Abstract
There is an immediate and critical need for a rapid, broad-based genotyping method that can evaluate multiple mutations simultaneously in clinical cancer specimens and identify patients most likely to benefit from targeted agents now in use or in late-stage clinical development. We have implemented a prospective genotyping approach to characterize the frequency and spectrum of mutations amenable to drug targeting present in urothelial, colorectal, endometrioid, and thyroid carcinomas and in melanoma. Cancer patients were enrolled in a Personalized Cancer Medicine Registry that houses both clinical information and genotyping data, and mutation screening was performed using a multiplexed assay panel with mass spectrometry–based analysis to detect 390 mutations across 30 cancer genes. Formalin fixed, paraffin-embedded specimens were evaluated from 820 Registry patients. The genes most frequently mutated across multiple cancer types were BRAF, PIK3CA, KRAS, and NRAS. Less common mutations were also observed in AKT1, CTNNB1, FGFR2, FGFR3, GNAQ, HRAS, and MAP2K1. Notably, 48 of 77 PIK3CA-mutant cases (62%) harbored at least one additional mutation in another gene, most often KRAS. Among melanomas, only 54 of 73 BRAF mutations (74%) were the V600E substitution. These findings demonstrate the diversity and complexity of mutations in druggable targets among the different cancer types and underscore the need for a broad-spectrum, prospective genotyping approach to personalized cancer medicine.
The identification of somatic mutations that cause aberrant activation of intracellular signaling pathways has transformed the diagnosis and treatment of cancer. Mutations in specific genes define distinct subtypes of cancer, and provide invaluable markers for disease diagnosis and prognosis. Many of the mutated proteins also represent targets for novel therapeutic agents that are more specific, more efficacious, and less toxic than broad-based chemotherapeutic regimens.1, 2, 3, 4, 5 Indeed, matching the right drug to the right cancer genotype is a proven model for improving treatment and outcome in patients with chronic myelogenous leukemia (CML), non–small-cell lung carcinoma (NSCLC), gastrointestinal stromal tumor (GIST), colorectal carcinoma, and, most recently, malignant melanoma.2, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16
The major successes from this therapeutic approach have been in diseases in which there is limited molecular heterogeneity, with all or most cases having a drug-sensitive mutation. Prime examples include BCR-ABL in CML9 and KIT in GIST.3, 7 There is increasing evidence that many common cancers similarly harbor potentially druggable targets, albeit at relatively lower frequencies. For example, subsets of NSCLC have oncogenic mutations in EGFR, KRAS, BRAF, PIK3CA, or HER2 or a translocation involving the ALK gene.17, 18, 19 In the more molecularly heterogeneous cancers, mutations in proteins other than the intended therapeutic target can profoundly affect response to therapy. Thus, in the case of EGFR inhibitor therapy in lung and colon cancer, KRAS and BRAF mutations strongly predict drug resistance.4, 5, 20, 21 Clearly, application of targeted therapy to these more molecularly complex cancers requires identification of the relevant molecular subtypes, for which high-throughput technologies are necessary.
Currently, new drugs are often tested against unselected patient populations, followed by retrospective analysis of molecular correlates. This is an ineffective process, likely to miss groups of patients who would truly benefit from the drug. For example, in four phase II trials of imatinib for the treatment of advanced melanoma, there was insufficient evidence of activity to support continued development of the drug for this indication22, 23, 24, 25 However, KIT gene mutations have since been identified in the acral and mucosal subsets of melanoma,26, 27, 28, 29 and a recent phase II trial that targeted these mutations yielded a 60% response rate.6 Using genomic mutation signatures predictive of sensitivity to targeted therapies is the most effective way to identify patients most likely to benefit. Moreover, because many of the compelling cancer drug targets are shared across several tumor types, new drug trials are likely to be most efficient when patient populations are recruited on the basis of genotype, as opposed to solely on the basis of cellular tumor type.
A transformative approach to drug development and routine clinical management of cancer patients would be to use broad-based prospective genotyping of clinical tumor specimens to identify relevant drug targets in individual patients. To this end, we have established a Personalized Cancer Medicine Registry (PCMR) with the goal of integrating prospective tumor genotyping into the care of cancer patients. The PCMR serves as a repository of both clinical information and genotyping data and is the focal point for the application of genotyping technologies that work on formalin-fixed, paraffin-embedded (FFPE) clinical material. Herein, we describe a mass spectrometry–based panel of multiplexed assays that can detect 390 mutations across 30 cancer genes. Evaluation of tumor specimens from 820 registry patients with urothelial, colorectal, endometrioid, or thyroid carcinoma, or melanoma using this approach yielded a previously unappreciated complexity and diversity of mutations in some cancers, particularly in regard to the druggable targets BRAF and PIK3CA. The patterns of oncogenic mutations emerging from these analyses have significant implications for the use of targeted therapeutics that are currently in clinical trials, and the methods described herein provide a robust means to identify these mutations in clinical specimens.
Materials and Methods
Patients
Participants were selected from patients receiving clinical care at Oregon Health & Science University for one of five cancer types, with an emphasis on late-stage disease: colorectal adenocarcinoma, endometrioid carcinoma of the uterus or ovary, thyroid carcinoma, urothelial (transitional cell) carcinoma of the bladder or kidney, and malignant melanoma. The study was conducted under full institutional review board approval, with consent or waiver of written documentation of consent per federal and institutional guidelines.
Tumor Specimens and DNA Preparation
Blocks of FFPE tumor tissue, or unstained sections of FFPE tissue, were obtained from the pathology archives of Oregon Health & Science University, or from 115 other health care institutions across 26 US states; one case was obtained from Korea. The diagnosis in each case was confirmed by a single pathologist (C.L.C.). Tumor-rich areas (>75%) were dissected from 5-μm unstained sections by comparison with a hematoxylin and eosin–stained slide, and genomic DNA was extracted using a QIAamp DNA Mini kit (Qiagen, Valencia, CA) in accordance with the manufacturer's instructions. In addition, buccal swabs or saliva samples were collected as a source of germline DNA from the majority of patients.
Mutation Screening
Mutation screening was primarily performed using a panel of 324 assays interrogating 390 mutations in 30 genes analyzed on a MassARRAY platform (Sequenom, San Diego, CA). A total of 500 ng FFPE-derived DNA was required to screen the 36-multiplex panel. This Solid Tumor Panel includes all of the assays that are part of the commercially available OncoCarta v01 panel (Sequenom), as well as 136 custom-designed assays that are now also commercially available (OncoCarta v02, Sequenom). Amplification primers and extension oligo sequences for the 136 custom-designed assays are available upon request.
Assay Designer 3.1 software (Sequenom) was used to design assay multiplexes targeting mutations in known cancer genes, and assays were performed using TypePLEX (Sequenom) chemistry. Initial PCR reactions were set up with an EpMotion 5075 liquid handler (Eppendorf), and used 10 ng DNA per multiplex in a total volume of 5 μl, with 100 nmol/L primers, 2 mmol/L MgCl2, 500 μmol/L dNTPs, and 0.1 units Taq polymerase. Amplification included one cycle of 94°C for 4 minutes, followed by 45 cycles of 94°C for 20 seconds, 56°C for 30 seconds, and 72°C for 1 minute, and one final cycle of 72°C for 3 minutes. Unincorporated nucleotides were inactivated by addition of 0.3 units shrimp alkaline phosphatase and incubation at 37°C for 40 minutes, followed by heat inactivation of shrimp alkaline phosphatase at 85°C for 5 minutes. Single base primer extension reactions were performed with 0.625 to 1.25 μmol/L extension primer and 1.35 units TypePLEX thermosequenase DNA polymerase. Extension cycling included one cycle of 94°C for 30 seconds, 40 cycles of 94°C for 5 seconds, five cycles of 52°C for 5 seconds and 80°C for 5 seconds, followed by one cycle of 72°C for 3 minutes. Extension products were purified by incubation for 30 minutes with an ion exchange resin (SpectroCLEAN, Sequenom), and approximately 10 nl of purified product was spotted onto SpectroChip II matrices. A Bruker matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometer (MassARRAY Compact, Sequenom) was used to resolve extension products. MassARRAY Typer Analyzer software (Sequenom) was used for automated data analysis, accompanied by visual inspection of extension products. Examples of all detected mutations were confirmed by standard, bidirectional Sanger sequencing.
