Abstract
Mounting evidence supports the importance of hormonal fluctuations in temporomandibular disorder (TMD) pain among women. Stabilizing influential hormones or having a plan and skills for coping with hormonally-related increases in TMD pain therefore may be beneficial for women with TMD pain. This randomized clinical trial evaluated the short- and long-term efficacy of three interventions for women with TMD pain: (1) dental hygienist-delivered pain self-management training (SMT; n = 59); (2) the same dental hygienist-delivered pain self-management training, but with a focus on menstrual cycle-related changes in pain and other symptoms (targeted SMT, or TSMT; n = 55); and (3) continuous oral contraceptive therapy (6 month trial), aimed at stabilizing hormones believed to be influential in TMD pain (COCT; n = 57). Study participants completed outcome (pain, activity interference, depression) and process (pain beliefs, catastrophizing, coping effectiveness) measures before randomization, and 6 and 12 months later. Intent-to-treat analyses supported the benefits of the SMT and TSMT interventions relative to COCT. Targeting the self-management treatment to menstrual cycle-related symptoms did not increase the treatment’s efficacy. The benefits of the self-management interventions relative to COCT for pain and activity interference were statistically significant at 12 months, but not at 6 months, whereas the benefits for the process measures generally were apparent at both timepoints. COCT was associated with multiple adverse events (none serious). The study provides further support for long-term benefits of a safe, low intensity (two in-person sessions and six brief telephone contacts), dental hygienist-delivered self-management treatment for TMD pain.
Keywords: temporomandibular disorders, chronic pain, randomized clinical trial, oral contraceptive, self-management
1. Introduction
Pain associated with temporomandibular disorders (TMD) is common, particularly among women, and frequently associated with activity interference and psychological distress [39]. Treatments are diverse and include various medications, occlusal appliances, surgery, and cognitive-behavioral therapies (CBT) [31]. In addition, we have demonstrated the efficacy of a dental hygienist-delivered self-management intervention [11].
Previous work by our group has also contributed to the accumulating evidence concerning the importance of hormonal fluctuations in TMD pain. We have shown that TMD pain varies systematically across the menstrual cycle, with highest pain during menses and the late luteal phase, times characterized by low or rapidly falling estradiol levels in normally cycling women and withdrawal of synthetic estrogen in women on oral contraceptives (OCs) [29]. In ovulating women, there is also a secondary pain peak around the time of ovulation, when estradiol levels fluctuate greatly. Laboratory studies have confirmed estradiol’s role as a modulator of facial pain [43] and clinical studies have found increased occurrence of headaches, a common comorbidity of TMD pain, during menses [30]. We therefore hypothesized that self-management interventions for women with TMD pain would be enhanced by attention to, and planning for, cyclic symptom changes.
Another option for reducing TMD pain increases associated with hormonal fluctuations might be continuous OC therapy (COCT). Women on standard OC regimens (hormone pills for 21 days, followed by seven days without hormones), like normally cycling women, experience cyclic patterns of headache associated with estrogen withdrawal [30,46]. COCT (i.e., eliminating the days without hormones) has been used for a number of years for contraception and to reduce the frequency of menstruation, and has been found to be safe and effective for contraception for up to two years [7,37]. We hypothesized that, if TMD pain increases with estrogen withdrawal, COCT, which would prevent estrogen withdrawal, might also prevent pain increases associated with changes in estrogen levels.
The aim of this randomized clinical trial (RCT) was to evaluate the efficacy of a dental hygienist-delivered pain self-management treatment targeted to menstrual cycle-related changes in pain (targeted self-management training, or TSMT). We compared this intervention to (1) a treatment of known efficacy - self-management training (SMT) - with the same format and intensity, but without attention to menstrual cycle-related changes in symptoms; and (2) a novel therapy focused on biological, but not psychosocial influences - COCT (for hormonal stability). We hypothesized that self-management skills training targeted to predicted menstrual cycle-related changes would result in greater improvement in TMD pain and activity interference, short-term and long-term, than would standard self-management training and stabilization of the hormonal environment by COCT. Based on previous work [39,1] demonstrating the importance of psychosocial factors in, and effectiveness of self-management and cognitive-behavioral therapies for, TMD pain, we also hypothesized that, at 12 months, both self-management interventions would be superior to COCT.
2. Methods
2.1. Setting and participants
The study was approved by the University of Washington (U.W.) institutional review board and all participants provided written informed consent. A Data Safety and Monitoring Committee provided oversight of the study.
Study participants were recruited from patients seeking care at the U.W. Orofacial Pain Clinic and by advertising between October 2005 and June 2009. Study inclusion criteria were: (1) female gender; (2) age 18–45 years; (3) a Research Diagnostic Criteria/Temporomandibular Disorders (RDC/TMD) Axis I TMD pain diagnosis [12] made by an oral medicine specialist based on a structured RDC/TMD clinical examination; (4) premenopausal; (5) characteristic pain intensity [55] ≥3 (0–10 scale, past 6 months timeframe); and (6) ability to communicate in English. Study exclusion criteria were lacking a menstrual cycle; pregnant, lactating, or planning to become pregnant in the next 7 months; unwilling to take a continuous OC; need for further diagnostic evaluation of facial pain (as determined by the oral medicine specialist); and major medical or psychiatric conditions that would interfere with ability to participate. In addition, for safety reasons, study participants randomized to the COCT group underwent a gynecological examination and were withdrawn from the study if they (1) had a medical contra-indication for COCT (e.g., history of or active thromboembolic disease; cerebrovascular or coronary artery disease; undiagnosed genital bleeding; estrogen-dependent cancer; acute liver disease; benign or malignant liver tumors; severe headaches or headaches with atypical neurological changes); (2) smoked cigarettes and were ≥35 years of age; (3) had used medication within the last three months that interfered with estrogen or progestin metabolism; (4) had an abnormal pelvic examination, abnormal cytology (Pap smear), or undiagnosed uterine bleeding; or (5) had no current mammogram and were ≥40 years of age.
2.2. Procedures
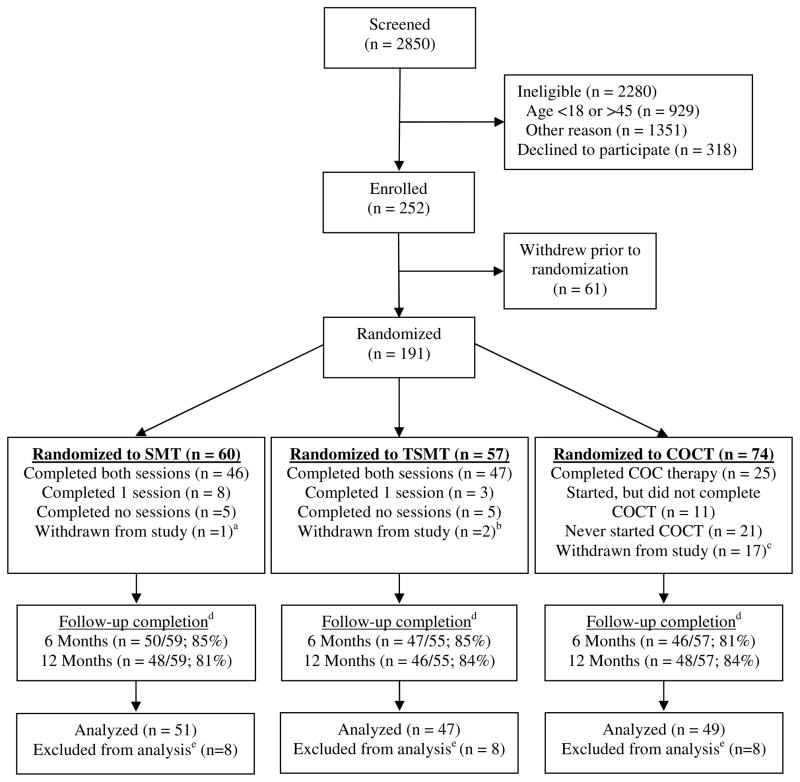
Figure 1 illustrates participant flow through the study. Patients at the U.W. Orofacial Pain Clinic were approached for study participation if they met basic study eligibility criteria. Individuals who called in response to advertising were first screened by telephone; if they met the initial inclusion criteria, they came to the clinic for a baseline session, during which their eligibility was confirmed. Potential participants were told that the study involved randomization to COCT or to one of two different versions of SMT. At no time before or during study participation were participants informed that one and only one version focused on menstrual cycle-related changes. All potential study participants completed a comprehensive clinical history questionnaire and had a comprehensive clinical dental examination to ensure there were no conditions that would preclude study participation and to derive an RDC/TMD Axis I diagnosis [12]. All clinical examiners were calibrated in the RDC/TMD examination protocols.
