Abstract
BACKGROUND
An enteric-coated levonorgestrel emergency contraceptive pill (E-LNG-ECP) is an improved formulation, in terms of side effects, which both dissolves and is absorbed in the intestine. Our aim was to evaluate the efficacy and safety of E-LNG-ECP as an over-the-counter (OTC) drug for emergency contraception (EC) in Chinese women.
METHODS
A Phase IV clinical trial was conducted in five family planning clinics in China. Women seeking EC within 72 h after unprotected sexual intercourse or contraceptive failure who met the inclusion criteria were recruited. The efficacy of contraception (primary end-point was pregnancy rate), side effects (i.e. safety) and the value of E-LNG-ECP for EC were investigated.
RESULTS
Of 2445 women (aged 15–48 years) who took E-LNG-ECP with follow-up to determine pregnancy, only five pregnancies (0.2%) occurred. The efficacy of contraception was 95.3%. In total, 6.5% of women reported at least one adverse event after taking E-LNG-ECP, and no serious adverse events were reported. Only four subjects (0.16%) reported vomiting. The incidence of menstrual cycle disturbance was 20.1% after taking E-LNG-ECP. Subjects who had previously taken ECPs (54.4% of these women) rated the acceptability of E-LNG-ECP at 9.36 (on a 10-point scale) higher (P<0.05) than the rating of other LNG-EC pills taken previously.
CONCLUSIONS
The study found that E-LNG-ECP was effective, safe and well tolerated as an OTC drug. However, an randomized controlled trial should be performed to compare standard LNG tablets with E-LNG-ECP.
Keywords: emergency contraception, levonorgestrel enteric-coated tablet, over-the-counter drug, clinical trial
Introduction
Emergency contraception (EC) methods, available in oral and intrauterine forms, are used to prevent pregnancy after unprotected intercourse or contraceptive failure. The most widely used EC drug is levonorgestrel (LNG), taken orally in repeated doses (0.75 mg × 2) or as a single dose (1.5 mg) within 72 h of sexual intercourse (Trussell et al., 2010). LNG EC is more effective and has fewer side effects than the Yuzpe regimen, which contains both estrogen and progestin (Cheng et al., 2004).
Several studies have shown that LNG acts by inhibiting or postponing ovulation (Gemzell-Danielsson, 2010; Leung et al., 2010; Noé et al., 2010). Higher doses of LNG taken after ovulation, or LNG administered by the vaginal route, have no effect on endometrial development or function (Gemzell-Danielsson, 2010; Meng et al., 2010; Palomino et al., 2010).
In recent years, the use of LNG has increased and it is now available for women seeking EC without a doctor's prescription in nearly 50 countries, including China (Pittrof et al., 2010; Trussell et al., 2010). However, extensive debate on overreliance on LNG as an over-the-counter (OTC) drug still occurs in many other countries, which is a barrier to switching the prescription status of LNG in these countries. As a result, prospective, multi-center, open-label clinical trials of LNG as an OTC drug are necessary to provide evidence to inform the discussion.
The LNG emergency contraceptive pill (ECP) was approved as an OTC drug by the State Food and Drug Administration (SFDA) in 1998 and has been widely available in China since that time. An enteric-coated LNG-ECP (E-LNG-ECP) is an improved formulation that both dissolves and is absorbed in the intestine rather than in the stomach so as to reduce the adverse events of nausea and vomiting. A pharmacokinetics study of E-LNG-ECP showed it to be bioequivalent with the previous, uncoated tablet with the use of the liquid chromatography–tandem mass spectrometry method, although the time to peak value in blood plasma was significantly longer than that for the previous tablet (Tmax: 3.4 versus 1.8 h) (Zhao et al., 2008). We conducted a Phase IV clinical trial in Chinese women to provide data on the efficacy and safety of the E-LNG-ECP as an OTC drug.
Materials and Methods
Subjects
This study was conducted in five family-planning clinics (Shanghai, Guangzhou, Changsha, Changchun and Wuhan) in China from August 2009 to October 2010. Ethical approval was obtained from the Ethics Committee of the medical college of Huazhong University of Science and Technology. The study was performed in full compliance with the Declaration of Helsinki and Good Clinical Practice of China.
Subjects were healthy women who sought ECP to avoid unwanted pregnancy. According to drug law and regulations in China, the minimum number of enrolled women subjects required in a Phase IV clinical trial of contraceptives is 2000. Given the high loss of follow-up in clinical trials of OTC drugs (20%), we planned to include 2400 women. Inclusion criteria were the following: 15–49 years old, regular menstrual cycle of 21–35 days, a usually menstrual period duration of 3–7 days and seeking EC within 72 h of unprotected sexual intercourse or contraceptive failure. Subjects were excluded if they had a contraindication to LNG, chronic disease with prolonged medication, a known or suspected pregnancy or if they were current or recent users of hormonal methods of contraception. All participants signed informed consent forms.
