Abstract
Developing amphibians need vision to avoid predators and locate food before visual system circuits fully mature. Xenopus tadpoles can respond to visual stimuli as soon as retinal ganglion cells (RGCs) innervate the brain, however, in mammals, chicks and turtles, RGCs reach their central targets many days, or even weeks, before their retinas are capable of vision. In the absence of vision, activity-dependent refinement in these amniote species is mediated by waves of spontaneous activity that periodically spread across the retina, correlating the firing of action potentials in neighboring RGCs. Theory suggests that retinorecipient neurons in the brain use patterned RGC activity to sharpen the retinotopy first established by genetic cues. We find that in both wild type and albino Xenopus tadpoles, RGCs are spontaneously active at all stages of tadpole development studied, but their population activity never coalesces into waves. Even at the earliest stages recorded, visual stimulation dominates over spontaneous activity and can generate patterns of RGC activity similar to the locally correlated spontaneous activity observed in amniotes. In addition, we show that blocking AMPA and NMDA type glutamate receptors significantly decreases spontaneous activity in young Xenopus retina, but that blocking GABAA receptor blockers does not. Our findings indicate that vision drives correlated activity required for topographic map formation. They further suggest that developing retinal circuits in the two major subdivisions of tetrapods, amphibians and amniotes, evolved different strategies to supply appropriately patterned RGC activity to drive visual circuit refinement.
Keywords: retinal waves, spontaneous activity, amphibia, amniotes, topographic maps, retinotectal projection
Introduction
The proper refinement of retinal projections is dependent upon RGC activity. In Xenopus laevis tadpoles, the retina is already capable of responding to light as retinal ganglion cell (RGC) axons begin to innervate their primary central target, the optic tectum (Holt and Harris, 1983). By contrast, RGCs in various amniote species including mammals, chicks, and turtles reach their CNS targets days, or even weeks, before the onset of vision (Wong, 1999; Huberman et al., 2008). In these species, activity-dependent refinement of retinotopic projections is thought to be mediated by waves of spontaneous activity that periodically sweep across the retina, so that action potential firing in correlated among neighboring RGCs (Huberman et al., 2008). To determine whether waves are a ubiquitous feature of immature vertebrate retinas or a specialization found only in species that must refine connectivity without visual experience, the structure of spontaneous activity in developing Xenopus retinas was examined. We tested whether Xenopus RGCs are spontaneously active at early stages of tadpole development and whether waves of spontaneous activity are present in both wild type pigmented and albino tadpoles. We further tested whether visual stimulation might provide correlated patterns of RGC activity similar to the spontaneous activity observed in amniotes. Our findings are relevant to the evolution of developmental strategies to supply appropriately patterned RGC activity to drive visual circuit refinement in both amphibians and amniotes.
Materials and Methods
All experiments were carried our in accordance with Institutional Animal Care and Use Committee standards. Tadpoles are generated from breedings of adults from our colony or purchased from Nasco (Fort Atkinson, WI). Embryos are reared at room temperature (20-22 °C). Dark-reared embryos were placed in the dark prior to stage 28. Partial water changes were performed under infrared illumination with the aid of a night vision scope (Find-R-Scope; FJW Optical Systems Inc.; Palatine, IL).
Retinal Dissections
Retinas were harvested from both wild type and albino Xenopus laevis ranging in age from stage 39-60. At all ages, animals were deeply anesthetized in 0.02% MS-222 and pithed with a 16 gauge needle prior to removal of their eyes. The eyes were placed in a chilled Ringer’s solution containing (in mM) NaCl (100), KCl (2.5), CaCl2 (2), MgCl2 (1), Na2HPO4 (1), NaHCO3 (20), and glucose (10). Under dim light, corneas were removed using a 30 gauge needle as a scalpel and the lens was removed with fine forceps. Except at stage 39-40, eyecups were incubated for 10-15 minutes in Ringer’s solution containing 0.5 mg/ml of hyaluronidase to digest the vitreous. After vitrectomy, eyecups were thoroughly rinsed with Ringer’s solution. To flatten the eyecup, 3-4 cuts were made at the periphery with a 30 gauge needle and the preparation was placed ganglion cell side down on a planar multielectrode array (MultiChannel Systems; Tubingen, Germany). The preparation was held in place over the array with a piece of tissue culture membrane (Corning Inc. Life Sciences; Acton, MA) and a platinum ring. The array was placed on the amplifier within a completely darkened light-tight faraday cage for a minimum of one hour prior to recording to allow the retina to fully dark adapt and to permit the RGC waveforms to stabilize. The preparation was continuously superfused with room temperature (20-22 °C) Ringer’s bubbled with 95% O2/5% CO2. Drugs were added to the Ringers to the final concentration stated. All chemical reagents were obtained from Sigma (St. Louis, MO).
