Abstract
Hepatocellular carcinoma (HCC) has a 5-year survival rate of <10% because it is difficult to diagnose early. Mutations in the TP53 gene are associated with approximately 50% of human cancers. A hotspot mutation, a G:C to T:A transversion at codon 249 (249T), may be a potential DNA marker for HCC screening because of its exclusive presence in HCC and its detection in the circulation of some patients with HCC. A locked nucleic acid clamp-mediated PCR assay, followed by melting curve analysis (using the SimpleProbe), was developed to detect the TP53 249T mutation. In this assay, the locked nucleic acid clamp suppressed 107 copies of wild-type templates and permitted detection of 249T-mutated template, with a sensitivity of 0.1% (1:1000) of the mutant/wild-type ratio, assessed by a reconstituted standard within 2 hours. With an amplicon size of 41 bp, it detects target DNA sequences in short fragmented DNA templates. The detected mutations were validated by DNA sequencing analysis. We then tested DNA isolated from urine samples of patients with HCC for p53 mutations and identified positive TP53 mutations in 9 of 17 samples. The possibility of using this novel TP53 249T assay to develop a urine or blood test for HCC screening is discussed.
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer and the third leading cause of cancer deaths worldwide. The 5-year survival rate is 26% among patients in whom cancer is found at an early stage, compared with only 2% when it is found after spreading to distant organs.1 A better method for detecting HCC at an early curable stage is needed to improve the prognosis of this disease.
Mutations in the TP53 gene are associated with approximately 50% of human cancers. Although the mutations have been found in many sites of the TP53 gene in cancers, a specific missense mutation, resulting from a guanine to thymine (G>T) transversion at the third position of codon 249 (249T) of the exon 7 of the gene, is the particular hotspot mutation found almost exclusively in patients with HCC. Approximately 50% of patients with HCC have this p53 hotspot mutation2–7; in some patients with HCC, this p53 hotspot mutation is also found in the circulation.3,8–11 Thus, the TP53 249T hotspot mutation could possibly be a DNA marker for HCC screening.
Researchers12–19 have shown that urine contains DNA from the circulation. We also showed that DNA in urine can be categorized into two species based on size: high-molecular-weight (HMW) urine DNA (>1 kb), derived mostly from sloughed-off cell debris from the urinary tract; and low-molecular-weight (LMW) urine DNA (approximately 150 to 250 bp), derived primarily from apoptotic cells.13 Researchers20–23 have also shown that the shorter the amplicon size, the higher the sensitivity is of the assay for detecting circulating DNA markers in the circulation or in urine. Sikora et al22 and Shekhtman et al23 further suggested that PCR assays targeting template sequences of ≤50 nucleotides (nt) are necessary to obtain a sensitivity >50% to detect circulation-derived DNA of interest in urine. Thus, one critical criterion for detecting the sequence of interest in the pool of small fragmented DNA from the circulation or urine is developing assays that produce amplicon sizes of <50 bp. We attempted to detect the HCC-derived TP53 249T hotspot mutation in the urine of patients with HCC so that a potential urine test for HCC screening could be explored.
Our previous studies13 suggested that an assay targeting a short amplicon with a sensitivity of 10 copies and a 1:100 mutant/wild-type (WT) sequence ratio might be needed to detect circulation-derived DNA markers in urine. The TP53 codon 249T hotspot mutation has been detected by various methods, including restriction fragment length polymorphism (RFLP) or RFLP-PCR when PCR sequencing was used to confirm or identify the mutation after RFLP,11,24–27 short oligonucleotide mass analysis,3,9,10,28 single-strand conformation polymorphism analysis of the PCR product,29 DNA sequencing of the PCR product,29 denaturing high-performance liquid chromatography,30 or PCR pyrosequencing.31 These assays are either targeting an amplicon size of >50 bp, requiring sophisticated instrumentation with multiple steps, or requiring less than desired sensitivity and specificity. In either case, the assay might not have sufficient sensitivity to detect circulation-derived TP53 249T in urine.
Previous studies30,32–34 have used a locked nucleic acid (LNA) clamp to inhibit the amplification of WT DNA in the PCR for detecting mutated sequences. The SimpleProbe (Roche Applied Science, Indianapolis, IN) has been used in real-time PCR assays as a sequence-specific reporting dye to quantitatively monitor the PCR amplification; it was also subsequently used to characterize the PCR product by melting curve analysis after the completion of the PCR amplification, such that the amplicon derived from the mutated and WT templates can be distinguished.35–40 We applied three strategies for the development of an assay for detecting the circulation-derived TP53 249T mutation in the urine of patients with HCC: i) an LNA clamp to suppress the amplification of the WT template to allow the detection of a small fraction of mutated sequences, ii) a SimpleProbe to characterize the PCR product by melting curve analysis at the completion of the PCR, and iii) an amplicon size of 41 bp to enhance the sensitivity of the assay for detecting circulating DNA markers. We demonstrate that this LNA–clamp-mediated PCR assay successfully detected the TP53 codon 249 mutation in the urine of patients with HCC, suggesting that a urine test for HCC screening could be possible.
Materials and Methods
Study Subjects
The HCC tissues and urine samples used in this study were obtained with informed consent from patients who underwent radical resection at National Cheng Kung University Medical Center, Taiwan, in accordance with the guidelines of the Institutional Review Board. The individual subject information is listed in Table 1. The urine samples of the healthy control group (with no symptom of any liver disease, n = 15) were collected at National Cheng Kung University Medical Center in accordance with the guidelines of the Institutional Review Board.
Table 1.
Study Subjects with HCC
| Subject | Sex | Age (years) | HBV | HCV |
|---|---|---|---|---|
| T1 | Male | 51 | + | NA |
| T2 | Male | 74 | − | NA |
| T3 | Male | 58 | + | − |
| T4 | Male | 29 | + | − |
| T5 | Female | 71 | + | NA |
| T6 | Male | 63 | + | − |
| T7 | Male | 65 | + | NA |
| T8 | Male | 43 | + | − |
| T9 | Male | 53 | + | − |
| T10 | Male | 68 | − | + |
| T11 | Female | 66 | − | + |
| T12 | Female | 70 | − | NA |
| T13 | Female | 67 | + | − |
| T14 | Male | 64 | + | NA |
| T15 | Male | 56 | + | NA |
| U1 | Male | 74 | − | NA |
| U2 | Male | 29 | + | − |
| U3 | Female | 71 | + | NA |
| U4 | Male | 53 | + | − |
| U5 | Female | 70 | − | NA |
| U6 | Female | 67 | + | − |
| U7 | Male | 64 | + | NA |
| U8 | Female | 64 | NA | − |
| U9 | Male | 48 | + | − |
| U10 | Male | 58 | + | NA |
| U11 | Male | 41 | + | + |
| U12 | Male | 77 | NA | NA |
| U13 | Male | 51 | + | − |
| U14 | Male | 61 | + | − |
| U15 | Female | 74 | + | NA |
| U16 | Female | 72 | − | + |
| U17 | Female | 52 | − | − |
HBV, hepatitis B virus; HCV, hepatitis C virus; NA, not available; T, tissue; U, urine; +, infected; -, not infected.
Oligonucleotides
Primers, probes, and the LNA clamp used in this study are listed in Table 2. Oligonucleotides were obtained from Sigma-Aldrich (St. Louis, MO). The SimpleProbes and LNA clamp were obtained from TIB Molbiol, LLC (Adelphia, NJ).
Table 2.
