Abstract
Background
To study the relationship between emphysema, airflow obstruction and lung cancer in a high risk population we performed quantitative analysis of screening computed tomography (CT) scans.
Methods
Subjects completed questionnaires, spirometry and low-dose helical chest CT. Analyses compared cases and controls according to automated quantitative analysis of lung parenchyma and airways measures.
Results
Our case-control study of 117 matched pairs of lung cancer cases and controls did not reveal any airway or lung parenchymal findings on quantitative analysis of screening CT scans that were associated with increased lung cancer risk. Airway measures including wall area %, lumen perimeter, lumen area and average wall HU, and parenchymal measures including lung fraction < −910 Hounsfield Units (HU), were not statistically different between cases and controls.
Conclusions
The relationship between visual assessment of emphysema and increased lung cancer risk could not be verified by quantitative analysis of low-dose screening CT scans in a high risk tobacco exposed population.
There is an association between airflow obstruction on spirometry and anatomic emphysema by visual assessment on low-dose screening CT scan and lung cancer, as reported by us [1] and others [2–7]. Two reports from the Mayo Clinic lung cancer screening study with small numbers of lung cancers (n = 24 and n = 64 respectively), utilized quantitative CT analysis to determine % emphysema and concluded that the quantity of radiographic emphysema was not found to be a significant risk for lung cancer [8, 9]. We report on 117 pairs of lung cancer subjects and matched controls from the Pittsburgh Lung Screening Study (PLuSS) analyzed by quantitative CT analysis for airway and parenchymal abnormalities. This is the first study to correlate quantitative CT measures of parenchymal disease (lung fraction < −910 HU) with visual emphysema, measures of airways disease (wall area and lumen measures) with FEV1, and lung cancer risk.
Methods
Participants
The Pittsburgh Lung Screening Study (PLuSS) involved 3,642 subjects [10] and was approved by the Institutional Review Board of the University of Pittsburgh (approval # 011171). Written informed consent was obtained from all participants. Between January 2002 and April 2005, volunteers with the following characteristics were recruited: (1) age 50 to 79 years; (2) no personal lung cancer history; (3) no participation in concurrent lung cancer screening studies; (4) no chest computed tomography (CT) within 12 months; (5) current or ex-cigarette smoker of at least one-half pack per day for at least 25 years, and, if quit, quit for no more than 10 years before study enrollment; and (6) body weight less than 400 pounds. Individuals were not excluded because of symptoms.
PLuSS participants performed the following baseline activities (T0) between March 2002 and September 2005: (1) completed a risk factor questionnaire, (2) provided peripheral blood samples, (3) underwent forced expiratory spirometry conducted and analyzed in accordance with American Thoracic Society standards [11], (4) underwent low-dose screening CT examination and physician referral for noncalcified lung nodules. Follow-up activities (T1) were performed between March 2002 and November 2006 and included repeat low-dose screening CT examination after 12 months and active surveillance for lung cancer–related endpoints. The current study includes 234 subjects selected from the PLuSS cohort, including 117 pathologically verified lung cancers and 117 control subjects, CT-screened lung cancer-free PLuSS subjects individually matched to the case group according to sex (men, women), year of birth category (before 1934, 1934-43, after 1943), year of baseline CT screening examination (2002, 2003, 2004, 2005), cigarette smoking status at time of PLuSS entry (current smokers and quit within three years, quit for more than three years), and pack-year smoking category (<40.0, 40–79.9, 80.0+ pack-years).
CT examinations
The initial or prevalence (T0) lung cancer screening CT examinations was used for analysis in this study. The CT examinations were performed on a GE LightSpeed Plus 4-detector (n=105) or GE LightSpeed Ultra 8-detector (n=129) (GE Healthcare, Waukesha, WI) with the subjects holding their breath at end-inspiration. The CT acquisition protocol was a non-contrasted, helical technique at 120 kVp (n=45) or 140 kVp (n=189), mean 24.4 (±8.3) mAs, and a HS (GE HealthCare “High Speed”) or 1.35:1 pitch. The CT images were contiguous and reconstructed at 2.5 mm thickness using the GE’s “lung” reconstruction kernel with a 512 × 512 pixel matrix.