Multielectrode Array Recordings
Data were acquired continuously on all 60 channels at 10 kHz. Spontaneous activity was recorded in complete darkness for 10-20 min, followed by recordings of light responses. As previously described (Kerschensteiner et al., 2008), visual stimuli, including full-field flashes, Gaussian white noise checkerboards and drifting bars were generated using the Cogent Graphics toolbox and Matlab (Mathworks Inc.; Natick, MA). Stimuli were presented by a white OLED microdisplay (eMagin Corp.; Bellevue, WA) projected onto the retina from below with a 20× 0.4 NA air objective. The irradiance at the retina was measured with a calibrated radiometer (International Light; Peabody, MA), and ranged from 30 mW/m2 to 5900 mW/m2 when the display was on. The mean irradiance during full-field flashes and Gaussian white noise checkerboard stimuli as well as the background irradiance during the drifting bar stimuli was 3000 mW/m2.
Spike Sorting and Data Analysis
Spikes were sorted into single units as described previously (Demas et al., 2003). At stage 59-62, and to a lesser extent at stage 46, single units were often detected on many electrodes. In these cases, we estimated their position as the vector average of the amplitude of the first, negative-going phase of the average waveform detected at each electrode where somatic spikes were detected (Meister et al., 1994). The correlation index between a pair of cells was computed as before (Wong et al., 1993). Briefly, we counted the number of spikes in the first train that fell within a time window, Dt, of each spike in the other train, and then normalized by the number of spikes predicted by a Poisson distribution parameterized by the mean firing rate of the first train. To estimate the intercellular distance we assigned each single unit the location of the electrode on which its waveform had the largest amplitude (Figure 1C). A comparison of intercellular distance computed this way to distances computed between receptive field centers (Demas and Cline, unpublished data) yielded comparable results, validating our approach. Least-squares exponential fits of correlation index versus estimated intercellular distance were then calculated. To test whether spike trains were Poisson, we calculated the Fano factor. The Fano factor was computed as the variance to mean ratio of spikes counted in 1s bins. For a homogeneous Poisson process, the Fano factor is 1.
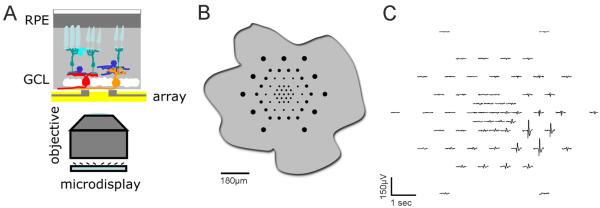
Figure 1. Microelectrode array recordings from Xenopus retina.
(A) Cartoon of the experimental setup. Retinal explants are placed ganglion cell down onto planar multielectrode arrays, which in turn are positioned above a 20× air objective through which visual stimuli can be presented from a microdisplay. (B) Relative size of the multielectrode array compared to an outline of a flattened retinal eyecup from a stage 43 tadpole. (C) Example of the waveforms recorded from a single unit from a stage 46 retina across the 60 electrodes of the array. Single units are often detected by many electrodes.
Results
Xenopus is an important model system for studying visual system development, however, the spatiotemporal pattern of activity in the tadpole retina has not been studied. To assess the spatiotemporal pattern of activity across the retina, it is necessary to monitor the activity of many RGCs at the same time. Planar multielectrode array recordings of wholemount retinas can detect action potentials from tens of RGCs simultaneously. The development of arrays with more densely packed electrodes (HexaMEA and HD-MEA; MultiChannel Systems, Tubingen, Germany) has made it possible to apply this technique to much smaller retinas, such as those of Xenopus tadpoles.
We used multielectrode arrays to record spikes from many RGCs simultaneously from Xenopus retinal explants. Dark adapted eyecup preparations were flattened ganglion cell side down onto a multielectrode array. Spontaneous activity was recorded in complete darkness. Patterned visual stimuli, generated by a microdisplay, were projected onto the flattened eyecup retinal preparation with a 20× air objection (Figure 1). We studied spontaneous activity during three distinct periods of visual system development. At stages 39-40 (2.25-2.75 dpf), innervation of the optic tectum begins (Holt and Harris, 1983). By stage 46 (4 dpf), retinotectal synaptic plasticity has become input specific (Tao et al., 2001) and activity-dependent modifications of this circuitry are widespread (Pratt and Aizenman, 2007; Dong et al., 2009). Finally, stages 59-62 (45-49 dpf) are around metamorphic climax when the retina is thought to be largely mature.Both albino and wild type (pigmented) Xenopus tadpoles are used widely to study visual system development and function. We compared spontaneous activity in retinas at several stages between wild type and albino animals.
