Abstract
Despite the widespread availability and use of dietary supplements, minimal work has been performed to assess the potential dangers many of these supplements may have on the host’s well-being, in particular the host’s ability to respond to infection. One supplement extensively used by both adolescents and adults is creatine. Using Real-time PCR, we examined the impact of short-term exposure of a mouse macrophage cell line (RAW 264.7 cells) to two readily available forms of creatine used in supplements – creatine monohydrate (CR) and creatine ethyl ester (CEE) as well as the end product of creatine metabolism, creatinine (CRN), on expression of toll-like receptor-2 (TLR-2), TLR-3, TLR-4, and TLR-7. CR down-regulated TLR-2, TLR-3, TLR-4 and TLR-7 mRNA levels in RAW cells. Similar results were observed following exposure of RAW cells to CRN. Conversely CEE appears to possess immunostimulatory properties and increases expression of TLR-2, TLR-3, TLR-4, and TLR-7 in RAW cells. These data are supported by immunostaining using antibodies specific for the individual TLRs before and after exposure of RAW cells to CR, CRN, or CEE. To extend these findings, we isolated murine splenocytes and exposed the cells to CR, CEE, or CRN for 24 hours and performed immunofluorescent staining for TLR-2, TLR-3, TLR-4 and TLR-7. The results obtained from this study with primary splenocytes were consistent with the studies using RAW cells. Together, these data suggest that creatine and creatine derivatives may impact the ability of immune cells to sense a wide array of viral and bacterial pathogens. Of great interest, CRN - largely considered to be a waste product of the argenine biosynthesis pathway may also have immunosuppressive properties similar to those of CR.
Keywords: creatine, creatine ethyl ester, creatinine, toll-like receptors
1. Introduction
Toll-like receptors (TLR) are expressed by many cell types including phagocytes and are involved in sensing the presence of foreign antigen in a host via binding of pathogen-associated molecular patterns (PAMPs) on the microbe. Binding of the PAMPs to the TLRs results in a cascade of events resulting in the generation of inflammation. Reactive oxygen intermediates generated during respiratory burst by neutrophils and macrophages are key mediators of the inflammatory process [1-4]. Oxidative stress and inflammation have been implicated as participants in a variety of disease processes including Alzheimer’s disease [4,5], cardiovascular disease [4,6], type 2 diabetes [4,6,7], and Parkinson’s disease [4,5]. Increased generation of reactive oxygen species by the mitochondria also contributes to the normal aging processes [5,8,9]. The mechanisms by which reactive oxygen species enhance inflammation is via the activation of NFκβ [10], resulting in the upregulation of cytokine gene transcription including interleukin-8 (IL-8) [3,11] and tumor necrosis factor-α (TNF-α) [12,13], and altering vascular tone in the host [9]. The signal generated by binding of the PAMPs to the TLRs results in activation of the NFκβ via the MyD88-dependant pathway.
Certain amino acids possess anti-inflammatory properties [14-19]. A variety of mechanisms are responsible for confirming these anti-inflammatory properties including myelotoxicity [17], an ability to recruit lymphocytes and neutrophils [17,18], altering cytokine production [20], and inhibiting T cell activation [17,21,22]. Histadine, an essential amino acid, has been postulated to reduce mucosal inflammation with Crohn’s disease via mechanism that reduces activation of NFκβ [20,23]. Another amino acid reported to possess anti-inflammatory properties is creatine [16,24-26], which is produced by the kidneys and liver. It is postulated that creatine stabilizes mitochondrial membranes and functions as an anti-oxidant [27].
In vivo, creatine is a product of argenine biosynthesis and metabolizes to creatinine [8]. Creatine is commonly used by athletes as a non-vitamin, non-mineral supplement that enhances athletic performance, reduces muscle inflammation, and speeds recovery following strenuous training [28-35]. Despite its extensive use as an unregulated nutritional supplement, relatively little work examining the impact of creatine on other host functions has been done, although there have been a few reports that suggest that immune function is altered by creatine supplementation [26,36]. Multiple forms of creatine are currently utilized by athletes and one form, creatine ethyl ester (CEE) is purported to contain the activity required to build strength without side effects such as water retention. In aqueous solutions (including plasma) [37], CEE is rapidly converted to creatinine (CRN), a compound that is generally viewed as inert. The rapid conversion of CEE to CRN brings into question the utility of CEE as an anti-inflammatory agent [37]. However, one study [16] suggests that CRN (at least under some circumstances) may also contain some anti-inflammatory properties.
We examined the impact of creatine monohydrate (CR) and some creatine derivatives on transcription of genes known to be involved in the sensing phase of the immune response. Specifically, we compared the ability of CR, CEE, and CRN to suppress transcription of the genes encoding toll-like receptor 2 (TLR-2), TLR-3, TLR-4, and TLR-7. Results were further confirmed using immunohistochemistry. These particular receptors are triggered by a wide array of pathogens including gram positive (TLR-2) and gram negative (TLR-4) bacteria, and viruses (TLR-3, TLR-7). Overall, these data support the hypothesis that CR and CRN possess anti-inflammatory properties that impact the sensing arm of the innate immune system.
2. Materials and methods
2.1. Cell culture
RAW 264.7 (American Tissue Type Culture Collection; Manassas, VA) were maintained in Dulbucco’s media supplemented with 10% FBS (Invitrogen; Carlsbad, CA), 100 μg/ml streptomycin (Invitrogen), and 100 U/ml penicillin (Invitrogen) at 37°C and 5% CO2. Dose response experiments were performed following exposure of the RAW 264.7 cells to varying doses of creatine ethyl ester hydrochloride (MW 195; Vireo Systems Inc, Madison TN) , creatinine hydrochloride (MW 149.58; Sigma-Aldrich; St. Louis, MO) and creatine monohydrate (MW 131.13; Creapure, Degussa AG, Germany). Control cultures were treated with media. A dose of 0.1 mM CEE, CRN, and CR were chosen for use in these studies based on results from the dose response studies, and are within the normal physiological levels of creatine in the blood [38]. CR, CEE, and CRN were added immediately to the cell cultures upon solubilization. No significant differences in pH of the media were detected between treatments.
