Abstract
Introduction
Measurement of tumor response by standard response criteria is challenging in thymic malignancies especially when the pleura is involved, as it often is in stage IV disease. In this study we aimed to determine the effectiveness of volumetric response evaluation criteria in solid tumors (volumetrics) for evaluating response in patients with thymic malignancies treated on a phase II study of belinostat.
Methods
We evaluated 25 thymic cancer patients' tumor responses using computed tomography (CT) based response evaluation criteria in solid tumors (RECIST), World Health Organization (WHO), modified RECIST, and volumetrics. As a control we assessed 37 NSCLC patients with RECIST and volumetrics.
Results
Agreement analyses in 23 thymic cancer patients at the time of RECIST-determined progressive disease (PD) compared volumetrics to RECIST, modified RECIST, and WHO criteria. Use of volumetrics was associated with 22% discordance compared to RECIST, 15% vs. modified RECIST, and 22% vs. WHO criteria. Volumetrics revealed PD 72 days earlier than RECIST (p=0.016). In another cohort of 35 NSCLC patients there was 9% discordance between volumetrics and RECIST at the time of PD. Volumetrics demonstrated PD 32 days earlier than RECIST in NSCLC (p=0.0078).
Conclusions
Our study suggests that volumetrics might improve detection of progressive disease. Prospective evaluation of this technique in a larger series of patients with thymic malignancies will be required.
Introduction
The WHO and Miller et al. developed criteria in the late 1970s and early 1980s to address the need for a common language to ensure consistent and objective reporting of results of treated cancer patients with solid tumors [1, 2]. More recently the response evaluation criteria in solid tumors (RECIST) were developed, which make use of unidimensional tumor measurements, in contrast to the bi-dimensional measurements of the WHO criteria. An updated version of the original RECIST criteria has very recently been introduced (RECIST 1.1) [3, 4]. RECIST 1.1 is now the gold standard for measuring disease burden in solid tumors in clinical trials. However, there are limitations in measuring tumors using only one dimension, which are dictated by the shape of the tumor and the sharpness by which edges can be defined on conventional imaging.
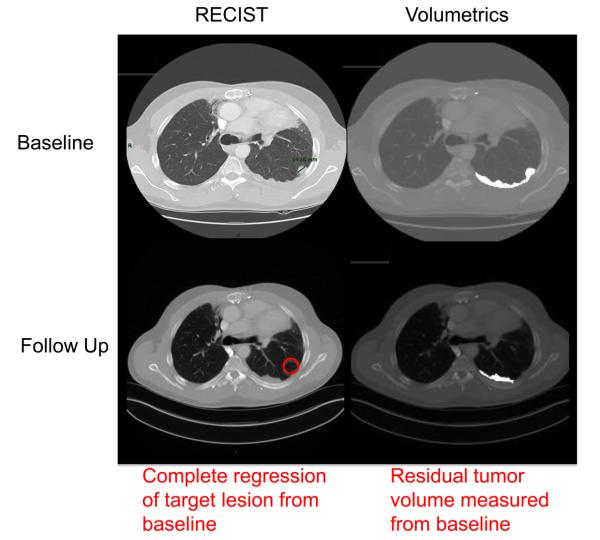
Thymic cancers are rare neoplasms accounting for 0.2% – 1.5% of all cancers [5]. It is not uncommon for these tumors to metastasize to the pleural cavity [6]. Given the anatomy of the thoracic cavity, metastases to the pleura often appear as curvilinear plaques on computed tomography (CT). These plaques are difficult to measure when utilizing RECIST (Figure 1), for which only one CT slice of the target lesion along the longest diameter is taken, which may not accurately reflect their non-cylindrical tumor growth pattern.
Fig. 1.
Metastatic thymoma patient's target lesion measured with RECIST and volumetrics at baseline and follow up restaging CT scans.
