Abstract
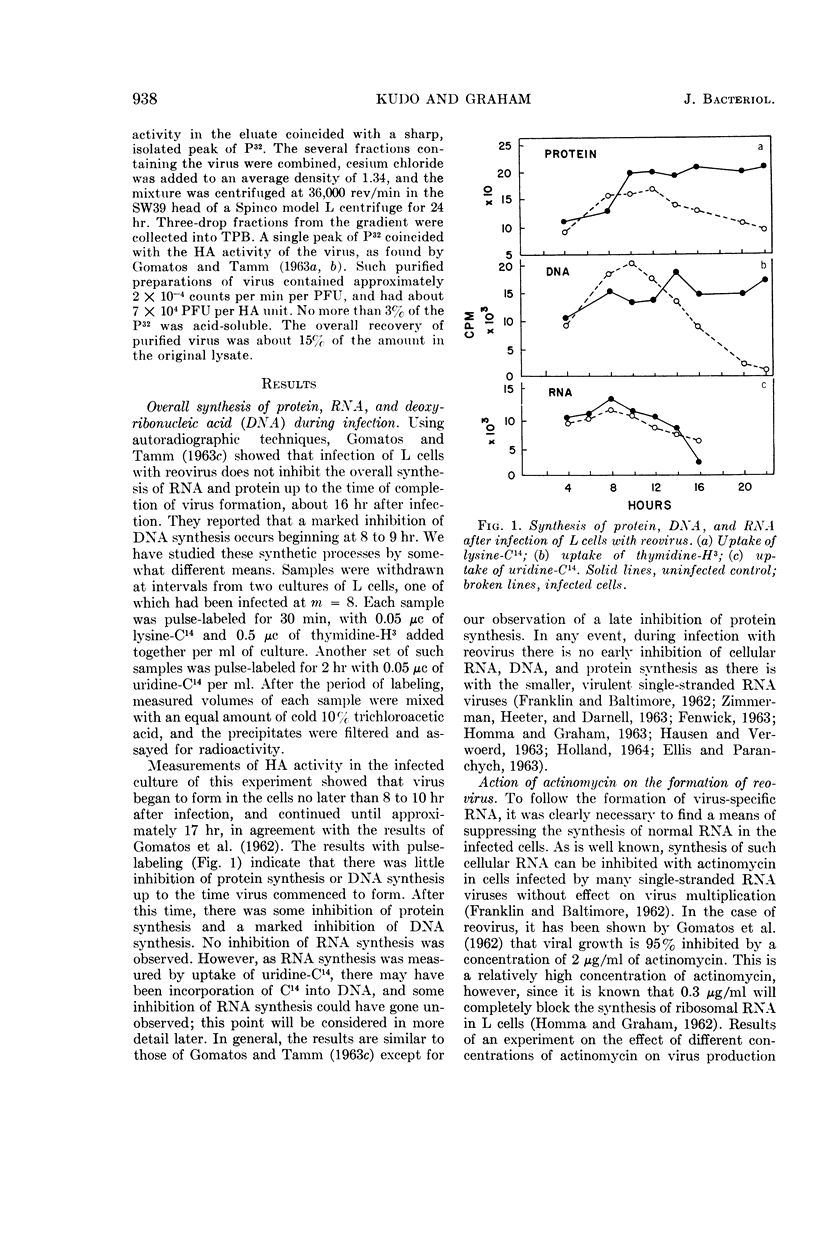
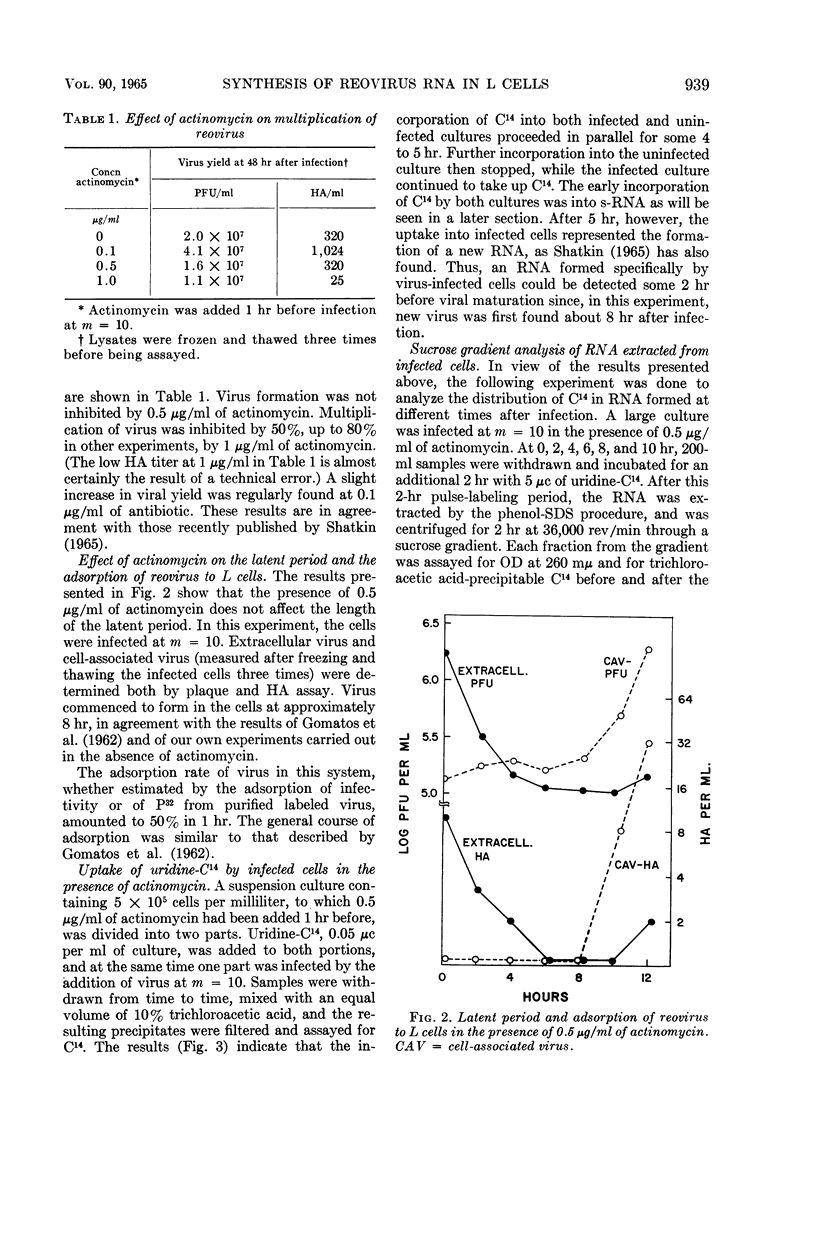
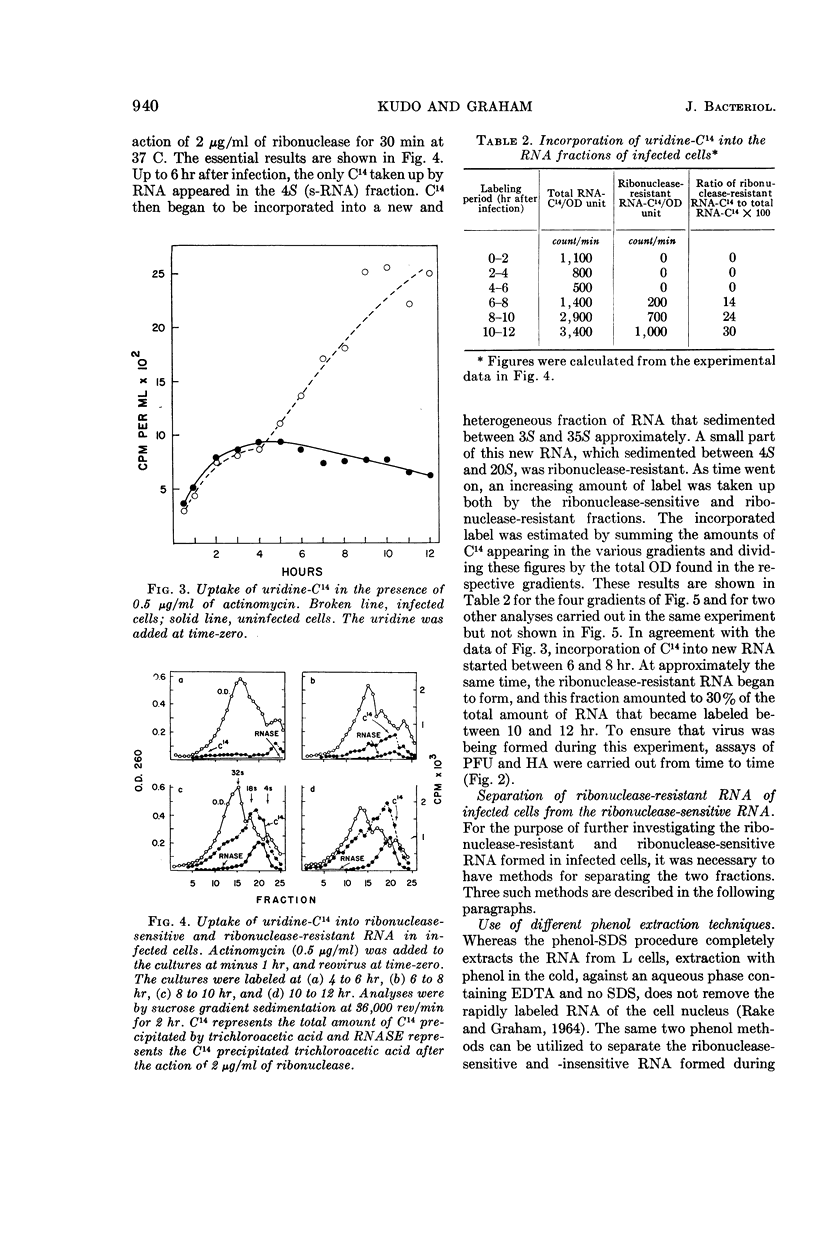
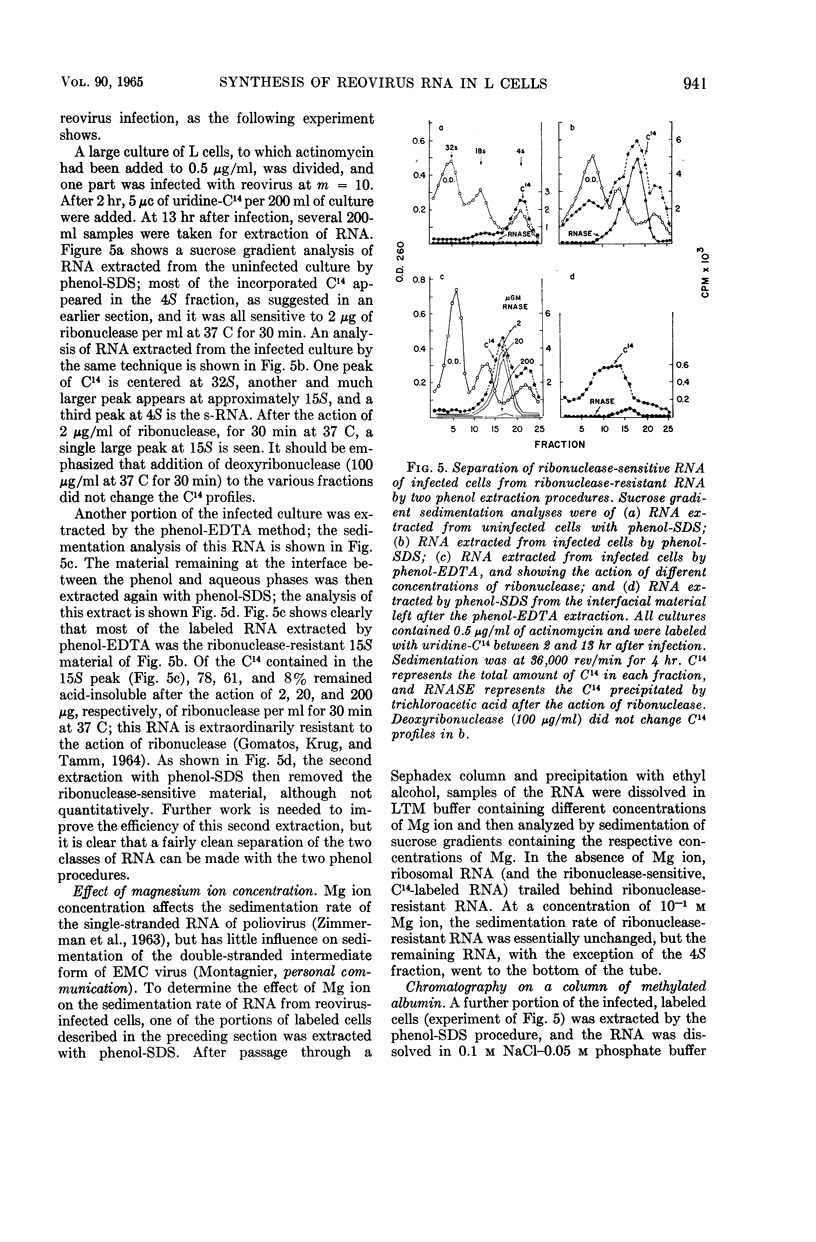
Kudo, Hajime (The Wistar Institute of Anatomy and Biology, Philadelphia, Pa.), and A. F. Graham. Synthesis of reovirus ribonucleic acid in L cells. J. Bacteriol. 90:936–945. 1965.—There is no inhibition of protein or deoxyribonucleic acid (DNA) synthesis in L cells infected with reovirus until the time that new virus starts to form about 8 hr after infection. At this time, both protein synthesis and DNA synthesis commence to be inhibited. Neither the synthesis of ribosomal ribonucleic acid (RNA) nor that of the rapidly labeled RNA of the cell nucleus is inhibited before 10 hr after infection. Actinomycin at a concentration of 0.5 μg/ml does not inhibit the formation of reovirus, although higher concentrations of the antibiotic do so. Pulse-labeling experiments with uridine-C14 carried out in the presence of 0.5 μg/ml of actinomycin show that, at 6 to 8 hr after infection, two species of virus-specific RNA begin to form and increase in quantity as time goes on. One species is sensitive to ribonuclease action and the other is very resistant. The latter RNA is probably double-stranded viral progeny RNA, and it constitutes