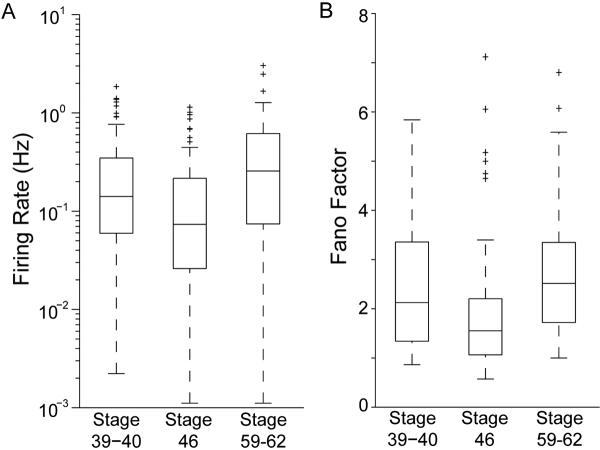
Raster plots of representative spike trains (Figure 2) and movies of the spatiotemporal pattern of activity (Supplemental Movies 1 and 2) from retinas at all of the ages studied show that the level and structure of spontaneous activity was highly variable from cell to cell in both albino and wild type retinas. The qualitative impression of heterogeneity in spike train temporal properties is demonstrated quantitatively by examining the distribution of firing rates at each age (Figure 3A). The median firing rate is not significantly different between different stages (stage 39-40: median firing rate = 0.141 Hz, n=7 retinas, n=93 cells; stage 46: median firing rate = 0.073 Hz, n=8 retinas, n=119 cells, stage 59-62: median firing rate = 0.256 Hz, n=5 retinas, n=52 cells). Xenopus RGC spike trains are extremely heterogeneous. To address this quantitatively, we computed the Fano factor for spike counts in 1s windows. For Poisson spike trains, the Fano factor is 1, regardless of the time window over which it is counted. At all ages, the median Fano factor for Xenopus RGC spike trains was less than three (Figure 3B: stage 39-40: median fano factor = 2.13; stage 46: median fano factor = 1.55; stage 59-62: median fano factor = 2.51) and increased slightly, but significantly (p<0.05; Mann-Whitney U test), between stage 46 and stage 60.
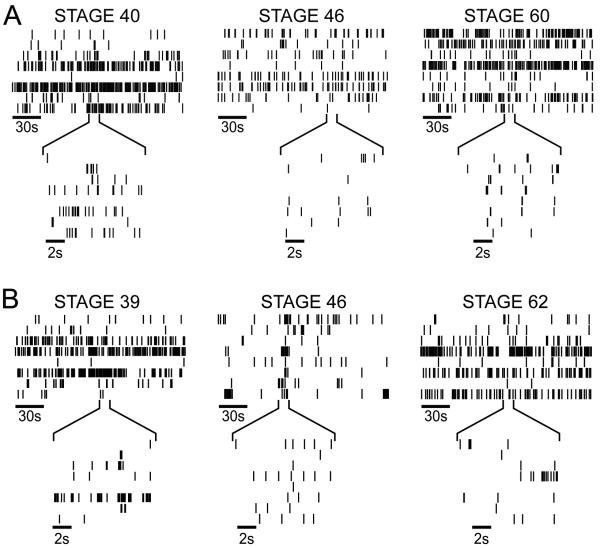
Figure 2. Spontaneous activity in Xenopus retinas.
Raster plots depicting RGC activity from albino (A) and pigmented wild type (B) Xenopus retinas. At each of three developmental stages indicated, 8 representative RGC spike trains that were recorded simultaneously are shown aligned in time. Insets expand 10s of activity. Activity patterns are comparable in albino and stage-matched wild type tadpoles. Stage 39/40: n=5 albino retinas, n=3 wild type retinas; Stage 46: n=6 albino retinas, n=3 wild type retinas; Stages 59-62: n=2 albino retinas, n=3 wild type retinas.
Figure 3. Distributions of temporal spike train properties.
(A) Whisker and box plots of the firing rate distributions across stages of tadpole development. Data from wildtype and albinos tadpoles are combined. Each box spans the inter-quartile range of RGC firing rates at the designated stages. The central bar indicates the median firing rate. The whiskers indicate the full range of the data excluding outliers, which are marked as crosses. Outliers are defined as any RGC whose firing rate is more than 1.5 times the inter-quartile range above or below the 75th and 25th percentile respectively. (B) Whisker and box plots of Fano factors. Fano factors for Xenopus RGC spike trains are relatively low, and are comparable to Fana factors determined from recordings of mammalian retinas after waves have disappeared.