To determine assay sensitivity, FFPE-derived samples with known mutations were diluted into FFPE-derived wild-type DNA at defined ratios. A total of 10 ng DNA was used per assay multiplex. In separate dilutions, the total amount of DNA in a multiplex was titrated from 20 ng to 0.312 ng. Mutant allelic ratios were determined from the peak area of each allele using Sequenom Typer Analyzer software.
Because the Solid Tumor Panel is incomplete for some genes, additional mutation screening was performed for FGFR3 exon 7, and KIT exons 11, 13 and 17 by high-resolution melting curve analyses on a Roche LightCycler 480. The primers used were FGFR3 exon 7 forward 5′-TGGCCCCTGAGCGTCATCTGC-3′, FGFR3 exon 7 reverse 5′-TCTGGTTGGCCGGCAGCCCC-3′, KIT exon 11 forward 5′-CCAGAGTGCTCTAATGACTG-3′, KIT exon 11 reverse 5′-CTCAGCCTGTTTCTGGGAAA-3′, KIT exon 13 forward 5′-GGAAGCCCTCATGTCTGAAC-3′, KIT exon 13 reverse 5′-ACACGGCTTTACCTCCAATG-3′, KIT exon 17 forward 5′-TCGGATCACAAAGATTTGTG-3′, and KIT exon 17 reverse 5′-GCAGGACTGTCAAGCAGAGA-3′. Reactions were performed with 100 ng DNA template in a total volume of 20 μl, with LC480 High Resolution Melting Master Mix (Roche) supplemented with 3 mmol/L MgCl2. FGFR3 primers were used at a final concentration of 200 nmol/L, and KIT primers were used at 500 nmol/L. The amplification conditions included one cycle of 95°C for 8 minutes, followed by 40 cycles of 95°C for 20 seconds, 58°C for 2 seconds, and 72°C for 10 seconds. Melt analysis was performed in one cycle of 95°C for 1 minute, 40°C for 1 minute, 65°C for 1 second, followed by continuous heating to 95°C, with fluorescence measured at 25 acquisitions per degree Celsius (°C).
RET-PTC1/PTC3 translocations were detected in cDNA samples from papillary thyroid carcinomas using hydrolysis probes as described previously.30 Briefly, RET-PTC1 and RET-PTC3 translocation products were detected in a multiplexed two-color RT-PCR assay with GAPDH as an internal positive control. PTC1- and PTC3-specific forward PCR primers were used with a common RET reverse primer and FAM-labeled Taqman probe, whereas GAPDH was detected with a Texas Red–labeled probe. The primer and probe sequences included H4 (PTC1) forward primer 5′-AAAGCCAGCGTGACCATC-3′, ELE1 (PTC3) forward primer 5′-TGGCTTACCCAAAAGCAGAC-3′, RET reverse primer 5′-GTTGCCTTGACCACTTTTC-3′, RET probe 5′-FAM-CCAAAGTGGGAATTCCCTCGGA-3′IABlkFQ, GAPDH forward primer 5′-AGCCGCATCTTCTTTTGC-3′, GAPDH reverse primer 5′-GCCCAATACGACCAAATCC-3′, and GAPDH probe 5′-/5TexRd-XN/TGG GGA AGG TGA AGG TCG GA/3IAbRQSp/−3′. PCR reactions were performed in a Roche LightCycler 480 instrument using a 20-μl reaction volume, with LightCycler 480 Probes Master reaction mix (Roche), and random-primed cDNA template derived from 40 to 200 ng total RNA. The GAPDH probe was used at 0.15 μmol/L, and all other primers and probes were used at a final concentration of 0.3 μmol/L each. Cycling conditions included an initial 10 minutes denaturing step at 95°C, followed by 40 cycles of 95°C for 10 seconds and 60°C for 20 seconds. Samples that scored positive for the RET-PTC1 and RET-PTC3 multiplex were re-tested with each primer pair individually to determine the RET fusion partner.
Results
Mass Spectrometry–Based Mutation Detection
The mass spectrometry–based assays consist of multiplexed single nucleotide primer extension reactions across known mutation sites, generating extension products that differ by the added mass of a wild-type or mutant nucleotide.31, 32 The Solid Tumor Panel consists of 324 assays covering 390 mutations across 30 cancer genes (see Supplemental Table S1 at http://jmd.amjpathol.org). The panel is necessarily biased toward activating mutations in oncogenes, as the design of MassARRAY assays requires foreknowledge of the mutations to be detected. For this reason, the MassARRAY approach is less suitable for screening inactivating mutations in tumor suppressor genes. As detailed in Table 1, the panel covers a substantial portion of the mutations reported in the Catalogue of Somatic Mutations in Cancer (COSMIC) database (http://www.sanger.ac.uk/genetics/CGP/cosmic; v48, released July 27, 2010) for these 30 genes, although rare mutations will still be missed by this approach. For example, in the case of BRAF, the Solid Tumor Panel covers 31 of the 131 distinct solid tumor amino acid mutations listed in the COSMIC database. Owing to the high frequency of specific mutations such as V600E, these 31 amino acid mutations account for 98% of the reported solid tumor BRAF mutations.
Table 1.
Mutations Covered in the Solid Tumor Panel
| Gene | No. of annotated mutant solid tumor cases in COSMIC | Percentage of COSMIC solid tumor cases covered in Solid Tumor Panel⁎ |
|---|---|---|
| ABL1 | 5 | 0% |
| AKT1 | 115 | 95% |
| AKT2 | 0 | NA |
| AKT3 | 2 | 0% |
| BRAF | 12870 | 98% |
| CDK4 | 0 | NA |
| CTNNB1 | 2667 | 76% |
| EGFR | 5292 | 80% |
| ERBB2 | 116 | 33% |
| FBX4 | 0 | NA |
| FBXW7 | 146 | 31% |
| FGFR1 | 10 | 20% |
| FGFR2 | 62 | 27% |
| FGFR3 | 2012 | 28% |
| FLT3 | 2 | 0% |
| GNAQ | 139 | 96% |
| HRAS | 626 | 91% |
| JAK2 | 5 | 0% |
| KIT | 2601 | 48% |
| KRAS | 14235 | 99% |
| MAP2K1 | 0 | NA |
| MAP2K2 | 0 | NA |
| MET | 140 | 51% |
| NRAS | 1353 | 98% |
| PDGFRA | 540 | 72% |
| PIK3CA | 2772 | 89% |
| PTPN11 | 15 | 7% |
| RET | 369 | 78% |
| SOS1 | 2 | 0% |
| TP53 | 11584 | 21% |
COSMIC, Catalogue of Somatic Mutations in Cancer (http://www.sanger.ac.uk/genetics/CGP/cosmic).
NA, not available.
Proportion of solid tumor cases in COSMIC with a mutation that would be detected by the Solid Tumor Panel.
