Abstract
A single G-to-T missense mutation in the gene for the JAK2 tyrosine kinase, leading to a V617F amino acid substitution, is commonly found in several myeloproliferative neoplasms. Reliable quantification of this mutant allele is of increasing clinical and therapeutic interest in predicting and diagnosing this group of neoplasms. Because JAK2V617F is somatically acquired and may be followed by loss of heterozygosity, the percentage of mutant versus wild-type DNA in blood can vary between 0% and almost 100%. Therefore, we developed a real-time PCR assay for detection and quantification of the low-to-high range of the JAK2V617F allele burden. To allow the assay to meet these criteria, amplification of the wild-type JAK2 was blocked with a peptide nucleic acid oligonucleotide. JAK2V617F patient DNA diluted in JAK2 wild-type DNA could be amplified linearly from 0.05% to 100%, with acceptable reproducibility of quantification. The sensitivity of the assay was 0.05% (n = 3 of 3). In 9 of 100 healthy blood donors, a weak positive/background signal was observed in DNA isolated from blood, corresponding to approximately 0.01% JAK2V617F allele. In one healthy individual, we observed this signal in duplicate. The clinical relevance of this finding is not clear. By inhibiting amplification of the wild-type allele, we developed a sensitive and linear real-time PCR assay to detect and quantify JAK2V617F.
In 2005, the involvement of the JAK2V617F somatic point mutation in myeloproliferative neoplasms (MPNs) became clear. JAK2V617F is caused by a G-to-T transversion in the Janus kinase 2 (JAK2) gene resulting in a valine-to-phenylalanine amino acid substitution at codon 617.1–4 JAK2 is a nonreceptor tyrosine kinase involved in the JAK-STAT signaling pathway.5 The autoinhibitory pseudokinase JH2 domain is thought to be by the mutation altered in such a way that the JAK-STAT signaling pathway is constitutively activated, resulting in growth factor–independent cell proliferation.6
Since the discovery of JAK2V617F, other mutations in JAK2 and other genes, such as Exon 12, MPL, and TET2, have been described. These mutations occur in a relatively small percentage of patients with MPN.7–10 JAK2V617F, in contrast, is found in many patients: 65% to 97% of polycythemia vera (PV) cases, 23% to 57% of essential thrombocythemia cases, and 35% to 57% of chronic idiopathic myelofibrosis cases.11,12 The diagnostic value of JAK2 mutational analysis in MPN is now well established and endorsed in the classification of hematologic malignancies by the World Health Organization.13
Patients with PV often carry a JAK2V617F homozygous burden, in contrast to those with essential thrombocythemia, in whom homozygosity is rare.14 This finding was also recapitulated in a large multicenter study: PV (n = 323: 67.8% heterozygous and 32.2% homozygous) and essential thrombocythemia (n = 639: 40.2% wild-type, 57.6% heterozygous, and 2.2% homozygous).15 It is, therefore, more common for patients with PV to have a mutant allele burden >50%. Although the clinical value of the JAK2V617F allele burden is not yet fully understood, a correlation between disease phenotype and the proportion of mutant alleles has been postulated.15,16 Splenomegaly, vascular events and pruritus in PV and splenomegaly, arterial thrombosis, and microvessel disease in essential thrombocythemia have been clinically correlated to mutant allele burdens.17,18 Patients in the lowest quartile of mutant allele burden in idiopathic myelofibrosis had, overall, significantly shortened overall and leukemia-free survival compared with those with higher allele burdens and those negative for JAK2V617F.19 In addition, the amount of mutant allele burden in correlation to therapy may be of increasing interest in future diagnostic and predictive tests.20
With the discovery of JAK2V617F, the detection of this mutation became routine procedure in many laboratories. Since 2005, many assays to detect, and sometimes quantify, JAK2V617F have been described, evaluated, and compared.4,6,21–51 Today, numerous techniques are used: PCR–restriction fragment length polymorphism (RFLP), direct sequencing, pyrosequencing, allele-specific PCR/amplification refractory mutation system, allelic discrimination, real-time PCR, high-resolution melting curve analysis, PCR, denaturing high-pressure liquid chromatography, PCR–matrix-assisted laser desorption/ionization–time of flight (PCR-MALDI-TOF) mass spectrometry, and sequence-specific primer–single molecule fluorescence detection. The techniques differ in a variety of characteristics, such as sensitivity (ranging from 0.01% to 20%, mutant allele diluted in wild-type allele), the potential for high-throughput diagnostics, and equipment required. An overview of currently available assays is presented in Table 1.
Table 1.
