Abstract
A hallmark of brain organization is the integration of primary and modulatory pathways by principal neurons. Primary sensory inputs are usually not plastic, while modulatory inputs converging to the same principal neuron can be plastic. However, the mechanisms determining this input specific expression of synaptic plasticity remain unknown. We investigated this problem in the dorsal cochlear nucleus (DCN), where principal cells integrate primary auditory nerve input with plastic, parallel fiber input. Our previous DCN studies have shown that parallel fiber inputs exhibit short- and long-term plasticities mediated by endocannabinoid signaling. Here we show that auditory nerve inputs to principal cells do not show short- or long-term endocannabinoid-mediated synaptic plasticity. Electrophysiological and electron microscopy studies indicate that input specificity arises from selective expression of presynaptic cannabinoid (CB1) receptors in parallel fiber terminals, but not in auditory nerve terminals. However, pairing of parallel fiber activity with auditory nerve activity elicits plasticity in parallel fiber inputs, thus suggesting a role for synaptic plasticity in multisensory integration.
Keywords: endocannabinoids, dorsal cochlear nucleus, plasticity, electron microscopy, electrophysiology
1. Introduction
DCN principal neurons (fusiform cells) integrate auditory signals (primary pathway) with multimodal signals (modulatory pathway) carried by descending parallel fibers (Fig. 1A). The integration of primary and modulatory inputs by a single neuron is a common anatomical motif found in many brain areas including the hippocampus (Amaral, 1993), cortex (Crick and Koch, 1998; Larkum et al., 2009; Petreanu et al., 2009), thalamus (Sherman and Guillery, 1998), cerebellum (Shepherd, 1990), and cerebellum-like circuits of the electrosensory system (Berman and Maler, 1999). Numerous studies have established that modulatory pathways show robust long-term synaptic plasticity such as LTP and LTD (Harvey-Girard et al.; Doller and Weight, 1985; Colbert and Levy, 1992; Bell et al., 1997; Ito, 2001; Fujino and Oertel, 2003; Bastian et al., 2004; Tzounopoulos et al., 2004). While it has been shown that in most cases primary inputs do not show any synaptic plasticity (Fujino and Oertel, 2003), the cellular mechanisms underlying input-specificity remain unknown.
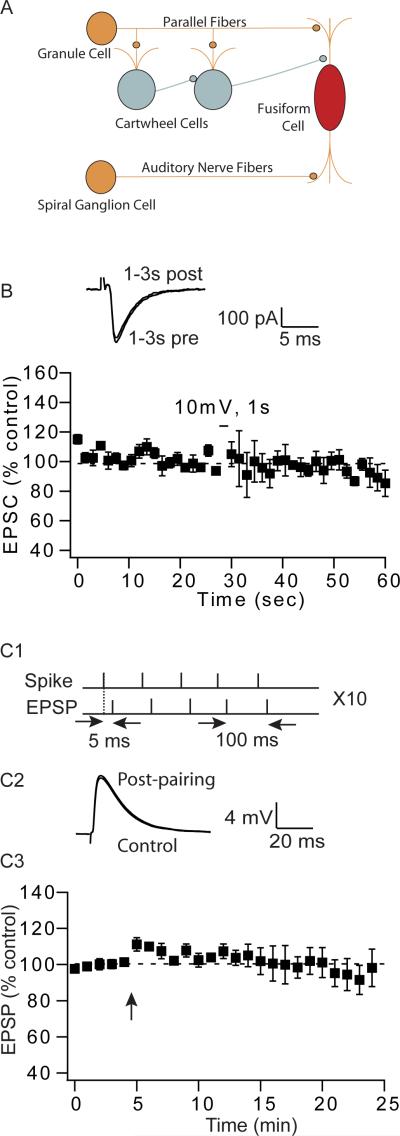
Figure 1.
Auditory nerve inputs that synapse onto fusiform cells do not show short- or long-term endocannabinoid-mediated synaptic plasticity. (A) Simplified circuitry of dorsal cochlear nucleus (DCN). (B) Depolarization to 10mV of fusiform cells for 1s fails to induce DSE in auditory nerve inputs (average DSE 3 s after depolarization: 0.7% ± 9.8%; n=6). Traces represent EPSCs taken 1–3 s before and after depolarization. (C1-3) Pairing of spikes and EPSCs fail to induce STDP in auditory nerve inputs. (C1) STDP induction protocol composed of 5 pairs, at 100ms interval, repeated 10 times at 0.2 Hz. (C2) Representative traces of EPSPs before and 15–20 min after the pairing protocol. (C3) Average time course of auditory nerve EPSPs before and after pairing (97% ± 8%, 15–20 min after pairing, n = 5); Arrow indicates when the STDP induction protocol was applied.
