prompt data, cross-sectional momentary relationships between stress and mood ratings were in the expected direction: stress was positively associated with negative mood and negatively associated with feelings of happiness and relaxation. These findings for ongoing stress (i.e., background stress not necessarily associated with a particular stressful event) are consistent with those of laboratory studies of responses to stressors in cocaine abusers. For example, cocaine abusers exposed to stress-imagery scripts or a speech stress task reported increases in fear, sadness, and anger and decreases in joy and neutral feelings (Brady et al. 2009; Sinha et al. 1999). The results are not consistent with the results of studies of stress and mood in college students (Watson 2000), in whom stress was strongly associated with negative affect but not with positive affect, whether or not stress was measured objectively.
Another positive cross-sectional momentary association we found was between ratings of stress and past-hour exposure to putative triggers in the natural environment. The relationship between stress and negative-mood-related triggers (such as having felt criticized by others) is consistent with what one might expect; it is also consistent with the relationship between stress and negative-mood ratings. Stress ratings were not associated with past-hour experience of the “good mood” trigger or with past-hour handling of cash, also as expected. The positive relationship between stress and drug-cue-related triggers was smaller than seen with negative-mood-related triggers but was nonetheless statistically significant. In terms of causality, one possibility is that exposure to drug cues is itself experienced as stressful, as has been seen in a laboratory setting (Sinha et al. 2000). If addicted humans experience stress in response to drug cues, then medications that block stress-induced but not cue-induced reinstatement of drug seeking in rats could block both in humans—an idea supported by findings that medications can block both stress-induced and cue-induced craving in human laboratory settings (Jobes et al. 2010; Sinha et al. 2007).
One surprising result is that when we examined stress ratings as a predictor of actual episodes of cocaine use, we found that the association was quite modest, not reaching statistical significance, even in cocaine-use episodes that participants attributed to stressful triggers. This contrasts with laboratory evidence that stress reactivity predicts subsequent drug craving and use (Back et al. 2010; Daughters et al. 2009; Hamidovic et al. 2010; Sinha et al. 2006) and with the finding by Shiffman and Waters (2004) that stress and negative mood increase in the hours prior to relapse to smoking, at least for those relapses that participants specifically attributed to stress or negative affect. It also seems, at first, to contrast with our own prior EMA findings of increases in past-hour exposure to negative-mood-related triggers before cocaine use (Epstein et al. 2009). We believe that this seeming failure to replicate is likely to be attributable to the framing of the questions in terms of “in the past hour” or “right now”: we found in prior analyses of the same data set that negative mood (including stress) “right now” did not predict cocaine use in the following hours (Preston et al. 2009). EMA ratings of past-hour mental events seem to be stronger predictors of cocaine use than are EMA ratings of possibly fleeting current mental events. With no item assessing stress “in the past hour” at randomly chosen moments, we do not have the data that would be most likely to confirm a prospective relationship between stress and cocaine use on a time scale of hours. This issue needs to be pursued if we are to identify objective, prospective measures of “stress-induced” cravings, uses, lapses, or relapses.
Any agreement between our natural-history EMA findings and prior laboratory findings should be viewed as a convergence of evidence from approaches with different limitations and strengths. Unlike laboratory studies, in which stress is manipulated in a controlled fashion, our natural-history study gathered EMA data from individuals who were being exposed to many stressors and drug cues, some of which were under the participants’ personal control and many of which were not. In addition, what our participants rated as stress may not have been the result of an acutely stressful event like those used in laboratory sessions but more likely reflected more general ongoing negative affect that participants labeled as stress. Subjective perceptions have been shown to differ between nonspecific stress and specific stressful events (Watson 2000). Our EMA method sampled only a small fraction of our participants’ experiences, which they may or may not have reported reliably, and because we imposed no experimental manipulations, no cause-and-effect relationships can be inferred. However, the ability to make causal inferences from EMA data is largely a function of study design, not an inherent limitation of the methodology; thus, associations detected in the current study can be tested in randomized trials.