Spleens were harvested from B6 × 129 mice, placed in cold HBSS, and dissociated into a single cell suspension. Cells were washed and resuspended at a concentration of 1 × 106 cells/ml in RPMI 1640 (Sigma-Aldrich), 5% FCS, 100 μg/ml streptomycin, 100 U/ml penicillin, and 0.1 mM CEE, CRN, or CR. Control splenocytes received only media. Cultures were incubated at 37°C and 5% CO2 for 24 hours. After 24 hours, cells were collected, washed in HBSS, resuspended to a concentration of 1 × 106 cells/ml in culture media. Cytospins were prepared and the slides fixed in acetone and air-dried. Slides were stored at -80°C until the time of immunofluorescent staining.
2.2. RNA isolation
Following treatment, RAW cells were lysed in Trizol (Invitrogen, Carlsbad, CA). After homogenization, samples were extracted with chloroform, centrifuged, and the aqueous phase was collected. RNA was precipitated with isopropyl alcohol. Following centrifugation, the pellets were washed with 70% ethanol and air dried. RNA was resuspended in dH2O. Residual DNA was removed from the samples by treatment with RQ1 DNase (Promega, Madison, WI) per the manufacturer’s protocol. After incubation with DNase at 37°C for 1 hr, samples were extracted with phenol:chloroform:isoamyl alcohol (25:24:1). RNA was precipitated with 1/10 volume of 3M sodium acetate (pH=5.2) and 3 volumes of 100% ethanol. Samples were stored at -80 °C until use.
2.3. Real time RT-PCR
Reverse transcription (Improm-II Reverse Transcription System, Promega, Madison, WI) followed by realtime PCR analysis was performed in 20 μL volumes containing 1 μl of cDNA corresponding to 40 ng of DNase-treated RNA, gene specific internal primers (0.5 μM of each primer), and 1X SsoFast EvaGreen Supermix (Bio-Rad, Hercules, CA). Primers used were synthesized by Integrated DNA Technologies (IDT, Coralville, IA) and have 100% sequence homology to the murine genome. Primer sequences are listed in TABLE 1. The PCR was performed using the following conditions: 95°C for 30 sec, and 40 cycles of 95°C for 5 sec and 57°C for 5 sec using a CFX96 Real-Time Detection System (Bio-Rad). GAPDH was used as a housekeeping gene to normalize mRNA levels between samples. Data were analyzed using the Bio-Rad CFX Manager V1.6.541.1028 software (Bio-Rad). The calibrator in the experiments was the sample obtained from control-treated RAW cells. Copy numbers of targeted mRNAs were expressed as ratios of GAPDH mRNA levels. The relative quantification (RQ) of gene expression in this method is determined by the formula: RQ = 2-ΔΔCT [39,40].
TABLE 1.
Primers used for realtime PCR studies
| Primer Name | Forward Primer 5′→3′ | Reverse Primer 5′→3′ | Accession # |
|---|---|---|---|
| mTLR-2 | TTGTTCCCTGTGTTGCTGGT | ACAAAGTGGTTGTCGCCTGCT | NM_011905 |
| mTLR-3 | CCTTGCGTTGCGAAGTGAAG | CAATTGTCTGGAAACACCCCG | NM_126166 |
| mTLR-4 | AGAAATTCCTGCAGTGGGTCA | TCTCTACAGGTGTTGCACATGTCA | NM_021297 |
| mTLR-7 | CGTGGACTGCACAGACAAG | GTTCAGCCTACGGAAGGAATC | NM_133211 |
| mGAPDH | GTGGCAAAGTGGAGATTGTTG | CATTCTCGGCCTTGACTGTG | NM_008084 |
2.4. TUNEL Assay
TUNEL was performed on RAW cell monolayers using the ApopTag Fluorescein In Situ Apoptosis Detection Kit per the manufacturer’s protocol (Millipore/Chemicon; Billerica, MA). Cells were fixed with cold acetone, air-dried, and treated with Proteinase K (20 μg/μl; Qiagen). Following incubation with equilibration buffer, terminal deoxynucleotidyl transferase (TdT) was applied to the slides. TdT binding was detected using a fluorescein-conjugated anti-digoxigenin antibody. Cells were counterstained and the slides mounted using Vectashield Mounting Media with DAPI (Vector Laboratories, Burlingame, CA).
2.5 Immunostaining
RAW cells were grown in chamber slides in the presence of 0.1 mM CEE, CRN, or CR. Control cells were cultured with only media. Immunohistochemical detection of TLRs was performed as described previously with minor modifications [41,42]. After fixation in cold acetone, the slides were air-dried and rehydrated in PBS (phosphate buffered saline). Blocking was performed at room temperature for 20 minutes. Following incubation with the primary antibody the slides were washed with PBS. The following primary antibodies were used in this study: mouse anti-TLR-2 (GeneTex; Irvine, CA), goat anti-TLR-3 (R&D Systems; Minneapolis, MN), rabbit anti-TLR-4 (Abcam; Cambridge, MA), rabbit anti-TLR-7 (Abcam), hamster anti-CD3ε (BD Biosciences, San Diego, CA), rat anti-B220 (BD Biosciences), and rat anti-F4/80 (Millipore). Detection was performed using Alexa Fluor conjugated secondary antibody (Invitrogen, Carlsbad, CA). After washing with PBS, slides were mounted using Vectashield Mounting Media with DAPI (Vector Laboratories). Staining was also performed on cytospins prepared from murine splenocytes. Images were captured using a Nikon i80 microscope (NY, USA) and a DigiFire camera and ImageSys Digital Imaging Software (Soft Imaging Systems GmbH, Munster, GER).