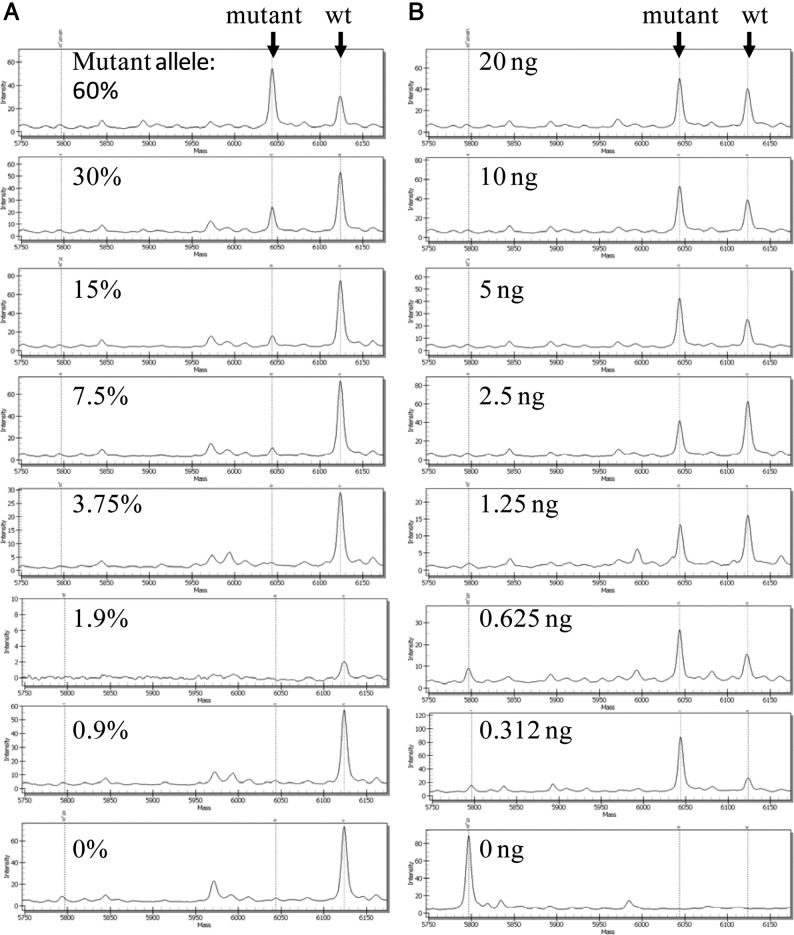
We performed dilution series for six different mutations from FFPE-derived DNA to assess the sensitivity of the system; the lower threshold for detection ranged between 5% and 10%. An example of a dilution series is depicted in Figure 1A. In general, salt adduct peaks, primarily sodium (+22 Da) and potassium (+38 Da), present the main source of background. Interference of these adducts was minimized by designing assays such that the wild-type allele was the high-mass allele, so that salt adducts of wild-type peaks would not overlap mutant allele peaks. In addition, we observed that the chips used for mass spectrometry in this study (SpectroChip II) showed only minimal salt adduct peaks in comparison with the previous version of these chips (SpectroChip I). The level of sensitivity that we observed is in keeping with other studies using the MassARRAY platform, and exceeds the sensitivity of standard DNA sequencing (approximately 20% mutant allele).33, 34, 35, 36, 37 Standard DNA input was 10 ng per multiplex well, but mutant alleles were readily detected with as little as 1 ng DNA per well (Figure 1B).
Figure 1.
Sensitivity of mass-spectrometry–based mutation detection. A: Specimen with 60% KIT K642E mutant allele frequency was diluted twofold into wild-type DNA, with 10 ng total DNA in each dilution. B: The amount of input DNA was titrated from 20 ng to 0.312 ng. Mutant and wild-type (wt) alleles are indicated by arrows.
For each sample analyzed, the mass spectrometry profiles for all 36 multiplexes were manually reviewed and the results were read as wild-type, “low confidence” for mutation (peak area between 10% and 20% of wild-type), or “high confidence” for mutation (peak area ≥20% of wild-type). Initially, all suspected mutations were either confirmed or ruled out by standard DNA sequencing. After sequencing to confirm a minimum of three high-confidence calls for a particular mutation, we accepted further high-confidence calls as positive. All low-confidence calls were followed up by sequencing, and the sequencing result was recorded as the final genotype. Thus, for low-frequency mutations, the limit of mutation detection was set by the sensitivity of the sequencing assay.
Of the 390 mutations represented on the panel, 85 were detected among the Registry tumors. These mutations were confirmed in 310 Registry specimens by standard DNA sequencing. Twelve additional samples were called mutant by the mass spectrometry assays but were deemed to be wild-type by standard sequencing. Notably, 11 of the 12 were low-confidence mass spectrometry calls, and it is not possible to unequivocally determine whether the mutant allele call was erroneous or whether the mutant allele frequency was below the detection limit of standard sequencing. In addition, mutations were sequence confirmed in 304 non-Registry specimens, covering 105 unique mutations. In total, 146 unique mutations detected by the mass spectrometry assay panel were confirmed by sequencing. It was not possible to directly confirm the remaining 244 mutations because we did not have these rare mutations in our tumor DNA archive. One of the strengths of the MassARRAY system, however, is that every assay yields at least one wild-type peak, indicating that the PCR, primer extension, and detection are all working properly. Of course, absence of a wild-type peak was deemed an assay failure.
The performance of the Solid Tumor Panel was robust on FFPE-derived DNA. On average, each of the 820 samples tested was successfully genotyped across >97.5% of the 324 assays in the panel, and 220 samples did not fail a single assay. Sample testing is performed in a 384-well format, and a single individual can screen 36 DNA samples with the 36-multiplex panel in 2 days, including data analysis.
Three assays originally included in the Solid Tumor Panel were found to detect SNPs, as the variants were observed in both tumor specimens and matched germline DNA from the same patients. These include MET R970C and T992I, which have been shown to lack transforming activity,38 and FBX4 S8R. These variants were not included in the total number of somatic mutations detected by the panel, and they were recorded as germline SNPs when detected in patient samples.
Mutation Frequencies
Tissue specimens were sought from 892 patients consented into the Personalized Cancer Medicine Registry (PCMR). In 67 cases, there was insufficient tumor tissue available for genotyping, including cases in which no tissue specimen was available, as well as cases in which a specimen was evaluated and deemed to contain insufficient tumor tissue. In another 5 cases, the yield of DNA was too low to proceed with genotyping. The remaining 820 cases were successfully genotyped, and included 85 urothelial carcinomas, 236 colorectal carcinomas, 90 endometrioid carcinomas, 164 melanomas, and 245 papillary thyroid cancers (Table 2; see also Supplemental Table S2 at http://jmd.amjpathol.org).
Table 2.
Registry Patient Characteristics
| Cancer type |
|||||
|---|---|---|---|---|---|
| Urothelial | Colorectal | Endometrioid | Melanoma | Thyroid | |
| Patient cases genotyped | 85 | 236 | 90 | 164 | 245 |
| Sex (%F/%M) | 21/79 | 42/58 | 100/0 | 46/54 | 73/27 |
| Average age (years) | 49 | 53 | 58 | 58 | 40 |
| Stage 0A | 2 | 0 | 0 | 0 | 0 |
| Stage I | 5 | 11 | 38 | 13 | 127 |
| Stage II | 19 | 14 | 14 | 61 | 8 |
| Stage III | 12 | 107 | 26 | 71 | 29 |
| Stage IV | 12 | 89 | 3 | 7 | 58 |
| Stage unknown | 35 | 15 | 9 | 12 | 23 |
F, female; M, male.