CT examination review
Readers used lung windows/level settings (1,496/−555) to view images (2.5 mm section thickness) on a PACS monitor display system (Stentor; Radiology Informatics Business Group of Phillips Medical Systems, Foster City, CA) and visually assessed the presence or absence of emphysema on a four-point scale as: (0) none, (1) trace, (2) mild (3), or (4) moderate-severe. The rating scale was based on a modified NETT rating that assigned using the percentages of emphysema: 0% - none, 1–10% trace, 11–25% mild, or 26% or greater moderate-severe [12]. Details, including measures of inter-reader reliability, appear in the online supplement to reference 1. (http://ajrccm.atsjournals.org/cgi/data/178/7/738/DC1/1)
Quantitative CT Analysis
Lung regions depicted on each CT image were segmented from the surrounding chest wall and mediastinal structures using a fully automated in-house computer algorithm [13], which has been improved to utilize 3-D connectivity information. Lung voxels meeting the following criteria were considered to be associated with the presence of emphysema: (1) a pixel value less than −910 HU and (2) a connection to ten or more other pixels with a value less than −910 HU based on 2-D, 8-neighbor connectivity [14]. The presence (or absence) of emphysema was quantified as the fraction (percent) of “emphysema” voxels relative to all of the lung voxels. Airways depicted on the CT images were analyzed using an in-house computer algorithm that automatically detects and quantifies airway characteristics of multiple airway sections for each CT examinations [15]. The airway quantification algorithm is based on a partial membership algorithm to compute lumen area, wall area, lumen perimeter, average HU values of the airway wall, and wall area as a percent of the total airway area. Airway parameters were computed for all airway sections automatically detected not at or near a bifurcation and for the smallest one-third of the airway sections detected for each subject [16]. For each subject we counted the airway sections analyzed and computed the mean of the airway variables across the airway sections. The airway analysis was only performed using the detection and quantification software without consultation from the radiologists or clinicians.
Statistical analysis
We used conditional logistic regression to control for matching factors and to estimate univariate lung cancer associations with cigarette dose exposure (pack-years), family history of cancer, visually assessed emphysema, and spirometry results categorized according to GOLD guidelines (At-a-Glance Pocket Reference for COPD Diagnosis, Management and Prevention; http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=2200). For quantitative CT measures, we tabulated median and interquartile range (IQR, 25th and 75th percentile) values and used the Kruskal Wallis rank sum tests to evaluate group differences for statistical significance. Statistical inferences used a p = 0.05 two-sided significance level.
Results
Subject characteristics
As shown in Table 1, 117 lung cancer cases from the PLuSS were well matched with 117 controls, with respect to sex, age, race, smoking status, and pack years. The only differences in the groups were a higher family history of lung cancer in the lung cancer group (p = 0.04) and visual emphysema in the lung cancer group (p < 0.0001), as we previously reported in a smaller subset of patients [1]. Approximately 45% of matched controls had some visual emphysema and 52% had some airflow obstruction (Table 1). Controlling for sex, year of birth, year of CT, smoking status, and pack-years, the presence of visually assessed emphysema associated with lung cancer more strongly than the presence of airflow obstruction (odds ratio (OR) 3.59, 95% confidence interval 1.98–6.50, p < 0.0001 vs. OR 2.02, 95% CI 1.13–3.58, p = 0.02). When evaluated as a dichotomous variable (GOLD I–IV vs. none), airflow obstruction achieved a statistically significant association with lung cancer (p = 0.02), but not when evaluated as a four category class variable (none, GOLD I, GOLD II, GOLD III–IV, p = 0.12, Table 1).
Table 1.