Here, we investigated the mechanisms underlying the input-specificity in the expression of endocannabinoid-mediated synaptic plasticity of DCN principal neurons. Previous studies have shown that parallel fibers exhibit long-term plasticity including classic LTP, LTD (Fujino and Oertel, 2003) and spike-timing dependent plasticity (STDP, Tzounopoulos et al., 2004). However, the same induction protocols that induced plastic changes in parallel fiber inputs failed to induce LTP or STDP in auditory nerve inputs (Fujino and Oertel, 2003; Zhao and Tzounopoulos, 2011). One of the main signaling pathways mediating synaptic plasticity in the DCN is the endocannabinoid signaling. Our results show that auditory nerve synapses do not exhibit any short- or long-term, endocannabinoid-mediated synaptic plasticity because auditory nerve fibers, unlike parallel fibers, do not express cannabinoid receptors (CB1Rs). In addition, our results show that pairing of auditory nerve input with parallel fiber input causes LTP in parallel fiber inputs, thus suggesting a role of synaptic plasticity in multisensory integration.
2. Materials and Methods
2.1. Electrophysiology
Coronal brain slices were made from ICR mice (P17–P25). The preparation and use of coronal slices containing DCN has been described in detail previously (Tzounopoulos et al., 2004). Animals were sacrificed according to methods approved by the Institutional Animal Care and Use Committee of University of Pittsburgh. Single cells were visualized with IR interference contrast optics and recorded using patch pipettes in either voltage- or current-clamp modes. Fusiform cells in the DCN were identified on the basis of morphological and electrophysiological criteria (Zhang and Oertel, 1993; Manis et al., 1994). The external solution contained (in mM): 130 NaCl, 3 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgSO4, 20 NaHCO3, 3 HEPES, and 10 glucose; saturated with 95% O2/5% CO2. For voltage clamp experiments, pipettes were filled with a Cs+-based solution containing (in mM): 130 CsGluconate, 10 CsCl, 2 MgCl2, 0.16 CaCl2, 0.5 EGTA, 10 HEPES, 4 Na-ATP, 0.4 Na-GTP, and 14 Tris-Creatine phosphate. For current clamp experiments, pipettes were filled with a K+-based internal solution containing (in mM): 113 K-gluconate, 4.5 MgCl2, 14 trisphosphocreatine, 9 HEPES, 0.1 EGTA, 4 Na-ATP, 0.3 tris-GTP, 10 sucrose. Glycinergic IPSCs were recorded with a pipette solution that had an elevated CsCl of 70 mM with parallel reduction in CsGluconate. All the internal solutions were adjusted to pH 7.3, ~300 mOsmol. Whole-cell recordings were performed at 31–33°C. For voltage-clamp experiments, series resistance was monitored throughout the experiment from the size and shape of the capacitive transient in response to a 5 mV hyperpolarization. Input resistance was calculated from the sustained response to the same step. Experiments were not included if the series and/or input resistance changed more than 20% throughout recording. Excitatory or inhibitory postsynaptic responses were evoked by stimulating parallel fiber tracts. Auditory nerve responses were evoked by stimulating the deep layer of the DCN. EPSCs were recorded in the presence of SR95531 (20 μM) and strychnine (0.5 μM). Glycinergic IPSCs (Fig. 2B) were recorded in the presence of NBQX (20 μM) and SR95531 (20 μM). Depolarization induced suppression of excitatory or inhibitory inputs (DSE or DSI) was induced by a depolarization of 1s to 10mV delivered to the postsynaptic cell. The amplitude of EPSCs or IPSCs was measured and averaged from 5–6 sweeps in each cell, then normalized to the average value before depolarization. EPSCs and IPSCs were evoked every 1.5 s. DSE or DSI was reported as a percentage of average EPSC or IPSC 3s after depolarization versus before depolarization (Zhao et al., 2009). Pairing of EPSPs with postsynaptic action potentials was used to induce long-term potentiation/depression (LTP/D). EPSPs were collected every 5 s (0.2Hz) before and after pairings. EPSP slope was measured and averaged every minute (12 sweeps), then normalized to baseline (Tzounopoulos et al., 2007). SR95531, NBQX and AM-251 were purchased from Ascent Scientific. Strychnine and muscarine were purchased from Sigma-Aldrich. WIN-55212-2 (WIN) was purchased from Tocris Cookson. Data were acquired and analyzed using pClamp10.1. All means are reported ± SEM. Statistical comparisons were made using unpaired two-tailed Student's t tests. Statistical significance was based on p values < 0.05.