A limitation of our data (in addition to those mentioned earlier, such as the nonexperimental design of the study) is that the word “stress” was not used in our items assessing past-hour trigger exposures and participants’ causal attributions for episodes of cocaine use. The wording of those items, as we said, was derived from a published taxonomy of triggers (Heather et al. 1991; Marlatt and Gordon 1985). Our reading of the literature on stress and negative moods suggests that the two terms refer to incompletely overlapping concepts: negative moods include low-arousal (and thus not prototypically stressful) states such as boredom and sadness, while responses to ostensible stressors can include positive moods (Larsen et al. 2001), especially as coping comes into play (Folkman 1997, 2008). Therefore, investigators need to be cautious about equating measures of stress with measures of negative mood or even with measures of high-arousal negative mood.
In addition, our EMA items did not include enough mood adjectives to support factor analyzing the mood ratings into composite indicators for positive and negative mood, which might have been more reliable than individual adjectives. Another possible limitation of our data is that we did not assess “stress right now” in the reports of cocaine use. However, ratings of stress in those reports would have been contaminated by the acute pharmacological effects of cocaine and by participants’ immediate responses to the fact of just having used. Stress ratings did not differ significantly overall during periods of two or more weeks when participants consistently tested cocaine-positive versus periods when they were not using cocaine (Epstein and Preston 2010).
Other limitations of our data include the fact that the findings may not generalize to populations of drug misusers differing from the one we studied: heroin and cocaine users in outpatient treatment. For example, although we suspect that cravings and exposure to drug cues (in an environment of drug availability) may have been stressful for our participants, the same might not be true for nontreatment seekers, especially early in their using careers. The absence of a nonsubstance-using control group in our study precludes any inferences about whether the levels of stress reported were outside general-population norms.
The EMA data showed that relationships between stress and negative mood and craving were internally consistent and generally agreed with findings from other methods (such as human laboratory studies and clinical trials). Our findings support the value of EMA as a tool for monitoring stress in the natural environment. The greatest surprise in this study was that background/ongoing stress showed, at best, only a weak predictive relationship with individual episodes of cocaine use. Additional EMA work is needed to determine whether the latter finding replicates when the stress questions are framed differently, along the lines of the negative-mood items that strongly predicted cocaine use in our prior analyses (Epstein et al. 2009).
Acknowledgments
Funding/support This research was supported by the NIDA Intramural Research Program and Genes and Environment Initiative Cooperative Agreement Z01-DA000499.
This research was supported by the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA), National Institutes of Health. We wish to thank the NIDA IRP Archway Clinic staff for data collection.
Footnotes
In this paper, we use the term mood synonymously with affect to refer to a transient feeling state (Watson 2000). Some authors distinguish mood from affect, but the distinction varies among authors.
Denominator degrees of freedom were expressed in rounded exponential form (“27E3”) in the SAS Proc Mixed output.
Financial disclosures/conflicts of interest None to declare.