Sequence and Location in the TP53 of Oligonucleotides Used in This Study
| Primer and probe name | Nucleotide location | Sequence |
|---|---|---|
| P53_qF | 14050–14065 | 5′-CTGCATGGGCGGCATG-3′ |
| P53_249R | 14076–14090 | 5′-TGATGGTGAGGATGG-3′ |
| P53_LNA | 14070–14076 | 5′-GGAGGCC-3′ |
| P53_249_T probe | 14067–14078 | 5′-ACCGGAGTCCCA-3′ |
| P53_249_C probe | 14067–14078 | 5′-ACCGGAGCCCCA-3′ |
| P53_249_WT probe | 14067–14078 | 5′-ACCGGAGGCCCA-3′ |
TP53 is GenBank no. X54156. The boldfaced and italicized bases denote LNA bases.
Urine Collection and DNA Isolation
The procedures for urine collection and urine DNA isolation were previously described.41 Briefly, 0.5 mol/L EDTA, pH 8.0, was added to a fresh urine sample to a final concentration of 10 mmol/L EDTA to inhibit possible nuclease activity and stored at −70°C. To isolate total urine DNA, the frozen urine sample was thawed at room temperature and then placed immediately in ice before DNA isolation. DNA was isolated from thawed urine within 1 hour.
Urine samples were mixed with 1 vol of 6 mol/L guanidine thiocyanate by inverting eight times. Then, 1 mL of resin (Wizard DNA purification kit; Promega, Madison, WI) was added to the urine lysate and incubated for 2 hours to overnight at room temperature, with gentle mixing. The resin-DNA complex was centrifuged, transferred to a minicolumn (provided in the kit), and washed with a buffer provided by the manufacturer; the DNA was then eluted with Tris-EDTA buffer. DNA from paraffin-embedded tissue sections was isolated using the MasterPure DNA kit (Epicenter, Madison, WI), per the manufacturer's instructions.
Fractionation of HMW and LMW DNA by CMBs
The HMW and LMW urine DNA fractions were obtained using carboxylated magnetic beads (CMBs; Agencourt Bioscience Corporation, Beverly, MA) and a binding method developed previously by our laboratory.42 Total urine DNA (resuspended in Tris-EDTA buffer) was mixed with 5 mol/L NaCl and 20% polyethylene glycol 8000 (AMRESCO Inc., Solon, OH) to final concentrations of 0.3 mol/L and 8%, respectively. The CMB suspension (Agencourt Bioscience Corporation) was washed and resuspended with Tris-EDTA buffer before use. A prewashed CMB suspension, 10 μL, was added to the DNA mix and incubated for 1 to 2 hours at room temperature to allow binding of HMW DNA to the beads. The beads bound with HMW DNA were then removed from the suspension using a magnetic plate (Agencourt Bioscience Corporation). The LMW DNA remaining in the suspension was collected by adding 10 μL of prewashed CMB in a solution of 1.2 mol/L NaCl and 10% polyethylene glycol 8000. The beads bound with LMW or HMW DNA were then washed with 75% ethanol, and the DNA was eluted in Tris-EDTA buffer.
Quantification of DNA Templates by Real-Time PCR
We developed the real-time PCR assay, targeting a 65-bp β-globin gene (GenBank no. M14695), to quantify DNA. A 10-μL reaction was assembled using the LightCycler FastStart DNA Master SYBR Green I system (Roche Applied Science, Mannheim, Germany). The reaction contained 1× SYBR Green Master Mix, 1.0 μmol/L primers [Glb-65: 5′- CTTCATCCACGTTCACCTTG-3′ (forward) and 5′-GCATCTGACTCCTGAGGAGA-3′ (reverse)], 2.5 mmol/L MgCl2, and the DNA template. By using the Roche LightCycler 2.0 Real-Time PCR system (Roche Applied Science, Indianapolis, IN), the PCR was performed under the following conditions: 95°C for 10 minutes to activate the FastStart Taq polymerase; then, 95°C for 10 seconds, 58°C for 20 seconds, and 72°C for 10 seconds for 45 cycles; and, finally, the melting curve at 95°C for 5 seconds, 65°C for 1 minute, and 97°C for continuous hold. Cooling occurred at 40°C for 30 seconds.
Construction of the Control Plasmids Containing p53 Codon 249 Mutations
To construct the mutation clone standards, the TP53 nt position 13975 to 14200 (GenBank no. X54156) was amplified from HepG2 cells, which contain the WT TP53 gene, and cloned into pPCR-Script Amp SK(+) vector using Stratagene's PCR-Script Amp Cloning Kit (Agilent Technologies, Santa Clara, CA) and designated as p53 WT. The sequence of the p53 WT clone was confirmed by DNA sequencing. The p53 WT plasmid was then used as a template to construct two mutant controls, 249T and codon 249 G>C mutation (249C), by two-step PCR site-directed mutagenesis. The DNA sequence of all of the control clones was confirmed by DNA sequencing by T3 and T7 primers.
Detection of the p53 Codon 249T Mutation
The p53 codon 249T mutation assay was developed using an LNA clamp-mediated PCR assay, followed by melting curve analysis using a SimpleProbe. We targeted a 41-bp DNA fragment from nt position 14048 to 14092 of the TP53 gene (GenBank no. X54156); the sequences and locations of primers are listed in Table 2. The PCR was assembled in a final volume of 10 μL containing 1.0 U Hot Start Taq (Qiagen, Valencia, CA), 1× PCR buffer, 2.5 mmol/L deoxynucleotide triphosphates, 1 μmol/L of each primer (P53_qF&P53_249R), and DNA templates. The cycle profile was 95°C for 15 minutes to activate Taq polymerase, followed by 40 cycles of 95°C for 30 seconds, 54°C for 30 seconds, and 72°C for 20 seconds. The PCR product was subsequently used for melting curve analysis. To perform the melting curve analysis, 0.2 μmol/L of the codon 249T–specific SimpleProbe was added, and the reaction was denatured at 95°C for 10 minutes and cooled to 30°C for 3 minutes. The temperature was then increased at a transition rate of 0.1°C/second to 70°C for continuous hold.
Results
Development and Characterization of an LNA Clamp-Mediated PCR Assay for p53 Codon 249 Mutations
Circulation-derived urine DNA is a collection of DNA fragments. We have shown that this circulation-derived DNA is part of an LMW urine DNA fraction that has primarily <300 bp.13 A tumor-derived DNA marker is assumed to be in a small fraction of LMW urine DNA isolated from patients with cancer; thus, it would require selective amplification to be detected. The LNA clamp assay has been used by researchers30,32–34 to suppress the amplification of the WT template during the PCR so that detection of a small fraction of mutated DNA from the pool of WT DNA templates could be accomplished. A 7-nt LNA clamp (Figure 1A) was designed to suppress the amplification of WT sequences. To prevent the LNA clamp from serving as a primer for polymerization, the 3′ end of the LNA oligonucleotide was phosphorylated to reduce the possibility of a false-positive result.
Figure 1.
