Abstract
Microarray analysis of cell-free RNA in amniotic fluid (AF) supernatant has revealed differential fetal gene expression as a function of gestational age and karyotype. Once informative genes are identified, research moves to a more focused platform such as quantitative reverse transcriptase-PCR. Standardized NanoArray PCR (SNAP) is a recently developed gene profiling technology that enables the measurement of transcripts from samples containing reduced quantities or degraded nucleic acids. We used a previously developed SNAP gene panel as proof of concept to determine whether fetal functional gene expression could be ascertained from AF supernatant. RNA was extracted and converted to cDNA from 19 AF supernatant samples of euploid fetuses between 15 to 20 weeks of gestation, and transcript abundance of 21 genes was measured. Statistically significant differences in expression, as a function of advancing gestational age, were observed for 5 of 21 genes. ANXA5, GUSB, and PPIA showed decreasing gene expression over time, whereas CASC3 and ZNF264 showed increasing gene expression over time. Statistically significantly increased expression of MTOR and STAT2 was seen in female compared with male fetuses. This study demonstrates the feasibility of focused fetal gene expression analysis using SNAP technology. In the future, this technique could be optimized to examine specific genes instrumental in fetal organ system function, which could be a useful addition to prenatal care.
Cell-free nucleic acids in second-trimester amniotic fluid (AF) supernatant derive almost exclusively from the fetus.1 Transcriptomic analyses of cell-free RNA from normal second-trimester AF supernatant reveals that the RNA represents a diverse assortment of fetal organ systems, with contributions from fetal skin, liver, lung, pancreas, and the central nervous system.2 In addition, differences in gene expression from AF supernatants are found in numerous functional pathways when comparing gestational ages3 and karyotypes.4 These studies suggest that analysis of gene expression from AF supernatant may be useful in assessing fetal organ system function.
The goal of a fetal gene expression panel would be to simultaneously assess tens to hundreds of gene transcripts to evaluate organ system function. Currently, the most common methods of RNA analysis are global gene-expression profiling (ie, micro-arrays) and the use of quantitative reverse transcriptase-PCR (qRT-PCR) amplification to measure a small number of genes. Although micro-array analysis allows for the simultaneous assessment of thousands of genes, this technology is costly, and the statistical and data mining aspects of global gene expression profiling are labor intensive. Alternatively, whereas qRT-PCR can measure the level of individual gene expression, multiple assays would need to be used for the assessment of a panel of genes to provide information about multiple organ systems or multiple genes within a specific organ system.
Standardized NanoArray PCR (SNAP) is a new technology that may provide the necessary attributes for the assessment of fetal organ system function. SNAP uses internal standard (IS) sequences to reproducibly measure the abundance of gene transcript. This technology uses a unique assay design for the simultaneous analysis of up to 3072 genes (or 384 samples at eight genes per sample). SNAP utilizes the sensitivity and specificity of qRT-PCR and the design of array technology for the assessment of a sufficient number of genes to evaluate fetal organ system function.5,6
The SNAP approach has several technical advantages. First, the IS sequences provide a control for analytical false-negative results, and the melting curve provides an additional source of specificity to reduce analytical false-positive results. Second, IS sequences are supplied by a single manufacturer, ensuring a high degree of interlaboratory concordance. Third, IS sequences control for PCR inhibitors that are common in clinical samples. Fourth, SNAP requires very little sample volume but is capable of delivering tens to hundreds of transcript measurements. The SNAP approach has been previously validated by comparison with standard qRT-PCR techniques.6 These qualities are highly desirable and necessary when developing and implementing molecular diagnostics.
We applied a previously developed gene panel with five additional genes (CASC3, GUSB, PPIA, TBP, and UBE2D2) to the SNAP array to examine the effectiveness of this technology in the prenatal setting (Table 1). The previously developed panel correlates with survival among patients with non-small-cell lung cancer.7 Genes associated with carcinogenesis also are associated with fetal development.4,8 Following the measurement of RNA isolated from amniocytes to assess the performance (eg, dynamic range and accuracy) of SNAP technology, the objective of the current study was to use this lung gene panel with SNAP as proof of concept to evaluate the effectiveness of this technique with cell-free AF supernatant.
Table 1.