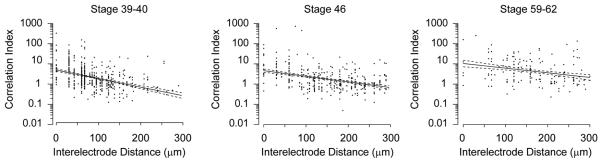
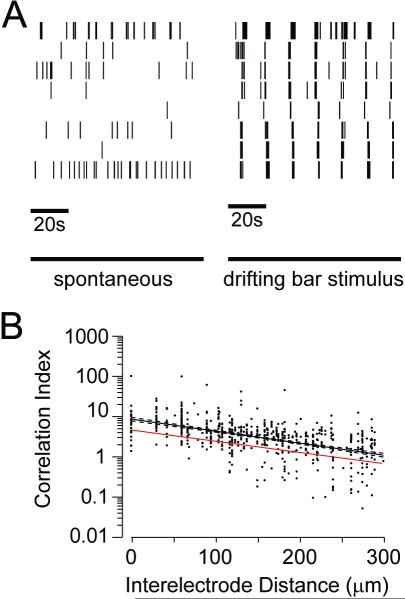
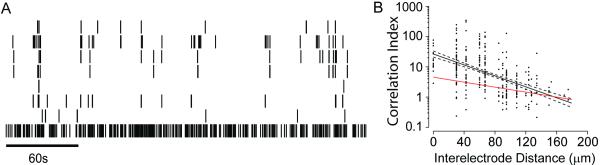
Waves are thought to be important for the retinotopic refinement of visual circuits because the degree to which activity is correlated in any pair of RGCs depends on the distance between the cells; however, the lack of waves in Xenopus retinas does not rule out the possibility that correlations in spontaneous activity in the tadpole retina change systematically with the distance between cell bodies. In other words, information about retinotopy could still be conveyed to target structures by correlations within the apparently random activity pattern of Xenopus RGCs. To examine this possibility we computed the correlation index, the ratio of how often a pair of RGCs fires within 50 ms of one another to how often they would fire together by chance if their activity were Poisson spike trains parameterized by their mean firing rate. A correlation index of 5 would mean that the pair of cells is 5 times more likely than chance to fire within 50ms of one another. A time window of 50 ms was chosen for comparison with results from other species (Lee et al., 2002; Demas et al., 2003). These correlation indices are then plotted as a function of the distance separating the RGCs (Figure 4). At all ages, correlations between nearby RGCs are low when compared to identical plots from mice and ferrets at ages when waves are present (Wong et al., 1993; Demas et al., 2003), and do not show large decreases as distances between RGCs increase. Rather, the y-intercepts of the least squares fits are comparable to those in mice following the loss of waves (Demas et al., 2003).
Figure 4. Lack of Correlations in Spontaneous Activity.
The correlation index for RGC pairs is plotted as a function of interelectrode distance, an estimate of the distance separating the cells. Note that the correlation index is plotted on a logarithmic scale. Data are pooled for albino and wildtype retinas. Even nearby cells are not much more likely than chance to fire within 50 ms of one another. Solid lines indicate the least squares best fit of an exponential to the data. Dashed lines indicate the 95% confidence intervals for the fits.
Xenopus are capable of vision throughout the development of retinotectal circuitry. Here we show that retinas from wild type and albino animals are already capable of responding to light at stage 39-40 (Figure 5). We assessed the role of visually evoked activity in instructing the development of visual circuitry. In natural scene statistics, points in visual space are correlated in a distance-dependent manner. If RGC activity during vision reflects these correlations, they could be used by target structures to decipher neighbor relations amongst RGCs. To test whether visual stimulation can induce robust, distance-dependent correlations we presented a moving bar stimulus to the isolated retinas on the multielectrode arrays. Black drifting bars on a light background were presented, one at a time, moving in one of 8 directions (0 to 315 degree in 45 degree increments) at 200 μm/s with each direction presented 5 times. The stimuli were presented in random order, and roughly 10 minutes were required to present the entire stimulus set. Comparable drifting bar stimuli have been previously shown to induce direction-selective responses in tectal neurons (Engert et al., 2002) and alter tectal receptive fields. Figure 6A shows rasters of spontaneous activity followed by responses to moving bars from 8 cells from a stage 46 retina. The drifting bar elicited light responses from RGCs and activity across the RGCs is correlated on a coarse timescale. It is interesting to note that RGCs are relatively silent after the stimulus, consistent with post-stimulus inhibition. The correlation indices during bar presentation are higher than during spontaneous activity and decay smoothly with increasing intercellular distance (Figure 6B). These results demonstrate that visual stimulation can induce correlated patterns of activity between neighboring RGCs that can be used for retinotopic refinement.
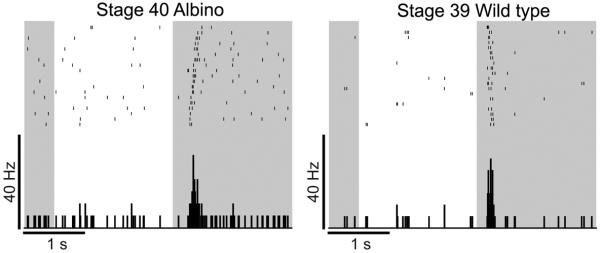
Figure 5. Retinas are light responsive at stages when RGC axons arrive in the optic tectum.
Rasters depict responses from a stage 39 wild type RGC (right) and a stage 40 albino RGC (left) to 20 repeats of a full field stimulus. The average response is shown below the rasters in the peristimulus time histograms (100 ms bins). The darkened background indicates when the full field stimulus was off. RGCs respond only to decrements in illumination and are therefore OFF cells.
Figure 6. Patterned visual stimuli induce RGC correlations.
(A) Raster plots from 8 RGCs in a stage 46 retina in the dark (left) and during the presentation of 7 moving bar stimuli (right). (B) Correlation indices from stage 46 retinas during presentation of moving bar stimuli. Red (grey) line indicates correlation index in the dark, and black line indicate correlation index during the moving bar stimulus. Solid black lines represent the median values for cell pairs binned in 50 μm increments.