Attempts to engineer MassARRAY assays for mutations in FGFR3 exon 7 codons 248 and 249 failed. We therefore performed supplemental screening of FGFR3 exon7 using a combination of real-time PCR and high-resolution melting curve analyses in cases of urothelial carcinoma. Melanomas were similarly screened for mutations in KIT exons 11, 13, and 17, because the diversity of KIT mutations in these tumors was not known at the time the Solid Tumor Panel was designed. RET-PTC1/PTC3 translocations in papillary thyroid carcinomas were screened in cDNA, requiring separate real-time assays.
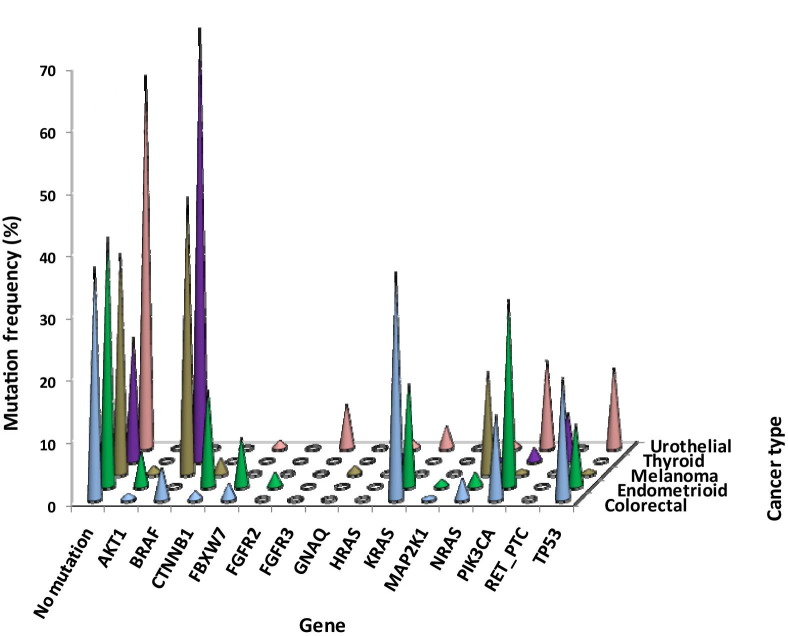
Genetic alterations were detected in 14 of the genes evaluated, and these define molecular subtypes of each of the cancers (Figure 2; see also Supplemental Table S3 at http://jmd.amjpathol.org). Several of these genes were mutated in many cancer types, whereas others exhibited a more narrow distribution of mutations. The genes most frequently mutated in multiple cancer types were BRAF (70% of thyroid carcinomas, 45% of melanomas, and 5.1% of colorectal carcinomas), PIK3CA (30% of endometrioid carcinomas, 14% each of urothelial and colorectal carcinomas, 2% of thyroid carcinomas, and 0.6% of melanomas), KRAS (36% of colorectal, 17% of endometrioid, and 3.5% of urothelial carcinomas), NRAS (17% of melanomas and 3.4% of colorectal, 2.2% of endometrioid, 1.2% of urothelial, and 0.4% of thyroid carcinomas), and TP53 (20% of colorectal, 13% of urothelial, and 10% of endometrioid carcinomas and 0.6% of melanomas). TP53 mutations are likely underrepresented, as the Solid Tumor Panel covers only 21% of known mutations in this tumor suppressor gene (Table 2). Mutations in AKT1, CTNNB1, FBXW7, HRAS, and MAP2K1 were also observed in two or more of the cancers. Genes that exhibited a cancer-specific pattern of alteration included FGFR2 in endometrioid carcinoma, FGFR3 in urothelial carcinoma, GNAQ in melanoma, and RET-PTC in thyroid carcinoma.
Figure 2.
Mutation frequencies for various genes. Mutation frequencies are displayed by gene as the percentage of total cases tested, for urothelial (n = 85), colorectal (n = 236), endometrioid (n = 90), melanoma (n = 164), and thyroid (n = 245) cancers.
Of note, no mutations were detected in KIT exons 11, 13, or 17 in the melanoma series, which included 154 cutaneous tumors, one mucosal primary, eight spindle cell variants, and one Spitzoid lesion. This was not surprising because KIT mutations are rare outside of the mucosal and acral subtypes.29 One BRAF mutation was identified among the spindle cell variants, and the Spitzoid case harbored mutations in both BRAF and CTNNB1.
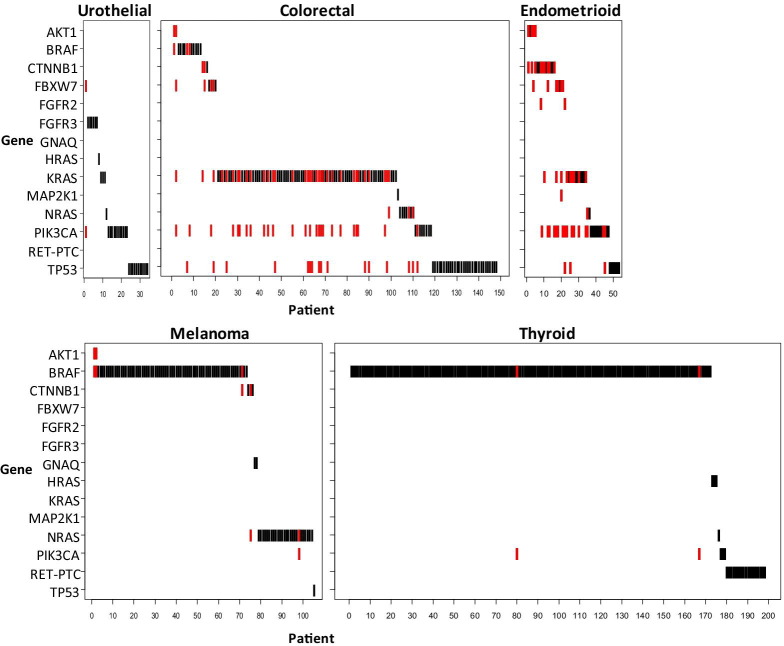
Multiple Mutations
Marked differences were observed in the frequency of multiple mutations in different cancer types, with substantially more mutation complexity in the colorectal and endometrioid cases compared with the urothelial, melanoma, and thyroid cases (Figure 3). Of 34 urothelial carcinomas in which a mutation was detected, only one case had multiple mutations (PIK3CA and FBXW7). Similarly, only two of 198 mutant thyroid specimens had two mutations, in PIK3CA and BRAF. Of 106 mutant melanomas, five exhibited multiple mutations: NRAS with PIK3CA, NRAS with CTNNB1, BRAF with CTNNB1, and two cases of BRAF with AKT1. In contrast, 196 mutations were detected in 148 colorectal specimens, with 42 cases (28%) exhibiting two or more mutations. In addition, among 54 endometrioid cases in which a mutation was detected, 24 cases (44%) had two or more mutations. Three mutations were detected in four colorectal and five endometrioid specimens, and one colorectal case harbored four mutations.
Figure 3.
Mutation overlap. Cases in which a single mutation was detected are depicted in black, whereas those with multiple mutations are depicted in red.
In terms of specific genes, eight BRAF-mutant cases harbored an additional mutation in either AKT1, CTNNB1, PIK3CA, or TP53. Of the 77 cases in which PIK3CA mutations were detected, 48 harbored additional mutations in other genes. The most common overlap was with KRAS mutations, which were observed in 22 colorectal and six endometrioid carcinomas. PIK3CA mutations also overlapped with mutations in AKT1 (case one), FGFR2 (case two), NRAS (case two), BRAF (case three), CTNNB1 (case four), and FBXW7 (case eight). Collectively, PIK3CA mutations were more likely to be found in combination with mutations in other genes than BRAF mutations (48 of 77 versus eight of 257, P < 0.001).