List of Genes Studied
| Gene symbol | RefSeq | UniGene | Entrez gene name |
|---|---|---|---|
| ANXA5 | NM_001154 | Hs.480653 | Annexin A5 |
| CASC3 | NM_007359 | Hs.725173 | Cancer susceptibility candidate 3 |
| CPEB4 | NM_030627 | Hs.127126 | Cytoplasmic polyadenylation element binding protein 4 |
| DLG2 | NM_001142699 | Hs.367656 | Discs, large homolog 2 |
| DUSP6 | NM_001946 | Hs.298654 | Dual specificity phosphatase 6 |
| ERBB3 | NM_001982 | Hs.118681 | V-erb-b2 erythroblastic leukemia viral oncogene homolog 3 |
| GUSB | NM_000181 | Hs.255230 | Glucuronidase, beta |
| HGF | NM_000601 | Hs.396530 | Hepatocyte growth factor |
| HMMR | NM_001142556 | Hs.72550 | Hyaluronan-mediated motility receptor |
| IRF4 | NM_002460 | Hs.401013 | Interferon regulatory factor 4 |
| LCK | NM_001042771 | Hs.470627 | Lymphocyte-specific protein tyrosine kinase |
| MMD | NM_012329 | Hs.463483 | Monocyte to macrophage differentiation-associated |
| MTOR | NM_004958 | Hs.338207 | Mechanistic target of rapamycin |
| NF1 | NM_001042492 | Hs.113577 | Neurofibromin 1 |
| PPIA | NM_021130 | Hs.356331 | Peptidylprolyl isomerase A |
| RNF4 | NM_002938 | Hs.724369 | Ring finger protein 4 |
| STAT1 | NM_007315 | Hs.642990 | Signal transducer and activator of transcription 1 |
| STAT2 | NM_005419 | Hs.530595 | Signal transducer and activator of transcription 2 |
| TBP | NM_003194 | Hs.590872 | TATA box binding protein |
| UBE2D2 | NM_003339.2 | Hs.108332 | Ubiquitin-conjugating enzyme E2D 2 |
| ZNF264 | NM_003417 | Hs.515634 | Zinc finger protein 264 |
Materials and Methods
Subjects
The Institutional Review Boards at Tufts Medical Center and Women and Infants Hospital approved the study. Amniocytes and residual AF supernatant obtained from euploid second-trimester pregnancies were collected anonymously; only the karyotype and gestational age of the samples were known. Amniocytes were cultured in Amniomax basal media (Gibco, Carlsbad, CA) with Amniomax supplement (Gibco) and penicillin-streptomycin at 37°C in 5% CO2. One flask of amniocytes was used for the initial dilution series to determine the SNAP dynamic range. Nineteen AF supernatant samples between 15 to 20 weeks of gestation were obtained. The karyotypes were 46,XX (n = 11) and 46,XY (n = 8).
Sample Preparation
RNA isolation of amniocytes after monolayer cell culture was done with TRIzol reagent using the “Isolation of RNA Using TRIzol Reagent Protocol” (Invitrogen, Carlsbad, CA) with the following changes: cells were lysed in 1 mL/cm2 TRIzol; the lysate was centrifuged at 12,000 × g for 15 minutes at 4°C; RNA was washed with 75% ethanol and centrifuged at 12,000 × g for 5 minutes at 4°C; and RNA was resuspended in ribonuclease-free water and incubated at 55°C for 5 minutes. Samples were stored at −80°C until further analysis.
RNA was extracted from 5 mL AF supernatant with use of the QIAamp Circulating Nucleic Acid Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol under the section “Purification of circulating RNA from 5 mL serum or plasma.”
RNA was converted to cDNA using primer-specific reverse transcription. Reverse transcription reaction components included the extracted RNA (400 ng amniocyte RNA or 10 μL AF supernatant RNA), 1× Omniscript RT buffer (Qiagen), 0.5 μmol/L deoxyribonucleoside triphosphate, random hexamer oligonucleotides (125 ng for amniocytes and 11 ng for AF supernatant) (Integrated DNA Technologies, Coralville, IA), and 100 nmol/L of each forward primer (Integrated DNA Technologies) (sequences in Table 2) in a 20-μL reaction volume. Samples were incubated at 80°C for 5 minutes and then were kept at room temperature for 5 minutes. One microliter Omniscript reverse transcription enzyme was then added to the reaction mixture and incubated at 37°C for 1 hour. Following cDNA synthesis, samples were stored at −20°C until further processing.
Table 2.
Oligonucleotide Sequences Used for Amplicons, Primers, Pleiades Probes, and IS
| Gene | Amplicon sequence |
|---|---|
| ANXA5 | 5′-CGAGACTTCTGGCAATTTAGAGCAACTACTCCTTGCTGTTGTGAAATCTATT(A)CGAAGT(A)ATACCTGCCTACCTTGCAGAGACC-3′ |
| CASC3 | 5′-TCCTGCAACCACGGGAACTTCGAGGTATGCCCAACCATATACACAT(A)GGGAGCA(T)GGACCTCCACCTCAGTTTAACCGG-3′ |
| CPEB4 | 5′-ACAGCCACTTGACCCACGAAAAACTATATTTGTTGGTGGTGTTCCTCGACCAT(A)TAC(G)GAGCTGTGGAGCTTGCGATGATAATGG-3′ |
| DUSP6 | 5′-GCTGCTGCTGATGGACTGCCGGCCGC(G)AGGAG(C)CTATACGAGTCGTCGCACATCGAGTCGGCCATCAACGTGG-3′ |
| ERBB3 | 5′-TGCTCACGGGACACAATGCCGACCTCTCCTTCCTGCAGT(C)GGATTCG(A)AGAAGTGACAGGCTATGTCCTCGTG-3′ |
| HMMR | 5′-TAAGGCGCCCTTGAAACGATTCAATGACCCTTCTGGTT(A)GTG(C)CACCATCTCCAGGTGCTTATGATGT-3′ |
| IRF4 | 5′-CAGGACTACAACCGCGAGGAGGACGCCGCGCTCTTCAAGGCTTGGGCACTGTTTAAAGGA(T)AAGTTCCGAGAAGGCATCGACA-3′ |
| MMD | 5′-ATGGCCGCTACAAGCCAACTTGCTATGAACATGCTGCTAACTGTTACACACACG(A)CAT(C)TCCTCATTGTTCCGGCCAT-3′ |
| NF1 | 5′-GGCCAATGTGGTTCCTTGTTCTCAGTGGGATGAACTAGCTCGAGTTCT(A)GGTT(A)ACTCTGTTTGATTCTCGGCATTTACTC-3′ |
| PPIA | 5′-TCCATGGCAAATGCTGGACCCAACACAAATGGTTCCCAGTTTTTCATCT(A)GCACTGCC(G)AAGACTGAGTGGTTGGATGGCAAGC-3′ |
| STAT2 | 5′-TCATACTAGGGACGGGAAGTCGCGACCAGAGCCATTGGAGGGCGCGGGGACTGCAACCCT(A)AAT(A)CAGCAGAGCCCAAATGGCGCAG-3′ |
| TBP | 5′-GCCCGAAACGCCGAATATAATCCCAAGCGGTTTGCTGCGGTAATCAT(A)GAGGAT(A)AAGAGAGCCACGAACCACGGCACTGATT-3′ |
| UBE2D2 | 5′-TGCCTGAGATTGCTCGGATCTACAAAACAGATAGAGAAAAGTACAACAGAAT(A)AGCT(A)CGGGAATGGACTCAGAAGTATGCGA-3′ |
| ZNF264 | 5′-TGTGGGCTCCTGGTGTCTCTGGGGTGTCCTGTTCCCAAAGCTGAGCTGA(T)TCTGC(G)CACCTAGAGCATGGGCAGGA-3′ |
| ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------- | |
| DLG2 | 5′-CGCAACTCGTCAGCCTTCAA(T)TGA(T)CTCTCCAACGGGCCGTCTCCCTGGAAGGAGAGCCTCGCAAGGTAGTCCTGCACAAAGGC-3′ |
| GUSB | 5′-CTCATTTGGAATTTTGCCGATTTCA(T)TGA(T)CTGAACAGTCACCGACGAGAGTGCTGGGGAATAAAAAGGGGATCTTCACTCGG-3′ |
| HGF | 5′-AAGTCTGTGACATTCCTCAGTGTTCAGAAGTTGA(G)A(G)TGCATGACCTGCAATGGGGAGAGTTATCGAGGTCTCATGGA-3′ |
| LCK | 5′-GAGCCCATCTACATCATCACTGAATACA(T)TGGA(T)GAATGGGAGTCTAGTGGATTTTCTCAAGACCCCTTCAGGCA-3′ |
| MTOR | 5′-AACAAGCGATCCCGAACGAGGA(T)CGGA(T)TTCCTACTCTGCTGGCCAGTCAGTCGAAATTTTGGACGGTGTGGAACTTGG-3′ |
| RNF4 | 5′-GCCTGTGGTGGTTGATCTGACTCAC(T)AATGA(G)CTCTGTTGTGATTGTTGACGAAAGAAGAAGACCAAGGAGGAATGCTAGGAGGCTG-3′ |
| STAT1 | 5′-GGGAAGGGGCCATCACATTCACATG(C)GGTGGA(T)GCGGTCCCAGAACGGAGGCGAACCTGACTTCCATGCGGTTG-3′ |
Single underline, forward primers; double underline, reverse primers; bold, pleiades probes; parentheses, nucleotide change in IS sequence to the left.
Above dashed line: forward primer correlates to limited primer; below dashed line: reverse primer correlates to limited primer.
Standardized Mixtures of Internal Standards (SMIS) were prepared by GeneExpress (Wilmington, NC) using IS sequences (Integrated DNA Technologies) for each of the 21 genes (Table 2). The gene symbols, names, and RefSeq and UniGene identifications for all genes in the panel are provided in Table 1. The IS sequences differed from each amplicon by alterations of one or two bases at the Pleiades binding sites (Table 2). The Pleiades probe is a fluorogenic, minor-groove binder probe with a contact-mediated quenching effect of the nonhybridized probe due to the interaction of the minor-groove binder and the quencher.9
The SMIS arrived from GeneExpress as restriction enzyme linearized plasmid pools consisting of 100 to 107 copies of each IS sequence per μL. SMIS strip-tube stocks were prepared with 20 to 2 × 106 copies of each IS sequence/sample, 70 nmol/L of each of the 21 forward/reverse primer sets (Sigma-Aldrich, St. Louis, MO) (Table 2), and 10 pg/μL yeast transfer RNA (Sigma-Aldrich) in 10 mmol/L Tris pH 8 and 100 μmol/L EDTA in a total volume of 5 μL. SMIS strip-tubes stocks were flash frozen in liquid nitrogen and stored at −20°C until further processing.
Seven serial dilutions of amniocyte cDNA and the entire sample of AF supernatant cDNA were pre-amplified by mixing 70 μL Fast SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) with 35 μL cDNA. Fifteen microliters of this mixture was distributed to the 5 μL SMIS strip-tube stock aliquots, and samples were pre-amplified at 50°C for 2 minutes and 95°C for 10 minutes, followed by 34 cycles of 92°C for 15 seconds and 60°C for 60 seconds.
SNAP Transcript Abundance Measurements
Each OpenArray through-hole contained the reagents needed to amplify 1 of the 21 genes in the panel. PCR amplification for the Pleiades detection probes requires an asymmetric primer ratio for detection. Each through-hole was preloaded with 250 nmol/L limited primer (Sigma-Aldrich), 1000 nmol/L excess primer (Sigma-Aldrich), and 250 nmol/L Pleiades probe (Epoch Biosciences, Bothell, WA) (Table 2). The pre-amplified sample and fresh master mix were distributed in an array-in-array 384-well plate and transferred into the OpenArray plate, and samples were amplified using the NT cycler at 92°C for 10 minutes, followed by 34 cycles of 55°C for 60 seconds and 92°C for 15 seconds according to the manufacturer's protocol (LifeTechnologies, Carlsbad, CA).
Statistical Analyses
All gene expression values were converted to log2 scale. Normalized gene expression values were corrected by subtracting the log2 mean of all 21 gene expression values for each sample from each individual gene's log2 gene expression value (similar to the ΔCT correction of qPCR).
A quantitative comparison of gene expression between sexes using Spearman correlation analysis correlations was performed. The association of the expression level of each gene and gestational age was determined by linear regression analysis. Statistical significance was assigned at P < 0.05. All statistical analyses were performed using SAS/STAT software (SAS Institute, Inc., Cary, NC).
Results
Dynamic range analysis of cultured cell cDNA showed that the majority of gene targets had sufficient starting copies to be measured in all dilutions. Evaluation of melting curves indicated that no native template was detected in the “no template” control samples. The background level of transcript detection was estimated to be ∼10 copies, defined as ∼10% of the lowest IS sequence quantity of 100 copies. To further minimize the potential for false-positive signal detection, any measurements <30 copies were considered undetectable. The analytic response to cDNA dilution was very linear, with a median r2 = 0.879. The dynamic range spanned more than five orders of magnitude (30 to 5 × 106 copies) (see Supplemental Figure S1 at http://jmd.amjpathol.org).