2.6. Statistical analysis
Data were analyzed by ANOVA with a Bonferroni’s test as the post-test. p values of ≤ 0.05 were considered statistically significant.
3. Results
We examined the impact of treatment of RAW cells with creatine derivatives on TLR-2, TLR-3, TLR-4, and TLR-7 mRNA expression. These TLRs were chosen for examination based on the array of microbial components recognized by these receptors. Pattern associated molecular patterns (PAMPs) associated with these receptors include lipotechic acid (TLR-2), double-stranded RNA (TLR-3), bacterial lipopolysaccharide (TLR-4), and single-stranded RNA (TLR-7) (reviewed in [43]). To ensure that differences in gene expression were not due to differences in the level of cell death, we performed a TUNEL assay to determine whether there were differences in apoptosis between the groups. Furthermore, we also performed flow cytometry following propidium iodine and Annexin V staining on cells from the various treatment groups, and compared the levels of cell death between the groups. No differences in cell death were noted between the treatment groups and the control-treated cells (data not shown).
3.1. TLR-2 mRNA levels are decreased following exposure to creatine monohydrate or creatinine hydrochloride
RAW cells were exposed to 0.1 mM CR, CEE, or CRN for periods of between 10 and 60 minutes. CEE exposure had a brief immunostimulatory effect on RAW cells after 30 minutes of co-culture (p≤0.01; Figure 1), but TLR-2 transcript levels returned to baseline by 60 minutes after the addition of CEE. CR was a robust suppressor of TLR-2 mRNA in the RAW cells. TLR-2 mRNA levels were reduced by ~70% within 10 minutes of exposure to creatine (p≤0.001; Figure 1) and remained suppressed through at least 30 minutes of exposure to CR. By 60 minutes post-exposure, mRNA levels of TLR-2 returned to control levels in the RAW cells. CRN suppressed TLR-2 transcript levels in RAW cells by approximately 50% within 10 minutes of exposure. TLR-2 mRNA levels remained decreased through at least 60 minutes post-exposure relative to control-treated cells (p≤0.05).
Figure 1.
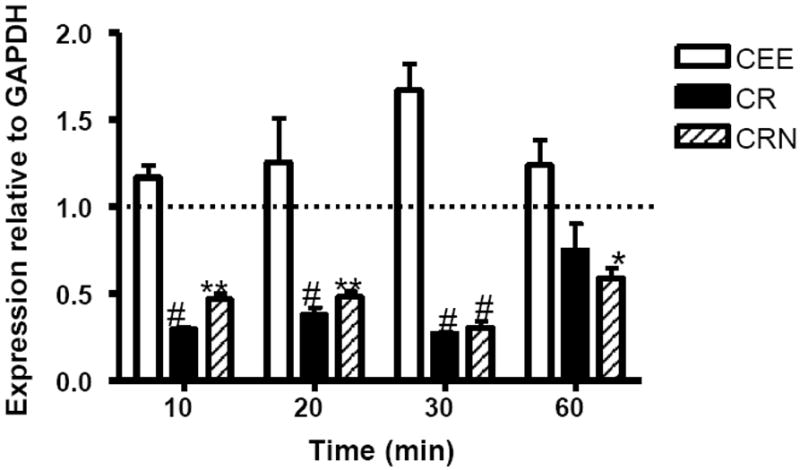
CR and CRN suppress TLR-2 transcript levels in RAW 264.7 cells. RAW 264.7 cells were cocultured with 0.1 mM of CEE (open bars), CR (filled bars), or CRN (striped bars) for 10 to 60 minutes. TLR-2 mRNA levels were measured by qRT-PCR. Values represent the mean transcript level ± the SEM, relative to GAPDH transcript levels. Statistical analysis was performed using a two-way ANOVA, with untreated cells as the control. *< 0.05; ** < 0.01; # < 0.001. Data are representative of three experiments.
3.2. TLR-3 is differentially expressed following exposure to creatine ethyl ester, creatine monohydrate or creatinine hydrochloride
CEE had stimulatory effects on TLR-3 transcripts at 20 minutes post-exposure (p≤0.001), but this observation was short-lived and control transcript levels were observed by 60 minutes after exposure (Figure 2). CR exposure reduced the levels of TLR-3 mRNA by RAW cells by 80% within 10 minutes of exposure (p≤0.05). This effect lasted through at least 30 minutes post-exposure to CR. By 60 minutes TLR-3 mRNAs were similar to control levels (p>0.05). Creatinine hydrochloride caused an increase in TLR-3 mRNA within 10 minutes of co-culture in the RAW cells however there was approximately an 80% reduction in TLR-3 message levels compared to the untreated controls by 30 minutes after exposure (p≤0.05), indicating a bimodal effect of the treatment on TLR-3 expression by RAW cells.
Figure 2.
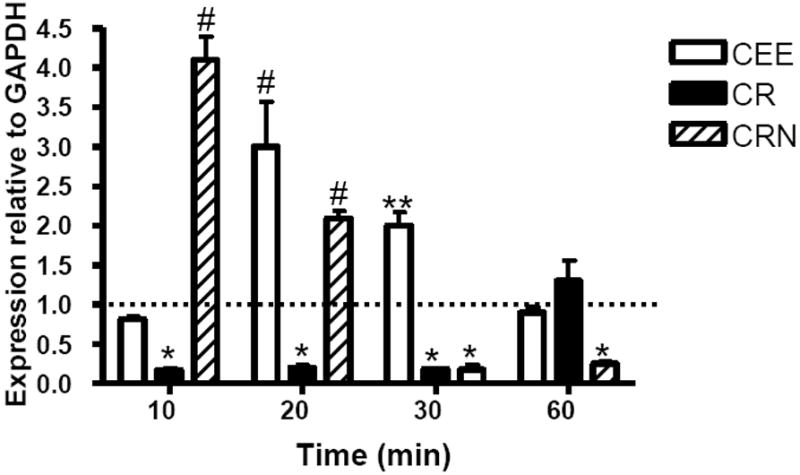
CR suppresses TLR-3 transcript levels in RAW 264.7 cells while CEE and CRN upregulate TLR-3 mRNA levels. RAW 264.7 cells were cocultured with 0.1 mM of CEE (open bars), CR (filled bars), or CRN (striped bars) for 10 to 60 minutes. TLR-3 mRNA levels were measured by qRT-PCR. Values represent the mean transcript level ± the SEM, relative to GAPDH transcript levels. Statistical analysis was performed using a two-way ANOVA, with untreated cells as the control. *< 0.05; ** < 0.01; # < 0.001. Data are representative of three experiments.