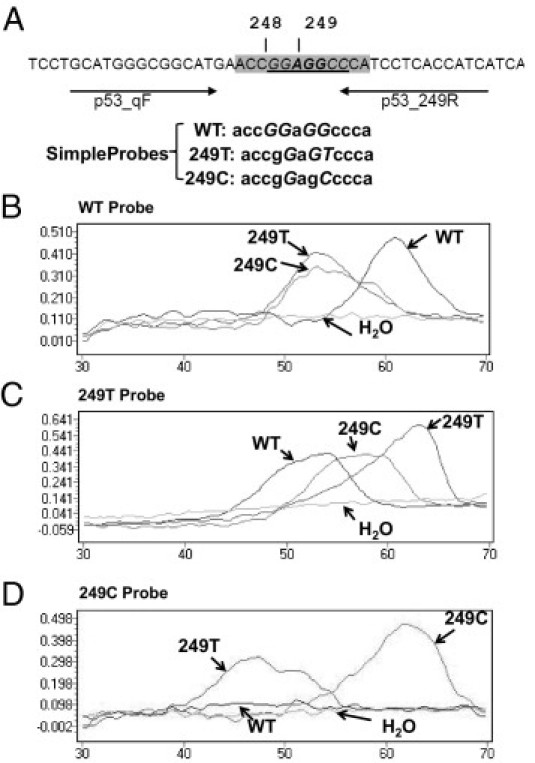
Detection of p53 codon 249 mutations by an LNA clamp-mediated PCR assay and melting curve analysis with SimpleProbes. A: Locations and sequences of primers (arrows), clamp (underlined), and probes (shaded) used in the assay. Codons 248 and 249 are indicated by vertical lines. LNAs are italicized. SimpleProbe sequences shown with LNA are italicized and capitalized. PCR products derived from plasmids p53 WT, p53_249T, p53_249C, or H2O (arrows) were determined by melting curve analysis with the SimpleProbes 249WT (B), 249T (C), and 249C (D).
As previously mentioned, the size of the amplicon should be ≤50 nt to have sufficient sensitivity to detect sequences of interest in a fragmented short DNA substrate. In this assay, we designed primers to amplify a 41-nt region (Figure 1A). The PCR was designed to amplify selective p53 templates that contained any mismatches with the LNA clamp. At the completion of the PCR, a SimpleProbe was added to each reaction to characterize the PCR product by melting curve analysis.
Characterization of PCR Products by Melting Curve Analysis Using SimpleProbes
Three SimpleProbes, SP_WT, SP_249T, and SP_249C, for the region of interest were designed (Figure 1A) for the melting curve analysis. The melting curves generated by different templates with SimpleProbes SP_WT, SP_249T, and SP_249C are shown in Figure 1, B–D. In this experiment, we performed PCR using the control plasmid DNA clones, p53_WT, p53_249T, and p53_249C, generated as described in Materials and Methods. The PCR products were analyzed by three different SimpleProbes. As expected, the PCR products generated from the p53_WT template had the highest Tm; (approximately 62°C) compared with templates p53_249T and p53_249C when a SimpleProbe SP_WT was used (Figure 1B). This occurred because of the 100% homology of the probe with the PCR product from the WT template and one mismatch existing between the PCR products generated from mutated templates and the probe. When the melting curve analysis was performed with SP_249T, the PCR products generated from the p53_249T-mutated sequence exerted the highest Tm of 63°C (Figure 1C) compared with that of WT and p53_249C. No melting curve was observed for the PCR performed with no template (H2O control). When the SimpleProbe SP_249C was used for melting curve analysis, the PCR products generated from the p53_249C exhibited a Tm of approximately 62°C; a Tm of approximately 47°C was observed for the PCR products generated from p53_249T. However, no clear peak was detected for the PCR products derived from the p53_WT template (Figure 1D). For subsequent experiments, we used the SimpleProbe SP_249T for melting curve analysis because 249T was the mutation of interest and it generated clear melting curves with the PCR products from all three templates.
Sensitivity of the TP53 249 Mutation Assay
To determine the sensitivity of the TP53 249 mutation assay, we first assessed the extent of the suppression of the PCR amplification of the WT template by the LNA clamp and the specificity of LNA suppression. Two sets of experiments were performed. First, we measured the degree of suppression of PCR amplification of the WT templates. The PCRs were performed in the presence or absence of the LNA clamp, with WT templates ranging from 101 to 107 copies. To control for PCR amplification in the presence of LNA, we added 10 copies of p53_249T templates to each reaction (Figure 2, A and B). After completion of PCR amplification, the PCR products were analyzed by melting curve analysis using a SimpleProbe SP_249T probe. In the absence of LNA, the PCR products generated by the WT templates ranging from 101 to 107 copies, were readily detected with a Tm of approximately 52°C. Only the 1:1 ratio of mutant/WT (10 copies of p53_249T and 10 copies of p53WT templates) was able to deliver a detectable PCR product of Tm of 63°C specifying the 249T-mutated sequence (Figure 2A). In contrast, amplification of WT templates was undetectable by melting curve analysis when 2.0 μmol/L of LNA clamp was used in the PCRs (Figure 2B), even with 107 copies of WT templates. Encouragingly, in the presence of LNA, a clear melting curve peak (Tm, approximately 63°C) of 10 copies of TP53 249T-mutated templates was detected even when it was mixed with 10,000 copies of WT templates, with a ratio of mutant/WT templates of 0.1% (1:1000).
Figure 2.
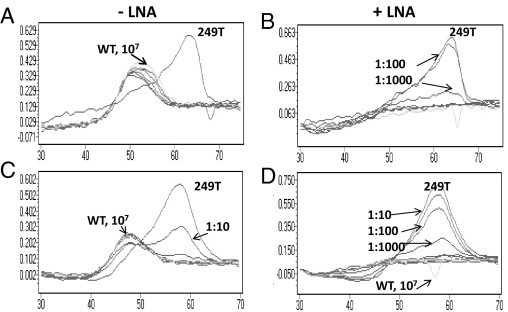
Selective suppression of PCR amplification of TP53 WT templates by the LNA clamp. Melting curve analysis of the PCR products generated from the p53_WT (gray) or p53_249T (black) template in the absence (-) or presence (+) of the LNA clamp using SimpleProbe SP_249T. A and B: The PCR products were generated from a range of 101 to 107 copies of WT (gray) templates, with 10 copies of the p53_249T (black) template in each reaction. The ratios of p53_249T/p53_WT are indicated. C and D: Melting curve analysis of the PCR products generated from a range of 101 to 107 copies of the mutated p53_249T template with 107 copies of the p53_WT template. The ratios of p53_249T/p53_WT are indicated.
Next, we determined the minimum amount of mutated templates that could be detected against the background of 107 copies of WT templates. Mutated templates, ranging from 1 to 107 copies, were mixed with 107 copies of WT template and subjected to the TP53 249 mutation assay. In the absence of the LNA clamp, the assay could detect only 106 copies of the p53_249T template (1:10 ratio of mutant/WT, Figure 2C). The assay was able to detect 104 copies of mutated templates (ie, a 1:1000 ratio of mutant/WT; Figure 2D). As expected, the amplification of 107 WT templates was undetectable by the SimpleProbe in the presence of the LNA clamp. Thus, the sensitivity of the TP53 249 mutation is 0.1%, as determined by both sets of experiments.
Validation of the TP53 249T Mutation Assay by DNA Sequencing Using HCC Tissues
To validate the assay, we performed the TP53 249 mutation assay on archived DNA isolated from HCC tissues, followed by DNA sequencing analysis to confirm the mutation detected by the LNA clamp-mediated TP53 249 mutation assay. The plasmid DNA, p53_WT, and p53_249T were used as controls for the assay. Fifteen HCC tissue DNA samples were tested in the absence or presence of the LNA clamp, followed by melting curve analysis with a SimpleProbe SP_249T. Of the 15 HCC tissue DNA samples tested, six contained p53 mutations (Table 3). The melting curves of the PCR products generated in the presence or absence of the LNA clamp of the samples that contained detectable mutations are shown in Figure 3. In the absence of the LNA clamp, PCR products generated from all of the reactions contained the WT sequence (Tm, 48°C) by melting curve analysis with a SimpleProbe SP_249T. A combination of two peaks was detected in two DNA samples (T9 and T13), suggesting that most of the DNA samples contained predominantly WT sequences and that only these two samples contained a sufficient amount of mutated sequences to be detected in the absence of LNA clamp suppression.