Risk factors, cancer cases vs. matched control cases
| Cancers n=117 |
Controls [1] n=117 |
||||||
|---|---|---|---|---|---|---|---|
| Attribute | N | % | N | % | OR | 95% CI | p-value |
| Sex [2] | |||||||
| men | 61 | 52.1 | 61 | 52.1 | |||
| women | 56 | 47.9 | 56 | 47.9 | |||
| Age (years) | |||||||
| 50–59 | 35 | 29.9 | 37 | 31.6 | |||
| 60–69 | 57 | 48.7 | 56 | 47.9 | |||
| 70+ | 25 | 21.4 | 24 | 20.5 | |||
| Race | |||||||
| White | 111 | 94.9 | 110 | 94.0 | |||
| Black | 6 | 5.1 | 7 | 6.0 | |||
| Smoking status [2] | |||||||
| Current or quit <3 years | 96 | 82.1 | 96 | 82.1 | |||
| Quit ≥3 years | 21 | 17.9 | 21 | 17.9 | |||
| Cigarette dose-duration index (pack years) [2] | |||||||
| <40 | 27 | 23.1 | 27 | 23.1 | |||
| 40–79 | 66 | 56.4 | 66 | 56.4 | |||
| 80+ | 24 | 20.5 | 24 | 20.5 | |||
| Family history of cancer | 0.04 | ||||||
| none or skin only | 38 | 32.5 | 46 | 39.3 | REF | ||
| non-lung | 49 | 41.9 | 56 | 47.9 | 1.05 | 0.59–1.85 | |
| lung only | 13 | 11.1 | 12 | 10.3 | 1.36 | 0.56–3.34 | |
| lung + non-lung | 17 | 14.5 | 3 | 2.6 | 6.43 | 1.78–23.2 | |
| Visual emphysema | <.0001 | ||||||
| none | 31 | 26.5 | 64 | 54.7 | REF | ||
| trace | 28 | 23.9 | 24 | 20.5 | 2.64 | 1.28–5.44 | |
| mild | 40 | 34.2 | 15 | 12.8 | 6.29 | 2.86–13.9 | |
| moderate-severe | 18 | 15.4 | 14 | 12.0 | 3.02 | 1.27–7.18 | |
| Airflow obstruction | 0.12 | ||||||
| none | 39 | 33.3 | 56 | 47.9 | REF | ||
| GOLD I | 20 | 17.1 | 17 | 14.5 | 1.82 | 0.84–3.96 | |
| GOLD II | 43 | 36.8 | 34 | 29.1 | 2.03 | 1.04–3.93 | |
| GOLD III–IV | 15 | 12.8 | 10 | 8.5 | 2.33 | 0.93–5.82 | |
Includes two subjects with lung cancer diagnosed during extended follow-up
Factor used to match cases and controls
Self-reported symptom count (cough, phlegm, and wheeze)
Self-reported count of physician diagnoses (asthma, bronchitis, and emphysema)
The lung cancer case series included 102 non small cell lung cancers (68 prevalent and 34 non prevalent) and 15 small cell lung cancers (2 prevalent and 13 non prevalent).
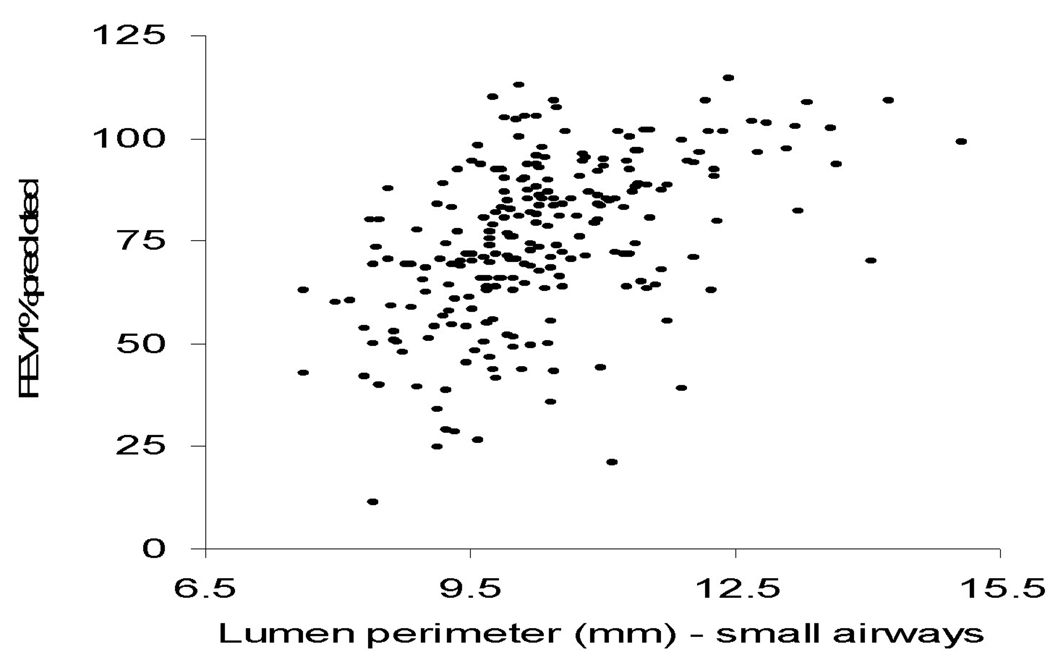
Table 2 shows the airway measures in cancer cases and controls. There were no significant differences between cases and controls in wall area %, lumen perimeter, lumen area, average airway wall HU, as measured for all airways or for the small airways subgroup. Figure 1 shows a correlation between one CT airway measure (small airway lumen perimeter) and one spirometry variable (FEV1 % predicted). As FEV1 declines, the airway measures get smaller, as expected (Figure 1).