3.3. TLR-4 mRNA levels are decreased by exposure to creatine monohydrate or creatinine hydrochloride and increased by exposure to creatine ethyl ester
TLR-4, a pattern recognition receptor for LPS [43], was not significantly down-regulated by CEE at any time-point. In contrast, CEE treatment of RAW cells appeared to either stabilize or upregulate expression of TLR-4 mRNA. TLR-4 transcripts were upregulated approximately 2.7-fold by 20 minutes post-exposure to creatinine ethyl ester (Figure 3; p≤0.001). TLR-4 mRNA levels returned to control levels by 60 minutes post-exposure to CEE. CR was a potent suppressor of the TLR-4 mRNA. Within 10 minutes of exposure of RAW cells to CR, TLR-4 mRNA levels decreased by almost 70%. TLR-4 mRNA levels remained suppressed through at least 60 minutes (p≤0.05). Within 20 minutes of exposure to CRN, TLR-4 transcripts were decreased by approximately 30%. This CRN-mediated mRNA transcriptional suppression was further enhanced at 30 minutes, where a 70% decrease in mRNA levels was measured. Decreased levels of TLR-4 mRNA were observed through 60 minutes (p<0.001).
Figure 3.

TLR-4 mRNA levels are repressed in RAW 264.7 cells by CR and CRN, while CEE enhances TLR-4 expression. RAW 264.7 cells were cocultured with 0.1 mM of CEE (open bars), CR (filled bars), or CRN (striped bars) for 10 to 60 minutes. TLR-4 mRNA levels were measured by qRT-PCR. Values represent the mean transcript level ± the SEM, relative to GAPDH transcript levels. Statistical analysis was performed using a two-way ANOVA, with untreated cells as the control. *< 0.05; ** < 0.01; # < 0.001. Data are representative of three experiments.
3.4. TLR-7 transcripts are decreased following exposure to either creatine monohydrate or creatinine hydrochloride
TLR-7 transcript levels were negatively impacted following exposure to either CR or CRN (Figure 4). These decreased transcript levels were observed within 10 minutes post-exposure to the creatine derivative and lasted at least through 30 minutes post-exposure in the instance of CR (p<0.001) and up to at least 60 minutes (CRN). While the suppressive effect on mRNA levels lasted longer in cells treated with CRN, the level of suppression observed in RAW cells was more profound following CR treatment. Interestingly, CEE was a potent upregulator of TLR-7 transcripts. Transcript levels were increased approximately 3-fold at 20 and 30 minutes of exposure, and returned to control levels by 60 minutes post-treatment (p>0.05).
Figure 4.
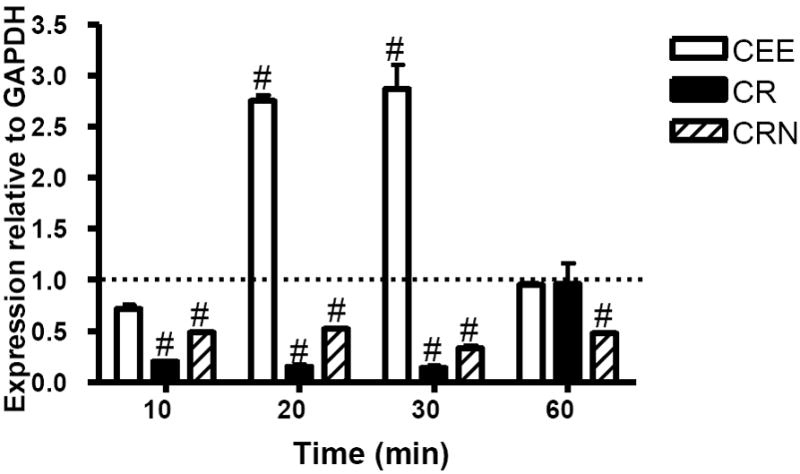
CR and CRN suppress TLR-7 transcript levels in RAW 264.7 cells while CEE upregulates TLR-7. RAW 264.7 cells were cocultured with 0.1 mM of CEE (open bars), CR (filled bars), or CRN (striped bars) for 10 to 60 minutes. TLR-7 mRNA levels were measured by qRT-PCR. Values represent the mean transcript level ± the SEM, relative to GAPDH transcript levels. Statistical analysis was performed using a two-way ANOVA, with untreated cells as the control. *< 0.05; ** < 0.01; # < 0.001. Data are representative of three experiments.
3.5. Immunofluorescent staining of RAW 264.7 cells reveal expression patterns reflecting alterations in mRNA levels
To assess whether changes in mRNA levels described following treatment of RAW cells with CR, CEE, or CRN resulted in altered protein expression of the TLRs, we performed immunohistochemical staining of RAW cells following exposure to 0.1 mM CR, CEE, or CRN. As changes in protein expression manifest later than alterations in mRNA levels, we performed the staining 24 hours after addition of the creatine derivatives to the cells. Untreated RAW cells served as controls. As shown in Figure 5, RAW cells expressed all TLRs studied under control conditions. Following exposure to CRN, the intensity of TLR-2 staining was reduced over that observed in control cells (Figure 5). CR treatment also resulted in decreased expression of TLR-2 (data not shown). Minimal changes in staining were observed following RAW cell treatment with CEE (data not shown).
