Abstract
Respiratory motoneurons provide the exclusive drive to respiratory muscles and therefore are a key relay between brainstem neural circuits that generate respiratory rhythm and respiratory muscles that control moment of gases into and out of the airways and lungs. This review is focused on postnatal development of fast ionotropic synaptic transmission to respiratory motoneurons, with a focus on hypoglossal motoneurons (HMs). Glutamatergic synaptic transmission to HMs involves activation of both non-NMDA and NMDA receptors and during the postnatal period co-activation of these receptors located at the same synapse may occur. Further, the relative role of each receptor type in inspiratory-phase motoneuron depolarization is dependent on the type of preparation used (in vitro versus in vivo; neonatal versus adult). Respiratory motoneurons receive both glycinergic and GABAergic inhibitory synaptic inputs. During inspiration phrenic and HMs receive concurrent excitatory and inhibitory synaptic inputs. During postnatal development in HMs GABAergic and glycinergic synaptic inputs have slow kinetics and are depolarizing and with postnatal development they become faster and hyperpolarizing. Additionally shunting inhibition may play an important role in synaptic processing by respiratory motoneurons.
Keywords: Motoneuron, Hypoglossal, Synaptic, Development, Inhibition, Excitation
1. Introduction
This review is focused on the postnatal development of fast ionotropic synaptic transmission to respiratory-related motoneurons, with an emphasis on studies of hypoglossal motoneurons (HMs) during the postnatal period. This review will not cover synaptic transmission involving modulatory neurotransmitters such as serotonin, norepinephrine and neuropeptides, nor the metabotropic receptors such as mGlu- and GABAB- receptors (see an earlier review by Rekling et al (2000) for information on these subjects).
Before discussing the development of fast ionotropic synaptic transmission to HMs it is important to understand the target muscle of these motoneurons. The tongue muscle is heterogeneous, containing 8 difference muscle groups, 4 are extrinsic and 4 are intrinsic (Sokoloff and Deacon, 1992). The tongue takes part in a number of basic functions including mastication, swallowing, suckling, and vocalization. It is also important in respiration due to its position in the upper airway, thereby having a role in upper airway patency. Regarding the latter function the main protrusor muscle group of the tongue, the genioglossus muscle, exhibits inspiratory-related electrical activity. Thus inspiratory-phase (I-phase) activation of this tongue muscle group promotes stabilization and patency of the upper airway posterior to the tongue. Failure of this activation to adequately occur is thought to be an important contributing factor to obstructive sleep apnea.
2. Morphology of hypoglossal motoneurons with respect to synaptic transmission
Brainstem motoneurons, such as HMs, are found in distinct bilaterally located nuclei within which the motoneurons are somatotopically organized. This is in contrast to spinal motoneurons which are found in longitudinally organized columns in the spinal cord ventral horn. A number of anatomical studies have shown that HM dendrites, while elaborate within the XII nucleus itself, also penetrate into reticular formation around the lateral and ventral sides of the XII nucleus (Altschuler et al., 1994; Nunez-Abades et al., 1994; Tarras-Wahlberg and Rekling, 2009; Van Brederode et al., 2011).
Although we do not know the relative distribution of inhibitory versus excitatory synapses onto HMs some indication of this may be gleaned from detailed studies at the ultrastructural level regarding this distribution in adult cat spinal motoneurons (Ornung et al., 1998). In spinal motoneurons it is the dendritic tree and not the somal membrane that dominates the synaptic receptive area by over 9 to 1. In primary first order (stem) dendrites of spinal motoneurons about 70% of synaptic boutons are γ-aminobutyric acid (GABA) and/or glycine containing, while only approximately 20% are glutamate containing. In non-primary dendrites approximately 55% are GABA and/or glycine containing, while approximately 40% are glutamate containing. Thus in the dendritic space close to the soma there is a strong inhibitory synaptic influence (Ornung et al., 1998). This may have importance with respect to synaptic integration that is described later in this review.
3. Glutamatergic synaptic transmission
There has been a recent overall review of ionotropic glutamate receptors by Traynelis, et al. (2010). Ionotropic glutamate receptors which mediate fast excitatory synaptic transmission have for the most part been separated into three broad classes based on structural, functional and pharmacological characteristics. These three classes are kainate receptors; AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors; and NMDA (N-methyl-D-aspartic acid) receptors. These are assembled from different combinations of four large glutamate receptor subunits (Traynelis et al., 2010). AMPA and kainate receptors have fast activation and deactivation rates as well as strong and rapid desensitization. NMDA receptors have slower gating kinetics and deactivate over a longer time-scale. NMDA receptors also exhibit modest and slow desensitization (Traynelis et al., 2010).
Developmental changes in glutamatergic synaptic transmission to HMs have been demonstrated. It has been shown that tritiated glutamate binding within the rat hypoglossal motor nucleus peaks in intensity at around nine days postnatal and then declines progressively at later postnatal ages. This likely reflects changes in glutamatergic receptor density and/or glutamate affinity (Rao et al., 1995).
Another feature of glutamatergic transmission is the presence at single postsynaptic sites on respiratory motoneurons of both non-NMDA and NMDA receptors. O’Brien at al. (1997) studied the properties of glutamatergic miniature excitatory postsynaptic currents (mEPSCs) recorded in neonatal rat HMs. They observed dual component mEPSCs that were generated by non-NMDA and NMDA receptors (Fig. 1). The separation of these components on the basis of their kinetics (decay times) led to the conclusion that non-NMDA and NMDA receptors are co-localized at individual synapses (O’Brien et al., 1997).
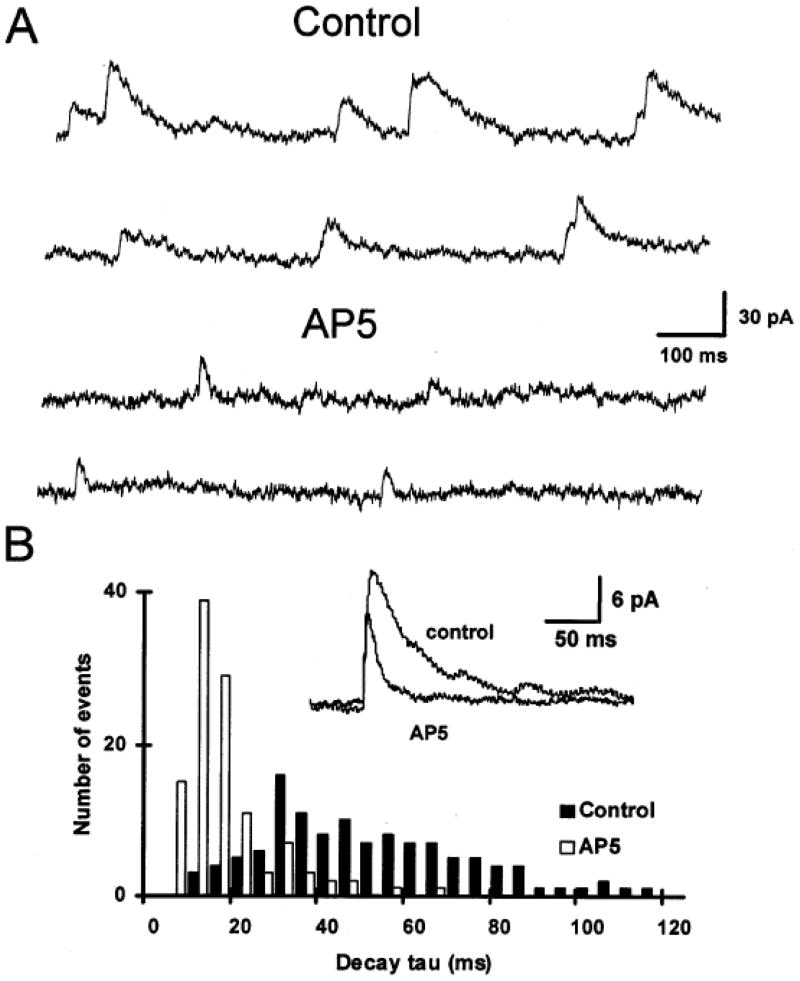
Fig. 1.