Fig. 1.
Participant flow through the study
COCT, Continuous Oral Contraceptive Therapy; SMT, Self-Management Training; TSMT, Targeted Self-Management Training
a 1 participant was unable to follow study protocol
b 1 participant moved out of state; 1 was discovered to be ineligible (due to psychiatric problems) after randomization
c 1 participant moved out of the country, 1 was found to be ineligible after randomization, 15 were ineligible upon medical evaluation after randomization due to medical contra-indications to COCT
d Follow-up completion rates based on number of participants randomized and not withdrawn
e Excluded from analysis due to no post-treatment or 12-month data, although included in the multiple imputation analyses
Study participants completed daily diaries of pain and other symptoms for one menstrual cycle prior to randomization (diary data are not the focus of this report). A few days prior to completion of the diaries, participants were called to reconfirm eligibility and interest in participating in the RCT. Only participants who returned completed diaries for at least 85% of the requested days were eligible for randomization. Participants who remained eligible and interested were randomized at the beginning of their next menstrual cycle.
Women randomized to the COCT group were seen by an Advanced Registered Nurse Practitioner (ARNP) who specialized in gynecology and was supervised by an obstetrician/gynecologist. The ARNP reviewed a medical history questionnaire completed by the study participant, completed a review of systems, and performed breast and pelvic examinations to determine the participant’s medical eligibility to receive COCT. A Pap test was obtained for women who could not provide the results of a Pap test done within the past year. Tests for pregnancy and, if indicated, gonorrhea and Chlamydia, were performed.
Participants completed the study measures at the time of study enrollment (baseline, prior to randomization) and at 6 and 12 months after randomization. The study protocol was designed so that the 6-month assessment would occur after the completion of all self-management in-person and telephone sessions (for participants assigned to SMT and TSMT), and just before ending the COCT (for participants assigned to COCT). Participants were compensated up to $325 for study participation (which included completion of diary and examination measures not reported here).
2.3. Measures
2.3.1. Outcome measures
2.3.1.1. Pain intensity and activity interference
The Graded Chronic Pain Scale (GCPS) [55,54] was used to assess pain intensity and interference with usual daily activities. The pre-specified primary outcome, characteristic pain intensity, was calculated by averaging 0–10 ratings of current facial pain and average and worst facial pain in the past month [15,55,54]. Activity interference [54] was calculated by averaging 0–10 ratings of facial pain interference with daily activities; work and housework activities; and recreational, social, and family activities in the past month. The characteristic pain intensity and activity interference scores have good internal consistency, test-retest reliability, and validity [53,54]. The GCPS can also be scored to categorize patients into chronic pain grades, which reflect level of pain and activity interference.
2.3.1.2. Clinically meaningful improvement in pain
As recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials-II (IMMPACT-II) [10], we also examined rates of clinically meaningful improvement in pain. We considered a reduction in characteristic pain intensity from baseline of ≥30% to be clinically meaningful and ≥50% to be substantial [17].
2.3.1.3. Pain quality
The Short Form McGill Pain Questionnaire (SF MPQ) [36] was used to assess sensory and affective dimensions of the pain experience. The SF MPQ consists of 11 sensory pain descriptors and four affective pain descriptors. Scores were calculated by averaging the responses on each scale. The measure has been shown to have good test-retest reliability and sensitivity to change [21,44].
2.3.1.4. Depression
The 21-item Beck Depression Inventory (BDI) [2,3] was used to assess depressive symptom severity. The BDI has high internal consistency, adequate test-retest reliability, and validity [4], and is a valid screening instrument for depression among patients with chronic pain [51,34,19]. We defined clinically meaningful levels of depressive symptoms as scores ≥21 [19].
2.3.1.5. Treatment helpfulness
At the 6-month assessment, study participants rated improvement in their facial pain as a result of participating in the study on a scale of 0–10, with 0 = ‘very much worse’ and 10 = ‘very much improved.’ Participants also used a 0–10 scale to rate to rate overall satisfaction with the treatment (0 = ‘not at all satisfied,’ 10 = ‘very satisfied’). Participants assigned to TSMT and SMT also used 0–10 scales to rate the helpfulness of specific components of their treatments, including information about TMD, learning to check and correct oral posture and jaw habits, jaw stretching, breathing and relaxation strategies, the personal plan, relapse prevention, and reading material. TSMT participants were also asked to rate the helpfulness of planning for increases in TMD pain at certain times in the menstrual cycle.
2.3.2. Process measures
2.3.2.1. Pain beliefs
We administered three scales from the Survey of Pain Attitudes (SOPA) [25]: Disability (belief that one’s pain is disabling), Harm (belief that pain signifies damage and that activity should be avoided), and Control (belief in one’s personal control over pain). These scales have demonstrated validity and good test-retest stability and internal consistency [24,45,25]. Scores on each scale can range from 0 to 4, with higher scores indicating greater agreement with the belief.
Participants also completed the 8-item TMD Self-Efficacy Scale (SES), which is a modification (by replacing the word ‘arthritis’ with ‘facial pain’) of the Arthritis Self-Efficacy Scale [20,33]. Participants rated from 0 = ‘very uncertain’ to 10 = ‘very certain’ their certainty that they can decrease their pain quite a bit, keep facial pain from interfering with their sleep, keep their pain from interfering with the things they want to do, regulate their activity so as to be active without aggravating their pain, keep the fatigue caused by pain from interfering with the things they want to do, do something to feel better if they are feeling blue, manage facial pain during their daily activities, and deal with the frustration of facial pain. Scale scores are calculated as the mean of the eight ratings, with higher scores indicating greater self-efficacy. This scale had excellent internal consistency (Cronbach’s alpha = 0.91) and validity in a previous sample of TMD patients [6]. Although the SOPA Control scale and the TMD SES are moderately correlated, the former scale assesses solely the belief in ability to control one’s pain, whereas the SES assesses confidence in ability not only to decrease pain but also to manage specific pain-related problems.
2.3.2.2. Pain catastrophizing
Pain catastrophizing was assessed by the Coping Strategies Questionnaire (CSQ) Catastrophizing scale [40]. This scale has excellent internal consistency [40,26] and has been shown to be associated with various measures of functioning in samples of patients with different pain conditions [27,26,23,9,35], including TMD [48]. Scores can range from 0 to 6, with higher scores indicating greater catastrophizing.
2.3.2.3. Perceived effectiveness of pain coping strategies
Perceived effectiveness of pain coping strategies was assessed by ratings on the CSQ of ability to control and decrease pain through the use of pain coping strategies [40]. These ratings have been demonstrated to be associated positively with better adjustment to chronic pain [28,18]. The mean of the two ratings (made on scales from 0 = ‘no control/can’t decrease it at all’ to 6 = ‘complete control/can decrease it completely’) was used in analyses.
2.4. Randomization
Randomization to the three treatment groups was stratified by participant’s baseline chronic pain grade and recruitment source (U.W. Orofacial Pain Clinic new patient, U.W. Orofacial Pain Clinic return patient, and non-U.W. sources), and with blocking to ensure that the groups would be (nearly) balanced across the study period and within each stratum. Block sizes were equal to 3 or 6, and were chosen randomly with 2/3 and 1/3 probability, respectively. In addition, the number of women randomized to the COCT group was increased by 25% to allow for some disqualification at the ARNP visit. The randomization list was prepared using the “sample” function of the S-PLUS® statistical software (Insightful Corporation, Seattle, WA). Treatment assignments were recorded on cards numbered consecutively within each stratum, and a study assistant not involved in the screening and randomization put the randomization assignments in sealed envelopes sequentially numbered by stratum. Randomization assignments were concealed to all study personnel with study participant contact until envelopes were opened by research staff at the time of randomization.