Figure 5.
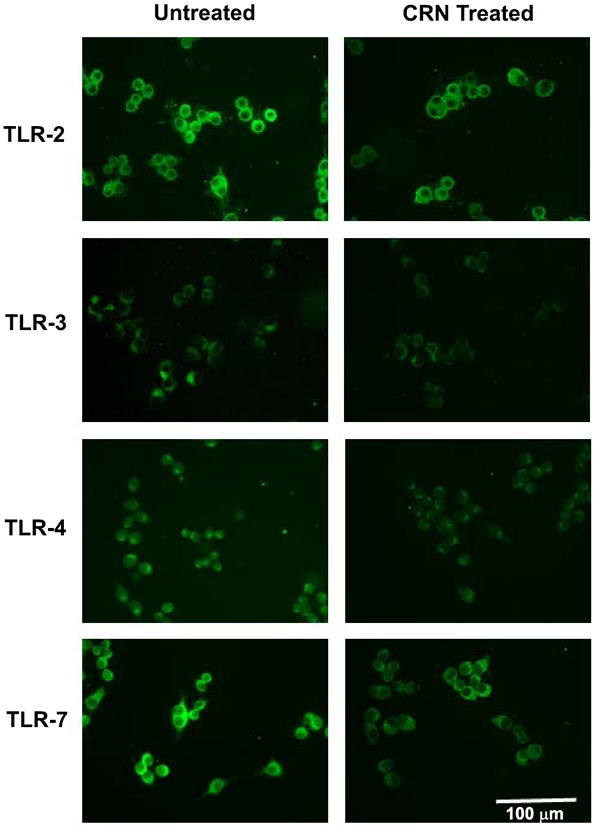
Altered expression of TLR-2, -3, -4 and -7 following exposure of RAW 264.7 cells to 0.1 mM of CRN as determined by immunofluorescent staining. Immunohistochemical staining revealing that exposure to CRN results in a decrease of TLR-2, TLR-3, TLR-4, and TLR-7 relative to the levels observed in the control-treated cells.
Immunostaining for TLR-3 suggests that there was a small decrease in protein expression of this protein following exposure to CRN (Figure 5), relative to the staining observed in the controls. Conversely, CRN and CR appear to reduce the intensity of staining for TLR-4 under similar conditions (Figure 5, data not shown). Very intense staining of RAW cells was observed using an antibody for TLR-7 (Figure 5). Staining intensity decreased upon incubation of RAW cells with CRN and dramatically decreased in the case of CR (Figure 5, data not shown), reflecting the mRNA data.
3.6 Immunofluorescent staining of primary splenocytes cultured with CEE, CR, and CRN demonstrate similar expression patterns to those observed in RAW 264.7 cells
To determine whether the impact of creatine and its derivatives on TLR expression is a RAW 264.7 cell-specific observation, we isolated splenocytes from mice and cultured the resultant cells in splenocyte media containing a 0.1 mM solution of CR, CRN, or CEE for 24 hours. Immunostaining for TLR-2, -3, -4, and -7 was performed on cytospins made from the cells. As observed with the RAW 264.7 cells, co-culture of murine splenocytes with CR or CRN downregulated expression of TLRs-2, -3, -4, and -7 (Figure 6). Dual immunofluorescent staining studies using antibodies to CD3ε, B220 and F4/80 indicate that T and B cells, as well as macrophages express TLR-2. -3, -4, and -7 as predicted (data not shown).
Figure 6.
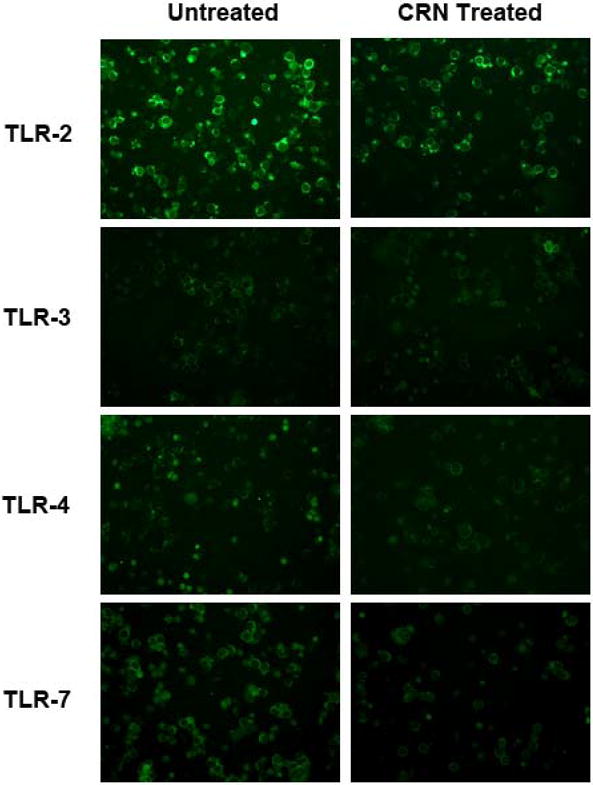
Reduced expression of TLR-2, -3, -4 and -7 following exposure of mouse splenocytes to 0.1 mM of CRN as determined by immunofluorescent staining. Immunohistochemical staining of cytospins prepared from splenocytes treated with CRN demonstrates that CRN results downregulates TLR-2, TLR-3, TLR-4, and TLR-7 relative to the levels observed in the control-treated cells.