Table 2.
Airway measures, cancer cases vs. matched control cases
| Cancers N=117 |
Controls [1] N=117 |
|||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | p-value | ||
| All airways | ||||||
| Wall area percent | mean | 53 | 50, 57 | 53 | 49, 56 | 0.24 |
| Lumen perimeter (mm) | mean | 13 | 12, 13 | 13 | 12, 14 | 0.21 |
| Lumen area (mm2) | mean | 13 | 12, 14 | 13 | 12, 16 | 0.24 |
| Average airway wall HU | mean | −183 | −204, −160 | −177 | −200, −157 | 0.24 |
| Small airways | ||||||
| Wall area percent | mean | 53 | 50, 56 | 52 | 49, 56 | 0.16 |
| Lumen perimeter (mm) | mean | 10 | 10, 11 | 10 | 10, 11 | 0.26 |
| Lumen area (mm2) | mean | 8 | 7, 9 | 8 | 8, 10 | 0.26 |
| Average airway wall HU | mean | −247 | −281, −223 | −246 | −266, −222 | 0.24 |
Includes two subjects with lung cancer diagnosed during extended follow-up
Figure 1.
FEV1 (% predicted) vs. small airway lumen perimeter (mm) in cases and controls combined (n=234). The rank order (Spearman) correlations between FEV1 (% predicted) vs. small airway lumen perimeter (mm) are 0.50 in the case group, 0.55 in the control group, and 0.53 in both groups combined.
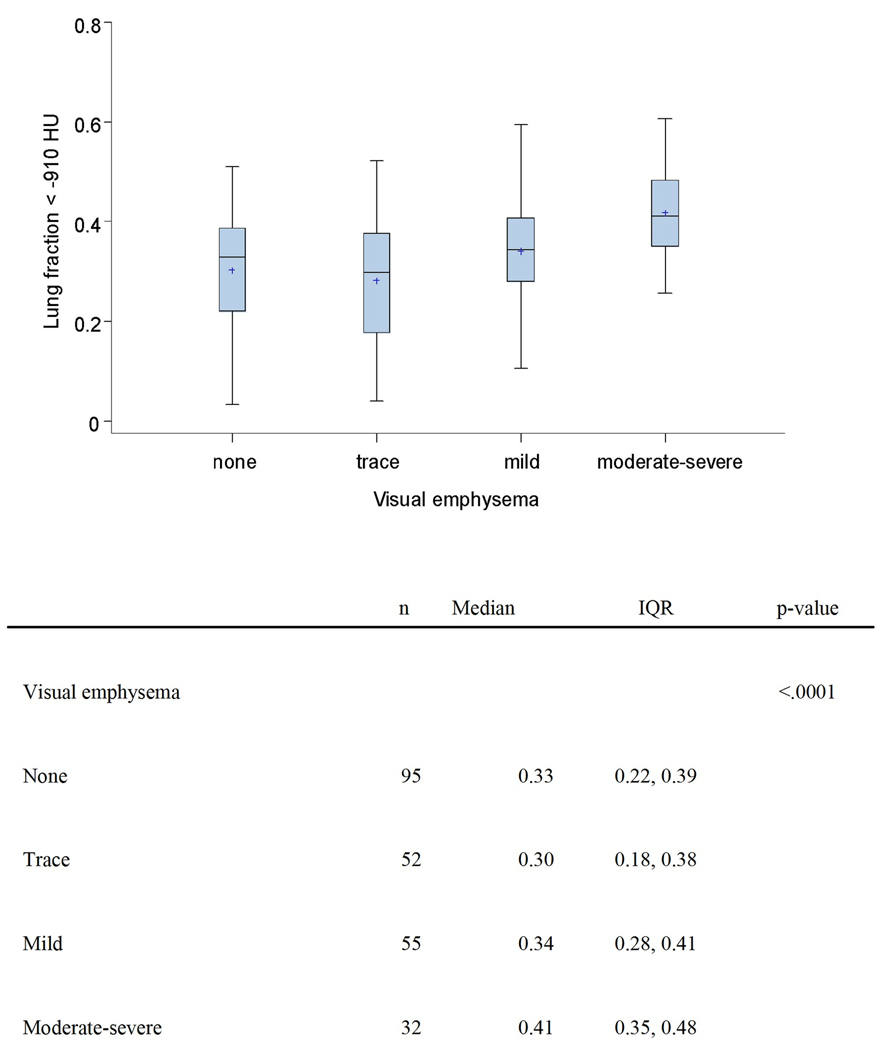
Table 3 and Figure 2 show the relationship between parenchymal measures of emphysema, the lung fraction < −910 HU and visual emphysema in cases and controls. There is no statistically significant relationship between quantitatively measured parenchymal measures (represented by lung fraction < −910 HU) and lung cancer risk. We observed a statistical association (p < 0.0001) between visual emphysema and lung fraction < −910 HU (Figure 2). When compared to subjects (cases and controls combined) with less than mild emphysema, lung fraction < −910 HU values were higher in subjects with