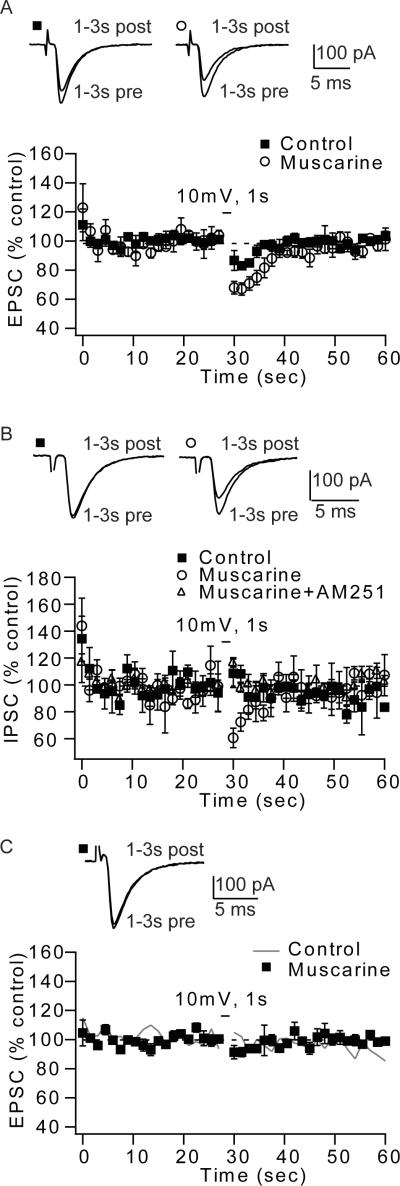
Figure 2.
Activation of muscarinic receptors enhanced endocannabinoid signaling at parallel fibers and at inhibitory terminals found in the molecular layer, but not at auditory nerve fibers. (A) Muscarine (mAChR agonist, 10 μM) increased DSE at parallel fiber to fusiform cell synapses (control; average DSE 3 s after depolarization: 15.1% ± 3.1%; n=6; muscarine; average DSE 3 s after depolarization: 31.4% ± 2.8%; n=6, p<0.05). (B) Application of mAChR agonist reveals DSI of molecular layer glycinergic inputs. Time course of DSI, induced by 1 s depolarization (control: average DSI 3 s after depolarization: −2.5% ± 5%; n=6; muscarine: average DSI 3 s after depolarization: 28.7% ± 6%; n=6, p<0.05; muscarine+AM-251: −5% ± 4%, n=5), Negative DSI values indicate that the average response was increased after depolarization. (C) Auditory nerve EPSCs do not show DSE, in control or in the presence of muscarine. Time course of DSE, induced by 1 s depolarization (control taken from 1B; average DSE 3 s after depolarization: 0.7% ± 9.8%; n=6; muscarine; average DSE 3 s after depolarization: 7.9% ± 4.2%; n=6, p>0.05). Traces represent parallel fiber EPSCs (A) or IPSCs (B) and auditory nerve EPSCs (C) taken 1–3 s before and after depolarization.
2.2. Electron Microscopy
2.2.1. Tissue procedure for immunohistochemistry
The handling of the animals prior to and during the experimental procedures was approved and supervised by the University of Connecticut IACUC and followed NIH guidelines. For structural analysis 2 mice (P22) were used. Mice were anesthetized with a mixture of ketamine 60 mg/kg and xylazine 6.5 mg/kg. After checking anesthetic depth, mice were perfused with 4% paraformaldehyde and 0.5% gluraldehyde in 0.12 M phosphate buffer (pH 7.2) for 10 min. Low glutaraldehyde fixation was followed by freeze-substitution as previously described (Rubio and Wenthold, 1997; Tzounopoulos et al., 2007).