approximately 40% of the RNA formed up to 16 hr after infection. The function of the ribonuclease-sensitive RNA is not yet known. Synthesis of both species of RNA is inhibited by 5 μg/ml of actinomycin added at early times after infection. Added 6 to 8 hr after infection, when virus-specific RNA has already commenced to form, 5 μg/ml of actinomycin no longer inhibit the formation of either species of RNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALTIMORE D., BECKER Y., DARNELL J. E. VIRUS-SPECIFIC DOUBLE-STRANDED RNA IN POLIOVIRUS-INFECTED CELLS. Science. 1964 Mar 6;143(3610):1034–1036. doi: 10.1126/science.143.3610.1034. [DOI] [PubMed] [Google Scholar]
- BURDON R. H., BILLETER M. A., WEISSMANN C., WARNER R. C., OCHOA S., KNIGHT C. A. REPLICATION OF VIRAL RNA, V. PRESENCE OF A VIRUS-SPECIFIC DOUBLE-STRANDED RNA IN LEAVES INFECTED WITH TOBACCO MOSAIC VIRUS. Proc Natl Acad Sci U S A. 1964 Sep;52:768–775. doi: 10.1073/pnas.52.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EGGERS H. J., GOMATOS P. J., TAMM I. Agglutination of bovine erythrocytes: a general characteristic of reovirus type 3. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:879–881. doi: 10.3181/00379727-110-27679. [DOI] [PubMed] [Google Scholar]