Overview and Some Characteristics of Currently Available JAK2V617F Detection (and Quantification) Techniques
| Technique | Sensitivity (%) | Advantages | Disadvantages | References |
|---|---|---|---|---|
| PCR-RFLP | 1–20 | No special equipment required, inexpensive | Risk of false positives due to incomplete digestion, labor intensive, post-PCR processing required | 4, 6, 21–26 |
| Direct sequencing | 5–20 | Simultaneous detection of other mutations | Labor intensive, sequencing equipment required, post-PCR processing required | 4, 6, 22, 23, 25, 27, 28 |
| Pyrosequencing | 2–10 | Simultaneous (real-time) detection of other mutations | Pyrosequencing equipment required, expensive | 6, 28, 29 |
| AS-PCR/ARMS | 0.01–5 | Highly sensitive | Post-PCR processing or real-time cycler required | 6, 21–25, 28, 30–33 |
| Real-time PCR | 0.01–5 | Highly sensitive, high-throughput, fast | Real-time cycler required | 6, 21, 24, 26, 27, 33–41 |
| HRM | 0.5–10 | Detection of other mutations | Real-time cycler required | 6, 21, 25, 27, 38, 42–45 |
| Allelic discrimination | 0.1 | Variable amplicon detection techniques possible | Real-time cycler required | 46, 47 |
| dHPLC | 1–2.5 | High-throughput, fast | Post-PCR processing required, technically challenging | 6, 23, 48 |
| PCR-MALDI-TOF | 0.01–1 | Highly sensitive, high-throughput, fast | MALDI-TOF equipment required, post-PCR processing required | 49, 50 |
| SSP-SMFD | 5 | High-throughput | Automated fluorescence cell sorter required, expensive | 50 |
AS-PCR/ARMS, allele-specific PCR/amplification refractory mutation system; dHPLC, denaturing high-pressure liquid chromatography; HRM, high-resolution melting curve analysis; PCR-MALDI-TOF, PCR -matrix-assisted laser desorption/ionization -time of flight; PCR-RFLP, PCR -restriction fragment length polymorphism; SSP-SMFD, sequence-specific primer–single molecule fluorescence detection.
To increase the sensitivity of the JAK2V617F diagnostics, several techniques have been used in our laboratory during the past years. We started with an RFLP assay and subsequently used a semiquantitative real-time PCR and allele-specific minor groove–binding probes.41 We then developed a quantitative real-time PCR using a locked nucleic acid (LNA) oligonucleotide to block amplification of the JAK2 wild-type allele. We report herein replacement of the LNA moiety by peptide nucleic acid (PNA) for better blocking.
Materials and Methods
JAK2V617F-Positive Blood Sample
JAK2V617F-positive blood containing 97% mutant allele, estimated by RFLP, the absence of a wild-type peak in the sequence chromatogram, the presence of minimal wild-type signal in real-time PCR, and a hematologic analysis showing 3% lymphoid cells and 97% myeloid cells together with a high white blood cell count (23.2 × 109/L)41 was derived from a patient with PV and generated a mean ± SD CT value of 24.13 ± 0.02. A 100-fold dilution from DNA from this sample in JAK2 wild-type DNA was used as a 1% JAK2V617F-containing positive control.
A second positive blood sample, used for serial dilutions in this study, was obtained from a 64-year-old man diagnosed as having possible PV, with a high number of erythrocytes [7.64 × 1012/L (reference range, 4.4 to 5.8 × 1012/L)] and iron deficiency. JAK2V617F real-time PCR using the PNA oligonucleotide on genomic DNA derived from this sample generated a mean ± SD CT value of 23.88 ± 0.07. Thus, the sample used in this study was considered to contain >97% JAK2V617F.
Cohort Healthy Donors
EDTA blood samples from 100 healthy blood donors—rendered anonymous—were used as a negative cohort. All the individuals were <30 years of age.
Genomic DNA Isolation from Blood
Genomic DNA was isolated from 200 μL of blood that had been anticoagulated with EDTA using the Qiagen blood mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. DNA was eluted in DNase- and RNase-free water (Fisher Emergo B.V., Landsmeer, The Netherlands), quantified by photo spectrometer (Eppendorf BioPhotometer; Eppendorf, Hamburg, Germany), and diluted to 10 ng/μL in DNase- and RNase-free TE (10 mmol/L Tris-HCl, pH 8; 0.1 mmol/L EDTA).
JAK2V617F Real-Time PCR Assay with PNA and LNA Blocking
Primers and probes were designed by Applied Biosystems (Foster City, CA) using TaqExpress software as previously described.41 The wild-type probe was altered to function as a blocking oligonucleotide with PNA or LNA. Oligonucleotide sequences are listed in Table 2. The PCR (25 μL) contained 20 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 3 mmol/L MgCl2 [prepared from 10× PCR buffer and 50 mmol/L MgCl2 solution delivered with Platinum Taq polymerase (Invitrogen, Breda, The Netherlands)], 0.75 U of Platinum Taq polymerase, 4% glycerol (molecular biology grade; CalBiochem, VWR International, Amsterdam, The Netherlands), 200 μmol/L of each deoxyribonucleotide triphosphate (Invitrogen), 0.5 μL of ROX reference dye (Invitrogen), 900 nmol/L primers and 200 nmol/L probes (Applied Biosystems), 1 μmol/L PNA oligonucleotide (PanaGene, Daejeon, Korea; alternate source: Biosynthesis Inc., Lewisville, TX) or LNA oligonucleotide (Sigma-Proligo, The Woodlands, TX), and 50 ng of target DNA. The ABI Prism 7500 Fast sequence detection system (Applied Biosystems) was used for amplification and detection (10 minutes at 95°C, 45 cycles of 3 seconds at 95°C, and 30 seconds at 60°C and an infinite holding step at 25°C in Fast 7500 mode). The threshold was set at 0.008, and baseline was set from cycles 6 to 15 for both reporters. CT values were calculated using SDS version 1.3.1 software. A real-time PCR targeting the human albumin gene was used to test whether DNA extraction and dilution to 10 ng/μL were successful and to verify that the PCR was not inhibited. Amplification was performed as described previously herein with 900 nmol/L both primers and 200 nmol/L probe. Oligonucleotide sequences were described earlier and are denoted in Table 2.52
Table 2.
Sequences of Primers, Detection Probes, and PNA and LNA Blocking Oligonucleotides to Detect and Quantify JAK2V617F and to Detect Albumin by Real-Time PCR
| JAK2 V617F: primers and mutant probe41 | Sequences |
|---|---|
| Forward primer | 5′-AAGCTTTCTCACAAGCATTTGGTTT-3′ |
| Reverse primer | 5′-AGAAAGGCATTAGAAAGCCTGTAGTT-3′ |
| Mutant probe | 5′-FAM-TCCACAGAAACATAC-MGB-BHQ-3′ |
| LNA wild-type blocking probe | 5′-ACAGACACATA-3′ |
| PNA wild-type blocking probe | 5′-Acetyl-CTCCACAGACACATAC-3′ |
| Albumin: primers and probe [53] | Sequences |
|---|---|
| Forward primer | 5′-TGAAACATACGTTCCCAAAGAGTTT-3′ |
| Reverse primer | 5′-CTCTCCTTCTCAGAAAGTGTGCATAT-3′ |
| Probe | 5′-FAM-TGCTGAAACATTCACCTTCCATGCAGA-TAMRA-3′ |
BHQ, black hole quencher; MGB, minor groove binder.
Sensitivity and Linearity Testing of PNA versus LNA Blocking
To compare PNA and LNA blocking oligonucleotides, a JAK2V617F real-time PCR was performed on twofold (v/v) serial dilutions of genomic DNA from the patient sample containing >97% JAK2V617F. JAK2V617F DNA (10 ng/μL) was diluted in genomic DNA (10 ng/μL) of a JAK2 wild-type individual. The lowest JAK2V617F concentration tested was 0.006% v/v. All the dilutions were tested in triplicate.
Results
Sensitivity and Linearity Testing of PNA versus LNA Blocking
LNA nucleotides are generally used as blocking oligonucleotides.26,39,53 Because our data using the LNA oligonucleotide showed suboptimal sensitivity of 0.2%, we introduced a PNA blocker approach. To determine the sensitivity and linearity of the real-time PCR test using the PNA oligonucleotide versus the LNA oligonucleotide, twofold (v/v) serial dilutions of genomic DNA from a patient with PV containing 0.006% to >97% JAK2V617F were tested. The assay combined with the PNA oligonucleotide linearly detected JAK2V617F with a for quantification acceptable (mean CT value ± 1 CT) reproducibility ranging to be approximately 97% to 0.05% (n = 3 of 3). This range was determined for the real-time PCR with the LNA oligonucleotide to be 97% to 0.2% (n = 3 of 3; Figure 1).
Figure 1.
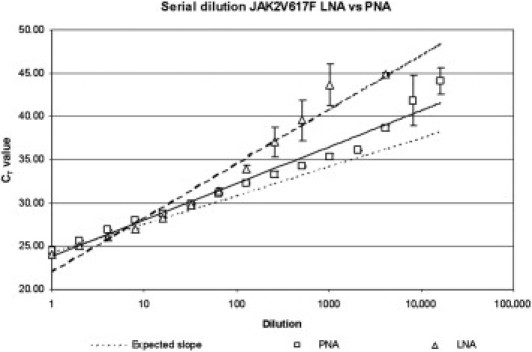
Standard curves (using twofold dilution steps) generated by JAK2V617F real-time PCR using PNA blocking (squares) and LNA blocking (triangles). Data are given as mean Ct ± SD.
Cohort Healthy Donors
One hundred healthy EDTA blood donors were tested in duplicate using the real-time PCR and the PNA blocking oligonucleotide. All individuals tested positive for the albumin housekeeping gene (CT: mean ± SD, 23.16 ± 0.19; range, 22.60 to 23.88). All DNA isolations were considered successful with this value, and none of the samples showed PCR inhibition. Ninety-one patients (91%) were JAK2V617F negative. In nine patients (9%), a background signal corresponding to ±0.01% mutant allele was observed in one or both duplicates (eight patients and one patient, respectively). Two representative amplification plots are depicted in Figure 2. The results are summarized in Table 3.
Figure 2.
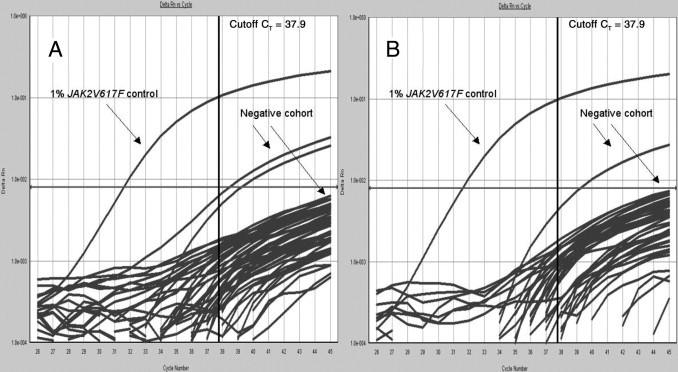
Amplification plots of JAK2V617F real-time PCR of 25 (A) and 22 (B) healthy individuals in duplicate (JAK2V617F negative cohort) and one 1% JAK2V617F positive control. The amplification plots shown are representative of the entire cohort. Gray horizontal line (at Delta Rn 0.8e-002) indicates threshold; black vertical line, cutoff CT value of 37.9.