Since the existence of RECIST there has been much debate about what type of tumor size assessment is the most informative. Recently, various methods of three-dimensional measurement criteria to evaluate cancer target lesions by CT have been under assessment including mathematical formulae [7], modified RECIST [8, 9], and computer-assisted systems [10-12] to estimate total tumor volume. These assessment methods have been investigated in various tumor types resulting in conflicting conclusions. Tumor volume can be calculated from RECIST-based measurements assuming that the tumor mass is cylindrical in nature. However, many tumor lesions in various cancer types do not appear as cylindrical masses on CT.
Three-dimensional measurements have been validated for the growth of plexiform neurofibromas in neurofibromatosis I patients via magnetic resonance imaging [13]. Also, preoperative three-dimensional tumor volumes < 51 cc have been associated with longer PFS in malignant pleural mesothelioma [14]. On the other hand, measurement of three-dimensional tumor volume was not demonstrated to have any prognostic significance compared to RECIST in patients with rhabdomyosarcoma treated with chemotherapy [15]. Also, no difference in progression free survival was noted in a comparison of RECIST against bi-dimensional and three-dimensional tumor response in adults with high grade gliomas [16].
We performed a retrospective study of patients with advanced thymic cancers enrolled in a phase II clinical trial treated with the histone deacetylase inhibitor, belinostat, aimed to investigate whether the clinical outcome of these patients would differ based on the method of evaluation used; i.e., RECIST, WHO, modified RECIST, and what we have termed volumetric response evaluation criteria in solid tumors (volumetrics). We also analyzed a separate cohort of patients with metastatic non-small cell lung cancer (NSCLC) enrolled in a phase II clinical trial of the single agent multi-kinase inhibitor sorafenib, where RECIST is the standard measurement method, as a control group to determine if there were any differences in objective response when using volumetrics or RECIST.
Patients and Methods
Measurement Methods
Tumor measurements were determined through axial CT scans or MRI scans when appropriate. Only one MRI was utilized in this study to follow a thymic cancer patient who had metastatic disease solely in the liver. In addition to the MRI scans, subsequent restaging CT scans were performed to assess for new lesions in this patient. Tumor measurements in this one patient were based off of lesions determined by MRI scans. Baseline scans were read and the 5 largest target lesions measuring > 1cm were chosen and measured using RECIST. All other target lesion measurements in this study were made retrospectively. Thymic cancer tumor burden was evaluated using RECIST [3], modified RECIST [8], and WHO criteria [1] [17]. Also, given the similarity in appearance on CT scans of metastatic pleural deposits of thymic tumors and malignant pleural mesothelioma we choose to utilize three-dimensional volumetric criteria described by Ak et al [18].
Assuming one is measuring a spherical lesion, mathematical formulae reveal that RECIST-defined partial response of 30% in the longest diameter and WHO-defined 50% decrease in two-dimensional measurements both equal to a 65% decrease in three-dimensional measurement. Also for a spherical lesion, the RECIST-defined disease progression of 20% increase in the longest diameter equals 77% increase in volume and the WHO-defined 25% increase in two-dimensional measurements equals 40% increase in volume (Table I) [12, 18]. Using ROC analysis Ak et al. showed that a ≥15% increase and ≥50% decrease in tumor volume were sensitive in detecting progression and response, respectively, and a significant correlation was demonstrated between median survival and response [18]. These measurement cut-off values of ≥15% increase and ≥50% decrease in three-dimensional tumor volume were thus shown to be sensitive in a non-spherical growing tumor and justified our use of these criteria.
Table I.