2.5. Interventions
Each study participant was given a personalized (by their own oral medicine specialist if a U.W. patient; otherwise, by the examining oral medicine specialist at their baseline visit) list of recommended TMD self-care strategies. These included some or all of the following: reduction of jaw parafunctional activities; instructions for jaw range of motion exercises; thermal packs; and food modifications (avoid chewy foods, small bites, etc).
2.5.1. SMT and TSMT
The SMT and TSMT interventions consisted of two 1.5-hour in-person sessions at the U.W. Orofacial Pain Clinic and six 10–15-minute telephone calls delivered by dental hygienists who were trained and supervised by a clinical psychologist. The two in-person sessions were conducted approximately four weeks apart, with two telephone contacts in between. The other telephone contacts were approximately 1, 2, 7, and 13 weeks after Session 2.
Two of the three dental hygienists had extensive prior experience providing a similar self-management treatment to patients with TMD pain. As part of a prior study, described by Dworkin et al. [11], these hygienists received eight hours of training in an earlier version of the SMT intervention and then provided the intervention for the study. The third hygienist received SMT training comparable to the prior training of the other two. This training was provided conjointly by one of the other hygienists (KHH) and a clinical psychologist (JAT) with extensive experience using self-management treatment and cognitive-behavioral therapy methods with patients with TMD and other chronic pain conditions. All hygienists also received training by the clinical psychologist in new materials added to the SMT and TSMT interventions for this study. In these in-person sessions, the treatment session protocols and patient manuals were reviewed systematically, and the psychologist and the dental hygienists engaged in role-playing the sessions. Finally, all three hygienists received education concerning the phases of the menstrual cycle and scientific evidence regarding the relationships between these hormonal changes and TMD pain. This education was provided by one of the authors (LLR), who has expertise in the relationships between hormones and TMD pain in women.
Both interventions were structured, manual-based treatments based on standard cognitive-behavioral pain therapies [52] and self-management interventions for chronic TMD pain that were previously developed and applied by our group [14,11,13]. Sessions included education about the biopsychosocial model of chronic pain, TMD etiology and treatments, and the rationale for self-management; relaxation [5,47] and stress management training; discussion of the role of stress and emotions as potential factors exacerbating and maintaining TMD symptoms; instruction and practice in self-monitoring of symptoms to identify factors that might be helpful to modify through self-care methods; practice of dentist-prescribed self-care treatments; and discussion of strategies to maintain gains and prevent relapse.
At each in-person and telephone session, study participants completed a ‘personal TMD health care plan’ for activities to complete between sessions. They identified potential barriers to the implementation of the plan and ways to overcome the barriers. Participants checked off activities as they completed them on a daily basis and had the completed plan available for discussion in each session. Certain activities were recommended to all participants (e.g., jaw posture monitoring, relaxation practice, breathing exercises) and other activities were added according to individual participants’ behavioral goals (e.g., increasing physical exercise). Jaw stretching exercises were included if they had been prescribed by the oral medicine specialist.
SMT and TSMT participants received a CD with relaxation exercises and a manual that included articles about TMDs and TMD treatments; acute versus chronic pain; stress reduction strategies, including abdominal breathing and progressive muscle relaxation; sleep hygiene; communication skills; and maintaining gains and managing setbacks. The manual also included instructions for TMD self-care activities (e.g., monitoring jaw posture, relaxing jaws, avoiding certain jaw movements and activities, application of heat or cold, diet modifications, and jaw stretching exercises if prescribed).
The TSMT intervention also included education about the potential effects of hormones on TMD pain, instructions to monitor the association of pain and other symptoms with menstrual cycle changes, and planning for times in participants’ menstrual cycles when symptoms might increase. At the first TSMT session, the dental hygienist and participant reviewed TMD pain data from the daily diary the participant completed for a full menstrual cycle prior to the session, and discussed whether pain seemed to fluctuate according to the phase of the cycle. The TSMT participants were encouraged to self-monitor stress and mood in critical periods during the menstrual cycle, and to increase their use of coping skills (e.g., relaxation) during these times.
In addition, TSMT participant contacts were timed according to each participant’s menstrual cycle. For example, for women with a 28-day cycle, the in-person sessions were scheduled to occur at approximately day 6 of the woman’s menstrual cycle, just after or at the end of menstruation, and before ovulation. The first telephone call occurred on day 13 (ovulatory period). The second telephone contact was scheduled to occur just prior to menses and included, in addition to the review and reinforcement of general pain coping skills as in the SMT call, focus on the fact that the woman would soon be entering a phase of the cycle that might be associated with increased TMD pain and other symptoms. The remaining TSMT telephone calls were also conducted at times targeted to critical phases of the menstrual cycle. If the sessions or calls could not be conducted on the targeted dates, the dental hygienist targeted the content to the participant’s current menstrual cycle status, with review or anticipation of cyclic symptoms.
2.5.2. COCT
At their clinic visit with the study ARNP, women who were randomized to, and met all eligibility criteria for, COCT received instructions on use of the medication, potential breakthrough bleeding problems, backup birth control methods in the event of missed doses (for participants who were also using the study medication for birth control), and contact information for any medication-related questions. A 4-month supply of the medication (20 mcg ethinyl estradiol and 100 mcg levonorgestrel) was dispensed. At a second visit three months later, they received additional medication, for a total of six months on the medication. Women returned for a final visit just prior to stopping the medication. They were queried about their future plans for contraception and informed that if they wanted to stay on the study medication they should contact their personal health care provider. The 3- and 6-month visits included a urine pregnancy test, medication use review, and adverse event assessment.
2.6. Statistical power
A priori power calculations were based on data from our previous studies. We expected characteristic pain intensity (our primary outcome) to have a mean of 5.9 at baseline and a standard deviation (SD) of 2.5 at each follow-up. At 12 months, an n of 68 participants per group would have 91% power to detect a 1.5-point difference between groups in mean characteristic pain intensity for a one-way analysis of variance (ANOVA) with a 0.05 significance level, assuming a decrease from baseline to 12-month follow-up by 35–45% in the SMT group, 55–65% in the TSMT group, and 30–40% in the COCT group. This sample size also allowed sufficient statistical power to detect a difference between groups in characteristic pain intensity (the primary outcome) at 6 months (>80% power), as well as in activity interference at 12 months (74% power; assuming a mean activity interference at baseline of 2.9 and a SD of 2.2 at each follow-up).
After beginning enrollment, we observed lower-than-expected enrollment and a somewhat lower-than-expected mean baseline pain score because participants recruited through advertising reported lower pain levels than did those recruited through the orofacial pain clinic. We recalculated the power based on the initial data of mean (SD) baseline characteristic pain intensity = 5.0 (2.0). A sample size of 45 per group at 12 months could detect a difference of 1.3 points between groups in mean characteristic pain intensity (assuming similar reductions in intensity as described above) with 80% power for a one-way ANOVA with a 0.05 significance level. However, power was insufficient (34%) to detect a difference between groups in activity interference, in part due to a low level of activity interference at baseline [mean (SD) of 2.0 (2.0)].
2.7. Statistical analysis
Examination of the distributions of the study measures revealed considerable skewness for activity interference and catastrophizing. Substantial proportions of study participants had scores of 0 on these measures, and no transformation of scores could make the distribution shapes normal and similar for the treatment groups. We therefore dichotomized scores on these measures into categories of 0 versus some. The distribution of BDI scores was also skewed, but normalized by a square root transformation of scores.
The primary analyses comparing the three study groups were intent-to-treat analyses, which included all randomized participants for whom follow-up data (either at 6 months or 12 months) were available, regardless of session attendance or medication adherence. However, we repeated the key analyses on the subsample of participants who attended both in-person treatment sessions or completed the study medication protocol to examine whether results differed for treatment completers (‘as-treated analyses’).
To assess comparability of the three treatment groups at baseline on the study measures, we used ANOVA (for continuous and ordinal variables) and chi-square tests (for categorical variables). To compare the three study groups on the outcome and process measures over time, we fit linear or logistic regression models, controlling for baseline values of the dependent variable examined. In the regression models, we tested for group differences separately at 6 months and 12 months, because a priori we had postulated that there would be differences in short-term and long-term efficacy of the interventions. We also compared the three study groups on rates of clinically meaningful improvement using logistic regression analyses.
In addition, we performed multiple imputation analyses for the outcomes of characteristic pain intensity and activity inference to assess the effect of missing data due to participants who provided no follow-up data. Baseline and follow-up values of characteristic pain intensity or activity inference, as well as a variable indicating assigned treatment, were used in the imputation of the missing values, which used a Markov Chain Monte Carlo (MCMC) method assuming an arbitrary missing data pattern and multivariate normality and a single chain to create five imputations using 200 burn-in iterations before the first imputation and 100 iterations between imputations. PROC MI was used to generate the imputed data sets and PROC MIANALYZE was used to combine the results of the linear and logistic regression analyses of imputed data sets and generate valid statistical inferences (SAS Version 9.1 software programs). We also conducted a sensitivity analysis to determine whether the results of the analyses for group differences in characteristic pain intensity and activity inference differed when adjusting for age, education, marital status, TMD diagnosis, and recruitment source (clinic versus advertising).
We did not make a statistical adjustment (e.g., Bonferroni method, which inflates type II error and reduces statistical power) for multiple testing. We expected moderate correlations of the outcome and process measures; patterns of significant differences among the study groups on multiple related measures would support an interpretation that the treatment had an effect on the constructs assessed by those measures. We included multiple measures within the outcome and process variable domains because it would be of interest to patients, clinicians, and researchers to know whether the interventions had a different impact on, for example, activity interference versus pain intensity. Similarly, it would be of interest to know if process variables change differentially with the different interventions. Given these considerations, we specified a priori the primary outcome, and as recommended for these circumstances [41,38,42], did not adjust for multiple comparisons of groups on secondary outcomes. However, in comparing specific treatments after finding an overall significant difference among treatments, we used Holm and Bonferroni methods [22] to control for the multiple group comparisons and to adjust confidence intervals for mean differences between groups in order to maintain an overall significance level of P ≤ 0.05.
3. Results
3.1. Study flow, treatment adherence, and follow-up assessment rates
Figure 1 shows the study flow and provides information concerning intervention session completion in the two self-management groups and medication adherence in the COCT group, as well as follow-up assessment completion. Among the 191 women who were randomized, 20 were withdrawn from the study (15 of whom were randomized to the COCT group but then withdrawn due to medical contra-indications). In each of the three groups, 86% of participants completed at least one follow-up assessment. In the analysis sample, the three treatment groups did not differ significantly in rates of completion of both follow-up assessments (92% SMT, 98% TSMT, 92% COCT; P = 0.47) and the two self-management groups did not differ significantly in rates of completion of both in-person sessions (88% SMT, 96% TSMT; P = 0.27) or of intervention phone calls (63% of the SMT group and 71% of the TSMT group completed at least 5 of the 6 calls; P = 0.64). Among the COCT analysis sample, 51% completed the COCT protocol, 33% never started the medication (most because they decided after randomization that they did not want to take the medication), and 16% started but did not complete the medication protocol (most because of side effects). Among the 25 women who completed the COCT protocol, 23 completed the 6-month questionnaire assessment prior to stopping the medication, one did not complete the 6-month assessment, and one completed the questionnaire six days after ending medication use. In sum, among 74 women randomized to the COCT arm of the study, only 25 (34%) completed the entire COC protocol. In contrast, both of the in-person self-management treatment sessions were completed by 46 (77%) of the 60 women randomized to SMT and 47 (82%) of the 57 women randomized to TSMT.
We compared the analysis sample (n = 147) to study participants excluded from analysis (i.e., no follow-up data; n = 24) on variables for which we had data for all participants. There were no significant (P < 0.05) differences in treatment group assignment; recruitment source; age; education; marital status; race; the three baseline GCPS [55] measures (characteristic pain intensity, pain-related interference, and chronic pain grade); or duration of facial pain.
3.2. Sample characteristics
The three study groups did not differ significantly in any sociodemographic characteristic examined, except for age (see Table 1). The TSMT group was significantly younger than the SMT group, although the average age in each group was 25–29 years. The study groups also did not differ significantly in duration of facial pain; SMT median (interquartile range [IQR]) = 5 years (2–12 years), TSMT median (IQR) = 5 years (2–10 years), COCT median (IQR) = 6 (3–14) years; Kruskal-Wallis test, P = 0.53).
Table 1.
Baseline sociodemographic characteristics of study participants (analysis sample)
| Characteristic | SMT (n = 51) | TSMT (n = 47) | COCT (n = 49) | P-value a |
|---|---|---|---|---|
| Age, mean (SD), years | 29.1 (7.4) | 25.4 (5.7) | 28.6 (6.9) | 0.02 |
| Education, % | 0.36 | |||
| High school or less | 6 | 18 | 10 | |
| Some college or vocational/technical | 39 | 27 | 38 | |
| College graduate | 55 | 55 | 52 | |
| Non-Hispanic White, % | 76 | 77 | 80 | 0.90 |
| Working full-time, % | 35 | 51 | 45 | 0.28 |
| Marital status, % | 0.72 | |||
| Married or living as married | 21 | 15 | 22 | |
| Never married | 67 | 79 | 70 | |
| Separated, divorced, or widowed | 12 | 6 | 8 | |
| Recruitment Source, % | 0.93 | |||
| Orofacial Pain Clinic | 24 | 32 | 27 |
COCT, Continuous Oral Contraceptive Therapy; SMT, Self-Management Training; TSMT, Targeted Self-Management Training
P-value from the overall group comparison (ANOVA for continuous measures and chi square test for categorical measures)
Patients may have more than one RDC/TMD diagnosis. Almost all study participants had an RDC/TMD Group I (muscle) diagnosis (98% in each of the 3 treatment groups), with more having a Ib (myofascial pain with limited opening) diagnosis (53–66% across groups) than a Ia (myofascial pain) diagnosis (32–45% across groups). The majority of the participants in each group (62–67% across groups) had a IIIa (arthralgia) diagnosis. There were no significant differences among groups in rates of any specific diagnosis.
3.3. Treatment group comparisons on outcome measures (intent-to-treat analyses)
Table 2 shows the scores on the outcome measures at each assessment. At baseline, pain intensity was moderate on average (5.0–5.3 across groups) and most participants (77%) reported that pain interfered with activities. The three study groups did not differ significantly at baseline on any outcome measure. There was a trend towards higher SF MPQ Sensory scores in the COCT group, although the clinical significance of the difference (less than 0.5 SD) is questionable and the difference was not statistically significant.
Table 2.
Outcomes for study participants in each treatment groupa
| Measure/Time | SMT | TSMT | COCT | P-value b |
|---|---|---|---|---|
| Characteristic pain intensity, mean (SD) | ||||
| Baseline | 5.0 (1.6) | 5.0 (1.5) | 5.3 (1.5) | 0.71 |
| 6 months | 3.1 (1.8) | 2.9 (2.0) | 3.6 (2.2) | 0.19 |
| 12 months | 2.8 (1.6) | 2.8 (2.0) | 3.9 (1.9) | 0.003 |
| Activity interf rence, % (n) with some | ||||
| Baseline | 77 (39) | 79 (37) | 76 (37) | 0.93 |
| 6 months | 42 (21) | 40 (19) | 54 (25) | 0.27 |
| 12 months | 40 (19) | 37 (17) | 63 (30) | 0.016 |
| SF MPQ Sensory, mean (SD) | ||||
| Baseline | 0.9 (0.5) | 0.9 (0.5) | 1.1 (0.5) | 0.056 |
| 6 months | 0.5 (0.4) | 0.6 (0.4) | 0.8 (0.5) | 0.057 |
| 12 months | 0.5 (0.4) | 0.5 (0.3) | 0.9 (0.5) | <0.001 |
| SF MPQ Affective, mean (SD) | ||||
| Baseline | 0.6 (0.6) | 0.4 (0.6) | 0.7 (0.6) | 0.20 |
| 6 months | 0.3 (0.4) | 0.3 (0.5) | 0.3 (0.3) | 0.68 |
| 12 months | 0.4 (0.5) | 0.2 (0.3) | 0.5 (0.5) | 0.04 |
| Beck Depression Inventory,c mean (SD) | ||||
| Baseline | 6.3 (5.7) | 5.4 (6.2) | 7.8 (6.8) | 0.18 |
| 6 months | 4.0 (5.4) | 2.9 (4.3) | 6.9 (7.3) | 0.006 |
| 12 months | 5.1 (5.3) | 3.0 (4.5) | 6.0 (7.6) | 0.13 |
COCT, Continuous Oral Contraceptive Therapy; SF MPQ, Short Form McGill Pain Questionnaire; SMT, Self-Management Training; TSMT, Targeted Self-Management Training;
Values in table are observed values for the analysis sample
P-values for baseline comparisons are from ANOVAs for continuous variables and chi-square tests for categorical variables, P-values for post and follow-up comparisons are from linear regression analyses for continuous variables and logistic regression analyses for the categorical variable adjusting for baseline values of the outcome variable.