- ELLIS D. B., PARANCHYCH W. SYNTHESIS OF RIBONUCLEIC ACID AND PROTEIN IN BACTERIA INFECTED WITH AN RNA BACTERIOPHAGE. J Cell Physiol. 1963 Oct;62:207–213. doi: 10.1002/jcp.1030620209. [DOI] [PubMed] [Google Scholar]
- ERIKSON R. L., FENWICK M. L., FRANKLIN R. M. REPLICATION OF BACTERIOPHAGE RNA: STUDIES ON THE FATE OF PARENTAL RNA. J Mol Biol. 1964 Dec;10:519–529. doi: 10.1016/s0022-2836(64)80070-x. [DOI] [PubMed] [Google Scholar]
- FENWICK M. L. The influence of poliovirus infection of RNA synthesis in mammalian cells. Virology. 1963 Mar;19:241–249. doi: 10.1016/0042-6822(63)90061-8. [DOI] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., KRUG R. M., TAMM I. ENZYMIC SYNTHESIS OF RNA WITH REOVIRUS RNA AS TEMPLATE. I. CHARACTERISTICS OF THE REACTION CATALYZED BY THE RNA POLYMERASE FROM ESCHERICHIA COLI. J Mol Biol. 1964 Jul;9:193–207. doi: 10.1016/s0022-2836(64)80100-5. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., STOECKENIUS W. ELECTRON MICROSCOPE STUDIES ON REOVIRUS RNA. Proc Natl Acad Sci U S A. 1964 Dec;52:1449–1455. doi: 10.1073/pnas.52.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I. Base composition of the RNA of a reovirus variant. Science. 1963 May 31;140(3570):997–998. doi: 10.1126/science.140.3570.997. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I., DALES S., FRANKLIN R. M. Reovirus type 3: physical characteristics and interaction with L cells. Virology. 