Diversity of PIK3CA Mutations
In PIK3CA, exon 9 and 20 mutations were most common, although many additional mutations were observed outside these exons, and substantial diversity was observed across cancer types. All of the mutations in the urothelial carcinomas and 94% of the mutations in the colorectal carcinomas were in either exon 9 or 20, with 10 of 12 urothelial mutations and 28 of 32 colorectal mutations occurring in exon 9. Two additional colorectal mutations were located in exon 7. By comparison, only 16 of 27 endometrioid mutations were located in exon 9 or 20, with eight additional mutations in exon 1 and three in exon 7. Although relatively few in number, PIK3CA mutations in thyroid carcinomas were diverse, with one each in exons 4, 13, and 20, and two in exon 9.
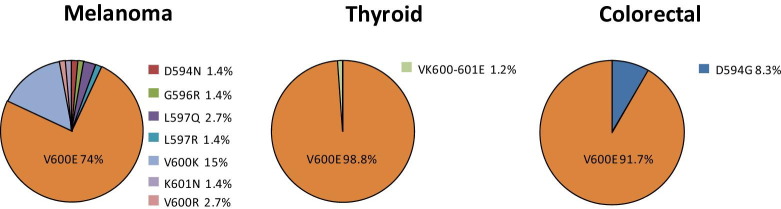
Spectrum of BRAF Mutations
BRAF mutations were identified in 12 colorectal and 172 thyroid carcinomas and in 73 melanomas. Among the colorectal and thyroid cases, the vast majority of the mutations were the V600E substitution, with only one D594G among the colorectal and two VK600-601E deletion/substitutions among the thyroid cases. In marked contrast, V600E comprised only 74% of the melanoma BRAF mutations, with 19 cases harboring other mutations across the region from codons 594 to 601 (Figure 4).
Figure 4.
Spectrum of BRAF mutations. Relative frequencies of different BRAF mutations are expressed as the fraction of all BRAF mutations detected in melanoma (n = 73), thyroid (n = 172), and colon (n = 12) cancers.
Discussion
The primary platform for genotyping tumors from the patients enrolled in the PCMR is the mass spectrometry–based MassARRAY system. Our Solid Tumor Panel covers mutations across 30 cancer genes, including several receptor tyrosine kinases, downstream signaling intermediates in both the MAP kinase and PI3 kinase/AKT pathways, and other genes that are important in cell cycle regulation. Mutation frequencies for the common oncogenic events were in the expected range across all five cancer types represented in the PCMR, validating the MassARRAY approach. For example, we found 45% of malignant melanomas to have BRAF mutations, and 36% of colorectal carcinomas to harbor KRAS mutations; these data are well in line with those in the literature.39, 40, 41, 42, 43
One of the goals of this study was to determine whether the MassARRAY system might be suitable for routine analysis of clinical tumor samples. We observed that high confidence calls are nearly always present on follow-up DNA sequencing; nevertheless, we believe that such sequencing would be necessary before reporting a suspected mutation in the clinical setting. In the current study, specimens were rejected if predissection would yield less than 75% pure tumor cells. However, the estimated sensitivity of the MassARRAY system (∼10%) is at least as good if not better than standard DNA sequencing. It should therefore be possible to lower the threshold for tumor content to 50%. Moreover, we have shown that laser-capture microdissection can be used to improve tumor yield and still generate sufficient material (500 ng) for testing.44
In addition to the common mutations in BRAF and KRAS, we uncovered a remarkable spectrum of mutations that were either unexpected or only rarely documented before. Endometrioid carcinomas are one example in which we observed PIK3CA, CTNNB1 and KRAS mutations at the expected frequencies but also found cases with activating mutations in FGFR2, AKT1, NRAS, or MAP2K1.45, 46, 47, 48, 49, 50 Moreover, a subset of the tumors had more than one mutation: eg, a CTNNB1 mutation paired with a mutation in KRAS or AKT1 or FGFR2, and cases with overlap of KRAS and PIK3CA mutations. These observations have two important implications. First, they demonstrate that among patients with advanced endometrioid carcinoma, there are multiple opportunities for the use of targeted therapeutics. AKT1 mutations are uncommon in these tumors, but such cases can be identified using a multiplex screening approach and the patients potentially entered into a clinical trial of an AKT1 inhibitor. The second implication of the data is that the broader screening approach afforded by the MassARRAY Solid Tumor Panel brings to light co-existing oncogenic alterations that would be missed by a single-gene assay and yet may have a significant impact on the response to a particular drug.
Malignant melanomas are another group of tumors analyzed through the PCMR that showed an unexpected diversity of oncogenic mutations. Among 164 cutaneous melanomas, we observed not only the expected BRAF and NRAS mutations but picked up rare mutations in AKT1, CTNNB1, GNAQ, and PIK3CA, all of which are components of potentially druggable signaling pathways.39, 40, 43, 51, 52, 53, 54, 55, 56 Equally important, we observed a greater diversity of BRAF mutations in melanomas than has previously been described. V600E mutations in our series comprised only 74% of total BRAF mutations compared with 85% to 100% in other series.43, 57, 58, 59, 60, 61 This difference likely reflects the more narrowly focused assays used in other studies, although the greater sensitivity of the MassARRAY platform compared with conventional sequencing may also be a factor. Ongoing trials of BRAF inhibitors for the treatment of melanoma are enrolling only patients with BRAF V600E (PLX4032, Roche/Plexxikon), or V600E and V600K mutations (GSK2118436, GlaxoSmithKline). The clinical implications of the other BRAF mutations, which represent 11% of the BRAF mutations in our melanoma series, remain to be determined. We also found several melanomas that harbored more than one mutation. Examples include overlap of NRAS and CTNNB1 mutations, BRAF and AKT1 mutations, and BRAF and PIK3CA mutations. In the latter two cases, the additional oncogenic hit could portend resistance to BRAF inhibitor therapy.
Three other cancers studied through the PCMR are thyroid carcinomas, urothelial carcinomas, and colorectal adenocarcinomas. In each of these cancers, we confirmed that there is a fraction of patients whose tumors have clinically significant mutations. For example, KRAS and BRAF mutations in colorectal cancer cases predict resistance to cetuximab treatment.20, 21 BRAF mutations and RET-PTC fusions are common in thyroid cancers and may be amenable to treatment with BRAF or RET inhibitors, respectively.62 FGFR3 and PIK3CA mutations are present in a subset of invasive urothelial cancers,63, 64 and we found PIK3CA mutations in 14% of PCMR colorectal cancers.