2.2.2. Freeze-substitution and postembedding immunogold labeling
Two mice were used for the detection CB1Rs with immunogold labeling after freeze-substitution, a protocol similar to that described in detail elsewhere was used (Rubio and Wenthold, 1997, 1999; Rubio, 2006; Tzounopoulos et al., 2007). Sections at the level of the cochlear nucleus were dissected and processed for freeze-substitution and low-temperature embedding. For postembedding immunocytochemistry, ultrathin sections (80 nm in thickness) on nickel grids were incubated in sodium borohydride and glycine in Tris-buffered saline solution with Triton X-100. After being pre-blocked with serum, the sections were incubated with the affinity purified primary polyclonal antibody for CB1R (gift from Dr. Ken Mackie, (Tzounopoulos et al., 2007). Primary antibody was detected with a secondary antibody conjugated to 5nm gold particles in diameter (1:20; Amersham GE Healthcare, Buckinghamshire, UK). No gold particles were observed on mitochondria and myelin sheets. Control sections were prepared either in the absence of the primary antibody during the incubation step. No gold particles were observed on the ultrathin sections after the control procedure (data not shown). Preadsorption for CB1R was done as previously described (Tzounopoulos et al., 2007). Ultrathin sections were analyzed with a TECNAI G2 Spirit Biotwin TEM. The images were captured with an AMT CCD camera at 68,000× magnification. Image processing was performed with Adobe Photoshop using only the brightness and contrast commands to enhance gold particles.
Identification of presynaptic nerve terminals in the deep layer of the DCN
We used previously established criteria to identify auditory nerve inputs terminating in the deep layer of the DCN (Kane, 1974; Smith and Rhode, 1985; Ryugo and May, 1993; Rubio and Juiz, 2004; Rubio, 2006; Whiting et al., 2009). These criteria include: 1) location in the nucleus (deep layers of the DCN), 2) ultrastructural characteristics including the size of presynaptic endings, 3) synaptic vesicles that are clear and round, 4) the presence of mitochondria and 5) the existence of multiple asymmetric synaptic contacts on the basal dendrites of fusiform cells. Here, we limited our histological studies to the auditory nerve endings.
3. Results
3.1. Input specific DSE/DSI in DCN fusiform cells
Whole-cell recordings were made from DCN fusiform cells. It is known that endocannabinoid signaling is a major retrograde neuromodulator, mediating short-term and long-term synaptic plasticities of DCN parallel fibers inputs (Tzounopoulos et al., 2007; Zhao et al., 2009; Zhao and Tzounopoulos, 2011). However, it remains unknown whether auditory nerve inputs exhibit endocannabinoid-mediated synaptic plasticity. Postsynaptic depolarization promotes endocannabinoid release, which leads to a transient depolarization-induced suppression of inhibitory and excitatory inputs (DSI and DSE, respectively) (Llano et al., 1991; Pitler and Alger, 1994; Kreitzer and Regehr, 2001a, b; Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001). Postsynaptic depolarization failed to reveal DSE in auditory nerve inputs, indicating the absence of endocannabinoid signaling at auditory nerve-fusiform cells synapses (Fig. 1B). Previous studies in the DCN have revealed that endocannabinoid signaling can be evoked by postsynaptic depolarization or by the pairing of EPSPs and action potentials (Tzounopoulos et al., 2007; Zhao et al., 2009). Pairing of EPSPs with action potentials leads to endocannabinoid-mediated LTD in parallel fiber synapses to cartwheel cells (Tzounopoulos et al., 2007). The same induction protocol when paired with synaptic activation of muscarinic acetylcholine receptors (mAChRs) leads to endocannabinoid-mediated LTD in parallel fiber-fusiform synapses (Zhao and Tzounopoulos, 2011). However, pairing of EPSPs with action potentials (EPSP-AP, at 5ms intervals) did not reveal any LTP or LTD in auditory nerve-fusiform synapses (Zhao and Tzounopoulos, 2011). When the temporal order of pairing was reversed such as that action potentials were followed by EPSPs (AP-EPSP, at 5ms intervals), no long- or short-term plasticity was induced (Figure 1C). Taken together, these data indicate that endocannabinoid-mediated short and long-term plasticities are not affecting synaptic strength of auditory nerve inputs to fusiform cells. In contrast, identical manipulations modulate synaptic strength of parallel fiber inputs to fusiform cells.
3.2. Enhancement of endocannabinoid synthesis by activation of mAChRs does not reveal endocannabinoid signaling in auditory nerve inputs
Endocannabinoids are synthesized and released from postsynaptic neurons and act as retrograde messengers that modulate synaptic transmission. This modulation occurs via activation of presynaptic CB1Rs (Freund et al., 2003; Piomelli, 2003; Chevaleyre et al., 2006; Hashimotodani et al., 2007). Therefore, absence of endocannabinoid signaling could be due to pre- or postsynaptic mechanisms. To determine whether the lack of endocannabinoid-mediated modulation in auditory nerve-parallel fiber synapses is due to insufficient release of endocannabinoids, we used muscarine (10µM, a muscarinic receptor agonist) to enhance endocannabinoid release from fusiform cells. Previous studies have reported that endocannabinoid signaling is enhanced by activation of muscarinic acetylcholinergic receptors (mAChRs) (Kim et al., 2002; Fukudome et al., 2004; Narushima et al., 2007; Uchigashima et al., 2007; Zhao and Tzounopoulos, 2011). Bath application of muscarine increased DSE of parallel fiber inputs (Fig. 2A). Furthermore, application of muscarine revealed DSI in the synaptic strength of glycinergic inhibitory inputs to fusiform cells (Fig. 2B), which is absent under control conditions (Zhao et al., 2009). DSI was mediated by endocannabinoids as it was blocked by AM251 (CB1R antagonist, 1 µM, Fig. 2B). However, identical bath application of muscarine did not reveal DSE at auditory nerve synapses to fusiform cells (Fig. 2C). Taken together, these results suggest that even under conditions that enhance endocannabinoid release and enhance endocannabinoid signaling in the molecular layer of the DCN, endocannabinoid signaling is not recruited in the auditory nerve inputs.