1962 Jul;17:441–454. doi: 10.1016/0042-6822(62)90139-3. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I. MACROMOLECULAR SYNTHESIS IN REOVIRUS-INFECTED L CELLS. Biochim Biophys Acta. 1963 Aug 20;72:651–653. [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSEN P., VERWOERD D. W. STUDIES ON THE MULTIPLICATION OF A MEMBER OF THE COLUMBIA SK GROUP (ME VIRUS) IN L CELLS. III. ALTERATION OF RNA AND PROTEIN SYNTHETIC PATTERNS IN VIRUS-INFECTED CELLS. Virology. 1963 Dec;21:617–627. doi: 10.1016/0042-6822(63)90235-6. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. INHIBITION OF HOST CELL MACROMOLECULAR SYNTHESIS BY HIGH MULTIPLICITIES OF POLIOVIRUS UNDER CONDITIONS PREVENTING VIRUS SYNTHESIS. J Mol Biol. 1964 Apr;8:574–581. doi: 10.1016/s0022-2836(64)80012-7. [DOI] [PubMed] [Google Scholar]
- HOMMA M., GRAHAM A. F. SYNTHESIS OF RNA IN L CELLS INFECTED WITH MENGO VIRUS. J Cell Physiol. 1963 Oct;62:179–192. doi: 10.1002/jcp.1030620207. [DOI] [PubMed] [Google Scholar]
- KELLY R. B., SINSHEIMER R. L. A NEW RNA COMPONENT IN MS2-INFECTED CELLS. J Mol Biol. 1964 Apr;8:602–605. doi: 10.1016/s0022-2836(64)80015-2. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., DUNNEBACKE T. H., SPENDLOVE R. S., SCHAFFER F. L., WHITCOMB R. F. ELECTRON MICROSCOPY OF RNA FROM REOVIRUS AND WOUND TUMOR VIRUS. J Mol Biol. 1964 Nov;10:282–288. doi: 10.1016/s0022-2836(64)80046-2. [DOI] [PubMed] [Google Scholar]