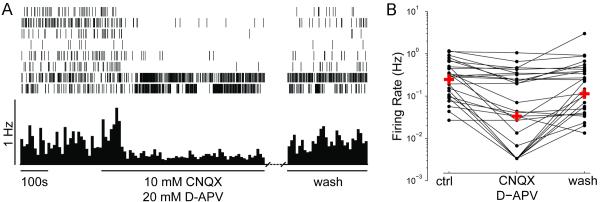
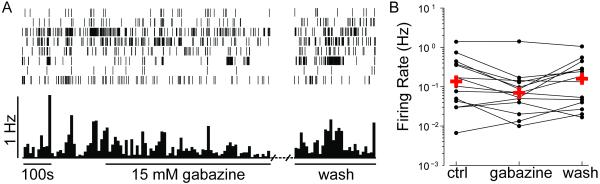
We further tested whether glutamatergic or GABAergic neurotransmission in the retina affects spontaneous firing activity of RGCs. We recorded from retinas in the presence of glutamate receptor blockers, CNQX (10 μM) and D-APV (20μM), to block AMPA- and NMDA-receptor-mediated transmission, respectively. Bath application of glutamate receptor antagonists significantly decreases spontaneous activity in RGCs as early as stage 40 (Figure 7; p<0.001 Wilcoxon Matched Pairs Test). The median firing rate was 0.247 Hz prior to drug application, 0.033 Hz following five minutes of CNQX and D-APV, and recovered to 0.113 Hz after washout of the drugs. As can be seen in the bottom two rasters, the drugs did not block activity in all RGCs (Figure 7A). Roughly 82% of the RGCs decreased their firing in response to the glutamate receptor antagonists, and of these cells more than 78% showed at least partial recovery upon washout (Figure 7B). To test whether GABAA receptor activity affects spontaneous activity, we bath applied gabazine (50μM), a GABAA receptor antagonist. Gabazine does not significantly alter RGC firing rates (Figure 8; p=0.17 Wilcoxon Matched Pairs Test), however, most cells (>71%) actually fired less during gabazine application, and 80% of these increased their activity following washout of GABAzine.
Figure 7. Glutamate drives spontaneous activity.
(A) Rasters of spontaneous activity of 8 RGCs recorded simultaneously from a stage 40 albino retina and their response to the bath application of the ionotropic glutamate receptor antagonists CNQX and D-APV. The mean firing rate (10s bins) of the 17 RGCs recorded from this retina are shown below the raster. (B) Changes in the firing rates of individual RGCs from retinas exposed to CNQX and APV are plotted on a logarithmic scale (n=3 retinas, n=34 RGCs). Crosses indicate the median firing rates for the population before drug treatment (ctrl), during drug treatment (CNQX, D-APV), and after drug washout (wash).
Figure 8. GABA blockers do not affect spontaneous activity.
(A) Spontaneous activity from 8 RGCs recorded simultaneously in a stage 40 albino retina in the presence of the GABAA receptor antagonist gabazine. The mean population firing rate of the 8 RGCs recorded from this retina are shown below the raster (bin=10s). (B) Individual firing rates from RGCs plotted on a logarithmic scale prior to application of gabazine (ctrl), during gabazine application (gabazine), and following washout of gabazine (wash). Crosses indicate the median firing rates for the RGCs recorded under each of these conditions (n=3 retinas, n=14 RGCs).
To test whether visual deprivation affects spontaneous activity in Xenopus retina, larvae were dark-reared (DR) from stage 28, when the first RGCs differentiate (Cima and Grant, 1980), until stage 40, about 34h later, when their axons have arrived at the tectum (Holt and Harris, 1983). Firing rates are significantly lower in dark-reared retinas (Figure 9A: control median=0.19 Hz; DR median=0.08 Hz; p=0.005; Mann-Whitney U test) as are Fano factors (control median=2.35; DR median=1.81; p=0.03; Mann-Whitney U test). Plots of correlation indices show that spontaneous activity is patterned differently in dark-reared retinas; a comparison of the fits indicates that correlations are significantly elevated in dark-reared retinas for RGCs separated by less than 120 mm (Figure 9B). Local bursts of activity are responsible for this increase in correlated activity rather than waves (see Supplemental Movie 4).
Figure 9. Early dark-rearing increases RGC correlations in stage 40 retina.
(A) Rasters depicting spontaneous activity of 8 RGCs from a dark-reared, stage 40 albino retina. (B) The correlation index for dark-reared RGC pairs (n=408 pairs; n=105 RGCs; n=3 retinas). The least squares fit (solid lines) and 95% confidence limits (dashed lines) are shown for the dark-reared stage 40 retinas (black) and stage 39-40 control retinas (red line; from Figure 3).