Table 3.
JAK2V617F Test Results from a Negative Cohort of 100 Healthy Individuals
| Result | n | Description |
|---|---|---|
| Total results | 200 | 100 healthy individuals tested in duplicate |
| Negative results | 190 | Negative for JAK2V617F/positive for albumin |
| Background CT ≳ 37.9 | 10 | Mean background signal ± 3 SD (99.7% CI); eight patients single background signal; one patient in duplicate background signal |
The samples were amplified in duplicate (n = 2 × 100). Signals were considered background when CT ≳ 37.9.
Discussion
We developed a highly sensitive JAK2V617F detection and quantification method by inhibiting amplification of the JAK2 wild-type allele by means of a PNA wild-type blocking oligonucleotide. All CT values generated during sensitivity and linearity testing that were higher than the cutoff CT value of 37.9 [determined with the cohort of healthy donors = mean CT value ±3 SD (99.7% CI)] were excluded. Compared with LNA blocking, PNA blocking resulted in a more sensitive and linear assay using the dilution series of JAK2V617F DNA in JAK2 wild-type DNA: 0.2% to ±97% JAK2V617F DNA versus 0.05% to ±97% JAK2V617F DNA (Figure 1). More efficient/stable blocking by the PNA oligonucleotide probably causes the difference in sensitivity and linearity of the real-time PCR using the PNA blocker versus the LNA blocker. Unlike DNA or DNA analogues such as LNA, PNAs do not contain any phosphate groups, resulting in a noncharged backbone. As a result, PNA-DNA complexes lack charge repulsion and are, therefore, more stable than are DNA-DNA and DNA-LNA complexes.54,55
Although the clinical significance of a low JAK2V617F allelic burden in newly diagnosed patients is not clear,38,40,52 highly sensitive mutant allele detection is currently used for minimal residual disease detection after allogeneic bone marrow transplantation.56 In addition, quantification of JAK2V617F will help in monitoring new therapeutic strategies. A prerequisite for reliable quantification is the linearity of the assays from high to low allele burdens.57
In the cohort of 100 healthy individuals (Table 3), 9% generated JAK2V617F background signals (Figure 2). A weak positive/background JAK2V617F signal was observed in duplicate in one healthy individual. First, these background signals could be due to a technical issue, such as incomplete blocking of wild-type amplification. Second, JAK2V617F could be present in these patients in an extremely small number of circulating cells. Although this phenomenon has been described for other JAK2V617F assays, its significance is not clear.38,40,52 To prevent false positives in the clinical setting, a cutoff CT value was determined. The mean CT value (CT values >40 are generally considered unreliable and were, therefore, excluded) ± 3 SD of the negative cohort was determined, and signals with a generated value of CT ≥ 37.9 (CI = 99.7%) were considered to be background. One patient generated duplicate JAK2V617F background signals (Table 3). Although both CT values were ≥37.9 and should be considered background, further analysis of this material may be of interest. Cloning and subsequent sequencing of these amplicons could be of value to determine whether the signals were nonspecific or that JAKV617F was, in fact, present. In addition, an assessment of the presence of erythropoietin-independent erythroid colonies might be useful in this and similar cases.58 In addition, such a patient could undergo testing for the presence of the JAK2V617F predisposing haplotype.59–61 In the routine diagnostic setting, a JAK2V617F allele burden of 0.1% to 1.0% is considered weak positive, and a remark stating that the clinical relevance of this finding is unclear is added to the test report. In addition, we have requested a follow-up sample after 6 months.
Although sequencing is the gold standard for mutation screening, it is less suitable for the detection of JAK2V617F because the mutation can be present at low levels relative to the wild-type sequence. PCR-RFLP, pyrosequencing, sequence-specific primer–single molecule fluorescence detection, high-resolution melting curve analysis, and denaturing high-pressure liquid chromatography are all less sensitive than is the real-time PCR described herein (Table 1), which has a sensitivity of 0.05% and, thereby, is comparable with techniques such as allele-specific PCR/amplification refractory mutation system, allelic discrimination, and PCR-MALDI-TOF (Table 1).4,21–51 In addition, interpretation of PCR-RFLP results is complicated by the risk of incomplete digestion, resulting in JAK2V617F false positives (Table 1).
Besides the higher or equal sensitivity, the real-time PCR using the PNA oligonucleotide has some additional advantages compared with other assays: i) it is fast (<2 hours for DNA extraction and real-time PCR); ii) it eliminates the need of post-PCR processing, a key step in techniques such as allele-specific PCR/amplification refractory mutation system, sequencing, PCR-RFLP, PCR, and PCR-MALDI-TOF, reducing the risk of contamination; and iii) it has a reduced hands-on time of approximately 30 minutes.