Drug
The enteric-coated tablet contained 1.5 mg LNG (Nevenol®, Regenex Pharmaceutical Corporation, Guangzhou, China). Participants were told to take one tablet orally within 72 h of unprotected intercourse or contraceptive failure.
Procedures
Participants filled in a registration form to provide basic information when they came to pharmacies to purchase emergency contraceptives. Throughout the study, participants were asked to keep a daily diary to record the time of sexual intercourse and administration of E-LNG-ECP, adverse effects, vaginal bleeding, concomitant medication and contraceptive use. A follow-up interview (either by telephone or in a clinic) was carried out within 3 days of the administration of E-LNG-ECP, with the content mainly focusing on adverse events. The second follow-up was 5–7 days after expected menses. If menses had occurred, the follow-up study ended. Women with negative pregnancy tests without menses on the expected date were contacted every 2 weeks, and periodic pregnancy testing was undertaken until menses resumed. Positive urinary pregnancy tests were confirmed by measurement of serum β-hCG and later by ultrasonography. The outcome of the pregnancies was obtained by further follow-ups.
The primary efficacy end-point was the rate of pregnancy in women who took E-LNG-ECP within 72 h of unprotected intercourse. The rate of side effects in women who received E-LNG-ECP within 72 h of unprotected intercourse was analyzed as a safety measurement. Serious side effects were defined in accordance with the female harmonization guidelines (Liao, 2004) and reported to the research center immediately by investigators. The principal investigator reviewed every pregnancy (enrolment and follow-up serum hCG concentrations, ultrasound dating, menstrual cycle and coital data) to establish whether conception clearly occurred before E-LNG-ECP was given or well after treatment (at least 10 days after treatment). Pregnancies that met these criteria were deemed to be incompatible with treatment failure. The efficacy of contraception (the fraction of expected pregnancies prevented) was estimated using the method of Trussell et al. (1998).
In addition, the subjects who had taken ECPs in the past rated E-LNG-ECP versus other ECPs on a scale ranging from 0 (poor) to 10 (excellent). The perceived advantages and disadvantages of E-LNG-ECP were also investigated in the follow-up interviews of participants.
Statistical analysis
Demographics, the efficacy of contraception and the frequency of adverse events in the observed cycle were described for the intention-to-treat population. The data were imputed and verified in two databases by two analyzers independently. Statistical analysis was performed using SAS version 9.0 (Cary, NC, USA). Statistical significance was set at P < 0.05.
Results
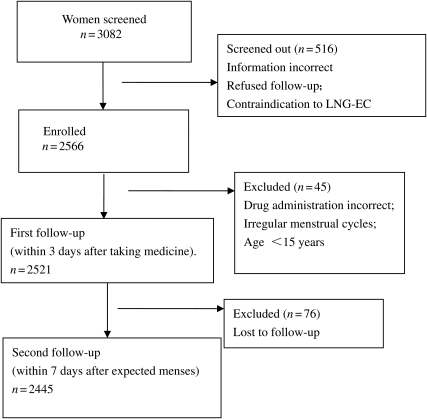
A total of 2566 subjects were enrolled in the study, 45 of whom were excluded because they did not meet the eligibility requirements (these 45 subjects were given E-LNG-ECP and there were no pregnancies). A total of 2521 were therefore eligible and treated with E-LNG-ECP; these women were all contacted for the first follow-up interview and were therefore included in the safety analysis. Of these, 2445 finished the study and were included in the efficacy analysis, while 76 women (with characteristics similar to the others) [3.0%, 95% confidence interval (CI) 2.4–3.8] were lost to follow-up (Fig. 1, trial profile). The ages of the enrolled participants ranged from 14 to 48 years, with a mean age of 27.4 years. Other characteristics of the participants are presented in Table I. Among the 2445 subjects, 91.2% of the follow-up interviews were conducted by telephone and 8.8% were completed in a clinic.
Figure 1.
Profile of a Phase IV clinical trial to investigate the efficacy and safety of a LNG enteric-coated tablet as an OTC drug for EC.
Table I.
Baseline characteristics of participants in a Phase IV clinical trial to study the efficacy and safety of an E-LNG-ECP as an OTC drug for emergency contraception in China (n= 2566).
| Mean | SD | Range | |
|---|---|---|---|
| Age (year) | 27.4 | 6.1 | 14–48 |
| Height (cm) | 160.45 | 4.06 | 145–186 |
| Weight (kg) | 52.39 | 6.56 | 34.5–86 |
| Length of menstrual cycle (days) | 29.1 | 1.7 | 20–40 |
| Duration of menstruation (days) | 5.0 | 1.1 | 1–11 |
| Marital status: total n (missing) 2410 (156) | n | % | |
| Married | 1075 | 44.61 | |
| Single | 1335 | 55.39 | |
| Education: total n (missing) 2451(115) | |||
| Primary school or less | 22 | 0.90 | |
| