Abstract
Multiple system atrophy (MSA) is a fatal oligodendrogliopathy characterized by prominent α-synuclein inclusions resulting in a neuronal multisystem degeneration. Until recently MSA was widely conceived as a nongenetic disorder. However, during the last years a few postmortem verified Mendelian pedigrees have been reported consistent with monogenic disease in rare cases of MSA. Further, within the last 2 decades several genes have been associated with an increased risk of MSA, first and foremost the SNCA gene coding for α-synuclein. Moreover, genes involved in oxidative stress, mitochondrial dysfunction, inflammatory processes, as well as parkinsonism- and ataxia-related genes have been implicated as susceptibility factors. In this review, we discuss the emerging evidence in favor of genetic players in MSA.
Keywords: Multiple system atrophy, Glial cytoplasmic inclusions, Alpha-synuclein, Neurodegeneration, Ataxia, Parkinsonism
1. Introduction
Multiple system atrophy (MSA) is a progressive neurodegenerative movement disorder characterized by autonomic failure, poorly levodopa-responsive parkinsonism, cerebellar ataxia, and pyramidal symptoms in variable combinations. Neuronal cell loss in the basal ganglia, cerebellum, pontine and inferior olivary nuclei, pyramidal tract, intermediolateral cell column, and Onuf's nucleus as well as gliosis are typically observed (Wenning et al., 2004). MSA is commonly regarded as a primary oligodendrogliopathy (Wenning et al., 2008) because of widespread glial cytoplasmic inclusions (GCIs; Papp-Lantos bodies) that are seen even in brain areas without evident neuronal loss. GCIs were first identified in 1989 using Gallyas silver impregnation (Gallyas and Wolff, 1986; Papp et al., 1989) and were later shown to be immunoreactive for α-synuclein (Lantos, 1998; Wakabayashi et al., 1998), thereby linking MSA with other synucleinopathies, such as Parkinson's disease (PD) and dementia with Lewy bodies (DLB) (Spillantini et al., 1998). Genetic studies have shown that variants in the SNCA gene, coding for α-synuclein, are major risk factors for MSA (see section 4.1). This recent finding represents a major breakthrough in our understanding of MSA.
Apart from the role of the SNCA gene, the etiopathogenesis of MSA is still enigmatic. Interactions of genetic and environmental factors, similar to other complex, sporadic neurodegenerative diseases, are likely (Brown et al., 2005). In a few controlled studies, an increased risk of developing MSA conferred by occupational and daily habits, such as exposure to solvents, additives, plastic monomers, metals, and various other toxins (Gilman et al., 1998; Nee et al., 1991; Vanacore, 2005), as well as a history of farming (Brown et al., 2005; Vidal et al., 2008) has been observed. A recent study, however, questioned some of these associations (Vidal et al., 2008). In general, convincing findings from environmental studies are hard to obtain due to limiting factors such as recall (overreporting of exposure) and selection bias (patients with severe diseases are less able to participate) (Stefanova et al., 2009). Thus the role of environmental factors is far from clear.
A disease-causing gene has not been identified in the few postmortem proven MSA pedigrees that will be reviewed here; however, these families indicate that monogenic MSA may occur (Hara et al., 2007; Wullner et al., 2004). In this article, we provide an update on genetic studies in MSA and discuss how they may increase our understanding of the pathogenesis of this devastating disorder.
2. Familial and monogenic MSA
Familial aggregation has been consistently documented for PD (Thacker and Ascherio, 2008) but only rare reports of familial MSA exist (Hara et al., 2007; Soma et al., 2006; Wullner et al., 2004). A recent study found a higher frequency of parkinsonism among first-degree relatives of MSA patients (Vidal et al., 2010). This is consistent with earlier findings reporting that 13% of MSA patients had at least 1 first-degree or second-degree relative with parkinsonism (Wenning et al., 1993) and that higher frequencies of neurological disease occur among first-degree relatives of MSA patients (Nee et al., 1991). In contrast, a positive family history for PD has not been shown to be a risk factor for MSA (Vanacore et al., 2005).
Only a few familial cases with MSA have been reported. One German family with probable MSA affecting 2 members in 2 successive generations was described (Wullner et al., 2004). Genetic testing excluded spinocerebellar ataxia (SCA) types 1–3, 6, 7, and 17 as well as mutations in the α-synuclein gene (Ozawa et al., 1999; Wullner et al., 2004). One affected patient died subsequently and the diagnosis was confirmed according to standard neuropathological criteria (Trojanowski and Revesz, 2007; Wullner et al., 2009).
A second family was reported in which a female patient with probable MSA had a father who was diagnosed at first with cortical cerebellar atrophy (CCA) and after developing orthostatic hypotension a few months later with probable MSA (Soma et al., 2006). Pedigree structure in both of these families was consistent with autosomal dominant inheritance.
In 2007, 4 Japanese MSA families with multiple affected siblings were reported (Hara et al., 2007). One patient had definite MSA, 5 patients were diagnosed with probable MSA, and the remaining 2 patients had possible MSA. Because a consanguineous marriage was noted for 1 family, both men and women were affected and because none of the affected individuals were ascertained in successive generations, an autosomal recessive mode of inheritance is likely. Mutational analysis of the coding regions of SNCA, however, failed to identify any mutations (Hara et al., 2007).
SNCA missense and multiplication mutations are a rare cause of parkinsonism. To date only a few families have been described with duplication or triplication mutations involving this locus (Fuchs et al., 2007; Polymeropoulos et al., 1997; Singleton et al., 2003). Clinical symptoms of SNCA multiplication patients sometimes resemble symptoms usually seen in MSA, suggesting that the clinical phenotype can be more variable and does not necessarily resemble that of idiopathic PD (Fuchs et al., 2007). Furthermore, neuropathological studies in SNCA triplication cases demonstrated GCI-like inclusions in addition to Lewy bodies (Farrer et al., 2004; Gwinn-Hardy et al., 2000; Muenter et al., 1998; Waters and Miller, 1994).
In order to shed more light on the genetic background and a possible hereditary component of MSA, it is therefore crucial to ascertain informative families for systematic genetic screening. Efforts are underway to sequence the exome, which is the entire coding sequence of the genome, in familial MSA cases in an attempt to identify disease-causing mutations.
3. Genocopies of familial MSA
MSA patients share some clinical features, such as prominent ataxia, dysmetria, and eye movement abnormalities, with autosomal dominant spinocerebellar ataxias (SCAs) (Schols et al., 2004). For instance, an MSA-C-like presentation has been reported for a family with SCA1 triplet repeat expansion (Gilman et al., 1996). Neuropathological changes involved not only cerebellum and brainstem, but also basal ganglia, thalamus, and intermediolateral columns of the spinal cord; furthermore tau- and ubiquitin-positive GCIs were reported. Several features of the index patient, however, were found to be unusual for MSA including early disease onset, cerebellar and autonomic features in the absence of any pyramidal or extrapyramidal signs, as well as sparse argyrophillic inclusions positive for tau and ubiquitin. Unfortunately, α-synuclein immunostaining was unavailable at the time of the study and therefore a definitive diagnosis of MSA could not be made (Lantos, 1998; Trojanowski and Revesz, 2007).
Berciano and Ferrer (1996) reported ubiquitin-positive GCIs in a patient with familial olivoponto-cerebellar atrophy (OPCA). A molecular genetic analysis in the patient's son showed CAG repeat expansion in the SCA2 gene. The proband's material was re-examined and immunohistochemistry showed ubiquitin-positive, α-synuclein-negative GCI-like inclusions (Berciano and Ferrer, 2005). Hence, in the absence of α-synuclein-positive GCIs, it is recommended to avoid the use of the term MSA to designate any familial ataxia (Gilman et al., 1996).
An intriguing case of SCA3 resembling MSA-C was reported recently (Nirenberg et al., 2007). Pathological changes met the consensus criteria for definite MSA (Gilman et al., 1999), including the presence of typical, α-synuclein containing GCIs, as well as neurodegenerative changes in the olivopontocerebellar, striatal, and pyramidal motor system. However, SCA3 expansions were not detected in a study of 80 Caucasian subjects with the clinical diagnosis of MSA indicating that SCA3 expansions are not a common cause of MSA (Bandmann et al., 1997). Despite this observation, SCA3 gene variants might act as susceptibility factors for the development of MSA-C (Nirenberg et al., 2007).
SCA6 accounts for less than 10% of patients with sporadic adult-onset ataxia (Abele et al., 2002), and it may rarely be confused with MSA because of associated levodopa-refractory parkinsonism (Khan et al., 2005). A screening study in Japanese patients, however, did not reveal any individuals with trinucleotide repeat expansions in the SCA6 gene, indicating that SCA6 is not commonly associated with MSA (Furiya et al., 2005).
Factor and colleagues reported an ataxia patient with unstable CTA/CTG repeats in the SCA8 allele and a brain pathology consistent with MSA (Lantos and Papp, 1994) including ubiquitin- and α-synuclein-positive GCIs (Factor et al., 2005). Repeat expansions in this gene were not observed in a Japanese study, suggesting that SCA8-related neurodegeneration is a rare genocopy of MSA (Furiya et al., 2005).
SCA17 is rare among the dominant ataxias (Schols et al., 2004). Interestingly, pathogenic trinucleotide repeat expansions have been reported in several patients with SCA17-related neurodegeneration, presenting with MSA-like features such as ataxia, cerebellar atrophy, urinary incontinence, postural instability, and bradykinesia (Kim et al., 2009; Lin et al., 2007). However, a subsequent candidate gene screening study in Japanese MSA patients did not reveal pathogenic repeat expansions (Furiya et al., 2005).
The SCAs and other hereditary ataxias such as Friedreich's ataxia (FA) and fragile X-associated ataxia syndrome (FXTAS) can present as an apparently sporadic disorder. It has been shown that even in ataxia patients with a negative family history there is a 15%–20% chance of a mutation (Abele et al., 2002). For instance, a study of 112 sporadic, late-onset ataxia patients found that 32 patients (29%) met the clinical criteria of possible (7%) or probable (22%) MSA. The Friedreich's ataxia mutation was found in 5 patients (4%), the SCA2 mutation in 1 (1%), the SCA3 mutation in 2 (2%), and the SCA6 mutation in 7 patients (6%) (Abele et al., 2002).
Clinical overlap with MSA has also been reported for FXTAS because both disorders are characterized by mid-to late-onset cerebellar ataxia, levodopa-unresponsive parkinsonism, and autonomic features (Kamm et al., 2005). This similarity led to the assumption that a premutation in the fragile X mental retardation gene 1 (FMR1) could be a susceptibility gene mutation of MSA (Yabe et al., 2004). A study performed in Japanese MSA patients failed to support an association of FMR1 premutations and MSA (Yabe et al., 2004). Data from the European MSA study group also suggest that probable MSA is only rarely associated with FMR1 premutations and confusing FXTAS with MSA is very unlikely (Kamm et al., 2005).
In summary, genetic testing for SCA genes in MSA patients should not be considered as a routine clinical procedure, particularly because SCAs are generally of early-onset, slow in progression, and mainly present in patients with a positive family history. Nevertheless, presence of “red flags” raising doubts about a diagnosis of MSA should alert to the possibility of an inherited ataxia, in which case genetic testing for the above discussed genes can be initiated.
4. Risk loci in MSA
4.1. SNCA
The presence of α-synuclein immunoreactive GCIs in MSA places the disease amongst the broad category of synucleinopathies including PD as well as DLB (Spillantini et al., 1998), and because of its fundamental role in MSA pathology, subsequent genetic approaches focused on the corresponding SNCA gene. Sequencing studies, gene dosage measurements, microsatellite testing, as well as a haplotype tagging approach failed to demonstrate a significant association of SNCA variants with MSA (Lincoln et al., 2007; Morris et al., 2000; Ozawa et al., 1999, 2006). This might have been because of the small sample size involved in these studies or because of common misdiagnosis in clinically ascertained cases.
In 2009, Scholz and colleagues demonstrated by a candidate single nucleotide polymorphism (SNP) association study that genetic variants within the SNCA locus are associated with an increased risk for developing MSA in Caucasian individuals (most significant single nucleotide polymorphism rs11931074: p-value under recessive model = 5.5 × 10−12, odds ratio for homozygous risk allele carriers = 6.2 [95% confidence interval, 3.4–11.2]) (Scholz et al., 2009). This result has been subsequently replicated in an independent set of autopsy-proven MSA cases (Ross et al., 2010). Furthermore, genetic risk variants in SNCA have also been replicated by Al-Chalabi and colleagues (Al-Chalabi et al., 2009), although this study was not independent, as a large proportion of the investigated samples have been previously tested in the original study by Scholz and colleagues.
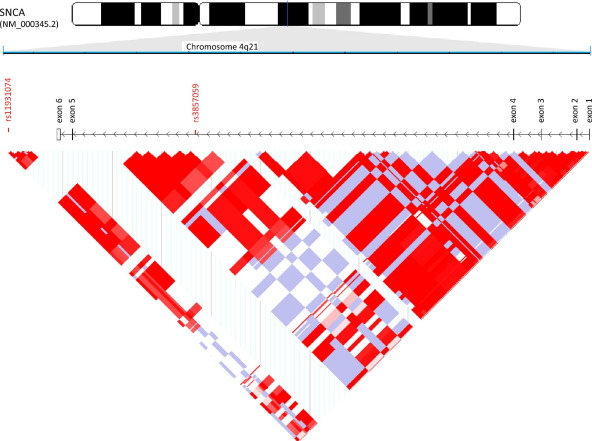
The identified risk variants are located in a haplotype block that extends from intron 4 to the 3′ untranslated region of the SNCA gene (Fig. 1) and are in strong linkage disequilibrium. It is of note, that the very same risk variants are also significantly associated with risk for PD (Satake et al., 2009; Simón-Sánchez et al., 2009), indicating shared pathogenic mechanisms between these 2 synucleinopathies.
Fig. 1.
Identified risk variants in a haplotype block extending from intron 4 to the 3′ untranslated region of the SNCA gene.
In contrast, a more recent South Korean study reported higher frequencies of the previously identified risk variants in their control cohort and failed to identify an association with disease risk among clinically diagnosed MSA patients (Yun et al., 2010). This observation suggests population heterogeneity at the SNCA locus similar to the heterogeneity described for the MAPT, LRRK2 or GBA loci in PD.
It is unclear how the identified SNCA risk haplotype confers risk to developing MSA, in particular because pathogenic mutations in the SNCA coding sequence could not be identified (Ozawa et al., 1999, 2006; Scholz et al., 2009). Although gene dosage measurements in MSA patients did not reveal SNCA duplications or triplications (Lincoln et al., 2007), modest changes in gene expression could still be possible. This notion is supported by the observation that duplication and triplication of SNCA in autosomal dominant PD families can lead to GCI-like inclusions and clinical features of MSA (Fuchs et al., 2007; Gwinn-Hardy et al., 2000; Singleton et al., 2003). Furthermore, transgenic animals overexpressing α-synuclein under oligodendroglial promoters have demonstrated neuropathological findings resembling MSA (Kahle et al., 2002; Shults et al., 2005; Yazawa et al., 2005).
4.2. Candidate gene studies
Until recently, the study of molecular genetic mechanisms involved in sporadic diseases was limited to candidate gene approaches. While many candidate gene studies have provided crucial insights into the pathogenesis of human disease, it is critical that the interpretation of results from candidate gene studies is handled with caution. This is particularly true for much of the past candidate gene research in MSA where most studies have a low power due to small sample sizes or lack replication stages. Table 1 provides a brief overview of candidate genes that have been tested to date. For the purpose of this review article, we will limit our discussion here to some of the major candidate genes.
Table 1.
Candidate gene studies in MSA
Key: ACT-A/A, α-1-antichymotrypsin; ADH, alcohol dehydrogenase; APOE, apolipoprotein E; ATF3, activating transcription factor 3; ATF4, activating transcription factor 4; BDNF, brain-derived neurotrophic factor; CARS, cysteinyl t-RNA synthetase; CEBPB, CCAAT/enhancer-binding protein-β; CHOP, CCAAT/enhancer-binding protein homologous protein; CNTF, ciliary neurotrophic factor; CYP1A1, cytochrome P450 1A1; CYP2D6, cytochrome P450 2D6; DAT1, dopamine transporter 1; DBH, dopamine-β-hydroxylase; DM2, myotonic dystrophy 2; EIF4EBP, eukaryotic translation initiation factor 4E-binding protein; FMR1, fragile X mental retardation 1; GSTM1, glutathione S-transferase M1; HLA, human leukocyte antigen; ICAM-1, intercellular adhesion molecule 1; IGF-1, insulin-like growth factor 1; IL, interleukin; LRRK2, leucine-rich repeat kinase 2; MAPT, microtubule-associated protein tau; MSA, multiple system atrophy; NAT2, N-acetyltransferase 2; PRGN, progranulin; SCA, spinocerebellar ataxia; SLC1A4, solute carrier family 1A4; SNCA, α-synuclein; SQSTM1, sequestosome 1; TGF-β1, transforming growth factor-β1; TNF-α, tumor necrosis factor-α; UCH-L1, ubiquitin carboxyl-terminal esterase L1.
Parkin and PTEN-induced putative kinase 1 (PINK1) mutations are the most common causes of autosomal recessive early-onset PD (Hatano et al., 2009; Nuytemans et al., 2010). A comprehensive mutation screening study investigating the role of genetic variants in parkin and PINK1 in pathology-proven MSA cases has been recently performed (Brooks et al., 2011). No clear pathogenic, homozygous mutations were identified, suggesting that genetic variants at these loci are not commonly associated with MSA (Brooks et al., 2011).
Genetic variability at the MAPT locus, coding for microtubule-associated protein tau, has been associated with a number of neurodegenerative diseases (Abraham et al., 2009; Poorkaj et al., 1998; Wider et al., 2010). Studies testing for a potential effect of MAPT variants on MSA failed to identify significant associations (Morris et al., 2000; Scholz et al., 2009). Along the same lines, mutations in the LRRK2 and GBA genes, known to be risk factors for PD (Lwin et al., 2004; Segarane et al., 2009; Sidransky, 2006), are not associated with MSA (Ozelius et al., 2007; Ross et al., 2006; Segarane et al., 2009; Tan et al., 2006).
Recently it has been suggested that oxidative and nitrative stress are associated with the onset and progression of α-synucleinopathies. This theory is based on the observation of nitrated α-synuclein in GCIs and neuronal cytoplasmic inclusions in MSA brain samples as well as in Lewy bodies and Lewy neurites of PD and DLB brains (Soma et al., 2008). Further, in vitro studies have revealed that nitric oxide and superoxide induce α-synuclein aggregation (Duda et al., 2000; Soma et al., 2008). Hence, genes involved in oxidative stress are interesting candidate genes for MSA. In a case-control study testing 8 candidate genes involved in oxidative stress significant associations were demonstrated for SLC1A4, SQSTM1, and finally EIF4EBPI (Soma et al., 2008). Replication studies testing these putative risk variants still need to be performed to draw final conclusions on the relevance of these findings.
Microglial activation has been reported to parallel the neuronal multisystem degeneration in MSA (Ishizawa et al., 2004), suggesting neuroinflammation as a key pathogenic mechanism. Activation of microglia produces cytokines, such as interleukin-1α (IL-1α), IL-1β, IL-6, tumor necrosis factor-α (TNF-α), chemokines such as IL-8, as well as inflammatory markers such as intercellular-adhesion molecule-1 (ICAM-1), all of which are known to contribute to tissue injuries (Wyss-Coray and Mucke, 2002). Thus, cytokine gene polymorphisms have been analyzed in several studies searching for genetic susceptibilities in MSA. Polymorphisms in IL-1α (Combarros et al., 2003), IL-1β (Nishimura et al., 2002), IL-8 (Infante et al., 2005), and ICAM-1 (Infante et al., 2005) were reported to be associated with an increased risk of MSA. A similar finding was also demonstrated for a polymorphism of the α-1-antichymotrypsin gene (Furiya et al., 2005), as well as for a promoter region polymorphism in the tumor necrosis factor gene (Nishimura et al., 2005b). These studies are important, because they point toward a possible role of neuroinflammation in disease pathogenesis. Nevertheless, they were performed with a small number of patients and should be repeated in larger, independent cohorts.
Other risk factors such as mutations in the alcohol dehydrogenase genes ADH1C and ADH7 have been analyzed in MSA patients. The ADHC1 G78X mutation was shown to be associated with MSA in the British, but not in the German population (Schmitt et al., 2006), whereas no significant associations were detected in ADH7 (Kim and Lee, 2003).