In 2006, Sidon et al39,40 described a similar real-time PCR as the assay described in this study using an LNA blocking oligonucleotide and claimed a sensitivity of their assay of 0.01% JAK2V617F diluted in wild-type allele. The sensitivity of this test, however, was determined using a HEL cell line, which is known to have more than two mutant alleles per cell, instead of patient DNA.29
Prospective evaluation of the real-time PCR with PNA oligonucleotide in a diagnostic setting for 1 year now included the analysis of 293 patients suspected of having MPN. Fourteen patients (4.8%) showed a JAK2V617F allele burden >50%, 16 (5.5%) between 1% and 50%, and 2 (0.7%) between 0.1% and 1%. The results generated during the performance of routine JAK2 diagnostics indicate a robust and rapid test with excellent reproducibility: the overall CV with different batches of controls, reagents, and various real-time PCR cyclers was 1.5% for the 1% control (n = 27) and 1.4% for the 50% control (n = 24). Reproducibility is essential for a reliable quantitative assay57; therefore, these observations further confirm that the developed assay is well suited for the application.
In summary, blocking of wild-type JAK2 with the PNA oligonucleotide resulted in a more sensitive real-time PCR assay to detect and quantify JAK2V617F with a longer range of linearity compared with the LNA oligonucleotide approach. Nine percent of healthy individuals in this negative cohort generated background JAK2V617F signals. Therefore, a cutoff CT value was introduced to prevent false-positive test results in a clinical setting.
Acknowledgments
We thank Kathelijn Geraats-Peters for critical reading of the manuscript and Colin Ingham for valuable comments.
References
- 1.Kralovics R., Passamonti F., Buser A.S., Teo S.S., Tiedt R., Passweg J.R., Tichelli A., Cazzola M., Skoda R.C. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 2.James C., Ugo V., Le Couédic J.P., Staerk J., Delhommeau1 F., Lacout C., Garçon L., Raslova H., Berger R., Bennaceur-Griscelli A., Villeval J., Constantinescu S.N., Casadevall N., Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 3.Levine R.L., Wadleigh M., Cools J., Ebert B.L., Wernig G., Huntly B.J., Boggon T.J., Wlodarska I., Clark J.J., Moore S., Adelsperger J., Koo S., Lee J.C., Gabriel S., Mercher T., D'Andrea A., Fröhling S., Döhner K., Marynen P., Vandenberghe P., Mesa R.A., Tefferi A., Griffin J.D., Eck M.J., Sellers W.R., Meyerson M., Golub T.R., Lee S.J., Gilliland D.G. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Baxter E.J., Scott L.M., Campbell P.J., East C., Fourouclas N., Swanton S., Vassiliou G.S., Bench A.J., Boyd E.M., Curtin N., Scott M.A., Erber W.N., Green A.R. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 5.Schindler C., Darnell J.E., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 6.Steensma D.P. JAK2 V617F in myeloid disorders: molecular diagnostic techniques and their clinical utility: a paper from the 2005 William Beaumont Hospital Symposium on Molecular Pathology. J Mol Diagn. 2006;8:397–411. doi: 10.2353/jmoldx.2006.060007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24:1128–1138. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott L.M., Tong W., Levine R.L., Scott M.A., Beer P.A., Stratton M.R., Futreal P.A., Erber W.N., McMullin M.F., Harrison C.N., Warren A.J., Gilliland D.G., Lodish H.F., Green A.R. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pikman Y., Lee B.H., Mercher T., McDowell E., Ebert B.L., Gozo M., Cuker A., Wernig G., Moore S., Galinsky I., DeAngelo D.J., Clark J.J., Lee S.J., Golub T.R., Wadleigh M., Gilliland D.G., Levine R.L. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tefferi A., Pardanani A., Lim K.H., Abdel-Wahab O., Lasho T.L., Patel J., Gangat N., Finke C.M., Schwager S., Mullally A., Li C.Y., Hanson C.A., Mesa R., Bernard O., Delhommeau F., Vainchenker W., Gilliland D.G., Levine R.L. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia. 2009;23:905–911. doi: 10.1038/leu.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson M.E., Steensma D.P. JAK2 V617F in myeloid disorders: what do we know now, and where are we headed? Leuk Lymphoma. 2006;47:177–194. doi: 10.1080/10428190500301348. [DOI] [PubMed] [Google Scholar]
- 12.Tefferi A., Gilliland D.G. The JAK2V617F tyrosine kinase mutation in myeloproliferative disorders: status report and immediate implications for disease classification and diagnosis. Mayo Clin Proc. 2005;80:947–958. doi: 10.4065/80.7.947. [DOI] [PubMed] [Google Scholar]
- 13.Vardiman J.W., Thiele J., Arber D.A., Brunning R.D., Borowitz M.J., Porwit A., Harris N.L., Le Beau M.M., Hellström-Lindberg E., Tefferi A., Bloomfield C.D. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;11:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 14.Scott L.M., Scott M.A., Campbell P.J., Green A.R. Progenitors homozygous for the V617F mutation occur in most patients with polycythemia vera, but not essential thrombocythemia. Blood. 2006;108:2435–2437. doi: 10.1182/blood-2006-04-018259. [DOI] [PubMed] [Google Scholar]