3.3. Input-specific endocannabinoid signaling is determined by input-specific expression of CB1Rs
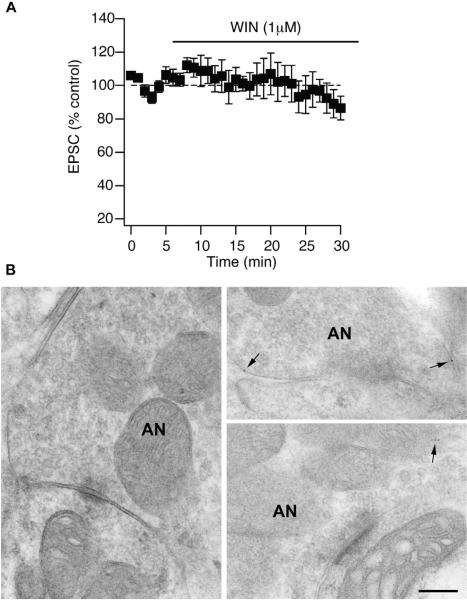
Next, we tested whether functional CB1Rs are expressed on auditory nerve fibers. We bath applied WIN-55,212-2 (WIN 1 µM, CB1R agonist); if there are CB1Rs localized on the presynaptic terminals, then WIN application is expected to lead to synaptic suppression. When WIN-55,212-2, was bath applied, no change in synaptic strength of auditory nerve inputs was observed (Fig. 3A), indicating that auditory nerve terminals are not expressing functional CB1Rs. This interpretation was confirmed through EM immunolocalization of CB1Rs (Fig. 3B). Postembedding immunolocalization was performed using antibodies directed against the entire C terminus (Fig. 3B). The number of gold particles for CB1Rs was very low on the auditory endings (1–4 range along the entire surface). The five-nanometer gold particles were found in the cytoplasm, and were not observed in areas facing the postsynaptic density. To discard the possibility of technical issues during the tissue preparation and the postembedding immunogold labeling, we analyzed the molecular layer that is enriched with CB1Rs expressed at the parallel fiber boutons terminating on cartwheel cells. As we previously reported (Tzounopoulos et al., 2007), gold particles were observed at the plasma membrane of parallel fibers facing the synapse and in the postsynaptic density of cartwheel cells (data not shown). Taken together, our data show that the input specificity of endocannabinoid-mediated synaptic plasticity is caused by input-selective expression of CB1Rs at terminals of parallel fibers, but not at the auditory nerve terminals.
Figure 3.
Auditory nerve terminals do not express CB1 receptors. (A) CB1R agonist (WIN, 1 μM) does not decrease EPSCs originating from activation of auditory nerve fibers, suggesting the lack of CB1R expression in auditory nerve inputs. Time course of 1 μM WIN-55212-2 block of transmission in fusiform cells when stimulating the deep layer of the DCN (WIN: 96% ± 10%, n = 5, 25–30 min after WIN application, p>0.05). (B) Auditory nerve terminals are not expressing CB1Rs. Electron micrographs show representative images of the auditory nerve (AN) terminals on fusiform cells of the DCN. Postembedding immunogold labeled for CB1Rs (5nm gold particles). Gold labeling is very low (1–4 particles along the entire surface of the ending) and it is observed in the cytoplasm (arrows). Scale bar: 0.2 μm.