NMDA and non-NMDA glutamatergic receptors are co-localized in the postsynaptic membrane of hypoglossal motoneurons. This is shown by the presence of spontaneous miniature excitatory postsynaptic currents (mEPSCs) that have dual temporal components. (A) When recorded in the presence of bicuculline to block GABAA-receptors, strychnine to block glycine receptors and TTX to block action potential generated synaptic events, the majority of mEPSCs show slow decay kinetics (Control – top two traces). With the addition of AP5 to block NMDA receptors the slow decaying component is abolished leaving only a fast decaying component due that is due to activation of non-NMDA receptors (AP5 - lower two traces). (B) Distribution of mEPSC decay time constants before and after application of AP5. Inset: Average of mEPSCs before and after addition of AP5. Data show that the AP5 sensitive component of the mEPSC has a significantly longer decay time constant. Data from HMs voltage clamped at +50 mV to remove the voltage-dependent Mg block of the NMDA receptor. (Adapted from O’Brien et al., 1997).
The role of glutamate-receptor mediated respiratory-cycle related excitation of respiratory motoneurons has been studied extensively. The excitatory synaptic input to respiratory motoneurons is a consequence of the synaptic release of glutamate from glutamatergic synaptic terminals apposed to these motoneurons. Receptor expression studies have revealed that the three classes of the glutamate receptor are present in HMs (Rekling et al., 2000). Functionally both non-NMDA and NMDA gated channels have been demonstrated on HMs (Berger et al., 1998; Funk et al., 1993; O’Brien et al., 1997; Rekling, 1992), and this is shown in Fig. 1.
The relative contribution of non-NMDA and NMDA receptor activation to the respiratory-related drive to respiratory motoneurons is controversial and depends on the preparation used. For example, Funk et al (1993), using the in vitro rhythmic rat medullary slice preparation, found that local blockade of NMDA-receptors in the hypoglossal nucleus did not alter the inspiratory burst amplitude observed in HMs, but blockade of non-NMDA-receptors caused a dose-dependent reduction in inspiratory burst amplitude. Thus even though HMs possess both non-NMDA and NMDA receptors they concluded that it was activation of primarily non-NMDA receptors that mediate transmission of inspiratory drive to HMs (Funk et al., 1993). Subsequently this group showed in an in vitro preparation from mutant neonatal mice lacking the important NMDA-receptor gene (they were unresponsive to application of exogenous NMDA), that respiratory-related activity recorded from either HMs or phrenic motoneurons was virtually identical with activity in control animals (Funk et al., 1997). Thus, these in vitro neonatal studies indicate that it is the non-NMDA receptor mediated I-phase excitatory synaptic input to these motoneurons that mediates the inspiratory-related drive. These in vitro neonatal results leave open the question of whether later in life there is a role for NMDA-receptors in the respiratory drive to motoneurons. Additionally, the in vitro nature of these experiments may influence the results.
Indeed a role for NMDA-receptors in mediating respiratory drive to respiratory motoneurons has been demonstrated in in vivo experiments involving mature animals. For example, it has been shown that in the adult rat both non-NMDA and NMDA receptors are involved in the inspiratory-drive that is seen in phrenic motoneurons (Chitravanshi and Sapru, 1996). Recently, it has been shown in the anesthetized adult rat, using microdialysis perfusion of non-NMDA and NMDA receptor antagonists into the hypoglossal nucleus, that activation of both non-NMDA and NMDA glutamate receptor types are involved in the excitatory synaptic I-phase drive to HMs (Steenland et al., 2006; Steenland et al., 2008). Significant reductions in respiratory-related genioglossus muscle activity with blockade of non-NMDA receptors by the antagonist CNQX (Fig. 2A), and separately the blockade of NMDA receptors by the antagonist D-APV (Fig. 2B), were observed. When this microdialysis perfusion technique was applied to intact free behaving rats it was found that the I-phase drive to HMs via activation of non-NMDA receptors in the hypoglossal motor nucleus was low. Interestingly local antagonism of NMDA receptors resulted in a significant depression of respiratory-related hypoglossal activity during wakefulness (Steenland et al., 2008). This importance of NMDA-receptor mediated I-phase drive to HMs in the intact awake animal may be a consequence of a tonic arousal-dependent depolarization of HMs. This depolarization relieves the voltage-dependent magnesium block of NMDA-receptors (Traynelis et al., 2010).
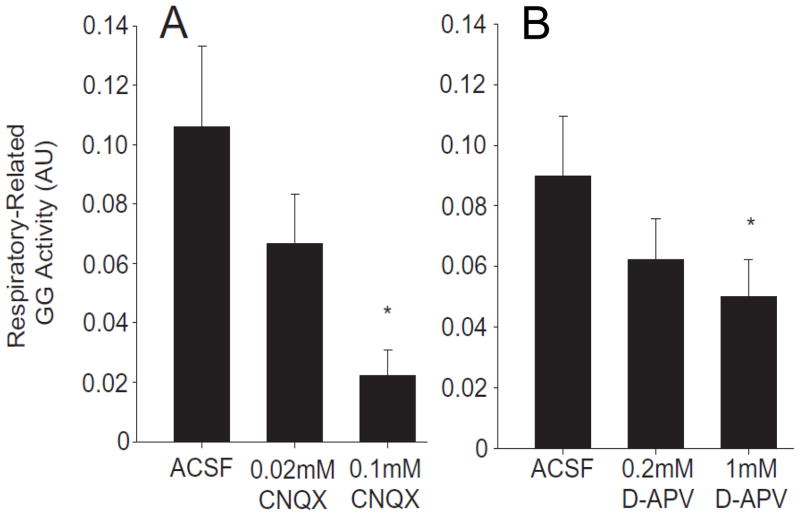
Fig. 2.
In the adult anesthetized rat both non-NMDA and NMDA receptors in the hypoglossal motor nucleus contribute to the excitatory synaptic I-phase drive to HMs. Shown are data for respiratory-related genioglossus muscle activity (GG activity) in response to microdialysis perfusion of the non-NMDA receptor antagonist CNQX and separately of the NMDA receptor antagonist D-APV (also known as AP5) into the hypoglossal motor nucleus. (A) Shows the dose-dependent reduction of respiratory-related activity with CNQX. (B) Shows the dose-dependent reduction with D-APV. Data reported at means ± SEM and * indicates a P< 0.05 level of significance compared to microdialysis perfusion of the artificial cerebrospinal fluid (ACSF) control solution. (Adapted from Steenland et al., 2006).
During early postnatal life the morphology of motoneurons can be affected by NMDA receptor activation. It has been shown that blockade of NMDA receptors acts to inhibit motoneuron somal growth and dendritic branching (Kalb, 1994). This important role of NMDA receptors in development maybe related to permeability of this receptor to calcium, intracellular calcium has a role in neuronal development. An interesting effect of NMDA-receptor activation by exogenously applied NMDA onto neonatal rat HMs in vitro, was the finding in many but not all motoneurons that this resulted in bursting behavior (Sharifullina et al., 2008). The similarity of the observed NMDA-evoked bursting pattern to that previously recorded during suckling led to the suggestion that suckling during the postnatal period may involve NMDA-receptor activation on HMs.