- 15.Vannucchi A.M., Antonioli E., Guglielmelli P., Rambaldi A., Barosi G., Marchioli R., Marfisi R.M., Finazzi G., Guerini V., Fabris F., Randi M.L., De Stefano V., Caberlon S., Tafuri A., Ruggeri M., Specchia G., Liso V., Rossi E., Pogliani E., Gugliotta L., Bosi A., Barbui T. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110:840–846. doi: 10.1182/blood-2006-12-064287. [DOI] [PubMed] [Google Scholar]
- 16.Passamonti F., Rumi E. Clinical relevance of JAK2 (V617F) mutant allele burden. Haematologica. 2009;94:7–10. doi: 10.3324/haematol.2008.001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vannucchi A.M., Antonioli E., Guglielmelli P., Longo G., Pancrazzi A., Ponziani V., Bogani C., Ferrini P.R., Rambaldi A., Guerini V., Bosi A., Barbui T., MPD Research Consortium Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952–1959. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- 18.Antonioli E., Guglielmelli P., Poli G., Bogani C., Pancrazzi A., Longo G., Ponziani V., Tozzi L., Pieri L., Santini V., Bosi A., Vannucchi A.M., Myeloproliferative Disorders Research Consortium (MPD-RC) Influence of JAK2V617F allele burden on phenotype in essential thrombocythemia. Haematologica. 2008;93:41–48. doi: 10.3324/haematol.11653. [DOI] [PubMed] [Google Scholar]
- 19.Tefferi A., Lasho T.L., Schwager S.M., Strand J.S., Elliott M., Mesa R., Li C.Y., Wadleigh M., Lee S.J., Gilliland D.G. The clinical phenotype of wild-type, heterozygous, and homozygous JAK2V617F in polycythemia vera. Cancer. 2006;106:631–635. doi: 10.1002/cncr.21645. [DOI] [PubMed] [Google Scholar]
- 20.Tefferi A. Mutational analysis in BCR-ABL-negative classic myeloproliferative neoplasms: impact on prognosis and therapeutic choices. Leuk Lymphoma. 2010;51:576–582. doi: 10.3109/10428191003605313. [DOI] [PubMed] [Google Scholar]
- 21.Cankovic M., Whiteley L., Hawley R.C., Zarbo R.J., Chitale D. Clinical performance of JAK2 V617F mutation detection assays in a molecular diagnostics laboratory: evaluation of screening and quantitation methods. Am J Clin Pathol. 2009;132:713–721. doi: 10.1309/AJCPFHUQZ9AGUEKA. [DOI] [PubMed] [Google Scholar]
- 22.Frantz C., Sekora D.M., Henley D.C., Huang C.K., Pan Q., Quigley N.B., Gorman E., Hubbard R.A., Mirza I. Comparative evaluation of three JAK2V617F mutation detection methods. Am J Clin Pathol. 2007;128:865–874. doi: 10.1309/LW7Q3739RBRMBXXP. [DOI] [PubMed] [Google Scholar]
- 23.Kannim S., Thongnoppakhun W., Auewarakul C.U. Two-round allele specific-polymerase chain reaction: a simple and highly sensitive method for JAK2V617F mutation detection. Clin Chim Acta. 2009;401:148–151. doi: 10.1016/j.cca.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Kremer M., Horn T., Koch I., Dechow T., Gattenloehner S., Pfeiffer W., Quintanilla-Martínez L., Fend F. Quantitation of the JAK2V617F mutation in microdissected bone marrow trephines: equal mutational load in myeloid lineages and rare involvement of lymphoid cells. Am J Surg Pathol. 2008;32:928–935. doi: 10.1097/pas.0b013e31815d6305. [DOI] [PubMed] [Google Scholar]
- 25.Lay M., Mariappan R., Gotlib J., Dietz L., Sebastian S., Schrijver I., Zehnder J.L. Detection of the JAK2 V617F mutation by LightCycler PCR and probe dissociation analysis. J Mol Diagn. 2006;8:330–334. doi: 10.2353/jmoldx.2006.050130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shammaa D., Bazarbachi A., Halas H., Greige L., Mahfouz R. JAK2 V617F mutation detection: laboratory comparison of two kits using RFLP and qPCR. Genet Test Mol Biomarkers. 2010;14:13–15. doi: 10.1089/gtmb.2009.0119. [DOI] [PubMed] [Google Scholar]
- 27.James C., Delhommeau F., Marzac C., Teyssandier I., Couédic J.P., Giraudier S., Roy L., Saulnier P., Lacroix L., Maury S., Tulliez M., Vainchenker W., Ugo V., Casadevall N. Detection of JAK2 V617F as a first intention diagnostic test for erythrocytosis. Leukemia. 2006;20:350–353. doi: 10.1038/sj.leu.2404069. [DOI] [PubMed] [Google Scholar]