3.4. Auditory nerve activity triggers plasticity of parallel fibers inputs
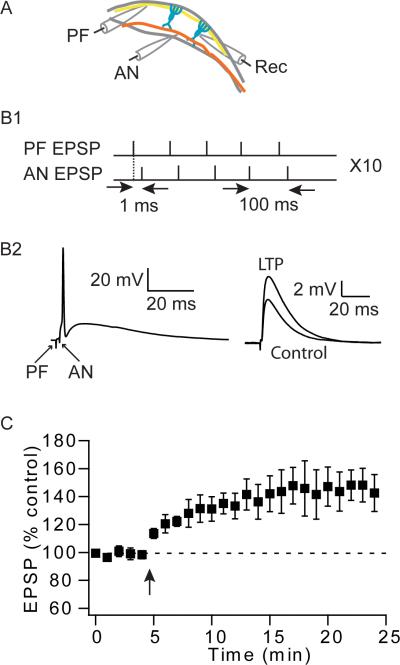
Although auditory nerve inputs to fusiform cells do not show short- or long-term plasticity, it is possible that the auditory nerve could modulate synaptic strength of parallel fiber inputs. To explore this possibility, we used a two-pathway pairing protocol. Parallel fiber and auditory nerve inputs were stimulated with two different stimulating electrodes (Fig. 4A). The amplitude of the stimulus to each group was adjusted so that both evoked EPSPs were subthreshold. Pairing was done in current clamp; a parallel fiber stimulus was followed by auditory nerve stimulus so that summation of the two responses always resulted to a spike. Five such pairs were delivered at 100 ms intervals followed by a 5-s pause, and repeated 10 times (Fig. 4B). This pairing protocol induced a significant amount of LTP at the parallel fiber EPSPs (Fig. 4C), indicating that coincident synaptic activation of auditory nerve inputs provides the trigger for the induction of LTP of parallel fiber inputs.
Figure 4.
Pairing of parallel fiber stimulation with auditory nerve stimulation induces LTP in parallel fiber inputs. (A) Schematic illustration of the location of recording (Rec) and stimulation electrodes activating parallel fiber (PF) and auditory nerve (AN) inputs. (B1) Long-term potentiation was induced with a protocol, composed of 5 pairs of parallel fiber and auditory nerve inputs, at 100 ms interval, repeated 10 times at 0.2 Hz. (B2) Representative responses of a pairing between parallel fiber and auditory nerve stimulation (left); examples of averaged traces before and 15–20 min after the pairing protocol (right). (C) Time course of induced long-term plasticity (LTP: 145% ± 13%, 20–25 min, n = 5, p < 0.05); Arrow indicates when the STDP induction protocol was applied.
4. Discussion
We used electrophysiological and anatomical techniques to study the mechanisms underlying input specificificity of endocannabinoid-mediated synaptic plasticity in the DCN. Our findings show that differences in endocannabinoid-mediated synaptic plasticity can be ascribed to input-specific differences in the targeting of presynaptic CB1Rs. The lack of CB1R expression in the auditory nerve fibers innervating the basal dendrites of principal neurons, determines the lack of endocannabinoid-mediated short- and long-term plasticities in these synapses. In contrast, parallel fibers innervating the apical dendrites of principal neurons express CB1Rs and thus express short- and long-term endocannabinoid-mediated synaptic plasticity. While auditory nerve inputs do not exhibit endocannabinoid-mediated synaptic plasticity, they provide the trigger for inducing synaptic plasticity of the parallel fiber inputs, analogous to the climbing fiber and parallel fiber in the cerebellum.
4.1 Input and cell-specific endocannabinoid signaling determines input- and cell-specific synaptic plasticity
Input-specific short- and long-term plasticities have been previously observed in different neural circuits. Target cells may determine the probability of release and short- term plasticity of synapses, thus allowing for the same fibers to influence their targets differentially (Thomson, 1997; Markram et al., 1998; Reyes et al., 1998; Toth et al., 2000; Rozov et al., 2001; Pouille and Scanziani, 2004). Here, we reveal that different fibers innervating the same principal neuron show differential expression of CB1Rs and thus only specific terminals can modify their Pr by postsynaptically released endocannabinoids. Previous studies have revealed that excitatory and inhibitory terminals innervating the same neuron express different amount of CB1Rs. In most brain areas, including the hippocampus, cerebellum, and striatum, inhibitory synapses are more sensitive to endocannabinoids than excitatory synapses, as a result of higher levels of CB1Rs at inhibitory inputs (Ohno-Shosaku et al., 2002; Brenowitz et al., 2006; Uchigashima et al., 2007). The arrangement and abundance of endocannabinoid signaling molecules in the DCN differs from other brain regions as previous studies have revealed that inhibitory terminals express lower levels of CB1Rs than excitatory terminals (Zhao et al., 2009). The lack of CB1Rs in auditory nerve terminals imposes complete lack of endocannabinoid signaling, thus ensuring that auditory nerve terminals are non-plastic even under neuromodulatory influence.