Concerning NMDA-receptors an important feature of their activation is the obligatory role that glycine has as a co-agonist at this receptor. This characteristic has important implications for NMDA-receptor activation of respiratory motoneurons. Work of Lim et al. (2004), showed that blockade of the glycine transporter-1 (GLYT-1) with NFPS potentiated NMDA-receptor-mediated synaptic transmission to HMs. Immunohistochemistry showed that there was GLYT-1 (primarily in glial cells) and GLYT-2 (primarily expressed in neurons) labeling in the hypoglossal motor nucleus. With postnatal development it was found that GLYT-1 becomes more intense and uniform, GLYT-2 labeling was observed throughout the motor nucleus and increased in intensity with postnatal age. Early work by Zafra et al (1995) showed that GLYT-1 and GLYT-2 mRNA and the corresponding protein levels rose in the brainstem during the first two weeks of postnatal life. The results of Lim et al. (2004) also demonstrated that the glycine binding site of the NMDA-receptor in HMs is not saturated during the first two weeks of postnatal life. Thus the level of glycine and its modulation by GLYT-1 can act to regulate NMDA-receptor-mediated synaptic transmission. The availability of the glycine binding site has been demonstrated in neonatal HMs studied in brainstem slices where NMDA-receptor-mediated spontaneous miniature excitatory postsynaptic currents (mEPSCs) and NMDA-induced responses are potentiated by addition of exogenous glycine (Berger et al., 1998; Berger and Isaacson, 1999).
4. Glycinergic and GABAergic synaptic transmission
There have been recent reviews of ionotropic glycine- and GABA-receptors (Ben-Ari et al., 2007; Lynch, 2009). Glycine and GABA are the primary inhibitory neurotransmitters in the central nervous system. Both activate different ionotropic ligand gated receptors whose primary permeable ion is chloride. While glycine is the predominant inhibitory neurotransmitter in spinal cord and brainstem, GABA is also present and phasic GABAergic synaptic transmission to motoneurons is readily observed. (Donato and Nistri, 2000; Gao et al., 1998; Jonas et al., 1998; O’Brien and Berger, 1999; O’Brien and Berger, 2001).
There is evidence that in general respiratory motoneurons, whether brainstem or spinal cord, receive both glycinergic and GABAergic inhibition. In vivo and in vitro studies have shown that HMs receive both GABAergic and glycinergic synaptic inputs (Altmann et al., 1972; Donato and Nistri, 2000; O’Brien and Berger, 1999; Rekling et al., 2000; Takata and Ogata, 1980). Synaptic inhibition of HMs has been shown to occur concurrently with I-phase synaptic excitation, but in contrast to phrenic motoneurons where expiratory-phase synaptic inhibition also occurs, rhythmic expiratory-phase inhibition is not present in HMs (Peever et al., 2002; Saywell and Feldman, 2004; Withington-Wray et al., 1988; Woch and Kubin, 1995). Thus for HMs it is disfacilitation that causes expiratory-phase repolarization, active synaptic inhibition does not contribute to the repolarization. During inspiration phrenic as well as HMs receive concurrent glutamatergic excitation and GABAergic inhibition (Parkis et al., 1999; Saywell and Feldman, 2004). While I-phase GABAergic inhibition of HMs may have an important function in gain modulation of HM activity, it appears that in the dog I-phase glycinergic inhibition may not have such a role (Sanchez et al., 2009). In contrast to these results in the dog, it has been shown in freely behaving rats, using the technique of local microdialysis perfusion of the glycine-receptor antagonist strychnine and the GABAA-receptor antagonist bicuculline into the XII nucleus, that functionally there is tonically active inhibition of HMs. This inhibition is mediated by both inhibitory transmitter systems and what has been observed with receptor blockade was that inspiratory-related genioglossus activity increased demonstrating that there is a functional glycinergic and GABAergic inhibition of genioglossus activity and this was shown to be dependent on the sleep-wake state of the animal (see Fig. 3 and (Morrison et al., 2003b; Morrison et al., 2003a)).
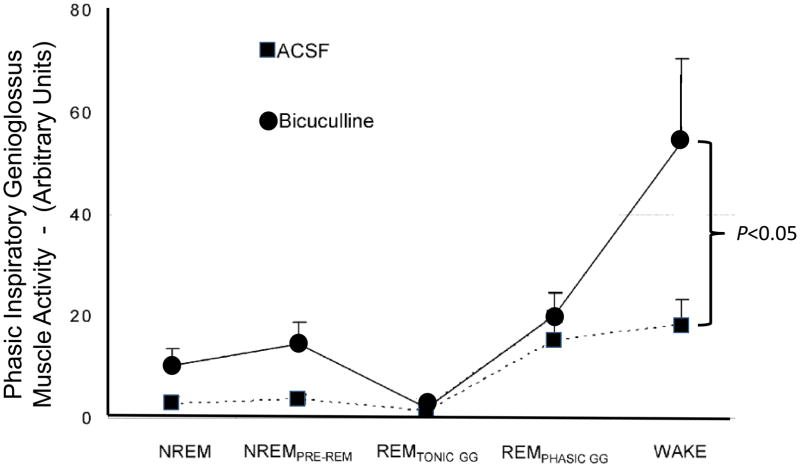
Fig. 3.
Microdialysis of bicuculline into the hypoglossal motor nucleus blocks GABAA-receptors and significantly increases inspiratory-phase related hypoglossal motoneuron activity in the awake rat. Shown are group data assessing changes in respiratory-related genioglossus muscle activity (GG activity) across sleep-wake states during microdialysis perfusion of artificial cerebrospinal fluid (ACSF) control solution and bicuculline into the hypoglossal motor nucleus during room-air breathing. Data reported at means ± SEM. (Adapted from Morrison et al., 2003b).
4.1 Inhibitory synaptic events change during postnatal development
During the postnatal period in many neurons there is a marked change in intracellular chloride concentration and this occurs due to maturation of the chloride ion transport system (Singer and Berger, 2000). As a consequence glycine and GABA-mediated synaptic transmission, which involves chloride ion movement across the cell membrane, changes from causing depolarization to hyperpolarization as animals mature. Whether one observes depolarization or hyperpolarization in response to activation of GABAA- or glycine-receptors is dependent on the sign of the driving force for chloride ion movement. The driving force is determined by the difference between a neuron’s membrane potential and the equilibrium potential for chloride ion. The latter being set by the Nernst relationship for chloride and is a function of the ratio of intracellular and extracellular chloride concentrations. These changes have has been studied in detail in HMs using the neonatal in vitro medullary slice preparation (Singer et al., 1998). Fig. 4 shows, using a gramicidin perforated-patch recording technique, that does not alter the intracellular chloride concentration, that the reversal potential for glycine-receptor-mediated currents recorded in HMs became more hyperpolarized from −37 mV (P0–3 rats) to −73 mV (P10–18 rats) with postnatal development (Singer et al., 1998) (see also confirming results of Marchetti et al. (2002)). Therefore, at birth in HMs glycinergic and GABAergic postsynaptic potentials in HMs are depolarizing, and become hyperpolarizing with postnatal development. It is of interest, and as stated above, that this maturation process appears to be dependent on the development of chloride transporters (Ren and Greer, 2006). For example, it has been shown based on recordings from fetal respiratory neurons in the ventrolateral medulla that prior to embryonic day 19 (in rat) that chloride-mediated conductances are depolarizing, and after this time and extending into the postnatal period they are hyperpolarizing. This change for ventrolateral medullary respiratory neurons is dependent on the early ontogenesis of chloride transporters in these neurons (Ren and Greer, 2006). Interestingly chloride transporters were strongly modulated by extracellular potassium concentration, thus depending on the preparation used to study in vitro respiratory rhythm (slice versus more intact preparations, and the corresponding extracellular potassium concentration used) one could obtain different results with respect to the effect of activating GABAA- or glycine receptors.