Because the distribution of Beck Depression Inventory scores was skewed, square root-transformed values were used in the analyses. For ease of interpretation, the mean and SD shown have been transformed back to the original scale.
On the primary outcome, characteristic pain intensity, the groups did not differ significantly at the 6-month assessment (P = 0.18). However, at 12 months, there was an overall treatment group difference (P = 0.003), with significantly lower pain intensity in the SMT and TSMT groups than in the COCT group (P < 0.05, Holm’s method). The adjusted (for baseline levels) mean difference in pain at 12 months was −1.0 (95% CI = −1.8, −0.2) between SMT and COCT and −1.0 (−1.9, −0.2) between TSMT and COCT.
A similar pattern was observed for activity interference and sensory and affective pain scores. There was no significant treatment group difference in activity interference at 6 months, but at 12 months, there was an overall difference (P = 0.016), with more TSMT (63%) and SMT (60%) participants than COCT participants (37%) reporting no interference (P < 0.05, Holm’s method). The odds of reporting no activity interference at 12 months were approximately three times greater in both the TSMT group (OR = 3.2; 95% CI = 1.1, 9.1) and the SMT group (OR = 2.9; 95% CI = 1.0, 8.3) than in the COCT group. Significant differences between groups at 12 months were also observed for the SF MPQ Sensory and Affective scores, with higher pain scores in the COCT group.
All groups showed low levels of depressive symptoms on average at baseline, as indicated by low scores on the BDI. BDI scores (adjusted for baseline levels) were significantly lower in the two self-management groups than in the COCT group at 6 months, but not significantly different among groups at 12 months. The groups did not differ at baseline in proportions with BDI scores indicating clinically significant levels of depressive symptoms (6%–8% across the 3 groups; logistic regression, exact P = 0.92). At 6 months, more COCT (11%) than SMT (4%) and TSMT (0%) participants had clinically significant levels of depressive symptoms (adjusted for baseline, exact P = 0.04). However, the treatment groups did not differ at 12 months (10% COCT, 4% SMT, 2% TSMT; logistic regression, adjusted for baseline, exact P = 0.27).
In the sensitivity analyses using multiple imputation for missing values, the primary conclusions remained unchanged; that is, there were significant overall group differences in characteristic pain intensity and activity interference at 12 months but not at 6 months. Further, the adjusted mean differences between groups in characteristic pain intensity at 12 months did not change meaningfully (−1.0 between COCT and both SMT and TSMT in the original analyses versus −1.0 in the comparison with SMT and −0.9 in the comparison with TSMT in the multiple imputation analyses). However, the odds ratios for comparisons of the SMT and TSMT groups relative to the COCT group in terms of no activity interference at 12 months were somewhat lower in the multiple imputation analyses than in the original analyses [SMT: 2.4 (adjusted 95% CI = 0.90, 6.67); TSMT: 2.6 (0.97, 6.67)].
In the sensitivity analyses that adjusted for age, education, marital status, RDC/TMD diagnosis, and recruitment source, there was a small attenuation of the treatment group differences for characteristic pain intensity, but the difference at 12 months, with greater pain intensity in the COCT group than in the other two groups, remained statistically significant. For activity interference, the significant treatment group difference at 12 months also remained statistically significant, but pairwise comparisons found a significant effect only for TSMT versus COCT.
3.4. Clinically significant change in pain (intent-to-treat analyses)
Table 3 shows rates of clinically meaningful improvement from baseline in characteristic pain intensity in the three treatment groups at each follow-up (intent-to-treat analyses). Patterns of findings were similar for rates of 30% and 50% improvement. The groups did not differ significantly at 6 months. However, at 12 months, there was a significant group difference, with the SMT and TSMT groups showing approximately double the rates in the COCT group of clinically meaningful and substantial improvement, but no significant differences between SMT and TSMT.
Table 3.
Rates of clinically meaningful improvement in pain in the 3 treatment groups (intent-to-treat analyses)
| Timepoint | SMT | TSMT | COCT | P-value a |
|---|---|---|---|---|
| ≥30% improvement in pain | ||||
| 6 months | 70% | 66% | 54% | 0.26 |
| 12 months | 67% | 70% | 35% | 0.001 |
| ≥50% improvement in pain | ||||
| 6 months | 46% | 43% | 28% | 0.18 |
| 12 months | 54% | 44% | 19% | 0.002 |
COCT, Continuous Oral Contraceptive Therapy; SMT, Self-Management Training; TSMT, Targeted Self-Management Training
P-values are for the overall treatment group effect and calculated using logistic regression analysis.
3.5. Treatment group comparisons on process measures (intent-to-treat analyses)
Table 4 shows the scores of each group on the process measures at each assessment. In general, there was an overall treatment group difference on the process measures at both 6 and 12 months. The exception was no significant difference at 12 months in catastrophizing. Pairwise contrasts generally indicated more maladaptive beliefs in the COCT group than in the self-management groups at 6 and 12 months, with no differences between the 2 self-management groups. This was the case for SOPA Harm, SOPA Control, and Self-Efficacy. For SOPA Disability and Catastrophizing, the difference at both follow-ups was significant only between COCT and TSMT. COCT participants’ perceived effectiveness of pain coping strategies was lower than that of participants in both self-management groups at 6 months, but significantly lower than that of only the SMT participants at 12 months.
Table 4.
Scores on the process measures in each treatment group at each timepointa
| Measure/Time | SMT | TSMT | COCT | P-value b |
|---|---|---|---|---|
| SOPA Disability, mean (SD) | ||||
| Baseline | 0.9 (0.6) | 0.9 (0.6) | 1.0 (0.7) | 0.62 |
| 6 months | 0.6 (0.6) | 0.6 (0.6) | 0.8 (0.6) | 0.04 |
| 12 months | 0.8 (0.7) | 0.7 (0.6) | 0.9 (0.6) | 0.02 |
| SOPA Harm, mean (SD) | ||||
| Baseline | 1.8 (0.6) | 1.8 (0.6) | 1.7 (0.5) | 0.57 |
| 6 months | 1.2 (0.6) | 1.4 (0.6) | 1.6 (0.6) | 0.002 |
| 12 months | 1.4 (0.6) | 1.3 (0.5) | 1.6 (0.5) | 0.001 |
| SOPA Control, mean (SD) | ||||
| Baseline | 2.2 (0.8) | 2.3 (0.5) | 2.0 (0.7) | 0.19 |
| 6 months | 3.0 (0.6) | 2.9 (0.7) | 2.4 (0.8) | 0.0003 |
| 12 months | 2.9 (0.6) | 2.9 (0.6) | 2.4 (0.6) | <0.001 |
| TMD Self-efficacy, mean (SD) | ||||
| Baseline | 6.0 (1.9) | 6.1 (1.7) | 5.6 (1.9) | 0.38 |
| 6 months | 8.1 (1.5) | 7.8 (2.0) | 6.5 (1.9) | <0.001 |
| 12 months | 8.1 (1.6) | 8.3 (1.4) | 6.6 (1.8) | <0.001 |
| CSQ Catastrophizing, % (n) with score of 0 | ||||
| Baseline | 10 (5) | 15 (7) | 12 (6) | 0.74 |
| 6 months | 36 (18) | 46 (21) | 22 (10) | 0.04 |
| 12 months | 44 (21) | 39 (18) | 38 (18) | 0.71 |
| Perceived effectiveness of pain coping strategies, mean (SD) | ||||
| Baseline | 2.7 (1.0) | 2.8 (0.9) | 2.6 (1.1) | 0.44 |
| 6 months | 4.2 (0.8) | 4.2 (1.1) | 3.4 (1.5) | 0.004 |
| 12 months | 4.2 (0.9) | 4.2 (1.1) | 3.7 (1.1) | 0.02 |
COCT, Continuous Oral Contraceptive Therapy; CSQ, Coping Strategies Questionnaire; PCS, Pain Catastrophizing Scale; SMT, Self-Management Training; SOPA, Survey of Pain Attitudes; TMD, temporomandibular disorder; TSMT, Targeted Self-Management Training Note: The SOPA was added to the questionnaires after the study began; as a result, data were missing for the SOPA for 15 participants at baseline and 13 at post-treatment. Statistical tests adjusted for baseline exclude these participants.