Comparison of tumor volume percent changes in RECIST (unidimensional), WHO (bidimensional), and volumetrics (three dimensional) relative to one another.
| % Change in Size of Spherical Tumors | ||
|---|---|---|
| 1D RECIST |
2D WHO |
3D Volumetrics |
| 6% | 13% | 20% |
| 12% | 25% | 40% |
| 20% | 44% | 73% |
Abbreviations: RECIST, response evaluation criteria in solid tumors; WHO, world health organization
Tumor volume was measured using MEDx software (Medical Numerics Inc, Germantown, MD), which calculates tumor volume by detecting pixels above −500 Hounsfield units from all CT slices containing manually outlined tumor lesions. A complete response (CR) was defined the same manner as RECIST [3]. Confirmed partial response (PR) was defined as a ≥50% decrease in the sum of volumetric measurements and having continuously stable non-target lesions from the baseline in at least two consecutive CT scans. Progressive disease (PD) was defined as a ≥15% increase in tumor volume of target lesions, or unequivocal increase in non-target lesions, and/or appearance of new lesions observed from the smallest measurement [18]. Patients were considered to have stable disease (SD) if their volumetric measurements were > PR and < PD.
Patients and Study Design
We analyzed tumor burden in 25 consecutive patients with advanced thymoma and thymic carcinoma enrolled in a phase II clinical trial treated with a histone deacetylase inhibitor, belinostat, at our institution [19]. Tumor burden was prospectively evaluated by RECIST. We retrospectively analyzed these patients' tumor burden using modified RECIST, WHO criteria, and volumetrics at baseline and at every restaging time point. For agreement analyses in the thymic cancer cohort we compared volumetrics against other measurement criteria mentioned above.
Initially we analyzed the data for agreement at the time of RECIST determined best response. We then assessed for agreement among the target lesion measurements alone at the time of RECIST designated progressive disease. We also performed an agreement analysis using the entire criteria of RECIST (target lesions, non-target lesions, and new lesions) at the time of progressive disease. If disagreement was observed between volumetrics and RECIST at the time of RECIST-designated progressive disease we determined which set of criteria revealed progression earlier. Additionally, overall survival beginning at the time of either volumetrics or RECIST date of progression to their date of death or date last known alive was assessed in those patients that had progressed by either of the two measurement criteria respectively.
Patient characteristics in the advanced thymic cancer cohort are presented in Table II A. At the time of writing this report 2 patients are on study. All twenty-five patients were evaluated for best response including the two patients on study. Since, progressive disease data points could not be determined until patients came off study, we did not include these two patients in the progressive disease analyses.
Table II.
Patient Characteristics
| A. Thymic cancer patient characteristics | |
|---|---|
| Characteristic | Number of Patients (%) |
| Age | |
| Median | 53 yrs |
| Range | 23-72 yrs |
|
| |
| Sex | |
| Male | 15 (60%) |
| Female | 10 (40%) |
|
| |
| Ethnicity | |
| Caucasian | 22 (88%) |
| African American | 2 (8%) |
| Asian | 1 (4%) |
|
| |
| Histopathology | |
| Thymoma | 14 (56%) |
| Thymic Carcinoma | 11 (44%) |
|
| |
| B. Non-small cell lung cancer patient characteristics | |
|
| |
| Characteristic | Number of Patients (%) |
|
| |
| Age | |
| Median | 63 yrs |
| Range | 30-85 yrs |
|
| |
| Sex | |
| Male | 18 (49%) |
| Female | 19 (51%) |
|
| |
| Ethnicity | |
| Caucasian | 25 (68%) |
| African American | 5 (13%) |
| Asian | 4 (11%) |
| Hispanic | 3 (8%) |
|
| |
| Histopathology | |
| Adenocarcinoma | 31 (84%) |
| Squamous Cell Carcinoma | 3 (8%) |
| Poorly Differentiate Carcinoma | 2 (5%) |
| Large Cell Neuroendocrine | 1 (3%) |
We chose NSCLC patients as a control group since imaging studies were readily available and RECIST is the standard measurement criteria. We evaluated tumor burden in 37 consecutive patients with metastatic NSCLC in a single center phase II clinical trial treated with the multi-kinase inhibitor, sorafenib [20]. Tumor burden was evaluated prospectively with RECIST. We retrospectively analyzed these patients' tumor burden with volumetrics at baseline and every restaging time point until they came off study.