Discussion
No Waves in Xenopus Retina
We have used multielectrode array recordings to characterize the spontaneous activity in RGCs of Xenopus tadpoles. We find that spontaneous activity in RGCs of all tadpole stages studies is extremely heterogeneous between cells within the same retinas. Some cells show consistently high levels of spontaneous activity, while other cells are relatively inactive. The rhythmic, synchronized bursting that is the hallmark of retinal waves in other species is absent at every age studied. In mammalian retinas, the range of RGC firing rates is relatively small when waves are present, however, even at the earliest stages examined in Xenopus, the inter-quartile range of firing rates is far larger than that found in mice when waves are present (Demas et al., 2003). Instead the distribution of firing rates is comparable to that found in 3 week old mice, after waves have disappeared and the retina is essentially mature. Xenopus RGC spike trains appear stochastic and unpredictable compared to those from species where retinal waves are present, as demonstrated by comparing the Fano factor between species. Fano factors are typically low for Xenopus RGC spike trains, comparable to mammalian retinas after waves have disappeared. By contrast, in P9 mouse retina, prior to the developmental loss of waves, the median Fano factor is high (>15) and decreases with development as the retina becomes visually responsive (Demas et al., 2003). Correlation indices show that activity in neighboring RGCs is not highly correlated at any stage studied, but are similar to those in mice after the loss of waves (Demas et al., 2003).
Visual Inputs Drive Correlated Activity during Retinotectal Map Formation
The multielectrode array recordings allow us to test specifically whether visually driven activity can provide patterned activity thought to be required to refine the retinotopic map. A number of features distinguish spontaneous activity in developing Xenopus retina from that seen in the amniote species examined to date. First, no RGCs exhibit rhythmic bursting; second, activity does not appear to be synchronized between RGCs; and third, activity rates and patterns vary dramatically within a single retina at all stages studied. Furthermore, retina of young tadpoles are visually responsive at stages when RGC axons first innervate the optic tectum, and visually-driven activity results in highly correlated activity patterns between neighboring RGCs. We did not investigate the possibility that spontaneous waves of activity are present in Xenopus retinas prior to the outgrowth of RGC axons at stage 39. Retinal waves have been seen prior to RGC axon outgrowth and retinal synaptogenesis in other species (Syed et al., 2004). Termed stage 1 waves, these early waves have been implicated in regulating neurogenesis within the retina. Regardless, our data indicate that the retinas of young Xenopus do not have spontaneous waves of activity that regulate refinement of their connectivity with central targets. Instead, visually-evoked activity provides cues that organize retinotopic projections.
Modulation of spontaneous RGC activity
Visual experience is required for the development of the retinotectal topographic map (Cline, 1991) as well as the structure and function of retinotectal circuitry (Aizenman et al., 2002; Engert et al., 2002; Sin et al., 2002; Aizenman et al., 2003; Ruthazer et al., 2003; Zhou et al., 2003; Tao and Poo, 2005; Ruthazer et al., 2006; Vislay-Meltzer et al., 2006; Aizenman and Cline, 2007; Cline and Haas, 2008; Li et al., 2011). Although dark-rearing is often used to test the requirement for visual experience during development on aspects of CNS visual system function, no studies have examined the potential role of visual experience on the early development of retinal circuitry in Xenopus. We find that dark-rearing Xenopus from stage 28 to stage 40, a period when RGCs are first differentiating in the retina, results in lower overall firing rates in RGCs than controls, and cells have spontaneous local bursting activity, which is not seen in retinas of normally-reared animals. Although this suggests that visual experience may affect the development of retinal circuitry in Xenopus, as previously reported in other species (Sernagor and Grzywacz, 1996), further studies would be required to address this topic (He et al., 2010). A previous study in Xenopus showed that the retinotopic map develops normally in dark-reared animals (Keating et al., 1986). Our results suggest that local bursting in dark-reared retinas may have compensated for the absence of vision-induced organized activity patterns in this earlier study.
In turtles, which have spontaneous waves of activity in the retina (Sernagor and Grzywacz, 1999), visual deprivation alters spontaneous activity such that patches of co-active RGCs persist after waves have disappeared in age-matched controls. The altered spontaneous activity in visually-deprived turtles appears to result from a delay in the maturation of the chloride gradient, such that GABA is predominately depolarizing in dark-reared turtle retinas and predominately hyperpolarizing in control animals (Sernagor et al., 2003). Although experience could affect the maturation of the chloride gradient in Xenopus retinal cells, as shown in tectal neurons (Akerman and Cline, 2006), our observations with bath application of gabazine, a GABAA receptor antagonist, indicate that GABAA receptor-mediated transmission does not appear to play a predominant role in controlling RGC firing. By contrast, glutamatergic transmission strongly regulates spontaneous RGC activity in Xenopus retina, as in other systems (Wong et al., 2000), where it could affect the development of the RGC dendritic arbor and RGC connectivity.
Evolutionary Perspective on Waves
There are two major subdivisions of tetrapods: amphibians and amniotes. We have shown that retinal waves are absent in the amphibian Xenopus laevis, the first example of a developing vertebrate retina without waves. Waves are present, however, in all amniotes examined to date including species of turtle, birds, and a variety of mammals (Huberman et al., 2008). The presence of retinal waves in this diverse group of amniotes suggests that they were likely a feature of early amniotes, prior to their diversification. Early amniotes were distinguished from their amphibian contemporaries by their capacity to lay eggs on land, which required, amongst other specializations, the evolution of a tough eggshell to protect the embryo and prevent desiccation (Gauthier et al., 1988; Stewart, 1997). This specialization left early amniotes visually-deprived prior to hatching. Amniotes may have evolved retinal waves as a surrogate for the visually-evoked activity that promotes synaptic and structural plasticity in the developing visual circuits of amphibian larvae. Although recordings of spontaneous activity have not been taken from retinas of fish, this hypothesis predicts that fish, which, like Xenopus, have functional retinas at the time when the retinal axons first project to the optic tectum, would not have waves of spontaneous retinal activity.