Values in table are observed values for the analysis sample
P-values for baseline comparisons are from ANOVAs for continuous variables and chi-square tests for categorical variables, P-values for post and 12-month comparisons are from linear regression analyses for continuous variables and logistic regression analyses for the categorical variable adjusting for baseline values of the measure.
3.6. Adverse events
No adverse events were reported in the two self-management groups. In the COCT group, among the 36 women who started the medication, 17 (47%) reported break-through bleeding or spotting, four (11%) reported increased appetite or weight gain, four (11%) reported increased moodiness, three (8%) reported breast tenderness, and three (8%) reported increased acne.
3.7. As-treated analyses
The results of the as-treated analyses for characteristic pain intensity and activity interference at 12 months were very similar to those of the intent-to-treat analyses. Among the participants in the analysis sample who completed treatment per protocol (both sessions of TSMT [n=45] or SMT [n=45], or the course of the OC [n=25]), there was a significant overall treatment group difference in pain at 12 months (P = 0.009), with significantly lower pain intensity in the SMT and TSMT groups than in the COCT group (P < 0.05, Holm’s method). The adjusted (for baseline levels) mean difference in pain at 12 months was −1.1 (95% CI = −2.1, −0.1) between SMT and COCT, and −1.2 (−2.2, −0.2) between TSMT and COCT. These differences were slightly greater than those in the intent-to-treat analyses.
Also similar to the intent-to-treat results, the as-treated analyses demonstrated a significant treatment difference at 12 months in activity interference (P = 0.017), with significantly fewer SMT and TSMT than COCT participants reporting activity interference (P < 0.05, Holm’s method). The differences between the self-management groups and the medication group were greater in the as-treated analyses than in the intent-to-treat analyses. In the as-treated analyses, the odds of reporting no activity interference were 4 times greater in the SMT group (OR = 4.0; 95% CI = 1.1, 14.9) and TSMT group (OR = 4.3; 95% CI = 1.1, 15.9) than in the COCT group, as compared with approximately 3.0 in the intent-to-treat analyses.
The as-treated analyses, as compared with the intent-to-treat analyses, found somewhat more improvement in the COCT group at 6 months in pain and activity interference. For example, adjusted (for baseline levels) mean differences and adjusted (for multiple CIs, using Bonferroni method) CIs for pain at 6 months were 0.1 (−0.8, 1.1) for both SMT and TSMT versus COCT in the as-treated sample, as compared with −0.4 (−1.3, 0.4) for SMT versus COCT and −0.7 (−1.5, 0.2) for TSMT versus COCT in the intent-to-treat sample. However, differences between groups were not significant at 6 months in either sample.
The pattern of findings in the as-treated analyses with respect to rates of clinically significant improvement in pain (as shown in Table 5) was similar to that of the intent-to-treat analyses; that is, these rates were significantly lower in the COCT group than in the two self-management groups at 12 months, but the groups did not differ significantly at 6 months. The COCT subgroup of women who completed the study medication protocol showed rates of clinically meaningful improvement in pain that were similar to those of the entire intent-to-treat sample. For example, in the as-treated versus the intent-to-treat sample, 61% versus 54% of the women had pain ratings that reflected 30% or greater improvement in pain at 6 months; at 12 months, the corresponding rates were 32% versus 35%.
Table 5.
Rates of clinically meaningful improvement in pain, as-treated sample (participants who completed both self-management training in-person sessions or the medication per protocol)
| Timepoint | SMT | TSMT | COCT | P-valuea |
|---|---|---|---|---|
| ≥30% improvement | ||||
| 6 months | 73% | 64% | 61% | 0.26 |
| 12 months | 67% | 71% | 32% | 0.0013 |
| ≥50% improvement | ||||
| 6 months | 51% | 42% | 36% | 0.18 |
| 12 months | 42% | 55% | 8% | 0.002 |
COCT, Continuous Oral Contraceptive Therapy; SMT, Self-Management Training; TSMT, Targeted Self-Management Training
P-values are for the overall treatment group effect and calculated using logistic regression analysis.
The as-treated analyses also yielded similar findings to those of the intent-to-treat analyses for the process measures. One exception was that statistically significant treatment group differences in the perceived effectiveness of pain coping strategies in the intent-to-treat analyses at 6 and 12 months were no longer significant in the as-treated analyses. Adjusted COCT group mean ratings on this 0–6 scale were slightly higher in the as-treated subsample than in the intent-to-treat sample (3.8 versus 3.4 at 6 months and 3.9 versus 3.7 at 12 months), and closer to the SMT and TSMT ratings (4.1 – 4.3 at both timepoints and in both samples).
3.8. 6-month ratings of treatment helpfulness
Among treatment completers (the “as-treated” subsample), 6-month ratings of overall satisfaction with the treatment (0–10 scale) were lower in the COCT group (mean = 6.5, SD = 2.6) than in the SMT (8.3, 1.7) and TSMT (7.9, 2.2) groups, P = 0.004. There was a trend towards a significant group difference in ratings of overall improvement in facial pain due to the treatment (P = 0.07), with the lowest mean improvement rating in the COCT group (7.2) and the highest in the SMT group (8.1) (TSMT = 7.6). There were no significant differences between the TSMT and SMT groups on the ratings of helpfulness of specific shared components of their treatments. In the TSMT group, there was a wide range of ratings of helpfulness of planning for TMD pain increases at certain times in the menstrual cycle, although most women rated this as at least moderately helpful (observed range 0–10, mean = 6.4, SD = 3.0, median = 6.0, IQR = 5.0–9.0).
3.9. OC use at 12 months
At 12 months, the groups did not differ significantly in rates of use of traditional OCs (21 days of active pill and 7 “spacer” pills) (SMT 33%, TSMT 44%, COCT 23%; chi-square test, P = 0.65). Only one participant (a COCT group participant) was taking a continuous OC.
4. Discussion
Consistently across outcome measures of pain and pain-related activity interference, the three treatment groups did not differ significantly at 6 months but did differ significantly at 12 months, with better outcomes in the two self-management groups than in the COCT group and no difference between the standard self-management intervention and the self-management intervention focused on menstrual cycle-related changes in symptoms. Inspection of group mean scores at each assessment point indicates that the COCT group improved less than the two self-management groups at 6 months, but the difference was not of sufficient magnitude to be statistically significant. From 6 to 12 months, the COCT group showed a worsening on measures of pain and function, whereas the two self-management groups showed maintenance of gains or improvement.
The results further support our previous findings that a brief dental hygienist-delivered self-management intervention, based on CBT principles, has long-lasting benefits for improving TMD pain and pain-related activity interference, with greater benefits at one year than immediately post-treatment [11]. Prior studies have also found that patients with TMD pain who participate in cognitive-behavioral treatments show continued improvement in pain from post-treatment to 1-year follow-up, whereas patients who receive only usual dental care do not [14,32]. Furthermore, we previously observed in an RCT [50] that patients with TMD pain who received CBT showed greater improvement in pain and activity interference from post-treatment to 12 months than did those who received an education/attention control intervention. These results are consistent with the conclusions of a systematic review of psychological therapies for all types of chronic pain (except headaches) that, compared with active control conditions, CBT has positive effects on disability immediately post-treatment and at 6–12 month follow-up, and positive effects on pain and mood at follow-up, but not immediately post-treatment [16]. We can only speculate as to the reasons for this, but it may be that CBT results in decreased worry about and focus on pain, increased confidence in ability to manage pain and engage in activities without harm, and regular use of cognitive and behavioral coping skills, all of which may in turn result in gradual improvement in pain and activity participation over time such that maximal benefits are seen some months after the end of formal treatment.