All agreement analyses in the NSCLC cohort were a comparison of volumetrics against RECIST. Evaluation of agreement, progression, and survival in the NSCLC cohort occurred precisely in the same manner as the thymic cancer cohort.
Patient characteristics in the metastatic NSCLC cohort are depicted in Table II B. At the time of writing this report 2 patients are on study. All thirty-seven metastatic NSCLC patients were evaluated for best response including the two patients on study. Since progressive disease information could not be determined until patients came off study, we did not include these two patients who remain on study in our progressive disease analyses.
Statistical Methods
A McNemar test for paired categorical data was used to determine the degree of the agreement between the various measurement methods with respect to their response classification when compared together. A small two-tailed p-value (p < 0.05) would indicate that there was a significant difference toward disagreement in one direction or the other when the two methods showed discrepant results. A Wilcoxon signed rank test was utilized to determine if one method detected disease progression earlier than the other method in the cases in which the two methods both detected progression. The probability of survival or of progression free survival as a function of time was determined by the Kaplan-Meier method. A Cox proportional hazards model was used, with the time of progressive disease by either volumetrics or RECIST criteria as a time-varying covariate, to determine if the classification of progression by either method was associated with the probability of survival. All p-values are two-tailed and presented without any adjustment for multiple comparisons.
Results
Tumor Response in Advanced Thymic Neoplasms
Initially, we identified the date of best response as determined by RECIST, and then compared the extent of agreement in declaring progressive disease or other than progressive disease at the RECIST designated best response date. We used volumetrics as the comparator arm at this time point and evaluated it against RECIST, modified RECIST, and WHO criteria in 25 metastatic thymic cancer patients treated with single agent belinostat. Compared to volumetrics, 56% of comparisons were not concordant with the findings from RECIST, 45% were not concordant with modified RECIST, and 52% were not concordant with WHO criteria. In each case, the disagreements tended to identify significantly greater progressive disease outcomes with volumetrics as opposed to RECIST (p=0.0001), modified RECIST (p=0.002), or WHO criteria (p=0.023; Table III A-C). At the time of best response as determined by RECIST, volumetrics revealed that overall tumor burden was increasing in 14/25 (56%) patients. This suggested that the patient would have been deemed to have progressive disease at an earlier time point and/or have a worse disease response if volumetrics were utilized instead of RECIST.
Table III.
Agreement analyses determined by McNemar's test in advanced thymic cancers comparing volumetrics against RECIST (A), modified RECIST (B), and WHO criteria (C) and volumetrics compared to RECIST in metastatic NSCLC (D) at the time of RECIST designated best response.
| A | RECIST | B | Modified RECIST | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Volumetrics | Frequency | CR/PR/SD | PD | Total | Volumetrics | Frequency | CR/PR/SD | PD | Total | ||
| CR/PR/SD | 8 | 0 | 8 | CR/PR/SD | 8 | 0 | 8 | ||||
| PD | 14* | 3 | 17 | PD | 10* | 4 | 14 | ||||
| Total | 22 | 3 | 25 | Total | 18 | 4 | 22 | ||||
| P = 0.0001 | P = 0.002 | ||||||||||
| C | WHO | D | RECIST | ||||||||
| Volumetrics | Frequency | CR/PR/SD | PD | Total | Volumetrics | Frequency | CR/PR/SD | PD | Total | ||
| CR/PR/SD | 6 | 2* | 8 | CR/PR/SD | 14 | 1* | 15 | ||||
| PD | 11* | 6 | 17 | PD | 9* | 11 | 20 | ||||
| Total | 17 | 8 | 25 | Total | 23 | 12 | 35 | ||||
| P = 0.023 | P = 0.0215 | ||||||||||
Underlined represent agreement and
represent disagreement.
Note: P < 0.05 indicates the two methods were not consistent with one another.
Abbreviations: RECIST response evaluation criteria in solid tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NSCLC, non-small cell lung carcinoma.
Additionally, we studied the target lesions alone at the time of RECIST- designated disease progression and examined the agreement of volumetrics to RECIST, modified RECIST, and WHO target lesions in the 23 metastatic thymic cancer patients who came off study. Compared to volumetrics, 48% of comparisons were not concordant with the findings from RECIST, 35% were not concordant with modified RECIST, and 44% were not concordant with WHO criteria. Again, in each case, the disagreements tended to identify greater progressive disease outcomes with volumetrics as opposed to RECIST (p=0.001), modified RECIST (p=0.016), or WHO criteria (p=0.11; Table IV A-C). Volumetrics target lesions alone detected disease progression in 11 cases when RECIST target lesions alone demonstrated these same patients to have stable disease or better. In other words, RECIST designated target lesions alone were less likely to detect disease progression compared to volumetrics target lesions alone when the patient had progressive disease.
Table IV.
Agreement analyses determined by McNemar's test in advanced thymic cancers comparing volumetrics against RECIST (A), modified RECIST (B), and WHO criteria (C) and volumetrics compared to RECIST in metastatic NSCLC (D) of the target lesions alone at the time of RECIST designated progressive disease.
| A | RECIST | B | Modified RECIST | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Volumetrics | Frequency | CR/PR/SD | PD | Total | Volumetrics | Frequency | CR/PR/SD | PD | Total | ||
| CR/PR/SD | 4 | 0 | 4 | CR/PR/SD | 4 | 0 | 4 | ||||
| PD | 11* | 8 | 19 | PD | 7* | 9 | 16 | ||||
| Total | 15 | 8 | 23 | Total | 11 | 9 | 20 | ||||
| P = 0.001 | P = 0.0156 | ||||||||||
| C | WHO | D | RECIST | ||||||||
| Volumetrics | Frequency | CR/PR/SD | PD | Total | Volumetrics | Frequency | CR/PR/SD | PD | Total | ||
| CR/PR/SD | 2 | 2* | 4 | CR/PR/SD | 3 | 1* | 4 | ||||
| PD | 8* | 11 | 19 | PD | 10* | 19 | 29 | ||||
| Total | 10 | 13 | 23 | Total | 13 | 20 | 33 | ||||
| P = 0.11 | P = 0.0117 | ||||||||||
Underlined represent agreement and
represent disagreement.
Note: P < 0.05 indicates the two methods were not consistent with one another.
Abbreviations: RECIST, response evaluation criteria in solid tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NSCLC, non-small cell lung carcinoma.
Finally, we analyzed the same 23 patients using the entire criteria (target lesions, non-target lesions, and new lesions) of RECIST at the RECIST determined time of progression and compared volumetrics against RECIST, modified RECIST, and WHO criteria for differences in agreement. In this case, there were fewer discrepant cases since 7 patients were deemed progressive disease via unequivocal increase in non-target lesions or appearance of new lesions. Compared to volumetrics, 22% of comparisons were not concordant with the findings from RECIST, 15% were not concordant with modified RECIST, and 22% were not concordant with WHO criteria. In this setting, the disagreements tended to identify relatively fewer additional progressive disease outcomes with volumetrics as opposed to RECIST (p=0.063), modified RECIST (p=0.25), or WHO criteria (p=1.00; Table V A-C). In addition, volumetrics identified progressive disease an average of 72 days earlier than RECIST when considering all lesions in the cases in which both methods identified progressive disease (p=0.0156). Thus, RECIST and volumetrics disagreed on disease progression classification in 22% of cases, and volumetrics detected disease progression significantly earlier than RECIST.
Table V.
Agreement analyses determined by McNemar's test in advanced thymic cancers comparing volumetrics against RECIST (A), modified RECIST (B), and WHO criteria (C) and volumetrics compared to RECIST in metastatic NSCLC (D) of the entire criteria (target lesions, non-target lesions, and new lesions) at the time of RECIST designated progressive disease.
| A | RECIST | B | Modified RECIST | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Volumetrics | Frequency | CR/PR/SD | PD | Total | Volumetrics | Frequency | CR/PR/SD | PD | Total | ||
| CR/PR/SD | 3 | 0 | 3 | CR/PR/SD | 3 | 0 | 3 | ||||
| PD | 5* | 15 | 20 | PD | 3* | 14 | 17 | ||||
| Total | 8 | 15 | 23 | Total | 6 | 14 | 20 | ||||
| P = 0.0625 | P = 0.25 | ||||||||||
| C | WHO | D | RECIST | ||||||||
| Volumetrics | Frequency | CR/PR/SD | PD | Total | Volumetrics | Frequency | CR/PR/SD | PD | Total | ||
| CR/PR/SD | 1 | 2* | 3 | CR/PR/SD | 0 | 0* | 0 | ||||
| PD | 3* | 17 | 20 | PD | 3* | 30 | 33 | ||||
| Total | 4 | 19 | 23 | Total | 3 | 30 | 33 | ||||
| P = 1.00 | P = 0.25 | ||||||||||
Underlined represent agreement and
represent disagreement.
Note: P < 0.05 indicates the two methods were not consistent with one another.
Abbreviations: RECIST, response evaluation criteria in solid tumors; VECIST, volumetric response evaluation in solid tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NSCLC, non-small cell lung carcinoma.
Progression by RECIST and volumetrics occurred in fifteen and twenty of the twenty-three thymic cancer patients, respectively. The remaining eight patients were taken off study secondary to clinical progression from pain (n=6) and voluntary removal from the clinical trial (n=2). In these eight cases RECIST never determined the patients as having PD, whereas volumetrics deemed them as PD 62.5% (5/8) of the time.
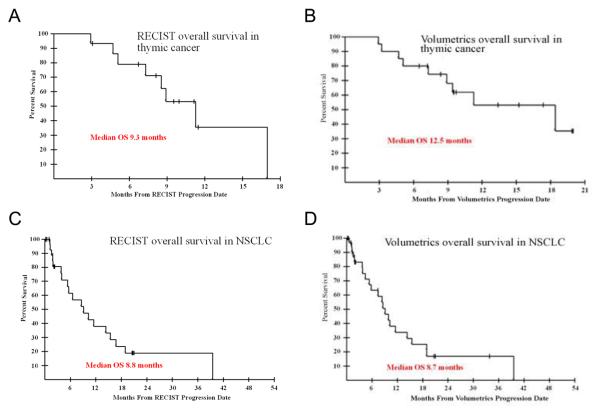
When survival probabilities were calculated starting from the date of progression by either method, the median survival from date of volumetric progression was 12.5 months, while it was 9.3 months from date of RECIST progression (Figure 2A-B). Since there are different patients in these two groups, formal comparison of survival times between these groups would not be appropriate.
Fig. 2.
Overall survival of thymic cancer patients beginning at dates of RECIST-designated progression (A) and volumetrics-designated progression (B). Overall survival of NSCLC patients beginning at dates of RECIST-designated progression (C) and volumetrics-designated progression (D).
Abbreviations: RECIST, response evaluation criteria in solid tumors
Tumor Response in Metastatic NSCLC
We evaluated NSCLC patients as a control group since RECIST is the current gold standard measurement method. This cohort was evaluated in a similar sequence to the thymic cancer cohort; although, only comparisons of volumetrics against RECIST were assessed as modified RECIST has not been validated in NSCLC and the WHO criteria have been shown to be no better than RECIST [8]. At the time of RECIST determined best response, 29% of subjects had a disagreement noted between the two criteria, with classifications favoring detection of progression by volumetrics (p = 0.0215; Table III D). Assessment of the target lesions alone at the time of RECIST designated progressive disease also revealed a 33% disagreement between the two methods and a large tendency toward finding more progressions by volumetrics (p = 0.0117; Table IV D). However, 9% disagreement was noted when the entire criteria (target lesions, non-target lesions, and new lesions) were assessed between RECIST and volumetrics at the time of RECIST-designated progressive disease, and only 3 discrepant cases were found—all classified as PD by volumetrics (p = 0.625; Table V D). Disease progression was also demonstrated to occur significantly earlier by volumetrics by on average 32 days (p = 0.0078). These results identified that there was no difference when using volumetrics or RECIST for determining whether there was disease progression or not in metastatic NSCLC patients, even though volumetrics identified patients as having progressive disease significantly earlier than RECIST.
Restricted to the patients who progressed by either RECIST or volumetrics in each case, we determined that there was a median overall survival of 8.7 months from the date of volumetrics progression compared to 8.8 months from the date of RECIST progression (Figure 2C-D). To evaluate if the time of determining progression could somehow impact the probability of survival, a Cox proportional hazards analysis was undertaken using the progression by RECIST as a time-varying covariate. The impact of having a progression determined by the RECIST criteria on survival had an associated p-value of 0.14. Thus, there was no significant evidence that having progression, as determined by RECIST, or not, as a function of when it was noted to occur, has any impact on the overall survival of the patient. Because of the fact that both patients who did not progress by volumetrics remain alive, the impact of this parameter could not be properly estimated.
Discussion
Two main findings stand out from our analyses. First, volumetrics tended to identify progressive disease more often than RECIST in advanced thymic cancers, but not in metastatic NSCLC. Second, volumetrics deemed patients to have progressive disease earlier than RECIST in thymic cancers and NSCLC. Thus, it seems that three-dimensional measurement criteria (volumetrics) may reveal disease progression in patients demonstrating clinical or radiological progression more often than uni-dimensional criteria (RECIST) in thymic cancers, but not in NSCLC.
Thymomas are slow growing tumors and these patients can live many years with their disease. Moreover, tumor deposits in most patients with advanced thymic malignancies are not spherical in shape, and when imaged by CT they appear as curvilinear plaques within the pleural cavity. Also, volumetrics seems to identify progressive disease more often and detects it earlier when compared to RECIST. Given this information, when the patient's slow growing, non-spherical disease burden is increasing, we feel that the use of volumetrics may aid clinicians considering alternative therapeutic strategies at an earlier time point.
Additionally, our data suggests that thymic malignancies may be treated with potentially suboptimal therapy for a longer duration if RECIST is used for assessment of tumor burden since progressive disease was detected 72 days earlier by volumetrics. However, in this retrospective study we are unable to answer the question of whether earlier detection of progressive disease affects overall survival of these patients. Making any definitive conclusion based on available survival data may be misleading, as the clinical trial that these patients were enrolled on was not designed to detect differences in survival between volumetrics and RECIST in a prospective manner. Therefore, a larger sample size of patients with thymic malignancies assessed with different measurement criteria in a prospective manner would be needed to address this important issue.
Previous studies have mimicked three-dimensional tumor volume measurements with the use of mathematical formulae [7]. Our study utilized software that calculated the tumor volume based on intensity via Hounsfield units. This is important because mathematical formulae assume tumor volumes are cylindrical, which is not the case with thymic tumors. We feel that volumetrics may ultimately provide a better assessment of objective response and occurrence of disease progression in thymic cancer patients.
We are aware of a number of limitations to our work. This is a single institutional retrospective study, in which only patients with complete three-dimensional assessment were included in our analysis; this may lead to selection bias. Our study has a low number of enrolled patients in either cohort. Also, there may be factors that can vary within and between CT-scanners, thus creating differences in the overall measurement of target lesions. Also differences in measurement may be introduced secondary to patient positioning or the phase of respiration when comparing restaging CT scans.
We feel that conclusions from this study should be utilized to stimulate further development into novel methods of detecting and prospectively following tumor burden. Our data suggests that volumetrics may provide a more accurate representation of tumor growth.