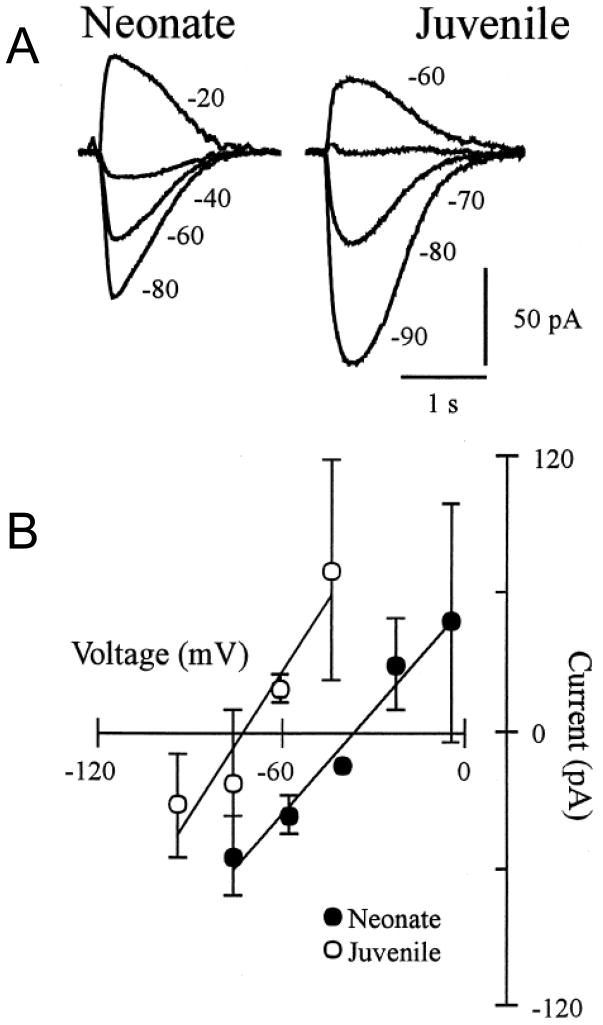
Fig. 4.
The reversal potential of glycine-receptor-mediated currents recorded in HMs becomes more hyperpolarized with postnatal development. (A) Local glycine application onto a voltage-clamped HM shows the reversal potential from a neonate (P2) and a juvenile (P15) rat. Various holding potentials indicated in mV. (B) Neonate and juvenile group data for the current-voltage relationship of the glycine-evoked responses. Results show the hyperpolarizing shift in the glycine-evoked response with postnatal development. All recordings were done using the gramicidin perforated-patch technique so as not to disturb the native intracellular chloride concentration. (Adapted from Singer et al., 1998).
In HMs it has been shown that the mean amplitude of spontaneous glycinergic inhibitory post synaptic currents (mIPSCs) increases by almost 100% over the course of the first 3 weeks of postnatal life (Singer and Berger, 1999). The reason for this increase is associated with a developmentally related increase in the number of glycine-receptors at each synapse (Singer and Berger, 1999).
Another feature of postnatal development is the speeding up of the time course of inhibitory synaptic events during this period (Singer et al., 1998). In the case of the glycine-receptor it has been demonstrated in HMs that expression of the fetal (α2) glycine-receptor subunit mRNA decreases while expression of the adult (α1) glycine-receptor subunit mRNA increases postnatally (Singer et al., 1998). It is this switch in subunit expression that is primarily responsible for the speeding up of glycinergic synaptic events in HMs during postnatal development. In the case of GABAA-receptor-mediated synaptic transmission to HMs, it was found that it also becomes faster with postnatal development (O’Brien and Berger, 2001). This manifested itself by a reduction in the decay time of single GABAergic synaptic events.
4.2 Co-transmission of glycinergic and GABAergic synaptic transmission
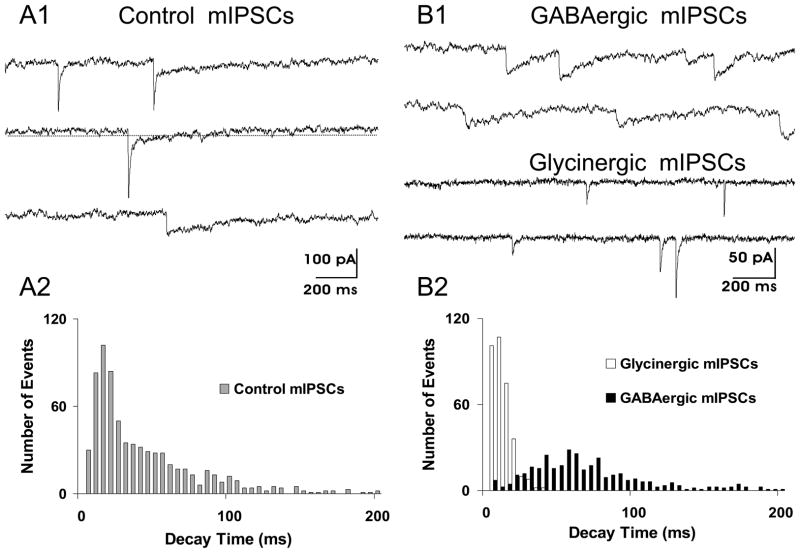
The transport of glycine and GABA by the same vesicular transporter (Burger et al., 1991) suggests the possibility that glycine and GABA maybe co-released by single synaptic vesicles. Further, if the postsynaptic cell membrane possesses both glycine- and GABAA-receptors at single synaptic sites, co-transmission of these two inhibitory transmitters may occur. Important anatomical evidence related to co-transmission to HMs of glycinergic and GABAergic synaptic transmission was provided by Muller et al. (Muller et al., 2004). They showed that there was co-clustering of glycine-receptor clusters and GABAA-receptor clusters on HMs. Importantly they demonstrated that these co-clusters increased during postnatal period into adulthood. Jonas et al. (1998) were the first to definitively show that GABA and glycine are co-released from single synaptic terminals in the spinal cord. Based on an analysis of the kinetics of spontaneous inhibitory miniature synaptic events they concluded at 44% of events were due to co-transmission, 41% were pure glycinergic events and 15% were pure GABAergic. Subsequently O’Brien and Berger (1999) showed that this also occurred in the case of inhibitory synaptic transmission to HMs (Fig. 5).
Fig. 5.
Co-transmission of GABA and glycine to a HM is shown by the presence of dual component spontaneous miniature inhibitory postsynaptic currents (mIPSCs). (A1) Control recording show the presence of three types of mIPSCs based on their temporal kinetics. Slow decaying GABAergic mIPSCs, fast decaying glycinergic mIPSCs and dual component mIPSCs, having both fast and slow decaying components. Presumably the dual component mIPSCs are due to co-release of both GABA and glycine from a single presynaptic vesicle. (A2) Distribution of mIPSC decay times in control conditions showing that the distribution is skewed toward longer decay times. (B1) In the presence of strychnine to block glycine receptors, isolated slow decaying GABAergic mIPSCs are recorded (top two traces). In the presence of bicuculline to block GABAA receptors, isolated fast decaying glycinergic mIPSCs are recorded (lower two traces). (B2) Distribution of mIPSC decay times of GABAergic and glycinergic mIPSCs shows two unique distributions (P<0.001) based on Kolmogorov-Smirnoff statistical testing of these distributions. (Adapted from O’Brien and Berger, 1999).