Contributor Information
Kenzie L. Preston, Email: kpreston@intra.nida.nih.gov, Treatment Section, Clinical Pharmacology and Therapeutics Branch, NIDA Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Room 01B-602, 251 Bayview Blvd., Suite 200, Baltimore, MD 21224, USA
David H. Epstein, Treatment Section, Clinical Pharmacology and Therapeutics Branch, NIDA Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Room 01B-602, 251 Bayview Blvd., Suite 200, Baltimore, MD 21224, USA
References
- Ariely D. Combining experiences over time: the effects of duration, intensity changes and on-line measurements on retrospective pain evaluations. J Behav Decis Mak. 1998;11:19–45. [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, McRae AL, Moran-Santa Maria MM, DeSantis SM, Simpson AN, Waldrop AE, Back SE, Kreek MJ. Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Arch Gen Psychiatry. 2009;66:422–430. doi: 10.1001/archgenpsychiatry.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Blendy JA. Molecular and genetic substrates linking stress and addiction. Brain Res. 2010;1314:219–234. doi: 10.1016/j.brainres.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann AF, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann US. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. J Psychopharmacol. 2010;24:247–255. doi: 10.1177/0269881108095716. [DOI] [PubMed] [Google Scholar]
- Colamussi L, Bovbjerg DH, Erblich J. Stress- and cue-induced cigarette craving: effects of a family history of smoking. Drug Alcohol Depend. 2007;88:251–258. doi: 10.1016/j.drugalcdep.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Richards JM, Gorka SM, Sinha R. HPA axis response to psychological stress and treatment retention in residential substance abuse treatment: a prospective study. Drug Alcohol Depend. 2009;105:202–208. doi: 10.1016/j.drugalcdep.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Lukas SE, Karlsgodt KH, Gasic GP, Breiter HC. Acute cortisol administration triggers craving in individuals with cocaine dependence. Psychopharmacol Bull. 2003;37:84–89. [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: craving and use during daily life. Addict Behav. 2010;35:318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. Daily life hour by hour, with and without cocaine: an ecological momentary assessment study. Psychopharmacology. 2010;211(2):223–232. doi: 10.1007/s00213-010-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S. Positive psychological states and coping with severe stress. Soc Sci Med. 1997;45:1207–1221. doi: 10.1016/s0277-9536(97)00040-3. [DOI] [PubMed] [Google Scholar]
- Folkman S. The case for positive emotions in the stress process. Anxiety Stress Coping. 2008;21:3–14. doi: 10.1080/10615800701740457. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Childs E, Conrad M, King A, de Wit H. Stress-induced changes in mood and cortisol release predict mood effects of amphetamine. Drug Alcohol Depend. 2010;109:175–180. doi: 10.1016/j.drugalcdep.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather N, Stallard A, Tebbutt J. Importance of substance cues in relapse among heroin users: comparison of two methods of investigation. Addict Behav. 1991;16:41–49. doi: 10.1016/0306-4603(91)90038-j. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong KI, Doebrick C, Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp Clin Psychopharmacol. 2007;15:134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. Effects of clonidine on cocaine craving in response to stress- and drug-related scripts. American College of Clinical Pharmacology; Baltimore: 2010. [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JT, McGraw AP, Cacioppo JT. Can people feel happy and sad at the same time? J Pers Soc Psychol. 2001;81:684–696. [PubMed] [Google Scholar]
- Marlatt GA, Gordon JRE. Relapse prevention: maintenance strategies in the treatment of addictive behaviors. Guilford; New York: 1985. [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology. 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM., III . The diagnostic interview schedule, version IV. Washington University; St. Louis: 1995. [Google Scholar]
- Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, Kassel JD. Remember that? A comparison of real-time versus retrospective recall of smoking lapses. J Consult Clin Psychol. 1997;65:292–300. doi: 10.1037/0022-006x.65.2.292.a. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J. Smoking patterns and dependence: contrasting chippers and heavy smokers. J Abnorm Psychol. 2006;115:509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology. 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Sutton S. Is wearing clothes a high risk situation for relapse? The base rate problem in relapse research. Addiction. 1993;88:725–727. doi: 10.1111/j.1360-0443.1993.tb02087.x. [DOI] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahabzadeh M, Epstein DH, Mezghanni M, Lin J-L, Preston KL. An electronic diary software for ecological momentary assessment (EMA) in clinical trials. Proceedings of the 17th IEEE symposium on computer-based medical systems (CBMS); 24–25 June 2004; Bethesda, MD, USA. 2004. pp. 167–172. [Google Scholar]
- Watson D. Mood and Temperament. Guilford Press; New York: 2000. Chapter 3. Situational and environmental influences on mood; pp. 62–103. [Google Scholar]