mild visual emphysema and higher yet in subjects with moderate-severe visual emphysema. Aside from perhaps slightly lower airway wall HU values in subjects with some visual emphysema vs. no emphysema, analyses failed to identify consistent, meaningful or statistically signification association between any airway measure and visual emphysema (Table 4).
Table 3.
Lung fraction < −910 HU [1], cancer cases vs. matched control cases
| Cancers | Controls | ||||||
|---|---|---|---|---|---|---|---|
| n | Median | IQR | n | Median | IQR | p-value | |
| All cases and controls | 117 | 0.33 | 0.23, 0.41 | 117 | 0.35 | 0.27, 0.40 | 0.54 |
| Visual emphysema | |||||||
| none | 31 | 0.31 | 0.22, 0.42 | 64 | 0.34 | 0.23, 0.39 | 0.58 |
| trace | 28 | 0.30 | 0.13, 0.40 | 24 | 0.30 | 0.23, 0.36 | 0.66 |
| mild | 40 | 0.33 | 0.24, 0.39 | 15 | 0.36 | 0.33, 0.44 | 0.05 |
| moderate-severe | 18 | 0.41 | 0.35, 0.48 | 14 | 0.41 | 0.34, 0.49 | 0.82 |
Blob 10 filter
Includes two subjects with lung cancer diagnosed during extended follow-up
Figure 2.
Box plots showing lung fraction < −910 HU in cases and control combined, sub-grouped according to visual emphysema. The line segment and plus (+) symbol within each box identify the median and mean, respectively. The lower and upper borders of each box identify the 25th and 75th percentiles, respectively. The lower whisker (lower fence) extends to the minimum observation greater than or equal to the 25th percentile minus 1.5 times the inter-quartile range. The upper whisker (upper fence) extends to the maximum observation less than or equal to the 75th percentile plus 1.5 times the inter-quartile range. No subject had a lung fraction < −910 HU measurement less than the lower fence or greater than the upper fence.
Table 4.