An important issue in regard to co-transmission of GABA and glycine is what might be its functional role. Since glycine synaptic events occur on a much faster time scale than GABA synaptic events, having co-transmission may enable these synaptic events to occur with a much longer duration. Given that early in the postnatal period glycinergic synaptic events are depolarizing due to the developmentally related change in intracellular chloride in HMs (Marchetti et al., 2002; Singer et al., 1998), and it is likely that GABAergic events are also depolarizing early in the postnatal period, then the large longer duration depolarizing synaptic events due to glycinergic and GABAergic co-transmission may lead to additional activation of voltage-gated calcium channels. Calcium channels are activated by membrane depolarization. A low-voltage-activated calcium current (so-called T-type current) is present in HMs (Umemiya and Berger, 1994). This current has a half-activation voltage that is near the reversal potential of the glycine-receptor-current in newborn rat HMs (see above). It was also found that the T-type calcium current decreases with postnatal age in HMs (Umemiya and Berger, 1994). Calcium influx is important in neuronal development (Cherubini et al., 1991). An additional function of the co-release of GABA and glycine may involve the feedback regulation of inhibitory transmitter release. This could be accomplished by synaptically released GABA-mediated feedback activation of presynaptic metabotropic GABAB-receptors located on inhibitory transmitter releasing terminals. Supporting this hypothesis we previously showed that activation of presynaptic GABAB–receptors inhibits GABA and glycine release from terminals that synapse on HMs (O’Brien et al., 2004).
4.3 Role of shunting inhibition
The dominance of inhibitory synaptic terminals on primary dendrites of motoneurons (see above) may have a function to shunt via inhibition more distally located synaptic inputs. Shunting inhibition is ordinarily seen when the reversal potential of a synaptic current is close to the resting membrane potential of a neuron. Activation of postsynaptic receptors causing shunting inhibition is the result of an increase in membrane conductance without a significant change in membrane potential. Modulation of this type may be particularly effective if it is present on the primary dendrites of a neuron. Thus, with the arrival of more distally located excitatory synaptic inputs and the near coincident arrival of more proximally located inhibition, the resultant shunting inhibition will cause the excitatory input as observed in the soma to be reduced compared with its magnitude if shunting inhibition were not present. Therefore, it is possible that one of the reasons for the prolonged time course of inhibitory synaptic events (especially GABA-receptor mediated events – or the longer duration glycinergic synaptic events seen early in postnatal development) compared with the time course of non-NMDA excitatory synaptic events is that it provides a mechanism whereby the prolonged IPSCs can modulate, via shunting inhibition, excitatory related inputs.
Marchetti et al. (2002) studied shunting inhibition in both spinal motoneurons and HMs. They used an in vitro slice preparation derived from early postnatal mice. In spinal motoneurons, where Renshaw inhibition of motoneurons is present, they observed glycinergic and GABAergic depolarizing synaptic events (due to the elevated levels of intracellular chloride in motoneurons at early postnatal ages discussed above) they found that these recurrent postsynaptic potentials consistently reduced synaptic excitation. They concluded that their observations were due to a GABA and glycine synaptically mediated increase in motoneuronal input conductance. In the case of HMs, which lack a recurrent Renshaw-like inhibitory pathway, they observed that focally stimulated GABAergic and glycinergic synaptic events in HMs inhibited in a majority of HMs evoked motoneuron firing. Again, this suggested to them that in the early neonatal period there is strong shunting inhibition of HMs due to the presence of depolarizing GABA and glycine mediated synaptic events, and these cause a large reduction in HM input resistance (Marchetti et al., 2002).
A key feature of a tonic shunting conductance is that it can have profound effects on both excitability as well as what is termed “gain control” of neurons (Bonin et al., 2007; Bright et al., 2007; Stell et al., 2003) and network excitability (Semyanov et al., 2003). In addition to phasic inhibition it has been shown that tonic inhibition of HMs occurs and that blockade of this results in an increase in HM input resistance (Nunez-Abades et al., 2000).
Recently in preliminary studies we showed that a tonic GABAA-receptor-mediated current is present in HMs (Numata et al., 2010). These studies were performed using whole-cell patch-clamp recordings on visually identified postnatal mouse HMs in an in vitro slice preparation. In the presence of blockers of glutamatergic, glycinergic and GABAB-receptor mediated transmission and blockade of GABA transporters, application of exogenous GABA to the bathing solution resulted in a tonic GABA current in HMs. Additional pharmacological studies showed that this tonic current was mediated by GABAA-receptors containing the δ subunit (Numata et al., 2010).
4.4 A role for inhibitory synaptic transmission in the generation of inspiratory motoneuronal synchronous activity
One of the ubiquitous findings regarding I-phase activity of all inspiratory motor pools, whether they be phrenic, laryngeal, external intercostals and hypoglossal, is the observation that there is synchronous discharge of inspiratory motoneurons during inspiration (Funk and Parkis, 2002). The frequency of the dominant spectral peak of these oscillations increases with postnatal development (Sebe et al., 2006). The functional relevance of these synchronous oscillations maybe related the input-output efficiency by which a respiratory motoneuron generates action potentials. It has been shown that in the absence of synchronous inputs to inspiratory motoneurons the number of I-phase spikes generated decreases significantly (Parkis et al., 2003). Additionally, neuromodulator induced changes in inspiratory motoneuron firing can be constrained by these synchronous oscillations (Parkis et al., 2003), and if we extrapolate from motoneuron-muscle computer modeling studies (Baker et al., 1999) to the HM-tongue muscle system then synchronized I-phase activity can increase tongue muscle force output.
Inhibitory synaptic transmission has been shown to be critical for the generation of synchronous oscillatory activity (Bou-Flores and Berger, 2001; Marchenko and Rogers, 2009; Sebe et al., 2006). For example, in the neonatal mouse brainstem spinal cord and the medullary rhythmic slice preparations blockade of GABAA- and glycine-receptors results in a dramatic reduction in the peak in the I-phase power spectra both for phrenic motoneurons and HMs (Bou-Flores and Berger, 2001; Sebe et al., 2006). This was found to not be due to the increased peak in integrated I-phase activity also seen with blockade of synaptic inhibition, because application of substance P alone, which also increased peak integrated hypoglossal activity, does not significantly affect oscillation power (Sebe et al., 2006). Thus, changes in oscillation power are not explained by changes in excitability, but are specific to blockade of synaptic inhibition. Consistent with this hypothesis is the observation that manipulation of the time course of GABAergic synaptic transmission can alter the median frequency of the power spectrum (Sebe et al., 2006). Further evidence for the role of GABAergic and glycinergic synaptic transmission comes from the observation that the time course of inhibitory synaptic transmission speeds up with postnatal development (see above), thus the speeding up of the inhibitory synaptic transmission time course with postnatal development may contribute to the observed postnatal developmental increase in HM synchronous oscillation frequency (Sebe et al., 2006).
Whether or not these I-phase synchronous oscillations are generated within or upstream of the inspiratory motor pool is an important question. Recent data showed that in the neonatal mouse rhythmic slice preparation that these inhibitory-synaptic transmission dependent synchronous oscillations are likely generated immediately upstream of HMs (Sebe and Berger, 2008). Additionally, in the adult rat in vivo it was recently shown that local injection of antagonists of GABAA- and glycine-receptors directly into the phrenic motor nucleus resulted in a marked reduction in I-phase phrenic motorneuronal synchrony (Marchenko and Rogers, 2009). Thus in both HMs in vitro and phrenic motoneurons in vivo local blockade of inhibitory synaptic transmission in or very close to the respective motor nuclei results in I-phase motor discharge becoming asynchronous or disorganized (Marchenko and Rogers, 2009; Sebe and Berger, 2008), this shows that local inhibitory synaptic transmission has a key role in the generation of motoneuronal I-phase synchrony.