- 28.Lippert E., Girodon F., Hammond E., Jelinek J., Reading N.S., Fehse B., Hanlon K., Hermans M., Richard C., Swierczek S., Ugo V., Carillo S., Harrivel V., Marzac C., Pietra D., Sobas M., Mounier M., Migeon M., Ellard S., Kröger N., Herrmann R., Prchal J.T., Skoda R.C., Hermouet S. Concordance of assays designed for the quantification of JAK2V617F: a multicenter study. Haematologica. 2009;94:38–45. doi: 10.3324/haematol.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jelinek J., Oki Y., Gharibyan V., Bueso-Ramos C., Prchal J.T., Verstovsek S., Beran M., Estey E., Kantarjian H.M., Issa J.P. JAK2 mutation 1849G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood. 2005;106:3370–3373. doi: 10.1182/blood-2005-05-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q., Lu P., Jones A.V., Cross N.C., Silver R.T., Wang Y.L. Amplification refractory mutation system, a highly sensitive and simple polymerase chain reaction assay, for the detection of JAK2 V617F mutation in chronic myeloproliferative disorders. J Mol Diagn. 2007;9:272–276. doi: 10.2353/jmoldx.2007.060133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan A.Y., Westerman D.A., Dobrovic A. A simple, rapid, and sensitive method for the detection of the JAK2 V617F mutation. Am J Clin Pathol. 2007;127:977–981. doi: 10.1309/1U61JVXTLPPQ7YP1. [DOI] [PubMed] [Google Scholar]
- 32.Vannucchi A.M., Pancrazzi A., Bogani C., Antonioli E., Guglielmelli P. A quantitative assay for JAK2(V617F) mutation in myeloproliferative disorders by ARMS-PCR and capillary electrophoresis. Leukemia. 2006;20:1055–1060. doi: 10.1038/sj.leu.2404209. [DOI] [PubMed] [Google Scholar]
- 33.Wolstencroft E.C., Hanlon K., Harries L.W., Standen G.R., Sternberg A., Ellard S. Development of a quantitative real-time polymerase chain reaction assay for the detection of the JAK2 V617F mutation. J Mol Diagn. 2007;9:42–46. doi: 10.2353/jmoldx.2007.060083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bousquet M., Le Guellec S., Quelen C., Rigal-Huguet F., Delsol G., Brousset P. Frequent detection of the JAK2 V617F mutation in bone marrow core biopsy specimens from chronic myeloproliferative disorders using the TaqMan polymerase chain reaction single nucleotide polymorphism genotyping assay: a retrospective study with pathologic correlations. Hum Pathol. 2006;37:1458–1464. doi: 10.1016/j.humpath.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Hammond E., Shaw K., Carnley B., P'ng S., James I., Herrmann R. Quantitative determination of JAK2 V617F by TaqMan: an absolute measure of averaged copies per cell that may be associated with the different types of myeloproliferative disorders. J Mol Diagn. 2007;9:242–248. doi: 10.2353/jmoldx.2007.060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kröger N., Badbaran A., Holler E., Hahn J., Kobbe G., Bornhäuser M., Reiter A., Zabelina T., Zander A.R., Fehse B. Monitoring of the JAK2-V617F mutation by highly sensitive quantitative real-time PCR after allogeneic stem cell transplantation in patients with myelofibrosis. Blood. 2007;109:1316–1321. doi: 10.1182/blood-2006-08-039909. [DOI] [PubMed] [Google Scholar]
- 37.Merker J.D., Jones C.D., Oh S.T., Schrijver I., Gotlib J., Zehnder J.L. Design and evaluation of a real-time PCR assay for quantification of JAK2 V617F and wild-type JAK2 transcript levels in the clinical laboratory. J Mol Diagn. 2010;12:58–64. doi: 10.2353/jmoldx.2010.090068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapado I., Albizua E., Ayala R., Hernández J.A., Garcia-Alonso L., Grande S., Gallardo M., Gilsanz F., Martinez-Lopez J. Validity test study of JAK2 V617F and allele burden quantification in the diagnosis of myeloproliferative diseases. Ann Hematol. 2008;87:741–749. doi: 10.1007/s00277-008-0512-x. [DOI] [PubMed] [Google Scholar]
- 39.Sidon P., Heimann P., Lambert F., Dessars B., Robin V., El Housni H. Combined locked nucleic acid and molecular beacon technologies for sensitive detection of the JAK2V617F somatic single-base sequence variant. Clin Chem. 2006;52:1436–1438. doi: 10.1373/clinchem.2006.066886. [DOI] [PubMed] [Google Scholar]
- 40.Sidon P., El Housni H., Dessars B., Heimann P. The JAK2V617F mutation is detectable at very low level in peripheral blood of healthy donors. Leukemia. 2006;20:1622. doi: 10.1038/sj.leu.2404292. [DOI] [PubMed] [Google Scholar]
- 41.Poodt J., Fijnheer R., Walsh I.B., Hermans M.H. A sensitive and reliable semi-quantitative real-time PCR assay to detect JAK2 V617F in blood. Hematol Oncol. 2006;24:227–233. doi: 10.1002/hon.800. [DOI] [PubMed] [Google Scholar]
- 42.Er T.K., Lin S.F., Chang J.G., Hsieh L.L., Lin S.K., Wang L.H., Lin C.W., Chang C.S., Liu T.C. Detection of the JAK2 V617F missense mutation by high resolution melting analysis and its validation. Clin Chim Acta. 2009;408:39–44. doi: 10.1016/j.cca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Olsen R.J., Tang Z., Farkas D.H., Bernard D.W., Zu Y., Chang C.C. Detection of the JAK2(V617F) mutation in myeloproliferative disorders by melting curve analysis using the LightCycler system. Arch Pathol Lab Med. 2006;130:997–1003. doi: 10.5858/2006-130-997-DOTJMI. [DOI] [PubMed] [Google Scholar]