In contrast to the lack of group differences on the pain and interference measures at 6 months, the study treatment groups differed significantly at 6 months on the depression and process measures. The two self-management groups had lower levels of depressive symptoms, higher scores on measures of adaptive beliefs, and lower scores on measures of maladaptive beliefs, as compared with the COCT group. These findings support the efficacy of the self-management interventions in changing beliefs in targeted ways, such that participants’ beliefs in their ability to control their pain increased and beliefs that they were disabled by their pain and that pain was a signal of harm decreased. The pattern of findings is consistent with changes in such beliefs mediating improvement in pain and function, as we have demonstrated in previous work [49].
We can only speculate as to why the self-management treatment targeted to menstrual cycle-related changes in pain was not superior to the standard treatment. Some women in the SMT group might have applied the SMT techniques on their own at critical times in their menstrual cycles and this might have diluted the benefits of TSMT over SMT. Anecdotally, the dental hygienists reported that some women in the SMT group spontaneously mentioned menstrual cycle-related fluctuations in their pain. Although the dental hygienists were instructed not to comment or focus on such observations, it is quite possible that some SMT participants became more aware of these relationships and applied self-management techniques at times in their cycles when symptoms increased or were about to increase.
It is also possible that some TSMT participants did not have fluctuations in pain related to their menstrual cycle or that the intervention was not successful in helping participants cope more effectively with menstrual cycle-related facial pain. There was a wide range of TSMT participants’ post-treatment ratings of helpfulness of planning for TMD pain increases at certain times in the menstrual cycle. Further research is needed to shed more light on the associations between hormonal fluctuations and TMD pain in women. Interesting questions to explore include whether hormonal fluctuations are more important in certain subtypes of TMD or certain subgroups of women with TMD pain, and whether such associations, when present, reflect direct influences of hormones on pain or are mediated by other symptoms, such as mood. Currently, it seems prudent to include assessment of menstrual cycle-related patterns of pain as part of any TMD pain self-management intervention for women with menstrual cycles, and to incorporate planning for such changes among women who observe them.
COCT participants who took the medication for the entire 6 months showed greater improvement in pain than did those who did not complete the protocol. The extent to which this reflects active specific effects of the medication on pain, nonspecific effects, or better outcomes for more adherent individuals is unclear. It is possible that the medication was more effective for a subgroup of women whose symptoms were more highly influenced by hormones and that this subgroup continued taking the medication because the benefits outweighed negative side effects.
We do not know whether we would have observed different results at 12 months had the COCT protocol called for 12 months on the medication. In designing the study, we decided to end each treatment by 6 months in order to equate the duration of the treatments; in each condition, women could continue using the therapy beyond 6 months by (in the COCT group) continuing on the medication on their own or (in the TSMT and SMT groups) continuing to apply the skills learned. Another reason for this design was the concern that women might not enroll in the study if the protocol called for a year on medication; we believed that a more appealing option might be 6 months, with the possibility of continuing on their own if desired. The fact that, at the 12-month follow-up, only one COCT participant had continued on COCT suggests that women did not find that the benefits of COCT outweighed the negative effects. Nonetheless, it is possible that, if the COCT group had continued on medication for 12 months, the worsening of outcomes observed in that group from 6 to 12 months, and the benefits of the self-management interventions over COCT at 12 months, might have been attenuated.
We encountered challenges in conducting a study involving hormonal manipulation for pain therapy. Many women declined to enroll in the study or withdrew after enrollment because they did not want to take the study medication. Furthermore, many women assigned to COCT decided after randomization that they did not want to take the medication, medication side effects were common, and many women who started the medication stopped prior to the end of the study protocol. Far more women preferred and adhered to self-management interventions as compared with medication - only about one-third of the women randomized to the COCT group completed the medication protocol, in contrast to the 77–82% rate of completion of both in-person sessions of the two self-management treatments. These observations, along with the superior benefits and safety of the self-management interventions relative to COCT, support the need for increased access to self-management interventions for TMD pain. It would be of interest to conduct cost-effectiveness studies of TMD pain self-management interventions as compared with usual TMD care and/or other specific therapies.
As with most RCTs, the extent to which the study findings can be generalized to patients with characteristics different from those in our sample is unclear, as is the extent to which the results might be replicated in community practice. In our sample at baseline, pain intensity scores were moderate and most participants had low scores on measures of activity interference, depression, and catastrophizing. In future studies enrolling individuals from non-tertiary care settings who are likely to report fairly low levels of depressive symptoms, it might be informative to include a measure of positive mood or wellbeing instead of, or in addition to, a measure of depression. Further research is needed to determine whether the interventions we studied would have different outcomes for patients reporting higher levels of pain, depression, and disability, and whether such patients might show greater benefits from more extensive self-management or cognitive-behavioral interventions.
Despite these limitations, the study makes a significant contribution by addressing what a comprehensive review [8] concluded was the greatest gap in human research on estrogenic modulation of pain: hormone manipulation studies. Finally, it is worth emphasizing that a limited contact (two in-person sessions plus six brief phone calls) treatment delivered by dental hygienists had clinically and statistically significant and long-lasting benefits. Implementation and evaluation of this intervention in other TMD clinical practice settings might be considered.
Synopsis.
Dental hygienist-delivered pain self-management training was superior to continuous oral contraceptive therapy for women with TMD pain; focusing on menstrually-related changes in symptoms did not increase its efficacy.
Acknowledgments
Funding for this study was provided by grant R01-DE16212 from the National Institute of Dental and Craniofacial Research. The authors gratefully acknowledge the invaluable assistance of Lauren Asaba, Sarah Cooley, Michel Daliva, Nancy Dorn, Melinda Lane, Heidi Matieri, Kathy Scott, and Drs. Leslie Miller and Edmond Truelove in the conduct of this study.