A substantial diversity of PIK3CA mutations was observed across the different cancers, in terms of both the site of mutation and the overlap with mutations in other genes. Across all cancer types, 47 of 77 (61%) PIK3CA mutations were located in “hotspots” in exon 9. However, 10 of 12 (83%) urothelial mutations and 28 of 32 (87%) colorectal mutations were located in exon 9, whereas only seven of 27 (26%) endometrioid mutations were located in this region. No additional mutations were identified in the urothelial exon 9 mutant cases, whereas 24 of 28 colorectal and six of seven endometrioid exon 9 mutant cases harbored a mutation in at least one other cancer gene. Across all PIK3CA exons, the most common additional mutation was in KRAS, which was observed in 22 colorectal and six endometrioid cases. Therapeutic strategies that target PIK3CA will need to account for the diversity of mutations, as well as the presence of multiple mutations in other genes. The cellular context is also important, as described in a recent report that ovarian cancer patients with PIK3CA and either KRAS or BRAF mutations respond to PI3 kinase pathway inhibitors, whereas colorectal cancer patients with PIK3CA and KRAS mutations do not.65
Collectively, our results substantiate the need for a broader approach in identifying the diversity of clinically significant mutations among cancer subtypes. Mass spectrometry–based screening is well suited to evaluate several hundred mutations per case in FFPE specimens, so that rare mutations can be readily discovered and multiple mutations can be detected in individual specimens. In addition, the prospective genotyping approach is essential to more effectively translate molecular cancer signatures into improved patient therapies, inasmuch as the genotyping data may be used to optimize the current and future care of enrolled patients, including selection into clinical trials of targeted agents.
Footnotes
This study was supported in part with funding from Novartis Pharmaceuticals.
CME Disclosure: None of the authors disclosed any relevant financial relationships.
Supplemental material for this article can be found at http://jmd.amjapthol.org or at doi: 10.1016/j.jmoldx.2011.04.003.
Supplementary data
References
- 1.Druker B.J. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112:4808–4817. doi: 10.1182/blood-2008-07-077958. [DOI] [PubMed] [Google Scholar]
- 2.Heinrich M.C., Corless C.L., Demetri G.D., Blanke C.D., von Mehren M., Joensuu H., McGreevey L.S., Chen C.J., Van den Abbeele A.D., Druker B.J., Kiese B., Eisenberg B., Roberts P.J., Singer S., Fletcher C.D., Silberman S., Dimitrijevic S., Fletcher J.A. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 3.Heinrich M.C., Owzar K., Corless C.L., Hollis D., Borden E.C., Fletcher C.D., Ryan C.W., von Mehren M., Blanke C.D., Rankin C., Benjamin R.S., Bramwell V.H., Demetri G.D., Bertagnolli M.M., Fletcher J.A. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: cALGB 150105 study by Cancer and Leukemia Group B and Southwest Oncology Group. J ClinOncol. 2008;26:5360–5367. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackman D.M., Miller V.A., Cioffredi L.A., Yeap B.Y., Janne P.A., Riely G.J., Ruiz M.G., Giaccone G., Sequist L.V., Johnson B.E. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5273. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sartore-Bianchi A., Di Nicolantonio F., Nichelatti M., Molinari F., De Dosso S., Saletti P., Martini M., Cipani T., Marrapese G., Mazzucchelli L., Lamba S., Veronese S., Frattini M., Bardelli A., Siena S. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS One. 2009;4:e7287. doi: 10.1371/journal.pone.0007287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvajal R.D., Chapman P.B., Wolchok J.D., Cane L., Teitcher J.B., Lutzky J., Pavlick A.C., Bastian B.C., Antonescu C.R., Schwartz G.K. A phase II study of imatinib mesylate (IM) for patients with advanced melanoma harboring somatic alterations of KIT. J Clin Oncol. 2009;27 abstr 9001 27 (No 15S):9001. [Google Scholar]
- 7.Demetri G.D., von Mehren M., Blanke C.D., Van den Abbeele A.D., Eisenberg B., Roberts P.J., Heinrich M.C., Tuveson D.A., Singer S., Janicek M., Fletcher J.A., Silverman S.G., Silberman S.L., Capdeville R., Kiese B., Peng B., Dimitrijevic S., Druker B.J., Corless C., Fletcher C.D., Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 8.Druker B.J., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., Sawyers C.L. Efficacy and safety of a specific inhibitor of the bcr-abl tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 9.Druker B.J., Sawyers C.L., Kantarjian H., Resta D.J., Reese S.F., Ford J.M., Capdeville R., Talpaz M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 10.Dummer R., Robert C., Chapman P.B., Sosman J.A., Middleton M., Bastholt L., Kemsley K., Cantarini M., Morris C., Kirkwood J. AZD6244 (ARRY-142886) versus temozolomide (TMZ) in patients (pts) with advanced melanoma: an open-label, randomized, multicenter, phase II study. J Clin Oncol. 2008;26 (No 15S):9033. [Google Scholar]
- 11.Flaherty K., Puzanov I., Sosman J., Kim K., Ribas A., McArthur G., Lee R.J., Grippo J.F., Nolop K., Chapman P. Phase I study of PLX4032: proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J Clin Oncol. 2009;27 (No 15S):9000. [Google Scholar]
- 12.Hodi F.S., Friedlander P., Corless C.L., Heinrich M.C., Mac R.S., Kruse A., Jagannathan J., Van den Abbeele A.D., Velazquez E.F., Demetri G.D., Fisher D.E. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26:2046–2051. doi: 10.1200/JCO.2007.14.0707. [DOI] [PubMed] [Google Scholar]
- 13.Jonker D.J., O'Callaghan C.J., Karapetis C.S., Zalcberg J.R., Tu D., Au H.J., Berry S.R., Krahn M., Price T., Simes R.J., Tebbutt N.C., van Hazel G., Wierzbicki R., Langer C., Moore M.J. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 14.Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W., Harris P.L., Haserlat S.M., Supko J.G., Haluska F.G., Louis D.N., Christiani D.C., Settleman J., Haber D.A. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 15.Sondak VK Smalley K. Targeting mutant BRAF and KIT in metastatic melanoma: ASCO 2009 meeting report. Pigment Cell Melanoma Res. 2009;22:386–387. doi: 10.1111/j.1755-148X.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty K.T., Puzanov I., Kim K.B., Ribas A., McArthur G.A., Sosman J.A., O'dwyer P.J., Lee R.J., Grippo J.F., Nolop K., Chapman P.B. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi J., Zhang J., Xie Y., Soh J., Shigematsu H., Zhang W., Yamamoto H., Peyton M., Girard L., Lockwood W.W., Lam W.L., Varella-Garcia M., Minna J.D., Gazdar A.F. Alterations in genes of the EGFR signaling pathway and their relationship to EGFR tyrosine kinase inhibitor sensitivity in lung cancer cell lines. PLoS One. 2009;4:e4576. doi: 10.1371/journal.pone.0004576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soda M., Choi Y.L., Enomoto M., Takada S., Yamashita Y., Ishikawa S., Fujiwara S., Watanabe H., Kurashina K., Hatanaka H., Bando M., Ohno S., Ishikawa Y., Aburatani H., Niki T., Sohara Y., Sugiyama Y., Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 19.Kwak E.L., Bang Y.J., Camidge D.R., Shaw A.T., Solomon B., Maki R.G., Ou S.H., Dezube B.J., Janne P.A., Costa D.B., Varella-Garcia M., Kim W.H., Lynch T.J., Fidias P., Stubbs H., Engelman J.A., Sequist L.V., Tan W., Gandhi L., Mino-Kenudson M., Wei G.C., Shreeve S.M., Ratain M.J., Settleman J., Christensen J.G., Haber D.A., Wilner K., Salgia R., Shapiro G.I., Clark J.W., Iafrate A.J. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loupakis F., Ruzzo A., Cremolini C., Vincenzi B., Salvatore L., Santini D., Masi G., Stasi I., Canestrari E., Rulli E., Floriani I., Bencardino K., Galluccio N., Catalano V., Tonini G., Magnani M., Fontanini G., Basolo F., Falcone A., Graziano F. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101:715–721. doi: 10.1038/sj.bjc.6605177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Cutsem E., Kohne C.H., Hitre E., Zaluski J., Chang Chien C.R., Makhson A., D'Haens G., Pinter T., Lim R., Bodoky G., Roh J.K., Folprecht G., Ruff P., Stroh C., Tejpar S., Schlichting M., Nippgen J., Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 22.Alexis J.B., Martinez A.E., Lutzky J. An immunohistochemical evaluation of c-kit (CD-117) expression in malignant melanoma, and results of imatinib mesylate (Gleevec) therapy in three patients. Melanoma Res. 2005;15:283–285. doi: 10.1097/00008390-200508000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Eton O., Billings L., Kim K. Phase II trial of imatinib mesylate (STI-571) in metastatic melanoma (MM) J Clin Oncol. 2004;22:114. –s114s. [Google Scholar]
- 24.Ugurel S., Hildenbrand R., Zimpfer A., La Rosee P., Paschka P., Sucker A., Keikavoussi P., Becker J.C., Rittgen W., Hochhaus A., Schadendorf D. Lack of clinical efficacy of imatinib in metastatic melanoma. Br J Cancer. 2005;92:1398–1405. doi: 10.1038/sj.bjc.6602529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyman K., Atkins M.B., Prieto V., Eton O., McDermott D.F., Hubbard F., Byrnes C., Sanders K., Sosman J.A. Multicenter phase II trial of high-dose imatinib mesylate in metastatic melanoma: significant toxicity with no clinical efficacy. Cancer. 2006;106:2005–2011. doi: 10.1002/cncr.21834. [DOI] [PubMed] [Google Scholar]
- 26.Antonescu C.R., Busam K.J., Francone T.D., Wong G.C., Guo T., Agaram N.P., Besmer P., Jungbluth A., Gimbel M., Chen C.T., Veach D., Clarkson B.D., Paty P.B., Weiser M.R. L576P KIT mutation in anal melanomas correlates with KIT protein expression and is sensitive to specific kinase inhibition. Int J Cancer. 2007;121:257–264. doi: 10.1002/ijc.22681. [DOI] [PubMed] [Google Scholar]
- 27.Curtin J.A., Busam K., Pinkel D., Bastian B.C. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 28.Torres-Cabala C.A., Wang W.L., Trent J., Yang D., Chen S., Galbincea J., Kim K.B., Woodman S., Davies M., Plaza J.A., Nash J.W., Prieto V.G., Lazar A.J., Ivan D. Correlation between KIT expression and KIT mutation in melanoma: a study of 173 cases with emphasis on the acral-lentiginous/mucosal type. Mod Pathol. 2009;22:1446–1456. doi: 10.1038/modpathol.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beadling C., Jacobson-Dunlop E., Hodi F.S., Le C., Warrick A., Patterson J., Town A., Harlow A., Cruz F I.I.I., Azar S., Rubin B.P., Muller S., West R., Heinrich M.C., Corless C.L. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14:6821–6828. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- 30.Sadow P.M., Heinrich M.C., Corless C.L., Fletcher J.A., Nose V. Absence of BRAF: NRAS, KRAS, HRAS mutations, and RET/PTC gene rearrangements distinguishes dominant nodules in Hashimoto thyroiditis from papillary thyroid carcinomas. Endocr Pathol. 2009;21:73–79. doi: 10.1007/s12022-009-9101-3. [DOI] [PubMed] [Google Scholar]
- 31.Oeth P., del Mistro G., Marnellos G., Shi T., van den Boom D. Qualitative and quantitative genotyping using single base primer extension coupled with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MassARRAY) Methods Mol Biol. 2009;578:307–343. doi: 10.1007/978-1-60327-411-1_20. [DOI] [PubMed] [Google Scholar]
- 32.van den Boom D., Ehrich M. Discovery and identification of sequence polymorphisms and mutations with MALDI-TOF MS. Methods Mol Biol. 2007;366:287–306. doi: 10.1007/978-1-59745-030-0_16. [DOI] [PubMed] [Google Scholar]
- 33.Fumagalli D., Gavin P.G., Taniyama Y., Kim S.I., Choi H.J., Paik S., Pogue-Geile K.L. A rapid, sensitive, reproducible and cost-effective method for mutation profiling of colon cancer and metastatic lymph nodes. BMC Cancer. 2010;10:101. doi: 10.1186/1471-2407-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janakiraman M., Vakiani E., Zeng Z., Pratilas C.A., Taylor B.S., Chitale D., Halilovic E., Wilson M., Huberman K., Ricarte Filho J.C., Persaud Y., Levine D.A., Fagin J.A., Jhanwar S.C., Mariadason J.M., Lash A., Ladanyi M., Saltz L.B., Heguy A., Paty P.B., Solit D.B. Genomic and biological characterization of exon 4 KRAS mutations in human cancer. Cancer Res. 2010;70:5901–5911. doi: 10.1158/0008-5472.CAN-10-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacConaill L.E., Campbell C.D., Kehoe S.M., Bass A.J., Hatton C., Niu L., Davis M., Yao K., Hanna M., Mondal C., Luongo L., Emery C.M., Baker A.C., Philips J., Goff D.J., Fiorentino M., Rubin M.A., Polyak K., Chan J., Wang Y., Fletcher J.A., Santagata S., Corso G., Roviello F., Shivdasani R., Kieran M.W., Ligon K.L., Stiles C.D., Hahn W.C., Meyerson M.L., Garraway L.A. Profiling critical cancer gene mutations in clinical tumor samples. PLoS One. 2009;4:e7887. doi: 10.1371/journal.pone.0007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas R.K., Baker A.C., Debiasi R.M., Winckler W., Laframboise T., Lin W.M., Wang M., Feng W., Zander T., MacConaill L., Lee J.C., Nicoletti R., Hatton C., Goyette M., Girard L., Majmudar K., Ziaugra L., Wong K.K., Gabriel S., Beroukhim R., Peyton M., Barretina J., Dutt A., Emery C., Greulich H., Shah K., Sasaki H., Gazdar A., Minna J., Armstrong S.A., Mellinghoff I.K., Hodi F.S., Dranoff G., Mischel P.S., Cloughesy T.F., Nelson S.F., Liau L.M., Mertz K., Rubin M.A., Moch H., Loda M., Catalona W., Fletcher J., Signoretti S., Kaye F., Anderson K.C., Demetri G.D., Dummer R., Wagner S., Herlyn M., Sellers W.R., Meyerson M., Garraway L.A. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 37.Arcila M., Lau C., Nafa K., Ladanyi M. Detection of KRAS and BRAF mutations in colorectal carcinoma roles for high-sensitivity locked nucleic acid-PCR sequencing and broad-spectrum mass spectrometry genotyping. J Mol Diagn. 2011;13:64–73. doi: 10.1016/j.jmoldx.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyner J.W., Fletcher L.B., Wang E.Q., Yang W.F., Rutenberg-Schoenberg M.L., Beadling C., Mori M., Heinrich M.C., Deininger M.W., Druker B.J., Loriaux M.M. MET receptor sequence variants R970C and T992I lack transforming capacity. Cancer Res. 2010;70:6233–6237. doi: 10.1158/0008-5472.CAN-10-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruz F., III, Rubin B.P., Wilson D., Town A., Schroeder A., Haley A., Bainbridge T., Heinrich M.C., Corless C.L. Absence of BRAF and NRAS mutations in uveal melanoma. Cancer Res. 2003;63:5761–5766. [PubMed] [Google Scholar]