5. Sources of synaptic inputs to hypoglossal motoneurons
An important question is where are the premotor neurons located that supply both excitatory and inhibitory monosynaptic inputs to HMs. Premotor neurons located in the medullary and pontine reticular formation constitute the greatest aggregation of hypoglossal premotor cells (Chamberlin et al., 2007; Dobbins and Feldman, 1995; Peever et al., 2002; Rekling et al., 2000). Additionally premotor neurons are located in the spinal trigeminal nucleus (Borke et al., 1983). These premotor cells have been shown to be GABAergic, glycinergic and glutamatergic (Li et al., 1997; Travers et al., 2005). It is likely that some of these neurons are involved in processing sensory information from the periphery and then project monosynaptically to HMs. For example, it has been shown that electrical stimulation of either the inferior alveolar nerve or the masseter nerve, both components of the trigeminal mandibular nerve and containing afferent fibers from oral structures, results in complex post-synaptic potentials in HMs (Sumino and Nakamura, 1974). These included various sequences of excitatory and inhibitory postsynaptic potentials. Other studies have shown that electrical stimulation of the lingual nerve (also a branch of the trigeminal) evokes at short latency complex depolarizing and hyperpolarizing synaptic potentials in HMs (Porter, 1967). These results are consistent with a disynaptic pathway between oral afferents, medullary interneurons and HMs. Thus HMs receive monosynaptic EPSPs (glutamatergic) and IPSPs (glycinergic and GABAergic) from medullary interneurons that are excited by oral sensory afferent fibers.
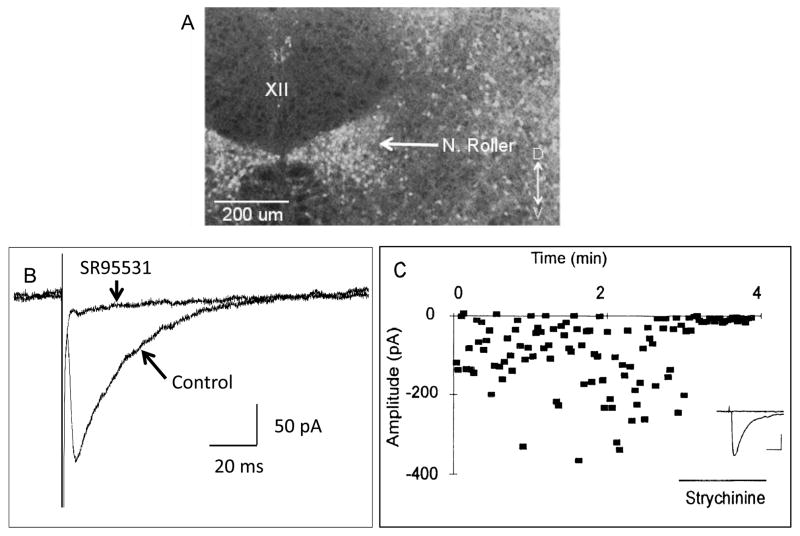
An important source of inhibitory inputs to the XII nucleus is located just ventral and ventrolateral to the XII nucleus, in a collection of inhibitory neurons called the Nucleus of Roller (Fig. 6A) (Aldes et al., 1988; van Brederode et al., 2011). For example electrical stimulation of the Nucleus of Roller produces both glycinergic and GABAergic evoked IPSCs in HMs (Umemiya and Berger, 1995; van Brederode et al., 2011) (Fig. 6B and C). Sumino and Nakamura in 1974 provided compelling evidence that interneurons in the Nucleus of Roller monosynaptically inhibited HMs and these interneurons were a part of the trigemino-hypoglossal reflex.
Fig. 6.
The Nucleus of Roller located just ventrolateral to the hypoglossal motor nucleus contains GABAergic and glycinergic neurons that monosynaptically inhibit HMs. (A) A confocal fluorescent photomicrograph of a transverse section of the medulla from a GAD67-GFP knock-in mouse showing the high density of GABAergic cells in the Nucleus of Roller, but an absence of GFP containing cells within the hypoglossal motor nucleus (XII). (B) Local electrical stimulation of the Nucleus of Roller produces GABAA receptor-mediated currents in a HM. Data shown are average traces of GABAA receptor-mediated evoked IPSCs before and during bath application of SR95531 (also known as gabazine) to block GABAA receptors. Application of SR95531 almost completely blocked the evoked response. (C) Local electrical stimulation of the Nucleus of Roller also can produce glycine-receptor-mediated currents in a HM. Data points are the amplitude of single evoked IPSCs plotted against time. Strychnine applied during the time indicated almost completely abolished the evoked glycinergic IPSCs. Inset: Shows average traces before and during application of strychnine. Calibration bars indicate 50 pA and 20 ms. (A and B adapted from van Brederode et al., 2011; C adapted from Umemiya and Berger, 1995).
Regarding inhibitory inputs studies based on immunohistochemistry have shown that the medullary reticular formation contain inhibitory neurons, both glycinergic and GABAergic, that bilaterally project to the hypoglossal nuclei (Li et al., 1997; Travers et al., 2005). Recent studies (van Brederode et al., 2011) using GAD67-GFP knock-in mice have shown that there is almost a complete absence of GABAergic neurons within the XII motor nucleus (Fig. 6A). The paucity of local inhibitory interneurons within the XII motor nucleus is compatible with the anatomical finding that axons of HM do not have collaterals (Laursen and Rekling, 1989; Withington-Wray et al., 1988). This suggests that, in contrast to spinal motoneurons, there is an absence of local feedback inhibition in HMs.
Early studies have shown that GABAergic synaptic terminals were located on both dendrites and to a lesser extent somata of HMs (Takasu et al., 1987). Other studies have shown that GABAergic innervation is not uniform across the XII motor nucleus, and this depends on the postnatal age of the animal. GABAergic innervation has been found to be densest in the ventral part of the nucleus that contains the somata of genioglossus motoneurons (Muller et al., 2004; O’Brien and Berger, 2001).
Although the tongue has a diversity of motor functions, Ono et al. (1994) and Peever et al. (2002) were able to find in the medullary reticular formation neurons that fired with inspiration and some of these glutamatergic neurons appeared to monosynaptically excite HMs.
6. Conclusions
Inhibitory and excitatory synaptic transmission to respiratory motoneurons as well as the interactions of these on a given motoneuron can result in significant effects on the output of these motoneurons to the target respiratory muscle. During the postnatal period there occur changes in synaptic inputs to motoneurons. As discussed these include but are not limited to the relative role of non-NMDA versus NMDA receptor-mediated respiratory related excitatory drive; the switch from depolarizing to hyperpolarizing GABAergic and glycinergic synaptic inputs; co-release of GABA and glycine from single synaptic vesicles and the developmentally dependent difference in the kinetics of these synaptic inputs; and the role of inhibition in contributing to the generation and developmentally dependent frequency of the synchronous activity that is seen during inspiration in all inspiratory motoneurons. Thus developmentally determined changes in various ionotropic synaptic inputs to respiratory motoneurons can have profound effects on their pattern of spike-firing and thereby ultimately on respiratory muscle contraction.