Table 3.
Detection of p53 Mutations in HCC Tissues by the TP53 249 Assay
| Tissue | Tm by SP_249T |
Sequence (5′-CCGGAGGCCCA-3′) | |
|---|---|---|---|
| Without LNA | With LNA | ||
| 1 | 48°C (WT) | – | ND |
| 2 | 48°C (WT) | 58°C | 5′-CCGGAGCCCCA-3′ |
| 3 | 48°C (WT) | 63°C | 5′-CCGGAGTCCCA-3′ |
| 4 | 48°C (WT) | –⁎ | ND |
| 5 | 48°C (WT) | – | ND |
| 6 | 48°C (WT) | – | ND |
| 7 | 48°C (WT) | – | ND |
| 8 | 48°C (WT) | – | ND |
| 9 | 48°C /57°C | 63°C | 5′-CCGGAGTCCCA-3′ |
| 10 | 48°C (WT) | 63°C | ND |
| 11 | 48°C (WT) | 60°C | 5′-CCGGAGTCCCA-3′ |
| 12 | 48°C (WT) | Insertion† | |
| 13 | 48°C (WT) | 63°C | 5′-CCGAAGGCCCA-3′ |
| 14 | 48°C (WT) | – | ND |
| 15 | 48°C (WT) | – | ND |
The boldfaced and underlined mutations are those detected by DNA sequencing, and the italicized and underlined sequence is that of the LNA clamp.
ND, not determined.
No peak detected.
Identified as underlined in the following sequences: 5′-CATGA-ACCGGAGGC-CCA-3′ and 5′-CATGA-TGTTCCCAGCAGGCTACACGCTCCTT-CCA-3′.
Figure 3.
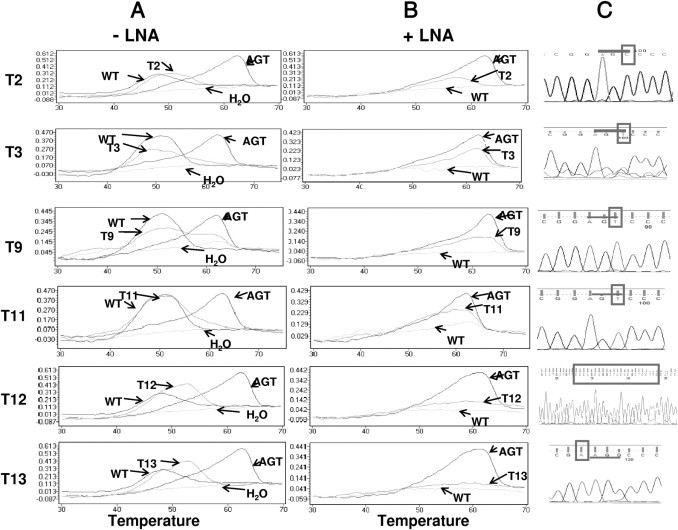
Comparison of melting curve analyses and DNA sequencing chromatograms of the HCC tissue DNA samples that contained the p53 mutation detectable by the p53_249 assay. Melting curves of the PCR products generated in the absence (-) or presence (+) of the LNA clamp using the SimpleProbe SP_249T are shown in A and B. The chromatograms of the sequence of interest, obtained by DNA sequencing analysis of the respective PCR clones, as described in the text, are shown in C. The sequences of the 249 codon are underlined, and the mutated or inserted sequences are boxed. In each reaction, p53_WT and p53_249T templates were used as controls.
To confirm that the p53 mutations detected by the TP53 249 PCR assay were not false-positive results, we performed DNA sequencing analysis of the PCR products that showed the Tm of the mutated sequences. Because a 41-bp PCR product is too short to perform DNA sequencing directly, we cloned the PCR products generated in the presence of the LNA clamp into the plasmid vector and then sent the cloned plasmid DNA for DNA sequencing analysis using a T7 primer. Five clones from each sample were analyzed. The mutated sequences identified are listed in Table 3, and the chromatograms of the sequencing data are shown in Figure 3.
Because the mutation was detected by inhibiting the amplification of WT sequences by the LNA clamp during the PCR, it is expected that the mutations occurring in the region of the LNA clamp would be detected. Table 3 indicates that we detected mutations in codons 248 and 249 and one insertion mutation in sample T12, which was not previously described. When we compared DNA sequencing analysis results with melting curve analysis results, we did not detect any false-positive results using the p53 mutation assay developed in this study. However, the melting curve analysis could accurately identify the 249T mutation only when a SimpleProbe SP_249T was used. For other codon 249 mutations such as p53 codon 249C, the assay could not detect only the existence of mutations in the region of interest other than the type of mutation.
Detection of a p53 Mutation in the Urine of Patients with HCC by the LNA Clamp-Mediated PCR Assay
Once the assay was validated for detection of p53 mutations, we used 17 urine samples from patients with HCC to see if it was able to detect the circulation-derived p53 mutation. Total urine DNA was isolated and fractionated into HMW and LMW DNA fractions, as described in Materials and Methods. The amount of the DNA template was quantified by a PCR assay we developed that targeted the β-globin gene, as described in Materials and Methods. The amount of DNA template (copy number per milliliter urine) in both HMW and LMW urine DNA fractions was determined and listed in Table 4. The amount of DNA from 200 μL of urine was subjected to the LNA clamp-mediated p53 mutation assay. The PCRs were performed with or without the LNA clamp. The results are shown in Figure 4 and summarized in Table 4. Of 17 urine samples, 10 contained detectable p53 mutations in the LMW urine DNA fraction, as shown by melting curve analysis of the PCR product generated in the presence of the LNA clamp. More strikingly, mutations were detected in five LMW urine DNA samples, U2, U3, U4, U7, and U14, even in the absence of the LNA clamp, suggesting that the amount of mutated DNA was clearly >1% because we were not able to detect 1% of mutated DNA in a reconstituted sample when the PCRs were preformed in the absence of the LNA clamp (Figure 2). With the exception of U7, no mutation was detected in the HMW urine DNA fractions that were matched with p53 mutation-positive LMW DNA fractions, suggesting that the p53 mutations detected in the LMW urine DNA fraction were clearly derived from the circulation, most likely from the HCC, because the TP53 249T hotspot mutation is rarely found in other cancers. In addition to U7, the HMW urine DNA fraction, which contained a detectable p53 mutation, the HMW urine DNA fraction of U15 also contained a non-WT melting curve in the absence of the LNA clamp. Interestingly, this non-WT melting curve was not detectable in the presence of the LNA clamp during the PCR amplification or in the LMW DNA fraction. As controls, LMW urine DNA isolated from 15 age- and sex-matched individuals was also subjected to p53 codon 249 mutation detection; we found no detectable p53 codon 249 mutation in any of the normal urine samples (data not shown). Detection of the p53 mutation in the LMW urine DNA fraction shows that the LNA clamp-mediated p53 PCR assay was able to detect a circulation-derived p53 DNA mutation in urine. It was of interest to see how many of the urine samples from patients with HCC who had TP53 249 mutations were positive for the mutation. However, only 7 of the 17 urine samples had corresponding tissue samples available, as listed in Table 4. These seven HCC tissues were part of the samples used to validate the TP53 249 mutations (Table 3). Three different scenarios were observed: i) tissue positive, urine positive (T9/U4 and T13/U6); ii) tissue positive, urine negative (T2/U1); and iii) tissue negative, urine positive (T4/U2, T5/U3, T12/U5, and T14/U7).
Table 4.
Detection of the p53 Mutation in the Urine of Patients with HCC
| HMW urine DNA |
LMW urine DNA |
HCC tissue |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Urine | Copy/mL⁎ | Without LNA | With LNA | Copy/mL⁎ | Without LNA | With LNA | Without LNA | With LNA | |
| 1 | 3.06 × 104 | WT | – | 1.43 × 105 | WT | – | T2 | WT | Mut |
| 2 | 9.74 × 103 | WT | – | 5.51 × 104 | Mix | Mut | T4 | WT | – |
| 3 | 7.60 × 100 | WT | – | 2.40 × 103 | Mix | Mut | T5 | WT | – |
| 4 | 3.28 × 102 | WT | – | 3.39 × 103 | Mix | Mut | T9 | Mix | Mut |
| 5 | 2.35 × 103 | WT | – | 1.02 × 103 | WT | Mut | T12 | WT | – |
| 6 | 1.19 × 103 | WT | – | 7.53 × 103 | WT | Mut | T13 | WT | Mut |
| 7 | 3.74 × 103 | WT | Mut | 3.09 × 103 | Mix | Mut | T14 | WT | – |
| 8 | 6.30 × 104 | WT | – | 8.57 × 104 | WT | Mut | NA | NA | NA |
| 9 | 1.88 × 104 | WT | – | 1.25 × 104 | WT | Mut | NA | NA | NA |
| 10 | 2.87 × 103 | WT | – | 8.88 × 102 | WT | Mut | NA | NA | NA |
| 11 | 9.06 × 103 | WT | – | 3.31 × 102 | WT | – | NA | NA | NA |
| 12 | 2.45 × 103 | WT | – | 3.54 × 103 | WT | – | NA | NA | NA |
| 13 | 6.60 × 103 | WT | – | 1.15 × 104 | WT | – | NA | NA | NA |
| 14 | 5.96 × 104 | WT | – | 5.83 × 104 | Mix | – | NA | NA | NA |
| 15 | 9.56 × 102 | Mix | – | 3.31 × 102 | WT | – | NA | NA | NA |
| 16 | 3.93 × 103 | WT | – | 2.58 × 103 | WT | – | NA | NA | NA |
| 17 | 7.20 × 103 | WT | – | 7.24 × 103 | WT | – | NA | NA | NA |
The PCR reaction of the p53 mutation assay was performed in the absence or presence of the LNA clamp. The plasmids DNA p53 WT and TP53 249T were used as WT and 249T mutation sequence controls in each assay to provide the Tm reference by melting curve analysis using the SP_249T SimpleProbe. The DNA equivalent of 200 μL urine was used in each assay.
WT, Tm of the PCR product is the WT sequence; Mut, Tm of the PCR product is not WT sequences; Mix, both WT Tm and non-WT Tm were observed; NA, not available.
DNA quantification was determined by the quantitative PCR assay, as described in Materials and Methods, and calculated as copy number/mL urine.
Figure 4.
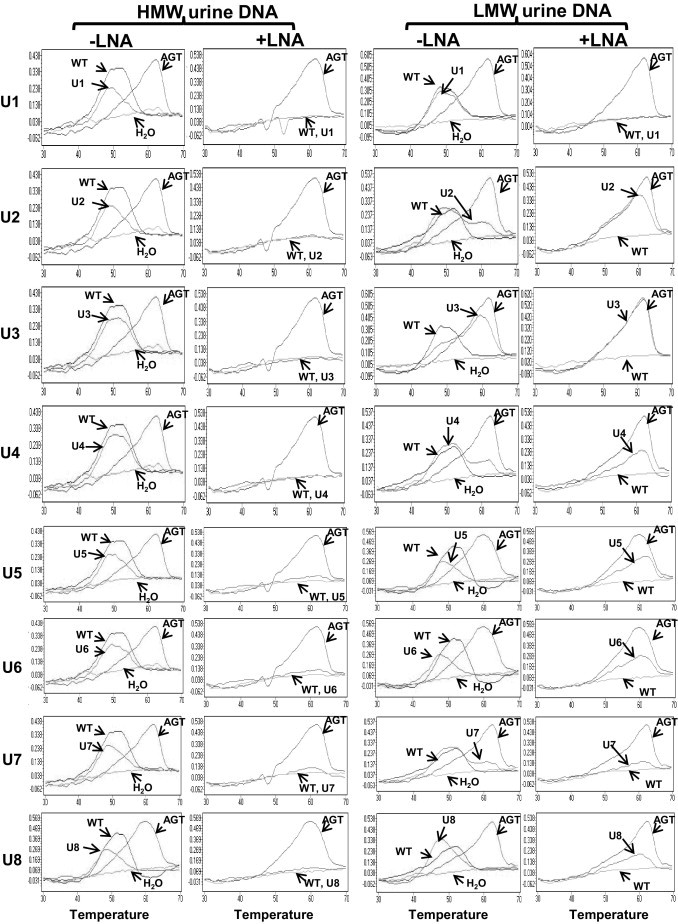
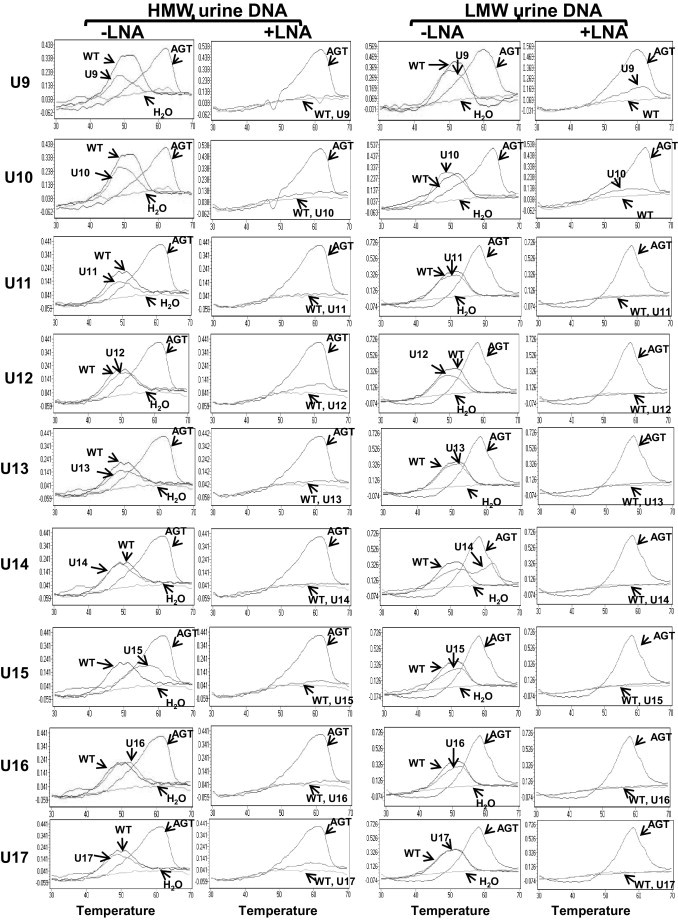
Detection of p53 mutations in urine samples from patients with HCC. HMW and LMW urine DNA samples (U1 to U17), isolated as described in Materials and Methods, were subjected to the LNA clamp-mediated PCR assay for the p53 mutation. PCRs were performed in the absence (-) or presence (+) of the LNA clamp; the melting curve analysis using the SimpleProbe SP_249T followed. In each reaction, p53_WT and p53_249T templates were used as controls.