Airway measures, according to visual emphysema
| None n=95 |
Trace n=52 |
Mild n=55 |
Moderate-severe n=32 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | p-value | ||
| All airways | ||||||||||
| Wall area percent | mean | 53 | 50, 56 | 52 | 48, 56 | 53 | 51, 56 | 53 | 50, 56 | 0.57 |
| Lumen perimeter (mm) | mean | 13 | 12, 14 | 12 | 12, 14 | 13 | 11, 13 | 12 | 12, 13 | 0.34 |
| Lumen area (mm2) | mean | 13 | 12, 16 | 13 | 12, 16 | 13 | 11, 15 | 12 | 12, 14 | 0.40 |
| Average airway wall (HU) | mean | −172 | −192, −153 | −189 | −218, −166 | −188 | −204, −167 | −179 | −208, −260 | 0.03 |
| Small airways | ||||||||||
| Wall area percent | mean | 52 | 49, 57 | 52 | 49, 55 | 54 | 50, 57 | 53 | 51, 56 | 0.41 |
| Lumen perimeter (mm) | mean | 10 | 10, 11 | 10 | 10, 11 | 10 | 09, 11 | 10 | 10, 11 | 0.18 |
| Lumen area (mm2) | mean | 9 | 08, 10 | 8 | 08, 10 | 8 | 07, 09 | 8 | 07, 09 | 0.19 |
| Average airway wall (HU) | mean | −242 | −265, −207 | −248 | −280, −228 | −254 | −275, −225 | −250 | −284, −289 | 0.13 |
Discussion
Our matched case control study (n = 117 pairs) evaluated quantitative CT airway and parenchymal measures. We also looked at airflow obstruction as measured on spirometry and visual assessment of emphysema, as we previously published. The relationship between airflow obstruction and lung cancer has been the subject of numerous studies [5–7, 9] which concluded that airflow obstruction is an independent risk factor for lung cancer. However, none of these studies controlled for the presence or absence of emphysema. One study by de Torres and colleagues found that the presence of emphysema on a CT scan, but not airflow obstruction, was associated with increased risk of lung cancer [2]. Our previously published study, as well as the current results, suggest that emphysema by visual assessment is superior to airflow obstruction as a single measure of lung cancer risk [1].
The quantitative analysis of low-dose screening CT scans did not reveal any airway or parenchymal findings that were associated with increased lung cancer risk. This finding is similar to the results of two prior studies by the Mayo clinic investigators [8, 9]. There is potential for bias in our study as 70/117 cancers are prevalent cancers and 34/70 prevalent cancers had cancer diagnosed from the same CT scan used in the emphysema analysis. The use of automated CT analysis for emphysema detection in low-dose screening CT scans is in its early stages. At trace and mild levels of emphysema by visual assessment, our automated CT analysis shows no difference in proportion of quantitative emphysema as measured by fraction < −910 HU compared to the no emphysema group (Table 3 and Figure 2). How can we reconcile the negative findings of the automated methodology of CT analysis for emphysema quantitation in the Mayo studies and our current analysis with the findings of visual emphysema as a significant predictor of increased lung cancer risk in de Torres and our own previous studies?
One limitation is that the CT examinations were acquired at a very low-dose and reconstructed using an over-enhancing, high-spatial frequency reconstruction kernel, which create CT images with a low signal-to-noise ratio that are not optimal for quantification of emphysema. In an effort to adjust (correct) for the increased image noise, we implemented a connectivity constraint to removes single (unconnected) or small isolated “emphysema” pixels. This correction may or may not have produced results that are close to the actual percentage of emphysema present in subjects. However, the CT examination for all subjects were performed under essentially the same CT protocol and, therefore, we believe that our quantitative emphysema measure, on a relative scale, reproducibly ranked subjects according to CT emphysema. Some subjects were scanned at lower x-ray tube energy. This could theoretically affect the quantification of emphysema, but we believe differences in tube energy were negligible and insignificant in terms of quantifying emphysema.
The visual assessment of emphysema is relatively straightforward. One looks at a CT scan and if emphysema is present the only debate is severity grading which is more subjective. The presence or absence of emphysema should be readily discernable. We would argue that visual assessment for the presence or absence of emphysema should be considered the standard until the algorithms and methodology for automated CT analysis are standardized and shown to be reproducible on low-dose screening CT scans. The use of quantitative methods for CT analysis is preliminary and an evolving methodology [17].
We would also suggest the possibility that quantitative CT parenchymal analysis and visual analysis for emphysema and quantitative CT airway analysis and FEV1 may be measuring different things. There are validated standards for spirometry [11] and visual emphysema analysis [12] that do not yet exist for quantitative CT.
This study advances the field by enhancing the discussion about the relationship of COPD, both airflow obstruction and emphysema, and lung cancer. There appears to be differences in the relationship between emphysema and lung cancer using an automated quantitative volumetric analysis compared to visual or semi-quantitative analysis, although the confounding results of the differing methodologies clearly require further study and clarification. The clinical implications of this study are significant, particularly in terms of lung cancer risk prediction in high risk patients, and the importance of methodology in emphysema determination of screening CT scans in high risk populations.