Acknowledgments
This work was supported by NIH grant HL-49657.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldes LD, Chronister RB, Marco LA. Distribution of glutamic acid decarboxylase and gamma-aminobutyric acid in the hypoglossal nucleus in the rat. J Neurosci Res. 1988;19:343–348. doi: 10.1002/jnr.490190309. [DOI] [PubMed] [Google Scholar]
- Altmann H, ten Bruggencate G, Sonnhof U. Differential strength of action of glycine and GABA in hypoglossus nucleus. Pflugers Arch. 1972;331:90–94. doi: 10.1007/BF00587194. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Bao X, Miselis RR. Dendritic architecture of hypoglossal motoneurons projecting to extrinsic tongue musculature in the rat. J Comp Neurol. 1994;342:538–550. doi: 10.1002/cne.903420404. [DOI] [PubMed] [Google Scholar]
- Baker SN, Kliner JN, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res. 1999;128:109–117. doi: 10.1007/s002210050825. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Dieudonne S, Ascher P. Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J Neurophysiol. 1998;80:3336–3340. doi: 10.1152/jn.1998.80.6.3336. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Isaacson JS. Modulation of motoneuron N-methyl-D-aspartate receptors by the inhibitory neurotransmitter glycine. J Physiol(Paris) 1999;93:23–27. doi: 10.1016/s0928-4257(99)80133-8. [DOI] [PubMed] [Google Scholar]
- Bonin RP, Martin LJ, MacDonald JF, Orser BA. α5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons. J Neurophysiol. 2007;98:2244–2254. doi: 10.1152/jn.00482.2007. [DOI] [PubMed] [Google Scholar]
- Borke RC, Nau ME, Ringler RL. Brain stem afferents of hypoglossal neurons in the rat. Brain Res. 1983;269:47–55. doi: 10.1016/0006-8993(83)90961-7. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Berger AJ. Gap junctions and inhibitory synapses modulate inspiratory motoneuron synchronization. J Neurophysiol. 2001;85:1543–1551. doi: 10.1152/jn.2001.85.4.1543. [DOI] [PubMed] [Google Scholar]
- Bright DP, Aller MI, Brickley SG. Synaptic release generates a tonic GABAA receptor-mediated conductance that modulates burst precision in thalamic relay neurons. J Neurosci. 2007;27:2560–2569. doi: 10.1523/JNEUROSCI.5100-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger PM, Hell J, Mehl E, Krasel C, Lottspeich F, Jahn R. GABA and glycine in synaptic vesicles: storage and transport characteristics. Neuron. 1991;7:287–293. doi: 10.1016/0896-6273(91)90267-4. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol(Lond) 2007;579:515–526. doi: 10.1113/jphysiol.2006.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain Res. 1996;715:104–112. doi: 10.1016/0006-8993(95)01565-5. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- Donato R, Nistri A. Relative contribution by GABA or glycine to Cl--mediated synaptic transmission on rat hypoglossal motoneurons in vitro. J Neurophysiol. 2000;84:2715–2724. doi: 10.1152/jn.2000.84.6.2715. [DOI] [PubMed] [Google Scholar]
- Funk GD, Johnson SM, Smith JC, Dong XW, Lai J, Feldman JL. Functional respiratory rhythm generating networks in neonatal mice lacking NMDAR1 gene. J Neurophysiol. 1997;78:1414–1420. doi: 10.1152/jn.1997.78.3.1414. [DOI] [PubMed] [Google Scholar]
- Funk GD, Parkis MA. High frequency oscillations in respiratory networks: functionally significant or phenomenological? Respir Physiol Neurobiol. 2002;131:101–120. doi: 10.1016/s1569-9048(02)00041-1. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Gao BX, Cheng G, Ziskind-Conhaim L. Development of spontaneous synaptic transmission in the rat spinal cord. J Neurophysiol. 1998;79:2277–2287. doi: 10.1152/jn.1998.79.5.2277. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kalb RG. Regulation of motor neuron dendrite growth by NMDA receptor activation. Development. 1994;120:3063–3071. doi: 10.1242/dev.120.11.3063. [DOI] [PubMed] [Google Scholar]
- Laursen AM, Rekling JC. Electrophysiological properties of hypoglossal motoneurons of guinea-pigs studied in vitro. Neuroscience. 1989;30:619–637. doi: 10.1016/0306-4522(89)90156-5. [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Kaneko T, Mizuno N. Distribution of GABAergic and glycinergic premotor neurons projecting to the facial and hypoglossal nuclei in the rat. J Comp Neurol. 1997;378:283–294. doi: 10.1002/(sici)1096-9861(19970210)378:2<283::aid-cne10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Lim R, Hoang P, Berger AJ. Blockade of glycine transporter-1 (GLYT-1) potentiates NMDA receptor-mediated synaptic transmission in hypoglossal motorneurons. J Neurophysiol. 2004;92:2530–2537. doi: 10.1152/jn.01123.2003. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–309. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Marchenko V, Rogers RF. GABAAergic and glycinergic inhibition in the phrenic nucleus organizes and couples fast oscillations in motor output. J Neurophysiol. 2009;101:2134–2145. doi: 10.1152/jn.91030.2008. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Pagnotta S, Donato R, Nistri A. Inhibition of spinal or hypoglossal motoneurons of the newborn rat by glycine or GABA. Eur J Neurosci. 2002;15:975–983. doi: 10.1046/j.1460-9568.2002.01927.x. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Liu X, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol(Lond) 2003a;552:975–991. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABAA receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J Physiol(Lond) 2003b;548:569–583. doi: 10.1113/jphysiol.2002.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller E, Triller A, Legendre P. Glycine receptors and GABAA receptor α1 and γ 2 subunits during the development of mouse hypoglossal nucleus. Eur J Neurosci. 2004;20:3286–3300. doi: 10.1111/j.1460-9568.2004.03785.x. [DOI] [PubMed] [Google Scholar]
- Numata JM, van Brederode H, Berger AJ. 2010 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2010. Tonic GABAA-receptor-mediated current in motoneurons. Online. Program No. 685.9. [Google Scholar]
- Nunez-Abades PA, He F, Barrionuevo G, Cameron WE. Morphology of developing rat genioglossal motoneurons studied in vitro: changes in length, branching pattern, and spatial-distribution of dendrites. J Comp Neurol. 1994;339:401–420. doi: 10.1002/cne.903390308. [DOI] [PubMed] [Google Scholar]
- Nunez-Abades PA, Pattillo JM, Hodgson TM, Cameron WE. Role of synaptic inputs in determining input resistance of developing brain stem motoneurons. J Neurophysiol. 2000;84:2317–2329. doi: 10.1152/jn.2000.84.5.2317. [DOI] [PubMed] [Google Scholar]
- O’Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol. 1999;82:1638–1641. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- O’Brien JA, Berger AJ. The nonuniform distribution of the GABAA receptor α1 subunit influences inhibitory synaptic transmission to motoneurons within a motor nucleus. J Neurosci. 2001;21:8482–8494. doi: 10.1523/JNEUROSCI.21-21-08482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JA, Isaacson JS, Berger AJ. NMDA and non-NMDA receptors are co-localized at excitatory synapses of rat hypoglossal motoneurons. Neurosci Lett. 1997;227:5–8. doi: 10.1016/s0304-3940(97)00293-0. [DOI] [PubMed] [Google Scholar]