Discussion
We have developed an assay that can detect an HCC-associated p53 codon 249T hotspot mutation with high sensitivity and specificity. The running time of the assay is <2 hours; it has the potential to be transformed to a high throughput format for a blood or urine test for HCC screening. This screening assay will be helpful in regions with high aflatoxin B1 exposure and high geographical prevalence for the TP53 codon 249T hotspot mutation, such as the Asian and African populations.43–45 For areas with a low prevalence of the mutation, this screening assay will have to be combined with other complementary screening tests, such as the α-fetoprotein blood test and ultrasonographic imaging. Three approaches were used for assay development. First, an LNA clamp was used to suppress the amplification of a WT DNA template, showing that the sensitivity and specificity of the assay could be enhanced. Second, the amplified region in the DNA template was only 41 bp. Third, SimpleProbes were used to characterize the PCR product by melting curve analysis.
The method described herein was benchmarked against other procedures available for the detection of TP53 249 mutations. First, in terms of high sensitivity and specificity, the LNA clamp-mediated PCR assay could detect up to a single copy of the mutated sequence with a high specificity ratio of 1:1000 (0.1%) of mutant/WT sequences. The short oligonucleotide mass analysis46 detected as little as 1% of mutant material47; the RFLP assay was marginally less sensitive than short oligonucleotide mass analysis, although RFLP was substantially more sensitive than other mutation detection methods. RFLP could detect mutant material at a minimum rate of approximately 3% to 6% compared with WT sequences.26
Next, the simplicity of the assay was achieved by suppressing PCR amplification of the WT templates using the LNA clamp instead of restriction endonuclease HaeIII digestion in the RFLP. HaeIII digestion was also often used to distinguish the PCR product generated from mutated templates from that of WT templates, because only the WT template contained the HaeIII recognition site at codon 249. In this assay, two molecules, SimpleProbe and LNA, were used to replace HaeIII digestion. The SimpleProbe was used to perform a 15-minute melting curve analysis of a PCR product in a 96-well PCR plate to characterize the PCR product, and the LNA clamp was used to selectively suppress the PCR amplification of WT templates. The application of these two molecules simplifies the assay procedure; the entire detection assay can be completed in <2 hours. Thus, this assay can be further developed to a high-throughput format.
The suppression of WT template amplification by the LNA clamp is based on the perfect match of the LNA clamp to the WT sequences. The base pairing of LNA to DNA exerts higher thermostability than that of DNA to DNA, thus providing a wide range of Tm differences (from 6°C to 10°C) between the perfect match and a single bp mismatch. This Tm difference allowed us to optimize a PCR condition to selectively amplify only the mutated sequence and not the WT sequence. We demonstrated the almost complete suppression of 107 copies of the WT sequence when PCR products were detected by the SimpleProbe (Figure 2). In fact, we noticed that the suppression was not complete because we detected WT sequences in some of our PCR clones by DNA sequencing. Nevertheless, the detection of mutated sequences was the purpose of the assay. The inability to visualize a low level of PCR products that leaked through the LNA suppression by the SimpleProbe did not hinder the purpose of the assay. More interestingly, the LNA suppression covers codons 248 to 250; thus, this assay is able to detect the 249T mutation and any mutation in the region of the LNA clamp. From the 15 HCC tissues we tested, we identified a 249T mutation, a 248A mutation, and a previously unpublished insertion mutation.
Our results are consistent with those from our previous studies (ie, the circulation-derived urine DNA is primarily in the LMW urine DNA fraction, and the HMW DNA is derived primarily from the cells sloughed off from the urinary tract).13 Of the 10 urine samples that contained detectable p53 mutations in the LMW urine DNA fraction, we detected the p53 mutation in only one HMW urine DNA fraction (ie, U7). Interestingly, U7 urine samples contained a high level of p53-mutated DNA because the mutated peak was clearly detected by the p53_249 mutation assay, even in the absence of the LNA clamp in the U7 LMW urine DNA. Thus, it is possible that the p53-mutated DNA detected in the U7 HMW DNA fraction was contaminated with small fragments of DNA (<1 kb) because the method of fractionation yielded approximately 95% removal of HMW DNA from the total DNA fraction.42 Another possibility is that some of the circulation-derived DNA was >1 kb. Nevertheless, it is clear that the p53 mutation detected in the urine of patients with HCC is derived from the circulation.
When we compared the ability of the assay to detect TP53 249 in urine and in matched tissue samples using a small sample size (n = 7, Table 4), we observed the three scenarios previously mentioned: i) tissue positive, urine positive (T9/U4 and T13/U6); ii) tissue positive, urine negative (T2/U1); and iii) tissue negative, urine positive (T4/U2, T5/U3, T12/U5, and T14/U7). The first two scenarios were expected because of the sensitivity of the urine test. The third scenario (ie, tissue negative, urine positive) is interesting. It is possible that the mutation detected in the urine samples was derived from tissue other than that sampled. HCC can occur in multiple sites of the liver, and each HCC nodule could be distinct.48–50 The p53 mutation detected in urine could be from an HCC nodule that was different from the tissue sampled. It is also possible that the p53 mutation detected was derived from another site because urine is a fluid collected from all parts of the body. A large-scale study to determine the concordance value and the sensitivity and specificity of the TP53 249 mutation assay to distinguish HCC from other liver diseases in a urine test is in progress.
The only mutation sequence this assay could identify was that with the sequence of the probe used. For instance, the 249T mutation could only be identified when a SimpleProbe 249T was used; although it detected the presence of other mutations, it was unable to identify the type of mutation. This defect can potentially be improved by performing a melting curve analysis using a different SimpleProbe. For instance, 248C could be clearly verified when we used the SimpleProbe 248C (data not shown). Because the 249T mutation is the hotspot mutation for HCC, we chose to use the SimpleProbe 249T in this assay for screening purposes. This assay is an end point analysis; thus, we were unable to determine the quantity of mutated sequences. The TP53 gene is an important tumor suppressor gene involved in at least four major cancer pathways: cell survival/apoptosis, cell proliferation, chromosome stability, and cell cycle.51 Mutation of the TP53 gene is detrimental to the cells. With an assay sensitivity of 0.1% or 10 copies per assay, we did not detect any p53 mutation in normal urine (n = 15, data not shown) or in the eight of nine HMW urine DNA fractions of HCC urine that contain a detectable p53 mutation, which suggests that the qualitative assay for TP53 249 might be sufficient to use in a urine or blood screening test for HCC.
Acknowledgment
We thank Pamela Fried for editorial help.
Footnotes
Supported by The Prevent Cancer Foundation (post-doctoral fellowship to S.J.), the Early Detection Research Network grant of The National Cancer Institute (T.M.B.), the Hepatitis B Foundation of America (T.M.B.), an appropriation from the Commonwealth of Pennsylvania (T.M.B.), NIH grant R01 CA125642 (Y.-H.S.), Department of Defense grant CA93176 (Y.-H.S.), and a translational research grant award from the Wallace Coulter Foundation (Y.-H.S.).