- LANGRIDGE R., BILLETER M. A., BORST P., BURDON R. H., WEISSMANN C. THE REPLICATIVE FORM OF MS2 RNA: AN X-RAY DIFFRACTION STUDY. Proc Natl Acad Sci U S A. 1964 Jul;52:114–119. doi: 10.1073/pnas.52.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGRIDGE R., GOMATOS P. J. The structure of RNA. Reovirus RNA and transfer RNA have similar three-dimensional structures, which differ from DNA. Science. 1963 Aug 23;141(3582):694–698. doi: 10.1126/science.141.3582.694. [DOI] [PubMed] [Google Scholar]
- MONTAGNIER L., SANDERS F. K. REPLICATIVE FORM OF ENCEPHALOMYOCARDITIS VIRUS RIBONUCLEIC ACID. Nature. 1963 Aug 17;199:664–667. doi: 10.1038/199664a0. [DOI] [PubMed] [Google Scholar]
- PONS M. INFECTIOUS DOUBLE-STRANDED POLIOVIRUS RNA. Virology. 1964 Nov;24:467–473. doi: 10.1016/0042-6822(64)90186-2. [DOI] [PubMed] [Google Scholar]
- RAKE A. V., GRAHAM A. F. KINETICS OF INCORPORATION OF URIDINE-C14 INTO L CELL RNA. Biophys J. 1964 Jul;4:267–284. doi: 10.1016/s0006-3495(64)86782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHIM J. S., JORDAN L. E., MAYOR H. D. Cytochemical, fluorescent-antibody and electron microscopic studies on the growth of reovirus (ECHO 10) in tissue culture. Virology. 