- O’Brien JA, Sebe JY, Berger AJ. GABAB modulation of GABAA and glycine receptor-mediated synaptic currents in hypoglossal motoneurons. Respir Physiol Neurobiol. 2004;141:35–45. doi: 10.1016/j.resp.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Ono T, Ishiwata Y, Inaba N, Kuroda T, Nakamura Y. Hypoglossal premotor neurons with rhythmical inspiratory-related activity in the cat: localization and projection to the phrenic nucleus. Exp Brain Res. 1994;98:1–12. doi: 10.1007/BF00229103. [DOI] [PubMed] [Google Scholar]
- Ornung G, Ottersen OP, Cullheim S, Ulfhake B. Distribution of glutamate-, glycine- and GABA-immunoreactive nerve terminals on dendrites in the cat spinal motor nucleus. Exp Brain Res. 1998;118:517–532. doi: 10.1007/s002210050308. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Dong XW, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: Phase-specific control of excitability. J Neurosci. 1999;19:2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkis MA, Feldman JL, Robinson DM, Funk GD. Oscillations in endogenous inputs to neurons affect excitability and signal processing. J Neurosci. 2003;23:8152–8158. doi: 10.1523/JNEUROSCI.23-22-08152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience. 2002;110:711–722. doi: 10.1016/s0306-4522(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Porter R. The synaptic basis of a bilateral lingual-hypoglossal reflex in cats. J Physiol(Lond) 1967;190:611–627. doi: 10.1113/jphysiol.1967.sp008231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao HW, Jean A, Kessler JP. Postnatal changes in glutamate binding in the lower medulla of the rat. Neurosci Lett. 1995;188:21–24. doi: 10.1016/0304-3940(95)11383-8. [DOI] [PubMed] [Google Scholar]
- Rekling JC. Interaction between thyrotropin-releasing hormone (TRH) and NMDA-receptor-mediated responses in hypoglossal motoneurones. Brain Res. 1992;578:289–296. doi: 10.1016/0006-8993(92)90260-g. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic central of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Greer JJ. Modulation of respiratory rhythmogenesis by chloride-mediated conductances during the perinatal period. J Neurosci. 2006;26:3721–3730. doi: 10.1523/JNEUROSCI.0026-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Mustapic S, Zuperku EJ, Stucke AG, Hopp FA, Stuth EAE. Role of inhibitory neurotransmission in the control of canine hypoglossal motoneuron activity in vivo. J Neurophysiol. 2009;101:1211–1221. doi: 10.1152/jn.90279.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saywell SA, Feldman JL. Dynamic interactions of excitatory and inhibitory inputs in hypoglossal motoneurones: respiratory phasing and modulation by PKA. J Physiol(Lond) 2004;554:879–889. doi: 10.1113/jphysiol.2003.054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebe JY, Berger AJ. Inspiratory-phase short time scale synchrony in the brainstem slice is generated downstream of the pre-Bötzinger complex. Neuroscience. 2008;153:1390–1401. doi: 10.1016/j.neuroscience.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebe JY, Van Brederode JF, Berger AJ. Inhibitory synaptic transmission governs inspiratory motoneuron synchronization. J Neurophysiol. 2006;96:391–403. doi: 10.1152/jn.00086.2006. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nature Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Sharifullina E, Ostroumov K, Grandolfo M, Nistri A. N-methyl-D-aspartate triggers neonatal rat hypoglossal motoneurons in vitro to express rhythmic bursting with unusual Mg2+ sensitivity. Neurosci. 2008;154:804–820. doi: 10.1016/j.neuroscience.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Singer JH, Berger AJ. Contribution of single-channel properties to the time course and amplitude variance of quantal glycine currents recorded in rat motoneurons. J Neurophysiol. 1999;81:1608–1616. doi: 10.1152/jn.1999.81.4.1608. [DOI] [PubMed] [Google Scholar]
- Singer JH, Berger AJ. Development of inhibitory synaptic transmission to motoneurons. Brain Res Bull. 2000;53:553–560. doi: 10.1016/s0361-9230(00)00389-0. [DOI] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J Neurophysiol. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Sokoloff AJ, Deacon TW. Musculotopic organization of the hypoglossal nucleus in the cynomolgus monkey, Macaca fascicularis. J Comp Neurol. 1992;324:81–93. doi: 10.1002/cne.903240107. [DOI] [PubMed] [Google Scholar]
- Steenland HW, Liu H, Horner RL. Endogenous glutamatergic control of rhythmically active mammalian respiratory motoneurons in vivo. J Neurosci. 2008;28:6826–6835. doi: 10.1523/JNEUROSCI.1019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland HW, Liu H, Sood S, Liu X, Horner RL. Respiratory activation of the genioglossus muscle Involves both non-NMDA and NMDA glutamate receptors at the hypoglossal motor nucleus in vivo. Neuroscience. 2006;138:1407–1424. doi: 10.1016/j.neuroscience.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumino R, Nakamura Y. Synaptic potentials of hypoglossal motoneurons and a common inhibitory interneuron in trigemino-hypoglossal reflex. Brain Res. 1974;73:439–454. doi: 10.1016/0006-8993(74)90668-4. [DOI] [PubMed] [Google Scholar]
- Takasu N, Nakatani T, Arikuni T, Kimura H. Immunocytochemical localization of γ-aminobutyric acid in the hypoglossal nucleus of the macaque monkey, Macaca fuscata: a light and electron microscopic study. J Comp Neurol. 1987;263:42–53. doi: 10.1002/cne.902630104. [DOI] [PubMed] [Google Scholar]
- Takata M, Ogata K. Two components of inhibitory postsynaptic potentials evoked in hypoglossal motoneurons by lingual nerve stimulation. Exp Neurol. 1980;69:299–310. doi: 10.1016/0014-4886(80)90213-7. [DOI] [PubMed] [Google Scholar]
- Tarras-Wahlberg S, Rekling JC. Hypoglossal motoneurons in newborn mice receive respiratory drive from both sides of the medulla. Neuroscience. 2009;161:259–268. doi: 10.1016/j.neuroscience.2009.02.064. [DOI] [PubMed] [Google Scholar]
- Travers JB, Yoo JE, Chandran R, Herman K, Travers SP. Neurotransmitter phenotypes of intermediate zone reticular formation projections to the motor trigeminal and hypoglossal nuclei in the rat. J Comp Neurol. 2005;488:28–47. doi: 10.1002/cne.20604. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, Mcbain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Properties and function of low- and high-voltage-activated Ca2+ channels in hypoglossal motoneurons. J Neurosci. 1994;14:5652–5660. doi: 10.1523/JNEUROSCI.14-09-05652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Presynaptic inhibition by serotonin of glycinergic inhibitory synaptic currents in the rat brain stem. J Neurophysiol. 1995;73:1192–1200. doi: 10.1152/jn.1995.73.3.1192. [DOI] [PubMed] [Google Scholar]
- van Brederode JFM, Yanagawa Y, Berger AJ. GAD67-GFP+ neurons in the Nucleus of Roller: a possible source of inhibitory input to hypoglossal motoneurons. I Morphology and firing properties. J Neurophysiol. 2011;105:235–248. doi: 10.1152/jn.00493.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience. 1988;25:1041–1051. doi: 10.1016/0306-4522(88)90057-7. [DOI] [PubMed] [Google Scholar]
- Woch G, Kubin L. Non-reciprocal control of rhythmic activity in respiratory-modulated XII motoneurons. Neuroreport. 1995;6:2085–2088. doi: 10.1097/00001756-199510010-00031. [DOI] [PubMed] [Google Scholar]
- Zafra F, Gomeza J, Olivares L, Aragon C, Gimenez C. Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur J Neurosci. 1995;7:1342–1352. doi: 10.1111/j.1460-9568.1995.tb01125.x. [DOI] [PubMed] [Google Scholar]