References
- 1.Farazi P.A., DePinho R.A. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 2.Kuang S.Y., Lekawanvijit S., Maneekarn N., Thongsawat S., Brodovicz K., Nelson K., Groopman J.D. Hepatitis B 1762T/1764A mutations, hepatitis C infection, and codon 249 p53 mutations in hepatocellular carcinomas from Thailand. Cancer Epidemiol Biomarkers Prev. 2005;14:380–384. doi: 10.1158/1055-9965.EPI-04-0380. [DOI] [PubMed] [Google Scholar]
- 3.Jackson P.E., Kuang S.Y., Wang J.B., Strickland P.T., Munoz A., Kensler T.W., Qian G.S., Groopman J.D. Prospective detection of codon 249 mutations in plasma of hepatocellular carcinoma patients. Carcinogenesis. 2003;24:1657–1663. doi: 10.1093/carcin/bgg101. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi H., Sugio K., Matsumata T., Adachi E., Takenaka K., Sugimachi K. The clinical significance of p53 gene mutation in hepatocellular carcinomas from Japan. Hepatology. 1995;22:1702–1707. [PubMed] [Google Scholar]
- 5.Honda K., Sbisà E., Tullo A. p53 Mutation is a poor prognostic indicator for survival in patients with hepatocellular carcinoma undergoing surgical tumour ablation. Br J Cancer. 1998;77:776–782. doi: 10.1038/bjc.1998.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain S.P., Schwank J., Staib F., Wang X.W., Harris C.C. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26:2166–2176. doi: 10.1038/sj.onc.1210279. [DOI] [PubMed] [Google Scholar]
- 7.Bressac B., Kew M., Wands J., Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- 8.Kirk G.D., Camus-Randon A.M., Mendy M., Goedert J.J., Merle P., Trépo C., Bréchot C., Hainaut P., Montesano R. Ser-249 p53 mutations in plasma DNA of patients with hepatocellular carcinoma from The Gambia. J Natl Cancer Inst. 2000;92:148–153. doi: 10.1093/jnci/92.2.148. [DOI] [PubMed] [Google Scholar]
- 9.Lleonart M.E., Kirk G.D., Villar S., Lesi O.A., Dasgupta A., Goedert J.J., Mendy M., Hollstein M.C., Montesano R., Groopman J.D., Hainaut P., Friesen M.D. Quantitative analysis of plasma TP53 249Ser-mutated DNA by electrospray ionization mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2005;14:2956–2962. doi: 10.1158/1055-9965.EPI-05-0612. [DOI] [PubMed] [Google Scholar]
- 10.Jackson P.E. Specific p53 mutations detected in plasma and tumors of hepatocellular carcinoma patients by electrospray ionization mass spectrometry. Cancer Res. 2001;61:33–35. [PubMed] [Google Scholar]
- 11.Hosny G., Farahat N., Tayel H., Hainaut P. Ser-249 TP53 and CTNNB1 mutations in circulating free DNA of Egyptian patients with hepatocellular carcinoma versus chronic liver diseases. Cancer Lett. 2008;264:201–208. doi: 10.1016/j.canlet.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein A.V., Melkonyan H.S., Tomei D., Umansky S.R. Circulating nucleic acids and apoptosis. Ann N Y Acad Sci. 2001;945:239–249. doi: 10.1111/j.1749-6632.2001.tb03892.x. [DOI] [PubMed] [Google Scholar]
- 13.Su Y.H., Wang M., Brenner D.E., Ng A., Melkonyan H., Umansky S., Syngal S., Block T.M. Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer. J Mol Diagn. 2004;6:101–107. doi: 10.1016/S1525-1578(10)60497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan A.K.C., Chiu R.W.K., Lo Y.M.D. Cell-free nucleic acids in plasma, serum and urine: a new tool in molecular diagnosis. Ann Clin Biochem. 2003;40:122–130. doi: 10.1258/000456303763046030. [DOI] [PubMed] [Google Scholar]
- 15.Pathak A.K., Bhutani M., Kumar S., Mohan A., Guleria R. Circulating cell-free DNA in plasma/serum of lung cancer patients as a potential screening and prognostic tool. Clin Chem. 2006;52:1833–1842. doi: 10.1373/clinchem.2005.062893. [DOI] [PubMed] [Google Scholar]
- 16.Diehl F., Schmidt K., Choti M.A., Romans K., Goodman S., Li M., Thornton K., Agrawal N., Sokoll L., Szabo S.A., Kinzler K.W., Vogelstein B., Diaz L.A., Jr Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anker P., Lyautey J., Lederrey C., Stroun M. Circulating nucleic acids in plasma or serum. Clin Chim Acta. 2001;313:143–146. doi: 10.1016/s0009-8981(01)00666-0. [DOI] [PubMed] [Google Scholar]
- 18.Jahr S., Hentze H., Englisch S., Hardt D., Fackelmayer F.O., Hesch R., Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 19.Stroun M., Maurice P., Vasioukhin V., Lyautey J., Lederrey C., Lefort F., Rossier A., Chen X.Q., Anker P. The origin and mechanism of circulating DNA. Ann N Y Acad Sci. 2000;906:161–168. doi: 10.1111/j.1749-6632.2000.tb06608.x. [DOI] [PubMed] [Google Scholar]
- 20.Su Y.-H., Wang M., Norton P.A., Brenner D.E., Block T.M. Detection of mutated K-ras DNA in urine, plasma and serum from patients with colorectal carcinoma or adenomatous polyps. Ann N Y Acad Sci. 2008;1137:197–201. doi: 10.1196/annals.1448.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan K.C.A., Leung S.F., Yeung S.W., Chan A.T.C., Lo Y.M.D. Quantitative analysis of the transrenal excretion of circulating EBV DNA in nasopharyngeal carcinoma patients. Clin Cancer Res. 2008;14:4809–4813. doi: 10.1158/1078-0432.CCR-08-1112. [DOI] [PubMed] [Google Scholar]
- 22.Sikora A., Zimmermann G., Rusterholz C., Birri D., Kolla V., Lapaire O., Hoesli I., Kiefer V., Jackson L., Hahn S. Detection of increased amounts of cell-free fetal DNA with short PCR amplicons. Clin Chem. 2010;56:136–138. doi: 10.1373/clinchem.2009.132951. [DOI] [PubMed] [Google Scholar]
- 23.Shekhtman E.M., Anne K., Melkonyan H.S., Robbins D.J., Warsof S.L., Umansky S.R. Optimization of transrenal DNA analysis: detection of fetal DNA in maternal urine. Clin Chem. 2009;55:723–729. doi: 10.1373/clinchem.2008.113050. [DOI] [PubMed] [Google Scholar]
- 24.Nogueira M.L., Wang V.E.H., Tantin D., Sharp P.A., Kristie T.M. Herpes simplex virus infections are arrested in Oct-1-deficient cells. Proc Natl Acad Sci U S A. 2004;101:1473–1478. doi: 10.1073/pnas.0307300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguilar F., Harris C.C., Sun T., Hollstein M., Cerutti P. Geographic variation of p53 mutational profile in nonmalignant human liver. Science. 1994;264:1317–1319. doi: 10.1126/science.8191284. [DOI] [PubMed] [Google Scholar]
- 26.Kirk G.D., Lesi O.A., Mendy M., Szymanska K., Whittle H., Goedert J.J., Hainaut P., Montesano R. 249Ser TP53 mutation in plasma DNA, hepatitis B viral infection, and risk of hepatocellular carcinoma. Oncogene. 2005;24:5858–5867. doi: 10.1038/sj.onc.1208732. [DOI] [PubMed] [Google Scholar]
- 27.Igetei R., Otegbayo J., Ndububa D., Lesi O., Anumudu C., Hainaut P., Gormally E. Detection of p53 codon 249 mutation in Nigerian patients with hepatocellular carcinoma using a novel evaluation of cell-free DNA. Ann Hepatol. 2008;7:339–344. [PubMed] [Google Scholar]