1962 Jun;17:342–355. doi: 10.1016/0042-6822(62)90125-3. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. ACTINOMYCIN AND THE DIFFERENTIAL SYNTHESIS OF REOVIRUS AND L CELL RNA. Biochem Biophys Res Commun. 1965 May 3;19:506–510. doi: 10.1016/0006-291x(65)90154-3. [DOI] [PubMed] [Google Scholar]
- SHIPP W., HASELKORN R. DOUBLE-STRANDED RNA FROM TOBACCO LEAVES INFECTED WITH TMV. Proc Natl Acad Sci U S A. 1964 Aug;52:401–408. doi: 10.1073/pnas.52.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUEOKA N., CHENG T. Y. Fractionation of nucleic acids with the methylated albumin column. J Mol Biol. 1962 Mar;4:161–172. doi: 10.1016/s0022-2836(62)80048-5. [DOI] [PubMed] [Google Scholar]
- VASQUEZ C., TOURNIER P. The morphology of reovirus. Virology. 1962 Aug;17:503–510. doi: 10.1016/0042-6822(62)90149-6. [DOI] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P., BURDON R. H., BILLETER M. A., OCHOA S. REPLICATION OF VIRAL RNA, III. DOUBLE-STRANDED REPLICATIVE FORM OF MSW PHAGE RNA. Proc Natl Acad Sci U S A. 1964 Apr;51:682–690. doi: 10.1073/pnas.51.4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN E. F., HEETER M., DARNELL J. E. RNA synthesis in poliovirus-infected cells. Virology. 1963 Mar;19:400–408. doi: 10.1016/0042-6822(63)90080-1. [DOI] [PubMed] [Google Scholar]



