Abstract
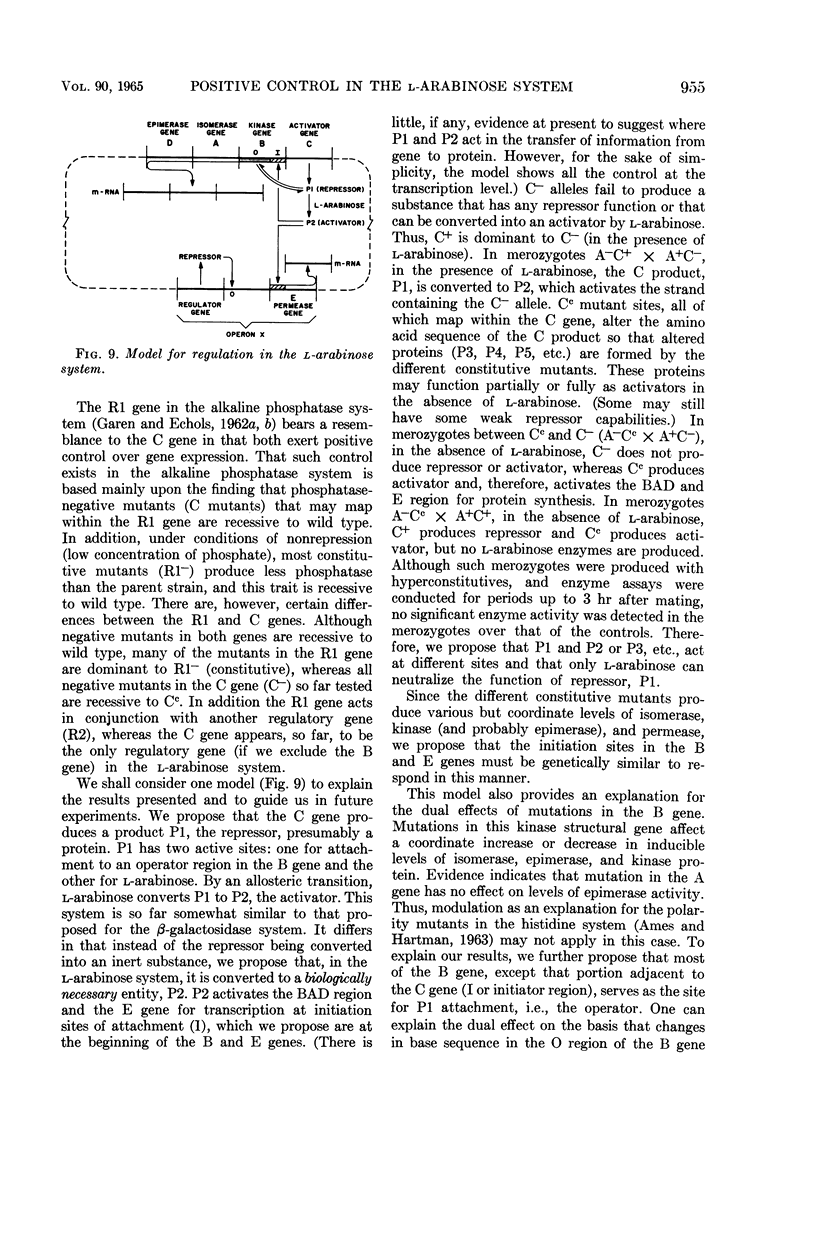
Englesberg, Ellis (University of Pittsburgh, Pittsburgh, Pa.), Joseph Irr, Joseph Power, and Nancy Lee. Positive control of enzyme synthesis by gene C in the l-arabinose system. J. Bacteriol 90:946–957. 1965.—The l-arabinose gene complex consists of genes D, A, B, and C, linked in that order between the markers thr and leu, and an unlinked gene E. Genes D, A, B, and E are the structural genes for three inducible enzymes and permease, respectively. Gene C, with two mutant alleles, C− and Cc, is the regulatory gene exhibiting positive and negative control. C− mutants are deficient and Cc mutants are constitutive for all three enzymes and permease. Complementation analysis, employing sexual merozygotes (A−C+ × A+C−), with six different C− mutants, demonstrates that C− is recessive to C+ (positive control). A total of 61 Cc mutants, isolated as clones resistant to d-fucose inhibition, are linked to the leu ara region of the chromosome, and the 22 Cc mutants that were analyzed in detail mapped within the C gene among the C− mutant sites. Cc mutants produce various but coordinate levels of the two enzymes measured, and permease. Complementation analysis (A−Cc × A+C−, A−Cc × A+C+) shows that Cc is dominant to C− (positive control) and recessive to C+ (negative control). Deletion mutants that extend into the C gene are l-arabinose permease-negative, thus supporting the positive regulatory role of the C gene. The name “activator gene” is proposed for genes of the C type to accentuate their positive role in gene expression. A working model consistent with these results is presented.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. RESTORATION OF OPERON ACTIVITY BY SUPPRESSORS. Biochim Biophys Acta. 1963 Sep 17;76:162–164. [PubMed] [Google Scholar]
- BOYER H., ENGLESBERG E., WEINBERG R. Direct selection of L-arabinose negative mutants of Escherichia coli strain B@rl. Genetics. 1962 Apr;47:417–425. doi: 10.1093/genetics/47.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER H. GENETIC CONTROL OF RESTRICTION AND MODIFICATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1652–1660. doi: 10.1128/jb.88.6.1652-1660.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRIBBS R., ENGLESBERG E. L-ARABINOSE NEGATIVE MUTANTS OF THE L-RIBULOKINASE STRUCTURAL GENE AFFECTING THE LEVELS OF L-ARABINOSE ISOMERASE IN ESCHERICHIA COLI. Genetics. 1964 Jan;49:95–108. doi: 10.1093/genetics/49.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., ANDERSON R. L., WEINBERG R., LEE N., HOFFEE P., HUTTENHAUER G., BOYER H. L-Arabinose-sensitive, L-ribulose 5-phosphate 4-epimerase-deficient mutants of Escherichia coli. J Bacteriol. 1962 Jul;84:137–146. doi: 10.1128/jb.84.1.137-146.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E. Enzymatic characterization of 17 L-arabinose negative mutants of Escherichia coli. J Bacteriol. 1961 Jun;81:996–1006. doi: 10.1128/jb.81.6.996-1006.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., WATSON J. A., HOFFEE P. A. The glucose effect and the relationship between glucose permease, acid phosphatase, and glucose resistance. Cold Spring Harb Symp Quant Biol. 1961;26:261–276. doi: 10.1101/sqb.1961.026.01.033. [DOI] [PubMed] [Google Scholar]