4.2 The origin of excitatory inputs terminating in the deep layer of the DCN
Electrical stimulation of deep layer activates excitatory auditory inputs arising from auditory nerve fibers and perhaps also from mulitipolar (D-type) cells or from descending inputs (Oertel et al., 1990; Doucet and Ryugo, 2003; Oertel and Young, 2004). In agreement with these reports, we also observed another type (not auditory nerve type) of presynaptic endings that is distributed within the deep layer of the DCN. These endings are larger, they show more electrondense axoplasm and they display darker synaptic vesicles compared to the auditory nerve endings (Rubio and Juiz, 2004). Thus, we hypothesize that these endings belong to D-type multipolar cells or to descending inputs. While our electrophysiological recordings cannot discriminate between these different types of inputs, our EM results on anatomically identified auditory nerve terminals further support the lack of CB1Rs on auditory nerve terminals.
4.3 Functional relevance of parallel fiber synaptic plasticity
Our results indicate that coordinated activation of parallel fiber inputs with auditory nerve inputs induces synaptic plasticity in the parallel fiber inputs, and thus this plasticity maybe involved in associative mediated learning or adaptation. Hebbian STDP has been linked with associative forms of learning such as the creation of a memory trace that sensitizes the circuit to particular profiles of subsequent sensory stimuli (Yao and Dan, 2001) and with training and deprivation-induced receptive field plasticity in sensory cortex (Feldman and Brecht, 2005; Dan and Poo, 2006). Therefore, Hebbian synaptic learning rules may create a memory trace (Yao and Dan, 2001) that sensitizes the DCN to particular profiles of subsequent auditory and nonauditory stimuli. In addition, our findings unmask a role for STDP in mediating multisensory integration in the DCN. Multisensory integration has been previously reported in the DCN (Shore, 2005). Our results suggest that an important factor in multisensory maturation is the temporal synchrony of different sensory inputs (Wallace and Stein, 2007). Presumably, experience with temporally coincident cross-modal stimuli leads to a physiological binding of these stimuli that is reflected through the strengthening of parallel fiber synapses.
Acknowledgment
This work was supported by National Institute for Deafness and Communication Disorders Grants R01 DC-007905 to T. Tzounopoulos and DC-006881 to M. Rubio.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG. Emerging principles of intrinsic hippocampal organization. Curr Opin Neurobiol. 1993;3:225–229. doi: 10.1016/0959-4388(93)90214-j. [DOI] [PubMed] [Google Scholar]
- Bastian J, Chacron MJ, Maler L. Plastic and nonplastic pyramidal cells perform unique roles in a network capable of adaptive redundancy reduction. Neuron. 2004;41:767–779. doi: 10.1016/s0896-6273(04)00071-6. [DOI] [PubMed] [Google Scholar]
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–281. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- Berman NJ, Maler L. Neural architecture of the electrosensory lateral line lobe: adaptations for coincidence detection, a sensory searchlight and frequency-dependent adaptive filtering. J Exp Biol. 1999;202:1243–1253. doi: 10.1242/jeb.202.10.1243. [DOI] [PubMed] [Google Scholar]
- Brenowitz SD, Best AR, Regehr WG. Sustained elevation of dendritic calcium evokes widespread endocannabinoid release and suppression of synapses onto cerebellar Purkinje cells. J Neurosci. 2006;26:6841–6850. doi: 10.1523/JNEUROSCI.1280-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Levy WB. Electrophysiological and pharmacological characterization of perforant path synapses in CA1: mediation by glutamate receptors. J Neurophysiol. 1992;68:1–8. doi: 10.1152/jn.1992.68.1.1. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C. Constraints on cortical and thalamic projections: the no-strong-loops hypothesis. Nature. 1998;391:245–250. doi: 10.1038/34584. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Doller HJ, Weight FF. Perforant pathway-evoked long-term potentiation of CA1 neurons in the hippocampal slice preparation. Brain Res. 1985;333:305–310. doi: 10.1016/0006-8993(85)91584-7. [DOI] [PubMed] [Google Scholar]