Gene expression analysis of AF supernatant was performed with normalized mean transcript abundances for each sample (see Supplemental Table S1 at http://jmd.amjpathol.org). No hepatocyte growth factor (HGF) transcript was detected in any of the AF supernatant samples; it is not known if this outcome was due to the absence of gene expression or a technical artifact. All other transcripts were present in all samples. All genes that were statistically significantly differentially expressed are shown in Table 3, including functional descriptions and gene expression levels in the fetus from the UniGene database.
Table 3.
Description of Statistically Significant Expression of Genes in Lung Panel
| Gene symbol | RefSeq description⁎ | Fetal gene expression pattern† | Basis of differential gene expression |
|---|---|---|---|
| ANXA5 | The protein encoded by this gene has been implicated in membrane-related events along exocytotic and endocytotic pathways; Annexin 5 is a phospholipase A2 and protein kinase C inhibitory protein with calcium channel activity and a potential role in cellular signal transduction, inflammation, growth and differentiation; Annexin 5 also has been described as a placental anticoagulant protein | 146 | Decreasing with gestational age |
| CASC3 | The product of this gene is a core component of the EJC and functions in NMD; the encoded protein binds RNA and interacts with two other EJC core components. | 130 | Increasing with gestational age |
| GUSB | This gene encodes a hydrolase that degrades glycosaminoglycans, including heparan sulfate, dermatan sulfate, and chondroitin-4,6-sulfate; mutations in this gene result in mucopolysaccharidosis type VII | 31 | Decreasing with gestational age |
| MTOR | The protein encoded by this gene belongs to a family of phosphatidylinositol kinase-related kinases; these kinases mediate cellular responses to stresses such as DNA damage and nutrient deprivation | 79 | Female > male |
| PPIA | This gene encodes an enzyme that catalyzes the cis-trans isomerization of proline imidic peptide bonds in oligopeptides and accelerates the folding of proteins; the encoded protein is a cyclosporin binding–protein and may play a role in cyclosporin A–mediated immunosuppression | 359 | Decreasing with gestational age |
| STAT2 | The protein encoded by this gene is a member of the STAT protein family; in response to cytokines and growth factors, STAT family members are phosphorylated by the receptor associated kinases and act as transcription activators; it is thought to be involved in the process of blocking IFN-alpha response by adenovirus | 22 | Female > male |
| ZNF264 | This gene encodes a zinc finger protein and belongs to the Kruppel C2H2-type zinc-finger protein family; it is thought to regulate transcription | 19 | Increasing with gestational age |
EJC, exon junction complex; IFN, interferon; NMD, nonsense-mediated mRNA decay.
RefSeq is available at http://www.ncbi.nlm.nih.gov/refseq/rsg.
Approximate level of gene expression based on expressed sequence tag (EST) profile from Unigene.
Correlation analysis showed that two genes, mechanistic target of rapamycin (MTOR) and signal transducer and activator of transcription 2 (STAT2), had expression levels that were statistically significantly different between male and female fetuses. Both genes had an 11% higher level of expression in females (P = 0.03 and 0.049, respectively) (Figure 1).
Figure 1.
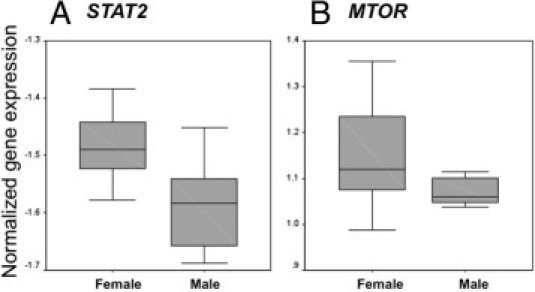
Box plots showing the genes among the panel with significant expression differences by sex. STAT2 is shown in A (P = 0.049), and MTOR (the gene encoding the mechanistic target of rapamycin) is shown in B (P = 0.030). The line within the box is the median; the box represents the 25th and 75th percentiles; and the whiskers represent the 10th and 90th percentiles.
Linear regression analysis showed that five genes had statistically significant different levels of expression as a function of increasing gestational age (Figure 2). Three genes showed decreasing gene expression: annexin A5 (ANXA5) (r = 0.40; P = 0.005), glucuronidase β (GUSB) (r = 0.23; P = 0.03), and peptidylprolyl isomerase A (PPIA) (r = 0.31; P = 0.018). This finding corresponds to percent decreases per week of gestation of 6% (ANXA5), 8% (GUSB), and 4% (PPIA). The other two genes showed increasing expression were cancer susceptibility candidate 3 (CASC3) (r = 0.65; P < 0.0001) and zinc finger protein 264 (ZNF264) (r = 0.39; P = 0.005). This finding corresponds to percent increases per week of gestation of 8% (CASC3) and 0.5% (ZNF264).