One of the latest discussions in neurological diseases center on the question of whether neurodegeneration can be caused in a cell-autonomous manner via independent formation of abnormal protein aggregates in affected brain cells, or whether propagation of protein misfolding occurs through mechanisms similar to those underlying prion diseases (Goedert et al., 2010). An intriguing case of MSA followed by sporadic prion disease has been published (Shibao et al., 2008). Genetic analysis of this patient did not reveal any mutations in the prion protein gene (PRNP), but it was noted that the proband was MM homozygous for the common M129V polymorphism (Shibao et al., 2008). A case-control study was initiated to investigate a possible connection between the M129V polymorphism and MSA (Shibao et al., 2008). The study revealed that the homozygotes are associated with an increased risk of MSA and earlier disease onset compared with PD subjects; however no association was observed when genotype frequencies were compared with matched controls.
Cardinal features of MSA include symptoms of progressive autonomic failure (Gilman et al., 1998). Internal body functions are maintained and regulated by the autonomic nervous system (ANS) with norepinephrine (NE) as its major neurotransmitter. Hence deficiency in NE, caused by mutations in the dopamine-β-hydroxylase (DBH) gene, may be associated with disorders of autonomic function (Cho et al., 2003). In an association study, patients with orthostatic intolerance, pure autonomic failure, MSA, and controls were genotyped for 7 mutations in the DBH gene. No mutations were found, suggesting that the major pathogenic mechanisms involved in NE deficiency and other autonomic disorders are fundamentally different (Cho et al., 2003).
Myotonic dystrophy type 2 (DM2), a slowly progressive multisystem disorder, has been recently associated with Parkinsonism (Annic et al., 2008). Lim and colleagues reported a case of clinically probable MSA-P, who developed muscle weakness and genetic testing confirmed 1 abnormal and 1 normal allele of the ZNF9 gene (Lim et al., 2009). This is so far the only report on a MSA patient with abnormalities in the ZNF9 gene.
Likewise, apolipoprotein E (APOE), associated with Alzheimer's disease (AD) and DLB, is not considered to be a player in MSA pathogenesis and does not contribute to an earlier disease onset (Cairns et al., 1997; Morris et al., 2000, 2001). No disease association has furthermore been detected for genetic variants of the progranulin (PRGN) gene (Yu et al., 2010).
5. General considerations and limitations of current studies
Genetic investigation in an apparently sporadic, rare disease comes with a number of challenges. First, large collections of DNA and tissue samples are required for sufficiently powered studies. This hurdle can be overcome by depositing samples to brain and DNA banks. Second, the availability of pathology-proven samples is crucial due to the high misdiagnosis rate in clinically ascertained cases. These samples are particularly important to validate potential risk variants in replication studies. Third, linkage studies, the standard genetic tool for disease gene identification, are not feasible in sporadic cases. However, recent advancements in neurogenomics have introduced genome-wide association studies and next-generation sequencing techniques as promising new tools for studying the genetic underpinnings of sporadic disease.
6. Conclusions
Within the last 2 decades, extensive studies investigating the role of genetic players in the pathogenesis of MSA have established variants in the SNCA locus as the only confirmed risk factor in the pathogenesis of MSA. Although other candidate genes have been implicated, independent replication studies are still necessary to confirm or refute these observations. To date, no protein-changing Mendelian gene mutations have been identified in rare families of MSA. More advanced genetic tools such as genome-wide association studies and next-generation sequencing are likely to unfold the mysterious nature of the beast (Quinn, 1989).
Disclosure statement
The authors declare that there are no actual or potential conflicts of interest.
Acknowledgements
This work has been supported in part by the Austrian Research Foundation graduate program SPIN (FWF W1206) and in part by the Intramural Research Program of the National Institute on Aging; project numbers Z01-AG000957-06 (SWS, ABS).
References
- Abele M., Bürk K., Schöls L., Schwartz S., Besenthal I., Dichgans J., Zühlke C., Riess O., Klockgether T. The aetiology of sporadic adult-onset ataxia. Brain. 2002;125:961–968. doi: 10.1093/brain/awf107. [DOI] [PubMed] [Google Scholar]
- Abraham R., Sims R., Carroll L., Hollingworth P., O'Donovan M.C., Williams J., Owen M.J. An association study of common variation at the MAPT locus with late-onset Alzheimer's disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009;150B:1152–1155. doi: 10.1002/ajmg.b.30951. [DOI] [PubMed] [Google Scholar]
- Al-Chalabi A., Dürr A., Wood N.W., Parkinson M.H., Camuzat A., Hulot J.S., Morrison K.E., Renton A., Sussmuth S.D., Landwehrmeyer B.G., Ludolph A., Agid Y., Brice A., Leigh P.N., Bensimon G., NNIPPS Genetic Study Group Genetic variants of the alpha-synuclein gene SNCA are associated with multiple system atrophy. PLoS One. 2009;4:e7114. doi: 10.1371/journal.pone.0007114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annic A., Devos D., Destée A., Defebvre L., Lacour A., Hurtevent J.F., Stojkovic T. Early dopasensitive Parkinsonism related to myotonic dystrophy type 2. Mov. Disord. 2008;23:2100–2101. doi: 10.1002/mds.22239. [DOI] [PubMed] [Google Scholar]
- Bandmann O., Sweeney M.G., Daniel S.E., Wenning G.K., Quinn N., Marsden C.D., Wood N.W. Multiple-system atrophy is genetically distinct from identified inherited causes of spinocerebellar degeneration. Neurology. 1997;49:1598–1604. doi: 10.1212/wnl.49.6.1598. [DOI] [PubMed] [Google Scholar]
- Bandmann O., Wenning G.K., Quinn N.P., Harding A.E. Arg296 to Cys296 polymorphism in exon 6 of cytochrome P-450-2D6 (CYP2D6) is not associated with multiple system atrophy. J. Neurol. Neurosurg. Psychiatry. 1995;59:557. doi: 10.1136/jnnp.59.5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berciano J., Ferrer I. Glial and neuronal cytoplasmic inclusions in familial olivopontocerebellar atrophy. Ann. Neurol. 1996;40:819–820. doi: 10.1002/ana.410400524. [DOI] [PubMed] [Google Scholar]
- Berciano J., Ferrer I. Glial cell cytoplasmic inclusions in SCA2 do not express alpha-synuclein. J. Neurol. 2005;252:742–744. doi: 10.1007/s00415-005-0747-6. [DOI] [PubMed] [Google Scholar]
- Berciano J., Infante J., García A., Polo J.M., Volpini V., Combarros O. Very late-onset Friedreich's ataxia with minimal GAA1 expansion mimicking multiple system atrophy of cerebellar type. Mov. Disord. 2005;20:1643–1645. doi: 10.1002/mds.20644. [DOI] [PubMed] [Google Scholar]
- Brooks J.A., Houlden H., Melchers A., Islam A.J., Ding J., Li A., Paudel R., Revesz T., Holton J.L., Wood N., Lees A., Singleton A.B., Scholz S.W. Mutational analysis of parkin and PINK1 in multiple system atrophy. Neurobiol. Aging. 2011;32:548.e5–548.e7. doi: 10.1016/j.neurobiolaging.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.C., Lockwood A.H., Sonawane B.R. Neurodegenerative diseases: an overview of environmental risk factors. Environ. Health Perspect. 2005;113:1250–1256. doi: 10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buervenich S., Sydow O., Carmine A., Zhang Z., Anvret M., Olson L. Alcohol dehydrogenase alleles in Parkinson's disease. Mov. Disord. 2000;15:813–818. doi: 10.1002/1531-8257(200009)15:5<813::aid-mds1008>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Cairns N.J., Atkinson P.F., Kovács T., Lees A.J., Daniel S.E., Lantos P.L. Apolipoprotein E e4 allele frequency in patients with multiple system atrophy. Neurosci. Lett. 1997;221:161–164. doi: 10.1016/s0304-3940(96)13316-4. [DOI] [PubMed] [Google Scholar]
- Cho J.W., Kim S.Y., Park S.S., Jeon B.S. Spinocerebellar ataxia type 12 was not found in Korean Parkinsonian patients. Can. J. Neurol. Sci. 2008;35:488–490. doi: 10.1017/s0317167100009161. [DOI] [PubMed] [Google Scholar]
- Cho J.W., Kim S.Y., Park S.S., Jeon B.S. The G2019S LRRK2 Mutation is Rare in Korean Patients with Parkinson's Disease and Multiple System Atrophy. Clin. Neurol. 2009;5:29–32. doi: 10.3988/jcn.2009.5.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S., Kim C.H., Cubells J.F., Zabetian C.P., Hwang D.Y., Kim J.W., Cohen B.M., Biaggioni I., Robertson D., Kim K.S. Variations in the dopamine beta-hydroxylase gene are not associated with the autonomic disorders, pure autonomic failure, or multiple system atrophy. Am. J. Med. Genet. A. 2003;120A:234–236. doi: 10.1002/ajmg.a.20194. [DOI] [PubMed] [Google Scholar]
- Combarros O., Infante J., Llorca J., Berciano J. Interleukin-1A (-889) genetic polymorphism increases the risk of multiple system atrophy. Mov. Disord. 2003;18:1385–1386. doi: 10.1002/mds.10540. [DOI] [PubMed] [Google Scholar]
- Duda J.E., Giasson B.I., Chen Q., Gur T.L., Hurtig H.I., Stern M.B., Gollomp S.M., Ischiropoulos H., Lee V.M., Trojanowski J.Q. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am. J. Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor S.A., Qian J., Lava N.S., Hubbard J.D., Payami H. False-positive SCA8 gene test in a patient with pathologically proven multiple system atrophy. Ann. Neurol. 2005;57:462–463. doi: 10.1002/ana.20389. [DOI] [PubMed] [Google Scholar]
- Farrer M., Kachergus J., Forno L., Lincoln S., Wang D.S., Hulihan M., Maraganore D., Gwinn-Hardy K., Wszolek Z., Dickson D., Langston J.W. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann. Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- Fuchs J., Nilsson C., Kachergus J., Munz M., Larsson E.M., Schüle B., Langston J.W., Middleton F.A., Ross O.A., Hulihan M., Gasser T., Farrer M.J. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology. 2007;68:916–922. doi: 10.1212/01.wnl.0000254458.17630.c5. [DOI] [PubMed] [Google Scholar]
- Furiya Y., Hirano M., Kurumatani N., Nakamuro T., Matsumura R., Futamura N., Ueno S. Alpha-1-antichymotrypsin gene polymorphism and susceptibility to multiple system atrophy (MSA) Brain Res. Mol. Brain Res. 2005;138:178–181. doi: 10.1016/j.molbrainres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Gallyas F., Wolff J.R. Metal-catalyzed oxidation renders silver intensification selective: Applications for the histochemistry of diaminobenzidine and neurofibrillary changes. J. Histochem. Cytochem. 1986;34:1667–1672. doi: 10.1177/34.12.3537114. [DOI] [PubMed] [Google Scholar]
- Gilman S., Low P.A., Quinn N., Albanese A., Ben-Shlomo Y., Fowler C.J., Kaufmann H., Klockgether T., Lang A.E., Lantos P.L., Litvan I., Mathias C.J., Oliver E., Robertson D., Schatz I., Wenning G.K. Consensus statement on the diagnosis of multiple system atrophy. J. Auton. Nerv. Syst. 1998;74:189–192. [PubMed] [Google Scholar]
- Gilman S., Low P.A., Quinn N., Albanese A., Ben-Shlomo Y., Fowler C.J., Kaufmann H., Klockgether T., Lang A.E., Lantos P.L., Litvan I., Mathias C.J., Oliver E., Robertson D., Schatz I., Wenning G.K. Consensus statement on the diagnosis of multiple system atrophy. J. Neurol. Sci. 1999;163:94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- Gilman S., Sima A.A., Junck L., Kluin K.J., Koeppe R.A., Lohman M.E., Little R. Spinocerebellar ataxia type 1 with multiple system degeneration and glial cytoplasmic inclusions. Ann. Neurol. 1996;39:241–255. doi: 10.1002/ana.410390214. [DOI] [PubMed] [Google Scholar]
- Goedert M., Clavaguera F., Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33:317–325. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Goker-Alpan O., Giasson B.I., Eblan M.J., Nguyen J., Hurtig H.I., Lee V.M., Trojanowski J.Q., Sidransky E. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67:908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- Gwinn-Hardy K., Mehta N.D., Farrer M., Maraganore D., Muenter M., Yen S.H., Hardy J., Dickson D.W. Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol. 2000;99:663–672. doi: 10.1007/s004010051177. [DOI] [PubMed] [Google Scholar]
- Hara K., Momose Y., Tokiguchi S., Shimohata M., Terajima K., Onodera O., Kakita A., Yamada M., Takahashi H., Hirasawa M., Mizuno Y., Ogata K., Goto J., Kanazawa I., Nishizawa M., Tsuji S. Multiplex families with multiple system atrophy. Arch. Neurol. 2007;64:545–551. doi: 10.1001/archneur.64.4.545. [DOI] [PubMed] [Google Scholar]
- Hatano T., Kubo S., Sato S., Hattori N. Pathogenesis of familial Parkinson's disease: new insights based on monogenic forms of Parkinson's disease. J. Neurochem. 2009;111:1075–1093. doi: 10.1111/j.1471-4159.2009.06403.x. [DOI] [PubMed] [Google Scholar]
- Healy D.G., Abou-Sleiman P.M., Quinn N., Ahmadi K.R., Ozawa T., Kamm C., Wullner U., Oertel W.H., Burk K., Dupont E., Pellecchia M.T., Tolosa E., Gasser T., Holton J.L., Revesz T., Goldstein D.B., Lees A.J., Wood N.W. UCHL-1 gene in multiple system atrophy: a haplotype tagging approach. Mov. Disord. 2005;20:1338–1343. doi: 10.1002/mds.20575. [DOI] [PubMed] [Google Scholar]
- Infante J., Llorca J., Berciano J., Combarros O. Interleukin-8, intercellular adhesion molecule-1 and tumour necrosis factor-alpha gene polymorphisms and the risk for multiple system atrophy. J. Neurol. Sci. 2005;228:11–13. doi: 10.1016/j.jns.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Ishizawa K., Komori T., Sasaki S., Arai N., Mizutani T., Hirose T. Microglial activation parallels system degeneration in multiple system atrophy. J. Neuropathol. Exp. Neurol. 2004;63:43–52. doi: 10.1093/jnen/63.1.43. [DOI] [PubMed] [Google Scholar]
- Iwahashi K., Miyatake R., Tsuneoka Y., Matsuo Y., Ichikawa Y., Hosokawa K., Sato K., Hayabara T. A novel cytochrome P-450IID6 (CYPIID6) mutant gene associated with multiple system atrophy. J. Neurol. Neurosurg. Psychiatry. 1995;58:263–264. doi: 10.1136/jnnp.58.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle P.J., Neumann M., Ozmen L., Muller V., Jacobsen H., Spooren W., Fuss B., Mallon B., Macklin W.B., Fujiwara H., Hasegawa M., Iwatsubo T., Kretzschmar H.A., Haass C. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm C., Healy D.G., Quinn N.P., Wüllner U., Moller J.C., Schols L., Geser F., Burk K., Børglum A.D., Pellecchia M.T., Tolosa E., del Sorbo F., Nilsson C., Bandmann O., Sharma M., Mayer P., Gasteiger M., Haworth A., Ozawa T., Lees A.J., Short J., Giunti P., Holinski-Feder E., Illig T., Wichmann H.E., Wenning G.K., Wood N.W., Gasser T., European Multiple System Atrophy Study Group The fragile X tremor ataxia syndrome in the differential diagnosis of multiple system atrophy: data from the EMSA Study Group. Brain. 2005;128:1855–1860. doi: 10.1093/brain/awh535. [DOI] [PubMed] [Google Scholar]
- Khan N.L., Giunti P., Sweeney M.G., Scherfler C., Brien M.O., Piccini P., Wood N.W., Lees A.J. Parkinsonism and nigrostriatal dysfunction are associated with spinocerebellar ataxia type 6 (SCA6) Mov. Disord. 2005;20:1115–1119. doi: 10.1002/mds.20564. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Lee M.S. Frequencies of single nucleotide polymorphism in alcohol dehydrogenase7 gene in patients with multiple system atrophy and controls. Mov. Disord. 2003;18:1065–1067. doi: 10.1002/mds.10500. [DOI] [PubMed] [Google Scholar]
- Kim J.Y., Kim S.Y., Kim J.M., Kim Y.K., Yoon K.Y., Kim J.Y., Lee B.C., Kim J.S., Paek S.H., Park S.S., Kim S.E., Jeon B.S. Spinocerebellar ataxia type 17 mutation as a causative and susceptibility gene in parkinsonism. Neurology. 2009;72:1385–1389. doi: 10.1212/WNL.0b013e3181a18876. [DOI] [PubMed] [Google Scholar]
- Lantos P.L. The definition of multiple system atrophy: a review of recent developments. J. Neuropathol. Exp. Neurol. 1998;57:1099–1111. doi: 10.1097/00005072-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Lantos P.L., Papp M.I. Cellular pathology of multiple system atrophy: a review. J. Neurol. Neurosurg. Psychiatry. 1994;57:129–133. doi: 10.1136/jnnp.57.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.Y., Wadia P., Wenning G.K., Lang A.E. Clinically probable multiple system atrophy with predominant parkinsonism associated with myotonic dystrophy type 2. Mov. Disord. 2009;24:1407–1409. doi: 10.1002/mds.22625. [DOI] [PubMed] [Google Scholar]
- Lin I.S., Wu R.M., Lee-Chen G.J., Shan D.E., Gwinn-Hardy K. The SCA17 phenotype can include features of MSA-C, PSP and cognitive impairment. Parkinsonism Relat. Disord. 2007;13:246–249. doi: 10.1016/j.parkreldis.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Lincoln S.J., Ross O.A., Milkovic N.M., Dickson D.W., Rajput A., Robinson C.A., Papapetropoulos S., Mash D.C., Farrer M.J. Quantitative PCR-based screening of alpha-synuclein multiplication in multiple system atrophy. Parkinsonism Relat. Disord. 2007;13:340–342. doi: 10.1016/j.parkreldis.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwin A., Orvisky E., Goker-Alpan O., LaMarca M.E., Sidransky E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol. Genet. Metab. 2004;81:70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Morris H.R., Schrag A., Nath U., Burn D., Quinn N.P., Daniel S., Wood N.W., Lees A.J. Effect of ApoE and tau on age of onset of progressive supranuclear palsy and multiple system atrophy. Neurosci. Lett. 2001;312:118–120. doi: 10.1016/s0304-3940(01)02190-5. [DOI] [PubMed] [Google Scholar]
- Morris H.R., Vaughan J.R., Datta S.R., Bandopadhyay R., Rohan De Silva H.A., Schrag A., Cairns N.J., Burn D., Nath U., Lantos P.L., Daniel S., Lees A.J., Quinn N.P., Wood N.W. Multiple system atrophy/progressive supranuclear palsy: alpha-Synuclein, synphilin, tau, and APOE. Neurology. 2000;55:1918–1920. doi: 10.1212/wnl.55.12.1918. [DOI] [PubMed] [Google Scholar]
- Muenter M.D., Forno L.S., Hornykiewicz O., Kish S.J., Maraganore D.M., Caselli R.J., Okazaki H., Howard F.M., Jr, Snow B.J., Calne D.B. Hereditary form of parkinsonism–dementia. Ann. Neurol. 1998;43:768–781. doi: 10.1002/ana.410430612. [DOI] [PubMed] [Google Scholar]
- Nee L.E., Gomez M.R., Dambrosia J., Bale S., Eldridge R., Polinsky R.J. Environmental-occupational risk factors and familial associations in multiple system atrophy: a preliminary investigation. Clin. Auton. Res. 1991;1:9–13. doi: 10.1007/BF01826052. [DOI] [PubMed] [Google Scholar]
- Nicholl D.J., Bennett P., Hiller L., Bonifati V., Vanacore N., Fabbrini G., Marconi R., Colosimo C., Lamberti P., Stocchi F., Bonuccelli U., Vieregge P., Ramsden D.B., Meco G., Williams A.C. A study of five candidate genes in Parkinson's disease and related neurodegenerative disorders: European Study Group on Atypical Parkinsonism. Neurology. 1999;53:1415–1421. doi: 10.1212/wnl.53.7.1415. [DOI] [PubMed] [Google Scholar]
- Nirenberg M.J., Libien J., Vonsattel J.P., Fahn S. Multiple system atrophy in a patient with the spinocerebellar ataxia 3 gene mutation. Mov. Disord. 2007;22:251–254. doi: 10.1002/mds.21231. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Kawakami H., Komure O., Maruyama H., Morino H., Izumi Y., Nakamura S., Kaji R., Kuno S. Contribution of the interleukin-1beta gene polymorphism in multiple system atrophy. Mov. Disord. 2002;17:808–811. doi: 10.1002/mds.10124. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Kuno S., Kaji R., Kawakami H. Brain-derived neurotrophic factor gene polymorphisms in Japanese patients with sporadic Alzheimer's disease, Parkinson's disease, and multiple system atrophy. Mov. Disord. 2005;20:1031–1033. doi: 10.1002/mds.20491. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Kuno S., Kaji R., Kawakami H. Influence of a tumor necrosis factor gene polymorphism in Japanese patients with multiple system atrophy. Neurosci. Lett. 2005;374:218–221. doi: 10.1016/j.neulet.2004.10.056. [DOI] [PubMed] [Google Scholar]
- Nuytemans K., Theuns J., Cruts M., Van Broeckhoven C. Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum. Mutat. 2010;31:763–780. doi: 10.1002/humu.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T., Healy D.G., Abou-Sleiman P.M., Ahmadi K.R., Quinn N., Lees A.J., Shaw K., Wullner U., Berciano J., Moller J.C., Kamm C., Burk K., Josephs K.A., Barone P., Tolosa E., Goldstein D.B., Wenning G., Geser F., Holton J.L., Gasser T., Revesz T., Wood N.W. The alpha-synuclein gene in multiple system atrophy. J. Neurol. Neurosurg. Psychiatry. 2006;77:464–467. doi: 10.1136/jnnp.2005.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T., Takano H., Onodera O., Kobayashi H., Ikeuchi T., Koide R., Okuizumi K., Shimohata T., Wakabayashi K., Takahashi H., Tsuji S. No mutation in the entire coding region of the alpha-synuclein gene in pathologically confirmed cases of multiple system atrophy. Neurosci. Lett. 1999;270:110–112. doi: 10.1016/s0304-3940(99)00475-9. [DOI] [PubMed] [Google Scholar]
- Ozelius L.J., Foroud T., May S., Senthil G., Sandroni P., Low P.A., Reich S., Colcher A., Stern M.B., Ondo W.G., Jankovic J., Huang N., Tanner C.M., Novak P., Gilman S., Marshall F.J., Wooten G.F., Chelimsky T.C., Shults C.W., North American Multiple System Atrophy Study Group G2019S mutation in the leucine-rich repeat kinase 2 gene is not associated with multiple system atrophy. Mov. Disord. 2007;22:546–549. doi: 10.1002/mds.21343. [DOI] [PubMed] [Google Scholar]
- Papp M.I., Kahn J.E., Lantos P.L. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J. Neurol. Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Planté-Bordeneuve V., Bandmann O., Wenning G., Quinn N.P., Daniel S.E., Harding A.E. CYP2D6-debrisoquine hydroxylase gene polymorphism in multiple system atrophy. Mov. Disord. 1995;10:277–278. doi: 10.1002/mds.870100307. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E.S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W.G., Lazzarini A.M., Duvoisin R.C., Di I.G., Golbe L.I., Nussbaum R.L. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Poorkaj P., Bird T.D., Wijsman E., Nemens E., Garruto R.M., Anderson L., Andreadis A., Wiederholt W.C., Raskind M., Schellenberg G.D. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Quinn N. Multiple system atrophy–the nature of the beast. J. Neurol. Neurosurg. Psychiatry Suppl. 1989:78–89. doi: 10.1136/jnnp.52.suppl.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross O.A., Toft M., Whittle A.J., Johnson J.L., Papapetropoulos S., Mash D.C., Litvan I., Gordon M.F., Wszolek Z.K., Farrer M.J., Dickson D.W. Lrrk2 and Lewy body disease. Ann. Neurol. 2006;59:388–393. doi: 10.1002/ana.20731. [DOI] [PubMed] [Google Scholar]
- Ross O.A., Vilariño-Güell C., Wszolek Z.K., Farrer M.J., Dickson D.W. Reply to: SNCA variants are associated with increased risk of multiple system atrophy. Ann. Neurol. 2010;67:414–415. doi: 10.1002/ana.21786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake W., Nakabayashi Y., Mizuta I., Hirota Y., Ito C., Kubo M., Kawaguchi T., Tsunoda T., Watanabe M., Takeda A., Tomiyama H., Nakashima K., Hasegawa K., Obata F., Yoshikawa T., Kawakami H., Sakoda S., Yamamoto M., Hattori N., Murata M., Nakamura Y., Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat. Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- Schmitt I., Wüllner U., Healy D.G., Wood N.W., Kölsch H., Heun R. The ADH1C stop mutation in multiple system atrophy patients and healthy probands in the United Kingdom and Germany. Mov. Disord. 2006;21:2034. doi: 10.1002/mds.21082. [DOI] [PubMed] [Google Scholar]
- Schöls L., Bauer P., Schmidt T., Schulte T., Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291–304. doi: 10.1016/S1474-4422(04)00737-9. [DOI] [PubMed] [Google Scholar]
- Scholz S.W., Houlden H., Schulte C., Sharma M., Li A., Berg D., Melchers A., Paudel R., Gibbs J.R., Simón-Sánchez J., Paisan-Ruiz C., Bras J., Ding J., Chen H., Traynor B.J., Arepalli S., Zonozi R.R., Revesz T., Holton J., Wood N., Lees A., Oertel W., Wullner U., Goldwurm S., Pellecchia M.T., Illig T., Riess O., Fernandez H.H., Rodriguez R.L., Okun M.S., Poewe W., Wenning G.K., Hardy J.A., Singleton A.B., Gasser T., del Sorbo F., Schneider S., Bhatia K.P. SNCA variants are associated with increased risk for multiple system atrophy. Ann. Neurol. 2009;65:610–614. doi: 10.1002/ana.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarane B., Li A., Paudel R., Scholz S., Neumann J., Lees A., Revesz T., Hardy J., Mathias C.J., Wood N.W., Holton J., Houlden H. Glucocerebrosidase mutations in 108 neuropathologically confirmed cases of multiple system atrophy. Neurology. 2009;72:1185–1186. doi: 10.1212/01.wnl.0000345356.40399.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibao C., Garland E.M., Gamboa A., Vnencak-Jones C.L., Van Woeltz M., Haines J.L., Yu C., Biaggioni I. PRNP M129V homozygosity in multiple system atrophy vs: Parkinson's disease. Clin. Auton. Res. 2008;18:13–19. doi: 10.1007/s10286-007-0447-7. [DOI] [PubMed] [Google Scholar]