- 40.Curtin J.A., Fridlyand J., Kageshita T., Patel H.N., Busam K.J., Kutzner H., Cho K.H., Aiba S., Brocker E.B., LeBoit P.E., Pinkel D., Bastian B.C. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 41.Barault L., Veyrie N., Jooste V., Lecorre D., Chapusot C., Ferraz J.M., Lievre A., Cortet M., Bouvier A.M., Rat P., Roignot P., Faivre J., Laurent-Puig P., Piard F. Mutations in the RAS-MAPK: PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer. 2008;122:2255–2259. doi: 10.1002/ijc.23388. [DOI] [PubMed] [Google Scholar]
- 42.Harada K., Hiraoka S., Kato J., Horii J., Fujita H., Sakaguchi K., Shiratori Y. Genetic and epigenetic alterations of Ras signalling pathway in colorectal neoplasia: analysis based on tumour clinicopathological features. Br J Cancer. 2007;97:1425–1431. doi: 10.1038/sj.bjc.6604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinozaki M., Fujimoto A., Morton D.L., Hoon D.S. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753–1757. doi: 10.1158/1078-0432.ccr-1169-3. [DOI] [PubMed] [Google Scholar]
- 44.Dunlap J., Le C., Shukla A., Patterson J., Presnell A., Heinrich M.C., Corless C.L., Troxell M.L. Phosphatidylinositol-3-kinase and AKT1 mutations occur early in breast carcinoma. Breast Cancer Res Treat. 2010;120:409–418. doi: 10.1007/s10549-009-0406-1. [DOI] [PubMed] [Google Scholar]
- 45.Fukuchi T., Sakamoto M., Tsuda H., Maruyama K., Nozawa S., Hirohashi S. Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res. 1998;58:3526–3528. [PubMed] [Google Scholar]
- 46.Hayes M.P., Wang H., Espinal-Witter R., Douglas W., Solomon G.J., Baker S.J., Ellenson L.H. PIK3CA and PTEN mutations in uterine endometrioid carcinoma and complex atypical hyperplasia. Clin Cancer Res. 2006;12:5932–5935. doi: 10.1158/1078-0432.CCR-06-1375. [DOI] [PubMed] [Google Scholar]
- 47.Moreno-Bueno G., Sanchez-Estevez C., Palacios J., Hardisson D., Shiozawa T. Low frequency of BRAF mutations in endometrial and in cervical carcinomas. Clin Cancer Res. 2006;12:3865–3866. doi: 10.1158/1078-0432.CCR-06-0284. [DOI] [PubMed] [Google Scholar]
- 48.Pollock P.M., Gartside M.G., Dejeza L.C., Powell M.A., Mallon M.A., Davies H., Mohammadi M., Futreal P.A., Stratton M.R., Trent J.M., Goodfellow P.J. Frequent activating FGFR2 mutations in endometrial carcinomas parallel germline mutations associated with craniosynostosis and skeletal dysplasia syndromes. Oncogene. 2007;26:7158–7162. doi: 10.1038/sj.onc.1210529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saegusa M., Hashimura M., Yoshida T., Okayasu I. beta-Catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer. 2001;84:209–217. doi: 10.1054/bjoc.2000.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shoji K., Oda K., Nakagawa S., Hosokawa S., Nagae G., Uehara Y., Sone K., Miyamoto Y., Hiraike H., Hiraike-Wada O., Nei T., Kawana K., Kuramoto H., Aburatani H., Yano T., Taketani Y. The oncogenic mutation in the pleckstrin homology domain of AKT1 in endometrial carcinomas. Br J Cancer. 2009;101:145–148. doi: 10.1038/sj.bjc.6605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curtin J.A., Stark M.S., Pinkel D., Hayward N.K., Bastian B.C. PI3-kinase subunits are infrequent somatic targets in melanoma. J Invest Dermatol. 2006;126:1660–1663. doi: 10.1038/sj.jid.5700311. [DOI] [PubMed] [Google Scholar]
- 52.Davies M.A., Stemke-Hale K., Tellez C., Calderone T.L., Deng W., Prieto V.G., Lazar A.J., Gershenwald J.E., Mills G.B. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer. 2008;99:1265–1268. doi: 10.1038/sj.bjc.6604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Omholt K., Platz A., Ringborg U., Hansson J. Cytoplasmic and nuclear accumulation of beta-catenin is rarely caused by CTNNB1 exon 3 mutations in cutaneous malignant melanoma. Int J Cancer. 2001;92:839–842. doi: 10.1002/ijc.1270. [DOI] [PubMed] [Google Scholar]
- 54.Van Raamsdonk C.D., Bezrookove V., Green G., Bauer J., Gaugler L., O'Brien J.M., Simpson E.M., Barsh G.S., Bastian B.C. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omholt K., Karsberg S., Platz A., Kanter L., Ringborg U., Hansson J. Screening of N-ras codon 61 mutations in paired primary and metastatic cutaneous melanomas: mutations occur early and persist throughout tumor progression. Clin Cancer Res. 2002;8:3468–3474. [PubMed] [Google Scholar]
- 56.Omholt K., Krockel D., Ringborg U., Hansson J. Mutations of PIK3CA are rare in cutaneous melanoma. Melanoma Res. 2006;16:197–200. doi: 10.1097/01.cmr.0000200488.77970.e3. [DOI] [PubMed] [Google Scholar]
- 57.Akslen L.A., Angelini S., Straume O., Bachmann I.M., Molven A., Hemminki K., Kumar R. BRAF and NRAS mutations are frequent in nodular melanoma but are not associated with tumor cell proliferation or patient survival. J Invest Dermatol. 2005;125:312–317. doi: 10.1111/j.0022-202X.2005.23788.x. [DOI] [PubMed] [Google Scholar]
- 58.Kumar R., Angelini S., Czene K., Sauroja I., Hahka-Kemppinen M., Pyrhonen S., Hemminki K. BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res. 2003;9:3362–3368. [PubMed] [Google Scholar]
- 59.Lang J., Mackie R.M. Prevalence of exon 15 BRAF mutations in primary melanoma of the superficial spreading, nodular, acral, and lentigo maligna subtypes. J Invest Dermatol. 2005;125:575–579. doi: 10.1111/j.0022-202X.2005.23833.x. [DOI] [PubMed] [Google Scholar]
- 60.Omholt K., Platz A., Kanter L., Ringborg U., Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483–6488. [PubMed] [Google Scholar]
- 61.Saldanha G., Potter L., Daforno P., Pringle J.H. Cutaneous melanoma subtypes show different BRAF and NRAS mutation frequencies. Clin Cancer Res. 2006;12:4499–4505. doi: 10.1158/1078-0432.CCR-05-2447. [DOI] [PubMed] [Google Scholar]
- 62.Henderson Y.C., Ahn S.H., Kang Y., Clayman G.L. Sorafenib potently inhibits papillary thyroid carcinomas harboring RET/PTC1 rearrangement. Clin Cancer Res. 2008;14:4908–4914. doi: 10.1158/1078-0432.CCR-07-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Platt F.M., Hurst C.D., Taylor C.F., Gregory W.M., Harnden P., Knowles M.A. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15:6008–6017. doi: 10.1158/1078-0432.CCR-09-0898. [DOI] [PubMed] [Google Scholar]
- 64.Kompier L.C., Lurkin I., van der Aa M.N., van Rhijn B.W., Van Der Kwast T.H., Zwarthoff E.C. FGFR3: HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS One. 2010;5:e13821. doi: 10.1371/journal.pone.0013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janku F., Tsimberidou A.M., Garrido-Laguna I., Wang X., Luthra R., Hong D.S., Naing A., Falchook G.S., Moroney J.W., Piha-Paul S.A., Wheler J.J., Moulder S.L., Fu S., Kurzrock R. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitor. Mol Cancer Ther. 2011;10:558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.