- Doucet JR, Ryugo DK. Axonal pathways to the lateral superior olive labeled with biotinylated dextran amine injections in the dorsal cochlear nucleus of rats. J Comp Neurol. 2003;461:452–465. doi: 10.1002/cne.10722. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:265–270. doi: 10.1073/pnas.0135345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Harvey-Girard E, Lewis J, Maler L. Burst-induced anti-Hebbian depression acts through short-term synaptic dynamics to cancel redundant sensory signals. J Neurosci. 30:6152–6169. doi: 10.1523/JNEUROSCI.0303-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Kane EC. Patterns of degeneration in the caudal cochlear nucleus of the cat after cochlear ablation. Anat Rec. 1974;179:67–91. doi: 10.1002/ar.1091790106. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001a;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001b;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science. 2009;325:756–760. doi: 10.1126/science.1171958. [DOI] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Manis PB, Spirou GA, Wright DD, Paydar S, Ryugo DK. Physiology and morphology of complex spiking neurons in the guinea pig dorsal cochlear nucleus. Journal of Comparative Neurology. 1994;348:261–276. doi: 10.1002/cne.903480208. [DOI] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci U S A. 1998;95:5323–5328. doi: 10.1073/pnas.95.9.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Fukaya M, Matsui M, Manabe T, Hashimoto K, Watanabe M, Kano M. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D, Young ED. What's a cerebellar circuit doing in the auditory system? Trends in Neurosciences. 2004;27:104–110. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wu SH, Garb MW, Dizack C. Morphology and physiology of cells in slice preparations of the posteroventral cochlear nucleus of mice. J Comp Neurol. 1990;295:136–154. doi: 10.1002/cne.902950112. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci. 2002;15:953–961. doi: 10.1046/j.1460-9568.2002.01929.x. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron. 1994;13:1447–1455. doi: 10.1016/0896-6273(94)90430-8. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nature Neuroscience. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol. 2001;531:807–826. doi: 10.1111/j.1469-7793.2001.0807h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ME. Redistribution of synaptic AMPA receptors at glutamatergic synapses in the dorsal cochlear nucleus as an early response to cochlear ablation in rats. Hear Res. 2006;216–217:154–167. doi: 10.1016/j.heares.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–950. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Differential distribution of intracellular glutamate receptors in dendrites. J Neurosci. 1999;19:5549–5562. doi: 10.1523/JNEUROSCI.19-13-05549.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ME, Juiz JM. Differential distribution of synaptic endings containing glutamate, glycine, and GABA in the rat dorsal cochlear nucleus. J Comp Neurol. 2004;477:253–272. doi: 10.1002/cne.20248. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, May SK. The projections of intracellularly labeled auditory nerve fibers to the dorsal cochlear nucleus of cats. J Comp Neurol. 1993;329:20–35. doi: 10.1002/cne.903290103. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. The Synaptic organization of the brain. 3rd Edition Oxford University Press; New York: 1990. [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci U S A. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE. Multisensory integration in the dorsal cochlear nucleus: unit responses to acoustic and trigeminal ganglion stimulation. Eur J Neurosci. 2005;21:3334–3348. doi: 10.1111/j.1460-9568.2005.04142.x. [DOI] [PubMed] [Google Scholar]
- Smith PH, Rhode WS. Electron microscopic features of physiologically characterized, HRP-labeled fusiform cells in the cat dorsal cochlear nucleus. J Comp Neurol. 1985;237:127–143. doi: 10.1002/cne.902370110. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Activity-dependent properties of synaptic transmission at two classes of connections made by rat neocortical pyramidal axons in vitro. J Physiol. 1997;502(Pt 1):131–147. doi: 10.1111/j.1469-7793.1997.131bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus.[see comment] Nature Neuroscience. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Early experience determines how the senses will interact. J Neurophysiol. 2007;97:921–926. doi: 10.1152/jn.00497.2006. [DOI] [PubMed] [Google Scholar]
- Whiting B, Moiseff A, Rubio ME. Cochlear nucleus neurons redistribute synaptic AMPA and glycine receptors in response to monaural conductive hearing loss. Neuroscience. 2009;163:1264–1276. doi: 10.1016/j.neuroscience.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yao H, Dan Y. Stimulus timing-dependent plasticity in cortical processing of orientation. Neuron. 2001;32:315–323. doi: 10.1016/s0896-6273(01)00460-3. [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Cartwheel and superficial stellate cells of the dorsal cochlear nucleus of mice: intracellular recordings in slices. J Neurophysiol. 1993;69:1384–1397. doi: 10.1152/jn.1993.69.5.1384. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tzounopoulos T. Physiological Activation of Cholinergic Inputs Controls Associative Synaptic Plasticity via Modulation of Endocannabinoid Signaling. J Neurosci. 2011;31(9):3158–3168. doi: 10.1523/JNEUROSCI.5303-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Rubio ME, Tzounopoulos T. Distinct functional and anatomical architecture of the endocannabinoid system in the auditory brainstem. J Neurophysiol. 2009;101:2434–2446. doi: 10.1152/jn.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]