- Shults C.W., Rockenstein E., Crews L., Adame A., Mante M., Larrea G., Hashimoto M., Song D., Iwatsubo T., Tsuboi K., Masliah E. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J. Neurosci. 2005;25:10689–10699. doi: 10.1523/JNEUROSCI.3527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E. Heterozygosity for a Mendelian disorder as a risk factor for complex disease. Clin. Genet. 2006;70:275–282. doi: 10.1111/j.1399-0004.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- Simón-Sánchez J., Schulte C., Bras J.M., Sharma M., Gibbs J.R., Berg D., Paisan-Ruiz C., Lichtner P., Scholz S.W., Hernandez D.G., Krüger R., Federoff M., Klein C., Goate A., Perlmutter J., Bonin M., Nalls M.A., Illig T., Gieger C., Houlden H., Steffens M., Okun M.S., Racette B.A., Cookson M.R., Foote K.D., Fernandez H.H., Traynor B.J., Schreiber S., Arepalli S., Zonozi R., van der Gwinn K., Lopez G., Chanock S.J., Schatzkin A., Park Y., Hollenbeck A., Gao J., Huang X., Wood N.W., Lorenz D., Deuschl G., Chen H., Riess O., Hardy J.A., Singleton A.B., Gasser T., Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat. Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton A.B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M.R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Soma H., Yabe I., Takei A., Fujiki N., Yanagihara T., Sasaki H. Heredity in multiple system atrophy. J. Neurol. Sci. 2006;240:107–110. doi: 10.1016/j.jns.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Soma H., Yabe I., Takei A., Fujiki N., Yanagihara T., Sasaki H. Associations between multiple system atrophy and polymorphisms of SLC1A4, SQSTM1, and EIF4EBP1 genes. Mov. Disord. 2008;23:1161–1167. doi: 10.1002/mds.22046. [DOI] [PubMed] [Google Scholar]
- Spillantini M.G., Crowther R.A., Jakes R., Cairns N.J., Lantos P.L., Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci. Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- Stefanova N., Bücke P., Duerr S., Wenning G.K. Multiple system atrophy: an update. Lancet Neurol. 2009;8:1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- Tan E.K., Skipper L., Chua E., Wong M.C., Pavanni R., Bonnard C., Kolatkar P., Liu J.J. Analysis of 14 LRRK2 mutations in Parkinson's plus syndromes and late-onset Parkinson's disease. Mov. Disord. 2006;21:997–1001. doi: 10.1002/mds.20875. [DOI] [PubMed] [Google Scholar]
- Thacker E.L., Ascherio A. Familial aggregation of Parkinson's disease: a meta-analysis. Mov. Disord. 2008;23:1174–1183. doi: 10.1002/mds.22067. [DOI] [PubMed] [Google Scholar]
- Trojanowski J.Q., Revesz T., Neuropathology Working Group on MSA Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol. Appl. Neurobiol. 2007;33:615–620. doi: 10.1111/j.1365-2990.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- Vanacore N. Epidemiological evidence on multiple system atrophy. J. Neural Transm. 2005;112:1605–1612. doi: 10.1007/s00702-005-0380-7. [DOI] [PubMed] [Google Scholar]
- Vanacore N., Bonifati V., Fabbrini G., Colosimo C., De Michele G., Marconi R., Stocchi F., Nicholl D., Bonuccelli U., De Mari M., Vieregge P., Meco G., ESGAP Consortium Case-control study of multiple system atrophy. Mov. Disord. 2005;20:158–163. doi: 10.1002/mds.20303. [DOI] [PubMed] [Google Scholar]
- Vidal J.S., Vidailhet M., Derkinderen P., Tzourio C., Alpérovitch A. Familial aggregation in atypical Parkinson's disease: a case control study in multiple system atrophy and progressive supranuclear palsy. J. Neurol. 2010;257:1388–1393. doi: 10.1007/s00415-010-5638-9. [DOI] [PubMed] [Google Scholar]
- Vidal J.S., Vidailhet M., Elbaz A., Derkinderen P., Tzourio C., Alpérovitch A. Risk factors of multiple system atrophy: a case-control study in French patients. Mov. Disord. 2008;23:797–803. doi: 10.1002/mds.21857. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Yoshimoto M., Tsuji S., Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci. Lett. 1998;249:180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- Waters C.H., Miller C.A. Autosomal dominant Lewy body parkinsonism in a four-generation family. Ann. Neurol. 1994;35:59–64. doi: 10.1002/ana.410350110. [DOI] [PubMed] [Google Scholar]
- Wenning G.K., Colosimo C., Geser F., Poewe W. Multiple system atrophy. Lancet Neurol. 2004;3:93–103. doi: 10.1016/s1474-4422(03)00662-8. [DOI] [PubMed] [Google Scholar]
- Wenning G.K., Stefanova N., Jellinger K.A., Poewe W., Schlossmacher M.G. Multiple system atrophy: a primary oligodendrogliopathy. Ann. Neurol. 2008;64:239–246. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- Wenning G.K., Wagner S., Daniel S., Quinn N.P. Multiple system atrophy: sporadic or familial? Lancet. 1993;342:681. doi: 10.1016/0140-6736(93)91789-o. [DOI] [PubMed] [Google Scholar]
- Wider C., Vilariño-Güell C., Jasinska-Myga B., Heckman M.G., Soto-Ortolaza A.I., Cobb S.A., Aasly J.O., Gibson J.M., Lynch T., Uitti R.J., Wszolek Z.K., Farrer M.J., Ross O.A. Association of the MAPT locus with Parkinson's disease. Eur. J. Neurol. 2010;17:483–486. doi: 10.1111/j.1468-1331.2009.02847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullner U., Abele M., Schmitz-Huebsch T., Wilhelm K., Benecke R., Deuschl G., Klockgether T. Probable multiple system atrophy in a German family. J. Neurol. Neurosurg. Psychiatry. 2004;75:924–925. doi: 10.1136/jnnp.2003.025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullner U., Schmitt I., Kammal M., Kretzschmar H.A., Neumann M. Definite multiple system atrophy in a German family. J. Neurol. Neurosurg. Psychiatry. 2009;80:449–450. doi: 10.1136/jnnp.2008.158949. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T., Mucke L. Inflammation in neurodegenerative disease–a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Yabe I., Soma H., Takei A., Fujik N., Sasaki H. No association between FMR1 premutations and multiple system atrophy. J. Neurol. 2004;251:1411–1412. doi: 10.1007/s00415-004-0546-5. [DOI] [PubMed] [Google Scholar]
- Yazawa I., Giasson B.I., Sasaki R., Zhang B., Joyce S., Uryu K., Trojanowski J.Q., Lee V.M. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45:847–859. doi: 10.1016/j.neuron.2005.01.032. [PubMed: 15797547] [DOI] [PubMed] [Google Scholar]
- Yu C.E., Bird T.D., Bekris L.M., Montine T.J., Leverenz J.B., Steinbart E., Galloway N.M., Feldman H., Woltjer R., Miller C.A., Wood E.M., Grossman M., McCluskey L., Clark C.M., Neumann M., Danek A., Galasko D.R., Arnold S.E., Chen-Plotkin A., Karydas A., Miller B.L., Trojanowski J.Q., Lee V.M., Schellenberg G.D., Van Deerlin V.M. The spectrum of mutations in progranulin: a collaborative study screening 545 cases of neurodegeneration. Arch. Neurol. 2010;67:161–170. doi: 10.1001/archneurol.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J.Y., Lee W.W., Lee J.Y., Kim H.J., Park S.S., Jeon B.S. SNCA variants and multiple system atrophy. Ann. Neurol. 2010;67:554–555. doi: 10.1002/ana.21889. [DOI] [PubMed] [Google Scholar]
- Zhang J., Montine T.J., Smith M.A., Siedlak S.L., Gu G., Robertson D., Perry G. The mitochondrial common deletion in Parkinson's disease and related movement disorders. Parkinsonism Relat. Disord. 2002;8:165–170. doi: 10.1016/s1353-8020(01)00041-4. [DOI] [PubMed] [Google Scholar]