Figure 2.
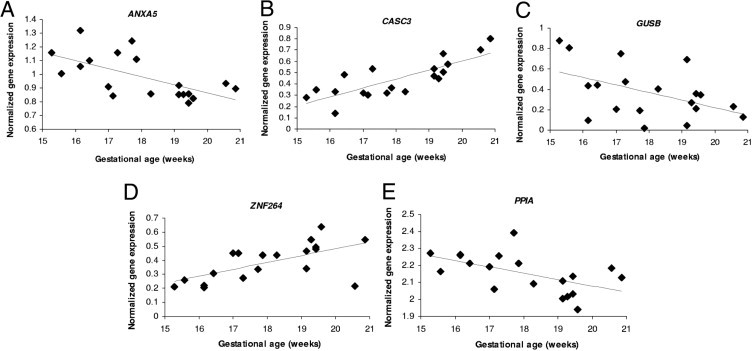
Regression curves showing the genes among the panel with significant expression differences by gestational age. A:ANXA5 (P = 0.005). B:CASC3 (P < 0.0001). C:GUSB (P = 0.03). D:ZNF264 (P = 0.005). E:PPIA (P = 0.018). ANXA5, GUSB, and PPIA show decreasing expression with increasing gestational age, and CASC3 and ZNF264 show increasing gene expression with increasing gestational age.
Discussion
In this feasibility study, we first demonstrated the dynamic range and accuracy of a novel SNAP technology on high-quality RNA extracted from amniocytes. The analytic performance and detection of all gene transcripts in the amniocyte cellular RNA led to the subsequent study of fetal gene expression in AF supernatant samples. The SNAP technology allows for the simultaneous quantitative assessment of tens to hundreds of transcripts from reduced and degraded nucleic acid samples. Gene expression that varies by up to five orders of magnitude can be quantified using a single assay. This technology may be an advance over existing approaches, including global gene expression profiling using micro-arrays. Not only are micro-arrays expensive, but they typically have a more restricted dynamic range, which prevents simultaneous detection of very high and low abundance molecules.10 One drawback of SNAP technology is the initial assay development cost due to the use of IS and Pleiades probes in the technology. However, after the assay is fully developed, the cost per sample becomes minimal. In addition, the benefits of SNAP include intrinsic quality assurance, quality control, and unbiased PCR, making SNAP an ideal platform for interlaboratory assessment with reduced and degraded nucleic acid samples.
The results of the AF supernatant analysis showed that SNAP technology is an effective method of gene expression measurement and that 7 of 21 genes in the panel were statistically significantly differentially expressed as a function of either fetal sex or gestational age. This finding suggests that the evaluation of fetal organs systems is not only possible but that some of the informative genes in the current panel may be useful to include in a modified fetal gene panel that incorporates additional genes. Nevertheless, of the seven differentially expressed genes, four genes (STAT2, MTOR, ANXA5, and PPIA) are associated with immunological function, including interferon signaling, T-cell differentiation, viral functioning, and cyclosporins.11–17 Interestingly, increased expression of at least two genes in the panel (GUSB and CASC3) are associated with rheumatoid or osteoarthritis, which also can be related to immune or autoimmune functions.18,19 Further investigation using this gene panel approach could contribute to the understanding of the complex immune pathways involved in the maternal-fetal relationship. Additionally, four of the seven statistically significant genes in the panel (STAT2, MTOR, PPIA, and CASC3) previously have been linked to different adult cancers.20–25 Because genes linked to carcinogenesis are present in studies of euploid fetal gene expression,8 these genes are most likely related to normal development of the fetus.
RNA in amniotic fluid supernatant is derived from multiple fetal organs; therefore, in the future, fetal gene expression panels could prove useful in prenatal care to evaluate function in cases of at-risk pregnancies and fetal pathologies. For example, fetal sonographic studies can detect a condition known as echogenic bowel.26 The differential diagnosis for echogenic bowel includes chromosomal anomalies, cystic fibrosis, infections, fetal growth restriction, and intra-amniotic bleeding. A platform based on fetal gene expression could include a panel of gastrointestinal genes and inflammatory genes, for example. These expression data could lead to a more rapid, detailed, and accurate diagnosis of the underlying etiology of the echogenic bowel.