Supplementary Material
Supplemental Movie 1. Spontaneous activity in a stage 40 retina. The spatiotemporal pattern of spontaneous activity is shown for RGCs from an albino retina. Each spot is the estimated location of an RGC, corresponding to the electrode on which it was recorded. The diameter of the spot corresponds to the firing rate of the RGC. The diameter is set to a minimum diameter when RGCs are inactive in order to visualize the RGCs locations. The firing rates are computed using a sliding window of 100 ms. Movie plays in real time at 25 frames/sec.
Supplemental Movie 2. Spontaneous activity in a stage 46 retina. The spatiotemporal pattern of spontaneous activity in stage 46 albino retina. Unlike the movies of stage 40 activity, the RGCs are located based on their receptive fields. RGCs are positioned at the center of least-squares elliptical fits to the spike triggered average gaussian white noise checkerboard stimulus. The diameter of the spot corresponds to the firing rate of the RGC. The firing rates are computed using a sliding window of 100 ms. Movie plays in real time at 25 frames/sec.
Supplemental Movie 3. Responses to drifting bar stimuli in a stage 46 retina. The same RGCs from Supplemental Movie 2 are shown here responding to a pair of drifting bar stimuli moving in opposite directions. Because RGC positions were determined using the same display that presented the drifting bars, it is possible to accurately depict the responses of the RGCs relative to the position of the drifting bar. Note that the spatial extent of RGC receptive fields is not shown to avoid confusion with changes in firing rates elicited by the stimuli; however, this omission creates the impression that RGCs fire in advance of the bar’s arrival.
Supplemental Movie 4. Spontaneous activity in dark-reared stage 40 retina. Same as Supplemental Movie 1, except spontaneous activity is from a stage 40 dark-reared retina.
Acknowledgements
This work was supported by the National Institutes of Health (ROI EY11261 to HTC and an NRSA EY180662 to JAD). We thank Dr. Rachel Wong for generously allowing us to perform an initial set of experiments in her lab and members of the Cline lab for valuable discussions.
References
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron. 2003;39:831–842. doi: 10.1016/s0896-6273(03)00527-0. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Cline HT. Enhanced visual activity in vivo forms nascent synapses in the developing retinotectal projection. J Neurophysiol. 2007;97:2949–2957. doi: 10.1152/jn.00452.2006. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Munoz-Elias G, Cline HT. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron. 2002;34:623–634. doi: 10.1016/s0896-6273(02)00674-8. [DOI] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci. 2006;26:5117–5130. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima C, Grant P. Ontogeny of the retina and optic nerve of Xenopus laevis. IV. Ultrastructural evidence of early ganglion cell differentiation. Dev Biol. 1980;76:229–237. doi: 10.1016/0012-1606(80)90375-9. [DOI] [PubMed] [Google Scholar]
- Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. J Physiol. 2008;586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT. Activity-dependent plasticity in the visual systems of frogs and fish. Trends Neurosci. 1991;14:104–111. doi: 10.1016/0166-2236(91)90071-2. [DOI] [PubMed] [Google Scholar]
- Demas J, Eglen SJ, Wong RO. Developmental loss of synchronous spontaneous activity in the mouse retina is independent of visual experience. J Neurosci. 2003;23:2851–2860. doi: 10.1523/JNEUROSCI.23-07-02851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Lee RH, Xu H, Yang S, Pratt KG, Cao V, Song YK, Nurmikko A, Aizenman CD. Visual avoidance in Xenopus tadpoles is correlated with the maturation of visual responses in the optic tectum. J Neurophysiol. 2009;101:803–815. doi: 10.1152/jn.90848.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Tao HW, Zhang LI, Poo MM. Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons. Nature. 2002;419:470–475. doi: 10.1038/nature00988. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Kluge AG, Rowe T. The early evolution of the Amniota. In: Benton MJ, editor. The Phylogeny and Classification of the Tetrapods. Volume 1: Amphibians, Reptiles, Birds. Clarendon Press; Oxford: 1988. [Google Scholar]
- He Q, Wang P, Tian N. Light-evoked synaptic activity of retinal ganglion and amacrine cells is regulated in developing mouse retina. Eur J Neurosci. 2010;33:36–48. doi: 10.1111/j.1460-9568.2010.07484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Harris WA. Order in the initial retinotectal map in Xenopus: a new technique for labelling growing nerve fibres. Nature. 1983;301:150–152. doi: 10.1038/301150a0. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating MJ, Grant S, Dawes EA, Nanchahal K. Visual deprivation and the maturation of the retinotectal projection in Xenopus laevis. J Embryol Exp Morphol. 1986;91:101–115. [PubMed] [Google Scholar]