4. Discussion
In the past decade, the use of nutritional supplements has grown rapidly [44-46]. Creatine is a readily available supplement that is widely used by adults and juveniles [28-30]. Despite its widespread use, potential side-effects related to the use of creatine or its derivates are largely undefined, although some studies have suggested that this supplement may have anti-inflammatory properties [24,36]. The impact of creatine supplementation on immune system function has not been well-studied. In the studies presented here, we examined the effects of low levels of creatine and other members of the argenine biosynthesis pathway on transcript levels of TLR receptors that are involved in the sensing phase of the immune response by recognizing PAMPs. TLRs, are one of the first receptors triggered in the host following infection, and can be located both on the cell (TLRs 1,2,4,5,6,10) or endosomal (3, 7, 8, 9) membranes. We examined the expression of TLRs known to be critical in sensing both bacterial (TLR-2, TLR-4) and viral (TLR-3, TLR-7) infections following short term exposure to creatine or its derivatives in vitro to determine whether exposure to these compounds could downregulate key molecules associated with triggering an immune response, thus having an adverse side effect on the host’s ability to respond to infection.
We determined that following exposure of a mouse macrophage cell line to creatine TLR-2, TLR-3, TLR-4 and TLR-7 levels were downregulated rapidly by the cells. We expected to observe minimal affects of creatine on the mouse macrophages, due to its rapid breakdown into creatinine [47]. Instead, creatine had profound immunosuppressive affects on all of the TLRs studied. The observation that creatinine also had a significant suppressive effect on TLR expression is surprising and novel since the molecule considered to be a biologically inert breakdown product of creatine. It has been accepted as dogma that creatinine is a pharmacologically inactive waste product of muscle metabolism devoid of biological activity which is excreted in the urine [48]. Creatinine dramatically decreased levels of TLR-2, TLR-3, TLR-4, and TLR-7 transcript levels in RAW cells. The decreased levels of mRNA levels could not be attributed to increased cell death, as apoptosis levels were similar to the level of apoptosis observed in the control cultures, regardless of the treatment (data not shown). In contrast to the observations in the cultures exposed to either CR or CRN, there were increased levels of TLRs observed at various time-points following exposure to of the RAW cells to CEE, a common over-the-counter formulation of creatine, suggesting that the precise formulation of this non-vitamin, non-mineral supplement may differentially impact the ability of the host to mount an innate immune response. Interestingly, despite decreased TLR-3 levels at 30 and 60 minutes post-exposure, TLR-3 levels were initially stimulated by the presence of CEE in the culture medium, as were TLR-2, TLR-4 and TLR-7. In fact, CEE was a potent inducer of TLR-7. This was unexpected since it was reported that CEE breaks down rapidly in aqueous solutions to CRN [49,50] which we demonstrated to be an inhibitor of TLR expression. It may be that CEE is rapidly taken up by RAW cells and/or stabilized in membranes in order to exert a stimulatory effect on TLR expression. It must be noted that the immunostimulatory effects of CEE occurred in the short-term (less than 40 minutes after exposure to CEE) and these effects are lost within 60 minutes of CEE exposure. It is likely, given the time-course data (Figs. 1-4), that longer incubation of CEE with RAW cells would demonstrate a decrease in TLR mRNA expression due increased levels of creatinine, a breakdown product of CEE in the cultures. TLRs receptor engagement is known to stimulate signaling NF-κβ-mediated signaling processes which can either directly or indirectly (via the production of cytokines) alter TLR expression levels [51]. We hypothesize that one mechanism by which TLR expression may be altered in our studies is via decreased pro-inflammatory mediator production (Leland, McDonald, and Drescher, manuscript in preparation).
The TLRs examined in this study are responsible for sensing a wide variety of pathogen components including lipoteiochoic acid (TLR-2), porin derived from Neisseria and Haemophoilis (TLR-2), lipopolysaccharide (TLR-4), and single- and double-stranded RNA (TLR-3 and TLR-7, respectively). These TLRs were chosen for study as they encompass sensors of a significant array of human pathogens including gram positive (TLR-2) and gram negative (TLR-4) bacteria, as well as viruses that cause significant disease in humans (i.e., influenza, coxsackieviruses, rotavirus). CR and CEE are supplements frequently used by athletes who are attempting to increase muscle mass, enhance performance, reduce recovery times, and reduce muscular inflammation [28-35]. Both short-term and long-term creatine supplementation regimens are common and often consist of a loading phase (20 grams/day for 5-7 days) followed by a maintenance phase (5 grams/day for up to 6 months). Loading protocols usually divide the 20 gram dose into four 5 gram doses/day [52,53]. Plasma creatine levels can increase up to 20-fold following ingestion of 5 grams of creatine [54]. While creatine in plasma has a relatively short half-life of approximately 3 hours [54], the increased plasma levels of creatine can be extended by ingesting multiple doses of creatine throughout the day. It is tempting to speculate that the extended elevation in creatine levels in vivo decreases TLR expression of cells involved in sensing infections and negatively impacts the host’s ability to respond to infections. Future studies in the laboratory will focus on the impact of CR, CRN, and CEE in animal models of viral and bacterial infections.
These studies support the hypothesis that CR and its metabolite, CRN may downregulate the innate immune response by decreasing TLR expression on macrophages, a key cell involved in the early phases of the immune response. In addition, these supplements may also influence cells of the adaptive immune response based on their ability to influence expression of TLR-2, -3, -4, and -7 on B cells, T cells, and macrophages. The observation that a derivative of CR, creatine ethyl ester, increased TLR expression is of interest and future studies need to explore whether this phenomenon is a property of other esters of CR (propyl, butyl, benzyl, etc.) or unique to CEE.
Highlights.
- The effect of creatine, creatinine, and creatine ethyl ester on
- Creatine and creatinine downregulate TLRs
- Creatine ethyl ester upregulate TLRs over the short-term
Acknowledgments
This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program (1 C06 RR17417-01) from the National Center for Research Resources, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Korey M. Leland, Email: KoreyLeland@creighton.edu.
Thomas L. McDonald, Email: tmcdonal@unmc.edu.