In conclusion, these results demonstrate that SNAP technology successfully detected differentially regulated genes in second-trimester AF supernatant. SNAP standardization of qPCR measurements from reduced and degraded nucleic acid samples enables development of gene expression profiling platforms for the evaluation of fetal organ system function. Some genes in the current panel may prove to be useful components of a fetal gene expression panel. Future studies are warranted to identify additional genes to be incorporated, including inflammatory, developmental, and gastrointestinal genes.
Footnotes
Supported by grants from the National Institutes of Health (T32 HD049341-05 and R01 HD42053-07 to D.W.B.; 1R21CA132806 to T.B.M.).
Supplemental material for this article can be found at http://jmd.amjpathol.org or at doi: 10.1016/j.jmoldx.2011.05.008.
Disclosure: T.B.M. has intellectual property rights at Biotrove, Inc., now known as Life Technologies, Inc.
Supplementary data
References
- 1.Hui L., Bianchi D.W. Cell-free nucleic acids in amniotic fluid. Hum Reprod Update. 2010;17:362–371. doi: 10.1093/humupd/dmq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui L., Johnson K.L., Slonim D.K., Bianchi D.W. Multiple fetal organs contribute to the normal midtrimester amniotic fluid transcriptome. Reprod Sci. 2011;18:269A. [Google Scholar]
- 3.Larrabee P.B., Johnson K.L., Lai C., Ordovas J., Cowan J.M., Tantravahi U., Bianchi D.W. Global gene expression analysis of the living human fetus using cell-free messenger RNA in amniotic fluid. JAMA. 2005;293:836–842. doi: 10.1001/jama.293.7.836. [DOI] [PubMed] [Google Scholar]
- 4.Slonim D.K., Koide K., Johnson K.J., Tantravahi U., Cowan J.M., Jarrah Z., Bianchi D.W. Functional genomic analysis of amniotic fluid cell-free mRNA suggests that oxidative stress is significant in Down syndrome fetuses. Proc Natl Acad Sci. 2009;106:9425–9429. doi: 10.1073/pnas.0903909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon J.M., Lubomirski M., Amaratunga D., Morrison T.B., Brenan C.J.H., Ilyin S.E. Nanoliter high-throughput RT-qPCR: a statistical analysis and assessment. Biotechniques. 2009;46:ii–viii. doi: 10.2144/000112838. [DOI] [PubMed] [Google Scholar]
- 6.Morrison T., Hurley J., Garcia J., Yoder K., Katz A., Roberts D., Cho J., Kanigan T., Ilyin S.E., Horowitz D., Dixon J.M., Brenan C.J. Nanoliter high throughput quantitative PCR. Nucleic Acids Res. 2006;34:e123. doi: 10.1093/nar/gkl639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H.Y., Yu S.L., Chen C.H., Chang G.C., Chen C.Y., Yuan A., Cheng C.L., Wang C.H., Terng H.J., Kao S.F., Chan W.K., Li H.N., Liu C.C., Singh S., Chen W.J., Chen J.J., Yang P.C. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med. 2007;356:11–20. doi: 10.1056/NEJMoa060096. [DOI] [PubMed] [Google Scholar]
- 8.Maron J.L., Johnson K.L., Slonim D., Lai C.-Q., Ramoni M., Alterovitz G., Jarrah Z., Yang Z., Bianchi D.W. Gene expression analysis in pregnant women and their infants identifies unique fetal biomarkers that circulate maternal blood. J Clin Invest. 2007;117:3007–3019. doi: 10.1172/JCI29959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukhtanov E.A., Lokhov S.G., Gorn V.V., Podyminogin M.A., Mahoney W. Novel DNA probes with low background and high hybridization-triggered fluorescence. Nucleic Acids Res. 2007;35:e30. doi: 10.1093/nar/gkl1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canales R.D., Luo Y., Willey J.C., Austermiller B., Barbacioru C.C., Boysen C., Hunkapiller K., Jensen R.V., Knight C.R., Lee K.Y., Ma Y., Maqsodi B., Papallo A., Peters E.H., Poulter K., Ruppel P.L., Samaha R.R., Shi L., Yang W., Zhang L., Goodsaid F.M. Evaluation of DNA microarray results with quantitative gene expression platforms. Nature Biotechnol. 2006;24:1115–1122. doi: 10.1038/nbt1236. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharya S., Eckner R., Grossman S., Oldread E., Arany Z., D'Andrea A., Livingston D.M. Cooperation of Stat2 and p300/CBP in signaling induced by interferon-alpha. Nature. 1996;383:344–347. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]