In conclusion, our quantitative analysis of low-dose screening CT scans in a high risk tobacco exposed population failed to reproduce the association of emphysema, as assessed visually on the same CT scans, with the diagnosis of lung cancer. Further research into the application of quantitative CT analysis to low-dose screening CT scans, and the relationship between emphysema, airflow obstruction and lung cancer is warranted.
Acknowledgments
Supported by the University of Pittsburgh Lung Cancer SPORE: NCI P50-CA90440, 1P50 HL084948, University of Pittsburgh Cancer Institute, and University of Pittsburgh Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no potential conflicts of interest for any of the authors.
References
- 1.Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008 Oct 1;178(7):738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007 Dec;132(6):1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno S, Takiguchi Y, Fujikawa A, et al. Chronic obstructive pulmonary disease and interstitial lung disease in patients with lung cancer. Respirology. 2009 Apr;14(3):377–383. doi: 10.1111/j.1440-1843.2008.01477.x. [DOI] [PubMed] [Google Scholar]
- 4.Petty TL. Are COPD and lung cancer two manifestations of the same disease? Chest. 2005 Oct;128(4):1895–1897. doi: 10.1378/chest.128.4.1895. [DOI] [PubMed] [Google Scholar]
- 5.Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann Intern Med. 1986 Oct;105(4):503–507. doi: 10.7326/0003-4819-105-4-503. [DOI] [PubMed] [Google Scholar]
- 6.Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987 Apr;106(4):512–518. doi: 10.7326/0003-4819-106-4-512. [DOI] [PubMed] [Google Scholar]
- 7.Wasswa-Kintu S, Gan WQ, Man SFP, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005 Jul;60(7):570–575. doi: 10.1136/thx.2004.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishi K, Gurney JW, Schroeder DR, Scanlon PD, Swensen SJ, Jett JR. The correlation of emphysema or airway obstruction with the risk of lung cancer: a matched case-controlled study. Eur Respir J. 2002 Jun;19(6):1093–1098. doi: 10.1183/09031936.02.00264202. [DOI] [PubMed] [Google Scholar]
- 9.Maldonado F, Bartholmai BJ, Swensen SJ, Midthun DE, Decker PA, Jett JR. Are airflow obstruction and radiographic emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest. doi: 10.1378/chest.09-2567. Prepublished online March 26, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Wilson DO, Weissfeld JL, Fuhrman CR, et al. The Pittsburgh Lung Screening Study (PLuSS): Outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med. 2008 Nov 1;178(9):956–961. doi: 10.1164/rccm.200802-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991 Nov;144(5):1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 12.National Emphysema Treatment Trial (NETT): Evaluation of lung volume reduction surgery for emphysema. [Accessed October 4, 2010]; http://www.nhlbi.nih.gov/health/prof/lung/nett/lvrsweb.htm.
- 13.Leader JK, Zheng B, Rogers RM, et al. Automated lung segmentation in X-ray computed tomography: development and evaluation of a heuristic threshold-based scheme. Acad Radiol. 2003 Nov;10(11):1224–1236. doi: 10.1016/s1076-6332(03)00380-5. [DOI] [PubMed] [Google Scholar]
- 14.Zheng B, Leader JK, Coxson HO, et al. CT acquisition technique and quantitative analysis of the lung parenchyma: variability and corrections. Proc SPIE. 2006;6143:61432S. [Google Scholar]
- 15.Zheng B, Leader JK, McMurray JM, et al. Automated detection and quantitative assessment of pulmonary airways depicted on CT images. Med Phys. 2007;34(7):2844–2852. doi: 10.1118/1.2742777. [DOI] [PubMed] [Google Scholar]
- 16.Leader JK, Zheng B, Sciurba FC, et al. The relation of airway size to lung function. Proc SPIE. 2008;6916:691623. [Google Scholar]
- 17.Avila RS, Zulueta JJ, Shara NM, et al. A quantitative method for estimating individual lung cancer risk. Acad Radiol. 2010;17(7):830–840. doi: 10.1016/j.acra.2010.03.012. [DOI] [PubMed] [Google Scholar]