References
- 1.Feng L, Xia Y, Garcia GE, Hwang D, Wilson CB. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-α, and lipopolysaccharide. J Clin Invest. 1995;95:1669–1675. doi: 10.1172/JCI117842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winrow VR, Winyard PG, Morris CJ, Blake DR. Free radicals in inflammation: second messengers and mediators of tissue destruction. Br Med Bull. 1993;49:506–522. doi: 10.1093/oxfordjournals.bmb.a072627. [DOI] [PubMed] [Google Scholar]
- 3.Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-kappaB. Int J Mol Med. 1999;4:223–230. doi: 10.3892/ijmm.4.3.223. [DOI] [PubMed] [Google Scholar]
- 4.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxident therapeutic options. Curr Neuropharm. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 6.Dandona P, Ghanim H, Chaudhuri A, Dhindsa S, Kim SS. Macronutrient intake induces oxidative and inflammatory stress: potential relevance to atherosclerosis and insulin resistance. Exp Mol Med. 2010;42:245–253. doi: 10.3858/emm.2010.42.4.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneto H, Katakami N, Matsuhisa M, Matsuoka T. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;2010:453892. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 10.Isreal N, Gougerot-Pocidalo MA, Aillet F, Virelizier JL. Redox status of cells influences constitutive or induced NF-kappa B translocation and HIV long terminal repeat activity in human T and monocytic cell lines. J Immunol. 1992;149:3386–3393. [PubMed] [Google Scholar]
- 11.DeForge LE, Preston AM, Takeuchi E, Kenney JS, Boxer LA, Remick DG. Regulation of interleukin-8 gene expression by oxidant stress. J Biol Chem. 1993;268:12–13. [PubMed] [Google Scholar]
- 12.Wang S, Leonard SS, Castranova V, Vallyathan V, Shi X. The role of superoxide radical in TNF-alpha induced NF-kappaB activation. Ann Clin Lab Sci. 1999;29:192–199. [PubMed] [Google Scholar]
- 13.Remick DG, Villarete L. Regulation of cytokine gene expression by reactive oxygen and reactive nitrogen intermediates. J Leuko Biol. 1996;59:471–475. doi: 10.1002/jlb.59.4.471. [DOI] [PubMed] [Google Scholar]
- 14.Lucotti P, Monti L, Setola E, La Canna G, Castiglioni A, Rossodivita A, et al. Oral L-arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after a aortocoronary bypass. Metabol Clin Exper. 2009;58:1270–1276. doi: 10.1016/j.metabol.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Ockenga J, Borchert K, Stüber E, Lochs H, Manns MP, Bischoff SC. Glutamine-enriched total parenteral nutrition in patients with inflammatory bowel disease. Eur J Clin Immunol. 2005;59:1302–1309. doi: 10.1038/sj.ejcn.1602243. [DOI] [PubMed] [Google Scholar]
- 16.Madan BR, Khanna NK. Effect of creatinine on various experimentally induced inflammatory models. 1979;23(1):1–7. [PubMed] [Google Scholar]
- 17.Burch RM, Weitzberg M, Blok N, Muhlhauser R, Martin D, Farmer SG, et al. N-(fluorenyl-9-methoxycarbonyl) amino acids, a class of antiinflammatory agents with a different mechanism of action. Proc Nat Acad Sci USA. 1991;88:359. doi: 10.1073/pnas.88.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dery RE, Mathison R, Davison J, Befus AD. Inhibition of allergic inflammation by C-terminal peptides of the prohormone submandibular rat 1 (SMR-1) Int Arch Allergy Appl Immunol. 2001;124:201–204. doi: 10.1159/000053710. [DOI] [PubMed] [Google Scholar]
- 19.Bruno O, Schenone S, Ranise A, Bondavalli F, Filippelli W, Falcone G, et al. Antiinflammatory agents: new series of N-substituted amino acids with complex pyrimidine structures endowed with antiphlogistic activity. Il Farmco. 1999;54:95–100. doi: 10.1016/s0014-827x(98)00109-8. [DOI] [PubMed] [Google Scholar]
- 20.Andou A, Hisamatsu T, Okamoto S, Chinen H, Kamada N, Kobayashi T, et al. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterol. 2009;136:564–574. doi: 10.1053/j.gastro.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez PC, Zea AH, DeSalvo J, et al. L-argenine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171:1232–1293. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 22.Sethi P, Lukiw WJ. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci Lett. 2009;459:100–104. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 23.Son DO, Satsu H, Shimizu M. Histidine inhibits oxidative stress- and TNF-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett. 2005;579:4671–4677. doi: 10.1016/j.febslet.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 24.Nomura A, Zhang M, Sakamoto T, Ishii Y, Morishima Y, Mochizuki M, et al. Anti-inflammatory activity of creatine supplementation in endothelial cells in vitro. Br J Pharm. 2003;139:715–720. doi: 10.1038/sj.bjp.0705316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawler JM. Direct antioxidant properties of creatine. Biochem Biophys Res Comm. 2002;290:47–52. doi: 10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- 26.Khanna NK, Madan BR. Studies on the anti-inflammatory activity of creatine. Arch Int Pharmacodyn Ther. 1978;231:340–350. [PubMed] [Google Scholar]
- 27.Lenz H, Schmidt M, Welge V, Schlattner U, Wallimann T, Elsässer H-P, et al. The creatine kinase system in human skin: protective effects of creatine against oxidative and UV damage in vitro and in vivo. J Invest Dermatol. 2004;124:443–452. doi: 10.1111/j.0022-202X.2004.23522.x. [DOI] [PubMed] [Google Scholar]
- 28.Perkin JE, Wilson WJ, Schuster K, Rodriguez J, Allen-Chabot A. Prevalence of nonvitamin, nonmineral supplement usage among university students. J Amer Diet Assn. 2002;102:412–414. doi: 10.1016/s0002-8223(02)90096-9. [DOI] [PubMed] [Google Scholar]