- FRANKLIN N. C., LURIA S. E. Transduction by bacteriophage P-1 and the properties of the lac genetic region in E. coli and S. dysenteriae. Virology. 1961 Nov;15:299–311. doi: 10.1016/0042-6822(61)90362-2. [DOI] [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Genetic control of induction of alkaline phosphatase synthesis in E. coli. Proc Natl Acad Sci U S A. 1962 Aug;48:1398–1402. doi: 10.1073/pnas.48.8.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., ENGLESBERG E. Determination of the order of mutational sites governing L-arabinose utilization in Escherichia coli B/r bv transduction with phage Plbt. Virology. 1959 Nov;9:314–331. doi: 10.1016/0042-6822(59)90125-4. [DOI] [PubMed] [Google Scholar]
- HELLING R. B., WEINBERG R. COMPLEMENTATION STUDIES OF ARABINOSE GENES IN ESCHERICHIA COLI. Genetics. 1963 Oct;48:1397–1410. doi: 10.1093/genetics/48.10.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E. Effect of metabolic activity on the glucose permease of bacterial cells. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1759–1765. doi: 10.1073/pnas.48.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- JACOB F., ULLMAN A., MONOD J. LE PROMOTEUR, 'EL'EMENT G'EN'ETIQUE N'ECESSAIRE 'A L'EXPRESSION D'UN OP'ERON. C R Hebd Seances Acad Sci. 1964 Mar 16;258:3125–3128. [PubMed] [Google Scholar]
- LEE N., ENGLESBERG E. COORDINATE VARIATIONS IN INDUCED SYNTHESES OF ENZYMES ASSOCIATED WITH MUTATIONS IN A STRUCTURAL GENE. Proc Natl Acad Sci U S A. 1963 Oct;50:696–702. doi: 10.1073/pnas.50.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE N., ENGLESBERG E. Dual effects of structural genes in Escherichia coli. Proc Natl Acad Sci U S A. 1962 Mar 15;48:335–348. doi: 10.1073/pnas.48.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ORIAS E., GARTNER T. K. SUPPRESSION OF A CLASS OF RII MUTANTS OF T4 BY A SUPPRESSOR OF A LAC -"OPERATOR NEGATIVE" MUTATION. Proc Natl Acad Sci U S A. 1964 Sep;52:859–864. doi: 10.1073/pnas.52.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ N. M. SUPPRESSION OF A LAC O-O MUTATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:996–1001. doi: 10.1128/jb.88.4.996-1001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLSON C., PERRIN D., COHN M., JACOB F., MONOD J. NON-INDUCIBLE MUTANTS OF THE REGULATOR GENE IN THE "LACTOSE" SYSTEM OF ESCHERICHIA COLI. J Mol Biol. 1964 Apr;8:582–592. doi: 10.1016/s0022-2836(64)80013-9. [DOI] [PubMed] [Google Scholar]
- WOLLMAN E. L., JACOB F., HAYES W. Conjugation and genetic recombination in Escherichia coli K-12. Cold Spring Harb Symp Quant Biol. 1956;21:141–162. doi: 10.1101/sqb.1956.021.01.012. [DOI] [PubMed] [Google Scholar]