- 44.Sutton B.C., Allen R.A., Zhao Z.J., Dunn S.T. Detection of the JAK2V617F mutation by asymmetric PCR and melt curve analysis. Cancer Biomark. 2007;3:315–324. doi: 10.3233/cbm-2007-3605. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka R., Kuroda J., Stevenson W., Ashihara E., Ishikawa T., Taki T., Kobayashi Y., Kamitsuji Y., Kawata E., Takeuchi M., Murotani Y., Yokota A., Hirai M., Majima S., Taniwaki M., Maekawa T., Kimura S. Fully automated and super-rapid system for the detection of JAK2V617F mutation. Leuk Res. 2008;32:1462–1467. doi: 10.1016/j.leukres.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 46.Nussenzveig R.H., Swierczek S.I., Jelinek J., Gaikwad A., Liu E., Verstovsek S., Prchal J.F., Prchal J.T. Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol. 2007;35:32–38. doi: 10.1016/j.exphem.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Passamonti F., Rumi E., Pietra D., Della Porta M.G., Boveri E., Pascutto C., Vanelli L., Arcaini L., Burcheri S., Malcovati L., Lazzarino M., Cazzola M. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006;107:3676–3682. doi: 10.1182/blood-2005-09-3826. [DOI] [PubMed] [Google Scholar]
- 48.Sattler M., Walz C., Crowley B.J., Lengfelder E., Jänne P.A., Rogers A.M., Kuang Y., Distel R.J., Reiter A., Griffin J.D. A sensitive high-throughput method to detect activating mutations of Jak2 in peripheral-blood samples. Blood. 2006;107:1237–1238. doi: 10.1182/blood-2005-07-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu J.F., Shi J.Y., Zhao W.L., Li G., Pan Q., Li J.M., Hu J., Shen Z.X., Jin J., Chen F.Y., Chen S.J. MassARRAY assay: a more accurate method for JAK2V617F mutation detection in Chinese patients with myeloproliferative disorders. Leukemia. 2008;22:660–663. doi: 10.1038/sj.leu.2404931. [DOI] [PubMed] [Google Scholar]
- 50.Koren-Michowitz M., Shimoni A., Vivante A., Trakhtenbrot L., Rechavi G., Amariglio N., Loewenthal R., Nagler A., Cohen Y. A new MALDI-TOF-based assay for monitoring JAK2 V617F mutation level in patients undergoing allogeneic stem cell transplantation (allo SCT) for classic myeloproliferative disorders (MPD) Leuk Res. 2008;32:421–427. doi: 10.1016/j.leukres.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Ohyashiki K., Hori K., Makino T., Ohyashiki J.H. Automated JAK2V617F quantification using a magnetic filtration system and sequence-specific primer-single molecule fluorescence detection. Cancer Genet Cytogenet. 2007;179:19–24. doi: 10.1016/j.cancergencyto.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Xu X., Zhang Q., Luo J., Xing S., Li Q., Krantz S.B., Fu X., Zhao Z.J. JAK2(V617F): Prevalence in a large Chinese hospital population. Blood. 2007;109:339–342. doi: 10.1182/blood-2006-03-009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rijnders R.J., Christiaens G.C., Bossers B., van der Smagt J.J., van der Schoot C.E., de Haas M. Clinical applications of cell-free fetal DNA from maternal plasma. Obstet Gynecol. 2004;103:157–164. doi: 10.1097/01.AOG.0000103996.44503.F1. [DOI] [PubMed] [Google Scholar]
- 54.Denys B., El Housni H., Nollet F., Verhasselt B., Philippé J. A real-time polymerase chain reaction assay for rapid, sensitive, and specific quantification of the JAK2V617F mutation using a locked nucleic acid-modified oligonucleotide. J Mol Diagn. 2010;12:512–519. doi: 10.2353/jmoldx.2010.090137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karkare S., Bhatnagar K. Promising nucleic acid analogs and mimics: characteristic features and applications of PNA, LNA, and morpholino. Appl Microbiol Biotechnol. 2006;71:575–586. doi: 10.1007/s00253-006-0434-2. [DOI] [PubMed] [Google Scholar]
- 56.Steckel N.K., Koldehoff M., Ditschkowski M., Beelen D.W., Elmaagacli A.H. Use of the activating gene mutation of the tyrosine kinase (VAL617Phe) JAK2 as a minimal residual disease marker in patients with myelofibrosis and myeloid metaplasia after allogeneic stem cell transplantation. Transplantation. 2007;15:1518–1520. doi: 10.1097/01.tp.0000263393.65764.f4. [DOI] [PubMed] [Google Scholar]
- 57.Bernard P.S., Wittwer C.T. Real-time PCR technology for cancer diagnostics. Clin Chem. 2002;48:1178–1185. [PubMed] [Google Scholar]
- 58.Prchal J.F., Axelrad A.A. Bone-marrow responses in polycythemia vera. N Engl J Med. 1974;290:1382. doi: 10.1056/nejm197406132902419. [DOI] [PubMed] [Google Scholar]
- 59.Olcaydu D., Harutyunyan A., Jäger R., Berg T., Gisslinger B., Pabinger I., Gisslinger H., Kralovics R. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41:450–454. doi: 10.1038/ng.341. [DOI] [PubMed] [Google Scholar]
- 60.Jones A.V., Chase A., Silver R.T., Oscier D., Zoi K., Wang Y.L., Cario H., Pahl H.L., Collins A., Reiter A., Grand F., Cross N.C. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41:446–449. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kilpivaara O., Mukherjee S., Schram A.M., Wadleigh M., Mullally A., Ebert B.L., Bass A., Marubayashi S., Heguy A., Garcia-Manero G., Kantarjian H., Offit K., Stone R.M., Gilliland D.G., Klein R.J., Levine R.L. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009;41:455–459. doi: 10.1038/ng.342. [DOI] [PMC free article] [PubMed] [Google Scholar]