- 28.Laken S.J., Jackson P.E., Kinzler K.W., Vogelstein B., Strickland P.T., Groopman J.D., Friesen M.D. Genotyping by mass spectrometric analysis of short DNA fragments. Nature Biotechnol. 1998;16:1352–1356. doi: 10.1038/4333. [DOI] [PubMed] [Google Scholar]
- 29.Katiyar S., Dash B.C., Thakur V., Guptan R.C., Sarin S.K., Das B.C. P53 tumor suppressor gene mutations in hepatocellular carcinoma patients in India. Cancer. 2000;88:1565–1573. doi: 10.1002/(sici)1097-0142(20000401)88:7<1565::aid-cncr10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Su Y.-H., Wang M., Aiamkitsumrit B., Brenner D.E., Block T.M. Detection of K-ras mutation in urine of patients with colorectal cancer. Cancer Biomark. 2005;1:177–182. doi: 10.3233/cbm-2005-12-305. [DOI] [PubMed] [Google Scholar]
- 31.Garcia C., Ahmadian A., Gharizadeh B., Lundeberg J., Ronaghi M., Nyrén P. Mutation detection by pyrosequencing: sequencing of exons 5–8 of the p53 tumor suppressor gene. Gene. 2000;253:249–257. doi: 10.1016/s0378-1119(00)00257-2. [DOI] [PubMed] [Google Scholar]
- 32.Arjomand-Nahad F., Diefenbach K., Landt O., Gaikovitch E., Roots I. Genotyping of the triallelic variant G2677T/A in MDR1 using LightCycler with locked-nucleic-acid-modified hybridization probes. Anal Biochem. 2004;334:201–203. doi: 10.1016/j.ab.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Oldenburg R.P., Liu M.S., Kolodney M.S. Selective amplification of rare mutations using locked nucleic acid oligonucleotides that competitively inhibit primer binding to wild-type DNA. J Invest Dermatol. 2009;128:398–402. doi: 10.1038/sj.jid.5700920. [DOI] [PubMed] [Google Scholar]
- 34.Ren X.D., Lin S.Y., Wang X., Zhou T., Block T.M., Su Y.H. Rapid and sensitive detection of hepatitis B virus 1762T/1764A double mutation from hepatocellular carcinomas using LNA-mediated PCR clamping and hybridization probes. J Virol Methods. 2009;158:24–29. doi: 10.1016/j.jviromet.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chase C.J., Ulrich M.P., Wasieloski L.P., Jr, Kondig J.P., Garrison J., Lindler L.E., Kulesh D.A. Real-time PCR assays targeting a unique chromosomal sequence of Yersinia pestis. Clin Chem. 2005;51:1778–1785. doi: 10.1373/clinchem.2005.051839. [DOI] [PubMed] [Google Scholar]
- 36.Loveless B.M., Yermakova A., Christensen D.R., Kondig J.P., Heine H.S., 3rd, Wasieloski L.P., Kulesh D.A. Identification of ciprofloxacin resistance by SimpleProbe, High Resolution Melt and Pyrosequencing nucleic acid analysis in biothreat agents: Bacillus anthracis, Yersinia pestis and Francisella tularensis. Mol Cell Probes. 2010;24:154–160. doi: 10.1016/j.mcp.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Valles-Ayoub Y., Saechao C., Haghighatgoo A., Neshat M.S., Esfandiarifard S., Pietruszka M., Darvish D. Validation of GNE: p.M712T identification by melting curve analysis. Genet Test. 2008;12:101–109. doi: 10.1089/gte.2007.0034. [DOI] [PubMed] [Google Scholar]
- 38.Klaassen C.H.W., van Aarssen Y.A.W.G., van der Stappen J.W.J. Improved real-time detection of the H63D and S65C mutations associated with hereditary hemochromatosis using a SimpleProbe assay format. Clin Chem Lab Med. 2008;46:985–986. doi: 10.1515/CCLM.2008.197. [DOI] [PubMed] [Google Scholar]
- 39.Monteros M.J., Ha B., Phillips D.V., Boerma H.R. SNP assay to detect the “Hyuuga” red-brown lesion resistance gene for Asian soybean rust. Theor Appl Genet. 2010;12:1033–1046. doi: 10.1007/s00122-010-1368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyon E., Wittwer C.T. LightCycler technology in molecular diagnostics. J Mol Diagn. 2009;11:93–101. doi: 10.2353/jmoldx.2009.080094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abner C.W., McKinnon P.J. The DNA double-strand break response in the nervous system. DNA Repair. 2004;3:1141–1147. doi: 10.1016/j.dnarep.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Su Y.-H., Song J., Wang Z., Wang X., Wang M., Brenner D.E., Block T.M. Removal of high molecular weight DNA by carboxylated magnetic beads enhances the detection of mutated K-ras DNA in urine. Ann NY Acad Sci. 2008;1137:82–91. doi: 10.1196/annals.1448.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirk G.D., Bah E., Montesano R. Molecular epidemiology of human liver cancer: insights into etiology, pathogenesis and prevention from The Gambia, West Africa. Carcinogenesis. 2006;27:2070–2082. doi: 10.1093/carcin/bgl060. [DOI] [PubMed] [Google Scholar]
- 44.Stern M.C., Umbach D.M., Yu M.C., London S.J., Zhang Z.Q., Taylor J.A. Hepatitis B, aflatoxin B(1), and p53 codon 249 mutation in hepatocellular carcinomas from Guangxi, People's Republic of China, and a meta-analysis of existing studies. Cancer Epidemiol Biomarkers Prev. 2001;10:617–625. [PubMed] [Google Scholar]
- 45.Denissenko M.F., Koudriakova T.B., Smith L., O'Connor T.R., Riggs A.D., Pfeifer G.P. The p53 codon 249 mutational hotspot in hepatocellular carcinoma is not related to selective formation or persistence of aflatoxin B1 adducts. Oncogene. 1998;17:3007–3014. doi: 10.1038/sj.onc.1202214. [DOI] [PubMed] [Google Scholar]
- 46.Soma M. Mice lacking serum amyloid p component do not necessarily develop severe autoimmune disease. Biochem Biophys Res Commun. 2001;286:200–205. doi: 10.1006/bbrc.2001.5364. [DOI] [PubMed] [Google Scholar]
- 47.Jackson P.E., Qian G.S., Friesen M.D., Zhu Y.R., Lu P., Wang J.B., Wu Y., Kensler T.W., Vogelstein B., Groopman J.D. Specific p53 mutations detected in plasma and tumors of hepatocellular carcinoma patients by electrospray ionization mass spectrometry. Cancer Res. 2001;61:33–35. [PubMed] [Google Scholar]
- 48.Choo K.B., Liu M.S., Chang P.C., Wu S.M., Su M.W., Pan C.C., Han S.H. Analysis of six distinct integrated hepatitis B virus sequences cloned from the cellular DNA of a human hepatocellular carcinoma. Virology. 1986;154:405–408. doi: 10.1016/0042-6822(86)90467-8. [DOI] [PubMed] [Google Scholar]
- 49.Ng I.O.L., Guan X.Y., Poon R.T., Fan S.T., Lee J.M. Determination of the molecular relationship between multiple tumor nodules in hepatocellular carcinoma differentiates multicentric origin from intrahepatic metastasis. J Pathol. 2003;199:345–353. doi: 10.1002/path.1287. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T., Kajino K., Kudo M., Sasaki Y., Arakawa Y., Hino O. Determination of the clonal origin of multiple human hepatocellular carcinomas by cloning and polymerase chain reaction of the integrated hepatitis B virus DNA. Hepatology. 1999;29:1446–1452. doi: 10.1002/hep.510290523. [DOI] [PubMed] [Google Scholar]
- 51.Whittaker S., Marais R., Zhu A.X. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]