- Kerschensteiner D, Liu H, Cheng CW, Demas J, Cheng SH, Hui CC, Chow RL, Wong RO. Genetic control of circuit function: Vsx1 and Irx5 transcription factors regulate contrast adaptation in the mouse retina. J Neurosci. 2008;28:2342–2352. doi: 10.1523/JNEUROSCI.4784-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Eglen SJ, Wong RO. Segregation of ON and OFF retinogeniculate connectivity directed by patterned spontaneous activity. J Neurophysiol. 2002;88:2311–2321. doi: 10.1152/jn.00372.2002. [DOI] [PubMed] [Google Scholar]
- Li J, Erisir A, Cline HT. In vivo time-lapse imaging and serial section electron microscopy reveal developmental synaptic rearrangements. Neuron. 2011 doi: 10.1016/j.neuron.2010.12.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Pine J, Baylor DA. Multi-neuronal signals from the retina: acquisition and analysis. J Neurosci Methods. 1994;51:95–106. doi: 10.1016/0165-0270(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci. 2007;27:8268–8277. doi: 10.1523/JNEUROSCI.1738-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Akerman CJ, Cline HT. Control of axon branch dynamics by correlated activity in vivo. Science. 2003;301:66–70. doi: 10.1126/science.1082545. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Li J, Cline HT. Stabilization of axon branch dynamics by synaptic maturation. J Neurosci. 2006;26:3594–3603. doi: 10.1523/JNEUROSCI.0069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Grzywacz NM. Influence of spontaneous activity and visual experience on developing retinal receptive fields. Curr Biol. 1996;6:1503–1508. doi: 10.1016/s0960-9822(96)00755-5. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Grzywacz NM. Spontaneous activity in developing turtle retinal ganglion cells: pharmacological studies. J Neurosci. 1999;19:3874–3887. doi: 10.1523/JNEUROSCI.19-10-03874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Young C, Eglen SJ. Developmental modulation of retinal wave dynamics: shedding light on the GABA saga. J Neurosci. 2003;23:7621–7629. doi: 10.1523/JNEUROSCI.23-20-07621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- Stewart JR. Morphology and evolution of the egg of oviparous amniotes. In: Sumida S, Martin K, editors. Amniote Origins-Completing the Transition to Land London. Academic Press; 1997. [Google Scholar]
- Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol. 2004;560:533–549. doi: 10.1113/jphysiol.2004.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao HW, Poo MM. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron. 2005;45:829–836. doi: 10.1016/j.neuron.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Tao HW, Zhang LI, Engert F, Poo M. Emergence of input specificity of ltp during development of retinotectal connections in vivo. Neuron. 2001;31:569–580. doi: 10.1016/s0896-6273(01)00393-2. [DOI] [PubMed] [Google Scholar]
- Vislay-Meltzer RL, Kampff AR, Engert F. Spatiotemporal specificity of neuronal activity directs the modification of receptive fields in the developing retinotectal system. Neuron. 2006;50:101–114. doi: 10.1016/j.neuron.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Wong RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Wong WT, Faulkner-Jones BE, Sanes JR, Wong RO. Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20:5024–5036. doi: 10.1523/JNEUROSCI.20-13-05024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Tao HW, Poo MM. Reversal and stabilization of synaptic modifications in a developing visual system. Science. 2003;300:1953–1957. doi: 10.1126/science.1082212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie 1. Spontaneous activity in a stage 40 retina. The spatiotemporal pattern of spontaneous activity is shown for RGCs from an albino retina. Each spot is the estimated location of an RGC, corresponding to the electrode on which it was recorded. The diameter of the spot corresponds to the firing rate of the RGC. The diameter is set to a minimum diameter when RGCs are inactive in order to visualize the RGCs locations. The firing rates are computed using a sliding window of 100 ms. Movie plays in real time at 25 frames/sec.
Supplemental Movie 2. Spontaneous activity in a stage 46 retina. The spatiotemporal pattern of spontaneous activity in stage 46 albino retina. Unlike the movies of stage 40 activity, the RGCs are located based on their receptive fields. RGCs are positioned at the center of least-squares elliptical fits to the spike triggered average gaussian white noise checkerboard stimulus. The diameter of the spot corresponds to the firing rate of the RGC. The firing rates are computed using a sliding window of 100 ms. Movie plays in real time at 25 frames/sec.
Supplemental Movie 3. Responses to drifting bar stimuli in a stage 46 retina. The same RGCs from Supplemental Movie 2 are shown here responding to a pair of drifting bar stimuli moving in opposite directions. Because RGC positions were determined using the same display that presented the drifting bars, it is possible to accurately depict the responses of the RGCs relative to the position of the drifting bar. Note that the spatial extent of RGC receptive fields is not shown to avoid confusion with changes in firing rates elicited by the stimuli; however, this omission creates the impression that RGCs fire in advance of the bar’s arrival.
Supplemental Movie 4. Spontaneous activity in dark-reared stage 40 retina. Same as Supplemental Movie 1, except spontaneous activity is from a stage 40 dark-reared retina.