- 12.Kraus T.A., Lau J.F., Parisien J.P., Horvath C.M. A hybrid IRF9-STAT2 protein recapitulates interferon stimulated gene expression and antiviral response. J Biol Chem. 2003;278:13033–13038. doi: 10.1074/jbc.M212972200. [DOI] [PubMed] [Google Scholar]
- 13.Araki K., Turner A.P., Shaffer V.O., Gangappa S., Keller S.A., Bachmann M.F., Larsen C.P., Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cejka D., Hayer S., Niederreiter B., Sieghart W., Fuereder T., Zwerina J., Schett G. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:2294–2302. doi: 10.1002/art.27504. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe Y., Hashimoto Y., Shiratsuchi A., Takizawa T., Nakanishi Y. Augmentation of fatality of influenza in mice by inhibition of phagocytosis. Biochem Biophys Res Commun. 2005;337:881–886. doi: 10.1016/j.bbrc.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 16.Yang F., Robotham J.M., Nelson H.B., Irsigler A., Kenworthy R., Tang H. Cyclophilin A is an essential cofactor for hepatitis C virus infection and the principle mediator of cyclosporine resistance in vitro. J Virol. 2008;82:5269–5278. doi: 10.1128/JVI.02614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braaten D., Luban J. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 2001;20:1300–1309. doi: 10.1093/emboj/20.6.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasztoi M., Nagy G., Geher P., Lakatos T., Toth K., Wellinger K., Pócza P., György B., Holub M.C., Kittel A., Pálóczy K., Mazán M., Nyirkos P., Falus A., Buzas E.I. Gene expression and activity of cartilage degrading glycosidases in human rheumatoid arthritis and osteoarthritis synovial fibroblasts. Arthritis Res Ther. 2009;11:R68. doi: 10.1186/ar2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janj J., Lim D.S., Choi Y.E., Jeong Y., Yoo S.A., Kim W.U., Bae Y.S. MLN51 and GM-CSF involvement in the proliferation of fibroblast-like synoviocytes in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2006;8:R170. doi: 10.1186/ar2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee I.N., Chen C.H., Sheu J.C., Lee H.S., Huag G.T., Yu C.Y., Lu F.J., Chow L.P. Identification of human hepatocellular carcinoma-related biomarkers by two-dimensional difference gel electrophoresis and mass spectrometry. J Proteome Res. 2005;4:2062–2069. doi: 10.1021/pr0502018. [DOI] [PubMed] [Google Scholar]
- 21.Choi K.J., Piao Y.J., Lim M.J., Kim J.H., Ha J., Choe W. Overexpressed cyclophilin A in cancer cells renders resistance to hypoxia- and cisplatin-induced cell death. Cancer Res. 2007;67:3654–3662. doi: 10.1158/0008-5472.CAN-06-1759. KSS. [DOI] [PubMed] [Google Scholar]
- 22.Treeck O., Wackwitz B., Haus U., Ortmann O. Effects of a combined treatment with mTOR inhibitor RAD001 and tamoxifen in vitro on growth and apoptosis of human cancer cells. Gynecol Oncol. 2006;102:292–299. doi: 10.1016/j.ygyno.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Sahin F., Kannangai R., Adegbola O., Wang J., Su G., Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10:8421–8425. doi: 10.1158/1078-0432.CCR-04-0941. [DOI] [PubMed] [Google Scholar]
- 24.Wang J., Pham-Mitchell N., Schindler C., Campbell I.L. Dysregulated Sonic hedgehog signaling and medulloblastoma consequent to IFN-alpha-stimulated STAT2-independent production of IFN-gamma in the brain. J Clin Invest. 2003;112:535–543. doi: 10.1172/JCI18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degot S., Regnier C.H., Wendling C., Chenard M.P., Rio M.C., Tomasetto C. Metastatic Lymph Node 51, a novel nucleocytoplasmic protein overexpressed in breast cancer. Oncogene. 2002;21:4422–4434. doi: 10.1038/sj.onc.1205611. [DOI] [PubMed] [Google Scholar]
- 26.Simon-Bouy B., Satre V., Ferec C., Malinge M.C., Girodon E., Denamur E., Leporrier N., Lewin P., Forestier F., Muller F. Hyperechogenic fetal bowel: a large French collaborative study of 682 cases. Am J Med Genet A. 2003;121A:209–213. doi: 10.1002/ajmg.a.20168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