Footnotes
clinicaltrials.gov identifier: NCT00237042
Conflict of interest statement
None of the authors have any financial or other relationships that might lead to a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aggarwal VR, Tickle M, Javidi H, Peters S. Reviewing the evidence: can cognitive behavioral therapy improve outcomes for patients with chronic orofacial pain? J Orofac Pain. 2010;24:163–171. [PubMed] [Google Scholar]
- 2.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. In: Pichot P, editor. Modern Problems in Pharmacopsychiatry. Vol. 7. Basel, Switzerland: Karger; 1974. pp. 151–169. [DOI] [PubMed] [Google Scholar]
- 3.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- 4.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 5.Bernstein DA, Borkovec TD, Hazlett-Stevens H. New directions in progressive relaxation training. Westport, Connecticut: Praeger; 2000. [Google Scholar]
- 6.Brister H, Turner JA, Aaron LA, Mancl L. Self-efficacy is associated with pain, functioning, and coping among patients with chronic temporomandibular disorder pain. J Orofac Pain. 2006;20:115–124. [PubMed] [Google Scholar]
- 7.Coutinho EM, O’Dwyer E, Barbosa IC, Zhi-Ping G, Shaaban MM, Aboul-Oyoon M, Aleem HA. Comparative study on intermittent versus continuous use of a contraceptive pill administered by vaginal route. Contraception. 1995;51:355–358. doi: 10.1016/0010-7824(95)00101-f. [DOI] [PubMed] [Google Scholar]
- 8.Craft RM. Modulation of pain by estrogens. Pain. 2007;132(Supplement 1):S3–S12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Dozois DJA, Dobson KS, Wong M, Hughes D, Long A. Predictive utility of the CSQ in low back pain: individual vs. composite measures. Pain. 1996;66:171–180. doi: 10.1016/0304-3959(96)03058-8. [DOI] [PubMed] [Google Scholar]
- 10.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite J, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne RA, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Dworkin SF, Huggins KH, Wilson L, Mancl L, Turner J, Massoth D, LeResche L, Truelove E. A randomized clinical trial using Research Diagnostic Criteria for Temporomandibular Disorders-Axis II to target clinic cases for a tailored self-care TMD treatment program. J Orofac Pain. 2002;16:48–63. [PubMed] [Google Scholar]
- 12.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. Journal of Craniomandibular Disorders: Facial & Oral Pain. 1992;6:301–355. [PubMed] [Google Scholar]
- 13.Dworkin SF, Turner JA, Mancl L, Wilson L, Massoth D, Huggins KH, LeResche L, Truelove E. A randomized clinical trial of a tailored comprehensive care treatment program for temporomandibular disorders. J Orofac Pain. 2002;16:259–276. [PubMed] [Google Scholar]
- 14.Dworkin SF, Turner JA, Wilson L, Massoth D, Whitney C, Huggins KH, Burgess J, Sommers E, Truelove E. Brief group cognitive-behavioral intervention for temporomandibular disorders. Pain. 1994;59:175–187. doi: 10.1016/0304-3959(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 15.Dworkin SF, Von Korff M, Whitney CW, Le Resche L, Dicker BG, Barlow W. Measurement of characteristic pain intensity in field research. Pain. 1990;Suppl 5:S290. [Google Scholar]
- 16.Eccleston C, Williams A, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD007407.pub2. Article No. CD007407. [DOI] [PubMed] [Google Scholar]
- 17.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 18.Geisser ME, Robinson ME, Henson CD. The Coping Strategies Questionnaire and chronic pain adjustment: a conceptual and empirical reanalysis. Clin J Pain. 1994;10(2):98–106. doi: 10.1097/00002508-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin J Pain. 1997;13(2):163–170. doi: 10.1097/00002508-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez VM, Stewart A, Ritter PL, Lorig K. Translation and validation of arthritis outcome measures into Spanish. Arthritis Rheum. 1995;38:1429–1446. doi: 10.1002/art.1780381010. [DOI] [PubMed] [Google Scholar]
- 21.Grafton KV, Foster NE, Wright CC. Test-retest reliability of the Short-Form McGill Pain Questionnaire: Assessment of intraclass correlation coefficients and limits of agreement in patients With osteoarthritis. Clin J Pain. 2005;21(1):73–82. doi: 10.1097/00002508-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 23.Jensen MP, Karoly P. Control beliefs, coping efforts, and adjustment to chronic pain. J Consult Clin Psychol. 1991;59:431–438. doi: 10.1037//0022-006x.59.3.431. [DOI] [PubMed] [Google Scholar]
- 24.Jensen MP, Karoly P. Pain-specific beliefs, perceived symptom severity, and adjustment to chronic pain. Clin J Pain. 1992;8:123–130. doi: 10.1097/00002508-199206000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Jensen MP, Turner JA, Romano JM, Lawler BK. Relationship of pain-specific beliefs to chronic pain adjustment. Pain. 1994;57:301–309. doi: 10.1016/0304-3959(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 26.Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37:51–56. doi: 10.1016/0304-3959(89)90152-8. [DOI] [PubMed] [Google Scholar]
- 27.Keefe FJ, Caldwell DS, Queen KT, Gil KM, Martinez S, Crisson JE, Ogden W, Nunley J. Pain coping strategies in osteoarthritis patients. J Consult Clin Psychol. 1987;55:208–212. doi: 10.1037//0022-006x.55.2.208. [DOI] [PubMed] [Google Scholar]
- 28.Keefe FJ, Williams DA. A comparison of coping strategies in chronic pain patients in different age groups. J Gerontol. 1990;45:P161–165. doi: 10.1093/geronj/45.4.p161. [DOI] [PubMed] [Google Scholar]
- 29.LeResche L, Mancl L, Sherman J, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Lieba-Samal D, Wöber C, Frantal S, Brannath W, Schmidt K, Schrolnberger C, Wöber-Bingöl Ç. Headache, menstruation and combined oral contraceptives: A diary study in 184 women with migraine. European Journal of Pain. doi: 10.1016/j.ejpain.2011.02.003. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 31.List T, Axelsson S. Management of TMD: evidence from systematic reviews and meta-analyses. J Oral Rehab. 2010;37(6):430–451. doi: 10.1111/j.1365-2842.2010.02089.x. [DOI] [PubMed] [Google Scholar]
- 32.Litt MD, Shafer DM, Kreutzer DL. Brief cognitive-behavioral treatment for TMD pain: Long-term outcomes and moderators of treatment. Pain. 2010;151(1):110–116. doi: 10.1016/j.pain.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorig K, Stewart A, Ritter PL, Gonzalez VM, Laurent D, Lynch J. Outcome measures for health education and other health care interventions. Thousand Oaks, CA: Sage Publications; 1996. [Google Scholar]
- 34.Love AW. Depression in chronic low back pain patients: diagnostic efficiency of three self-report questionnaires. J Clin Psychol. 1987;43:84–89. [PubMed] [Google Scholar]
- 35.Martin MY, Bradley LA, Alexander RW, Alarcon GS, Triana-Alexander M, Aaron LA, Alberts KR. Coping strategies predict disability in patients with primary fibromyalgia. Pain. 1996;68(1):45–53. doi: 10.1016/S0304-3959(96)03179-X. [DOI] [PubMed] [Google Scholar]
- 36.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 37.Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol. 2003;101:653–661. doi: 10.1016/s0029-7844(03)00014-0. [DOI] [PubMed] [Google Scholar]
- 38.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rollman GB, Gillespie JM. The role of psychosocial factors in temporomandibular disorders. Curr Rev Pain. 2000;4:71–81. doi: 10.1007/s11916-000-0012-8. [DOI] [PubMed] [Google Scholar]
- 40.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 41.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 42.Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet. 2005;365:1591–1595. doi: 10.1016/S0140-6736(05)66461-6. [DOI] [PubMed] [Google Scholar]
- 43.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. Journal of Neuroscience. 2006;26:5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strand LI, Ljunggren AE, Bogen B, Ask T, Johnsen TB. The Short-Form McGill Pain Questionnaire as an outcome measure: Test-retest reliability and responsiveness to change. European Journal of Pain. 2008;12(7):917–925. doi: 10.1016/j.ejpain.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Strong J, Ashton R, Chant D. The measurement of attitudes towards and beliefs about pain. Pain. 1992;48:227–236. doi: 10.1016/0304-3959(92)90062-G. [DOI] [PubMed] [Google Scholar]
- 46.Sulak PJ, Scow RD, Preece C, Riggs MW, Kuehl TJ. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol. 2000;95:261–266. doi: 10.1016/s0029-7844(99)00524-4. [DOI] [PubMed] [Google Scholar]
- 47.Syrjala KL. Relaxation and imagery techniques. In: Loeser JD, editor. Bonica’s Management of Pain. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1779–1788. [Google Scholar]
- 48.Turner JA, Dworkin SF, Mancl L, Huggins KH, Truelove EL. The roles of beliefs, catastrophizing, and coping in the functioning of patients with temporomandibular disorders. Pain. 2001;92:41–51. doi: 10.1016/s0304-3959(00)00469-3. [DOI] [PubMed] [Google Scholar]
- 49.Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain. 2007;127:276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Turner JA, Mancl L, Aaron LA. Short- and long-term efficacy of brief cognitive-behavioral therapy for patients with chronic temporomandibular disorder pain: a randomized, controlled trial. Pain. 2006;121:181–194. doi: 10.1016/j.pain.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 51.Turner JA, Romano JM. Self-report screening measures for depression in chronic pain patients. J Clin Psychol. 1984;40:909–913. doi: 10.1002/1097-4679(198407)40:4<909::aid-jclp2270400407>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 52.Turner JA, Romano JM. Cognitive-behavioral therapy for chronic pain. In: Loeser JD, editor. Bonica’s Management of Pain. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1751–1758. [Google Scholar]
- 53.Underwood MR, Barnett AG, Vickers MR. Evaluation of two time-specific back pain outcome measures. Spine. 1999;24:1104–1112. doi: 10.1097/00007632-199906010-00010. [DOI] [PubMed] [Google Scholar]
- 54.Von Korff M. Epidemiological and survey methods: assessment of chronic pain. In: Turk DC, Melzack R, editors. Handbook of pain assessment. New York: The Guilford Press; 2001. pp. 603–618. [Google Scholar]
- 55.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]