- 29.Juhn MS, O’Kane JW, Vinci DM. Oral creatine supplementation in male collegiate athletes: a survey of dosing habits and side effects. J Amer Diet Assn. 1999;99:593–595. doi: 10.1016/s0002-8223(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez NR, DiMarco NM, Langley S. Position of the American dietetic association, dietitians of Canada and the American college of sports medicine: nutrition and athletic performance. J Amer Diet Assn. 2009;109:509–527. doi: 10.1016/j.jada.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Bosco C, Tihanyi J, Pucspk J, Kovacs I, Gabossy A, Colli R, et al. Effect of oral creatine supplementation on jumping and running performance. Int J Sports Med. 1997;18:369–372. doi: 10.1055/s-2007-972648. [DOI] [PubMed] [Google Scholar]
- 32.Dawson B, Cutler M, Moody A, Lawrence S, Goodman C, Randall N. Effects of oral cretine loading on single and repeated maximal short sprints. Aust J Sci Med Sport. 1995;27:56–61. [PubMed] [Google Scholar]
- 33.Warthin AS. Hereditary with reference to carcinoma as shown by the study of the cases examined in the pathological laboratory of the University of Michigan, 1895-1913. Arch Intern Med. 1913;12:546–555. doi: 10.3322/canjclin.35.6.348. [DOI] [PubMed] [Google Scholar]
- 34.Prevost MC, Nelson AG, Morris GS. Creatine supplementation enhances intermittent work performance. Res Quart Exer Sport. 1997;68:233–240. doi: 10.1080/02701367.1997.10608002. [DOI] [PubMed] [Google Scholar]
- 35.Cooke WH, Grandjean PW, Barnes WS. Effect of oral creatine supplemention on power output and fatigue during bicycle ergometry. J Appl Physiol. 1995;78:670–673. doi: 10.1152/jappl.1995.78.2.670. [DOI] [PubMed] [Google Scholar]
- 36.Vieira RP, Duarte ACS, Claudino RC, Perini A, Santos ABG, Moriya HT, et al. Creatine supplementation exacerbates allergic lung inflammation and airway remodeling in mice. Am J Respir Cell Mol Biol. 2007;37:660–667. doi: 10.1165/rcmb.2007-0108OC. [DOI] [PubMed] [Google Scholar]
- 37.Katseres NS, Reading DW, Shayya L, DiCesare JC, Purser GH. Non-enzymatic hydrolysis of creatine ethyl ester. Biochem Biophys Res Comm. 2009;386:363–367. doi: 10.1016/j.bbrc.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 38.Seenge GR, Simpson EJ, Greenhaff PL. Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans. J Appl Physiol. 2000;89:1165–1171. doi: 10.1152/jappl.2000.89.3.1165. [DOI] [PubMed] [Google Scholar]
- 39.Khademi M, Illes Z, Gielen AW, Marta M, Takazawa N, Baecher-Allan C, et al. T cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerecerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J Immunol. 2004;172:7169–7176. doi: 10.4049/jimmunol.172.11.7169. [DOI] [PubMed] [Google Scholar]
- 40.Medhurst AD, Harrison DC, Read SJ, Campbell CA, Robbins MJ, Pangalos MN. The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J Neurosci Methods. 2000;98:9–20. doi: 10.1016/s0165-0270(00)00178-3. [DOI] [PubMed] [Google Scholar]
- 41.Tracy S, Drescher KM, Chapman NM, Kim KS, Carson SD, Pirruccello S, et al. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J Virol. 2002;76:12097–12111. doi: 10.1128/JVI.76.23.12097-12111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drescher KM, Kono K, Bopegamage S, Carson SD, Tracy S. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology. 2004;329:381–394. doi: 10.1016/j.virol.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 43.Beutler B. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wold RS, Lopez ST, Yau CL, Butler LM, Pareo-Tubbeh SL, Waters DL, et al. Increasing trends in elderly persons’ use of nonvitamin, nonmineral dietary supplements and concurrent use of medications. J Amer Diet Assn. 2005;105:54–63. doi: 10.1016/j.jada.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Timbo BB, Ross MP, McCarthy PV, Lin C-TJ. Dietary supplements in a national survey: prevalence of use and reports of adverse events. J Amer Diet Assn. 2006;106:1966–1974. doi: 10.1016/j.jada.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Piccano MF. Dietary supplement use by US adults: data from the national health and nutrition examination survey, 1999-2000. Am J Epidemiol. 2004;160:339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 47.Harris RA, Crabb DW. Metabolic interrelationships. In: Devlin TM, editor. Textbook of Biochemistry with Clinical Correlations. New York: Wiley-Liss; 2005. p. 884. [Google Scholar]
- 48.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 49.Giese MW, Lecher CS. Qualitative in vitro NMR analysis of creatine ethyl ester pronutrient in human plasma. Int J Sports Med. 2009;30:766–770. doi: 10.1055/s-0029-1231045. [DOI] [PubMed] [Google Scholar]
- 50.Giese MW, Lecher CS. Non-enzymatic cyclization of creatine ethyl ester to creatinine. Biochem Biophys Res Comm. 2009;388:252–255. doi: 10.1016/j.bbrc.2009.07.151. [DOI] [PubMed] [Google Scholar]
- 51.Zarember KA, Godowski PJ. Tissue expression of human toll-like receptors and differential regulation of toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 52.Kreider RB. Effects of creatine supplementation on performance and training adaptations. Mol Cell Biochem. 2003;244:89–94. [PubMed] [Google Scholar]
- 53.Bemben MG, Lamont HS. Creatine supplementation and exercise performance. Sports Med. 2005;35:107–125. doi: 10.2165/00007256-200535020-00002. [DOI] [PubMed] [Google Scholar]
- 54.Jäger R, Harris RC, Purpura M, Francauz M. Comparison of new forms of creatine in raising plasma creatine levels. J Int Soc Sports Nutr. 2007;4:17. doi: 10.1186/1550-2783-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]