In conclusion, our findings show that three-dimensional measurement criteria (volumetrics) may detect progressive disease earlier and more often than uni-dimensional measurement criteria (RECIST) in advanced thymic cancer patients. However, in diseases such as heavily pre-treated metastatic NSCLC patients undergoing third or fourth line therapy, there is no difference in determination of progressive disease whether one chooses RECIST or volumetrics measurement methods. The use of three-dimensional measurement criteria may be more beneficial in the management of relatively slow growing, non-spherical tumors such as thymic malignancies. To better understand, confirm, and validate our findings a larger prospective study of thymic cancer patients utilizing volumetrics and RECIST for assessment of tumor burden is warranted.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health. This work was supported by the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer Inc).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization . WHO handbook for reporting results of cancer treatment. World Health Organization; Geneva Albany, N.Y.: p. 45. (WHO offset publication no. 48. 1979). sold by WHO Publications Centre USA. [Google Scholar]
- 2.Miller AB, et al. Reporting results of cancer treatment. Cancer. 1981;47(1):207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Bogaerts J, et al. Individual patient data analysis to assess modifications to the RECIST criteria. Eur J Cancer. 2009;45(2):248–60. doi: 10.1016/j.ejca.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Rajan A, Giaccone G. Treatment of advanced thymoma and thymic carcinoma. Curr Treat Options Oncol. 2008;9(4-6):277–87. doi: 10.1007/s11864-009-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. The Journal of Thoracic and Cardiovascular Surgery. 2009;138(1):26–31. doi: 10.1016/j.jtcvs.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorensen AG, et al. Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol. 2001;19(2):551–7. doi: 10.1200/JCO.2001.19.2.551. [DOI] [PubMed] [Google Scholar]
- 8.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15(2):257–60. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 9.Nowak AK. CT, RECIST, and malignant pleural mesothelioma. Lung Cancer. 2005;49(Suppl 1):S37–40. doi: 10.1016/j.lungcan.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 10.van Klaveren RJ, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361(23):2221–9. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, et al. Assessment of therapy responses and prediction of survival in malignant pleural mesothelioma through computer-aided volumetric measurement on computed tomography scans. J Thorac Oncol. 2010;5(6):879–84. doi: 10.1097/JTO.0b013e3181dd0ef1. [DOI] [PubMed] [Google Scholar]
- 12.Sohaib SA, et al. CT assessment of tumour response to treatment: comparison of linear, cross-sectional and volumetric measures of tumour size. Br J Radiol. 2000;73(875):1178–84. doi: 10.1259/bjr.73.875.11144795. [DOI] [PubMed] [Google Scholar]
- 13.Solomon J, et al. Automated detection and volume measurement of plexiform neurofibromas in neurofibromatosis 1 using magnetic resonance imaging. Comput Med Imaging Graph. 2004;28(5):257–65. doi: 10.1016/j.compmedimag.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Pass HI, et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1998;115(2):310–7. doi: 10.1016/S0022-5223(98)70274-0. discussion 317-8. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari A, et al. Comparison of the prognostic value of assessing tumor diameter versus tumor volume at diagnosis or in response to initial chemotherapy in rhabdomyosarcoma. J Clin Oncol. 2010;28(8):1322–8. doi: 10.1200/JCO.2009.25.0803. [DOI] [PubMed] [Google Scholar]
- 16.Shah GD, et al. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro Oncol. 2006;8(1):38–46. doi: 10.1215/S1522851705000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James K, et al. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91(6):523–8. doi: 10.1093/jnci/91.6.523. [DOI] [PubMed] [Google Scholar]
- 18.Ak G, et al. Three-dimensional evaluation of chemotherapy response in malignant pleural mesothelioma. Eur J Radiol. 74(1):130–5. doi: 10.1016/j.ejrad.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Giaccone G, et al. Phase II study of the histone deacetylase inhibitor belinostat in thymic malignancies. J Clin Oncol. 2009;27(15s) [Google Scholar]
- 20.Kelly RJ, et al. Evaluation of KRAS mutations, angiogenic biomarkers and DCE-MRI in patients with advanced non-small cell lung cancer receiving sorafenib. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-10-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]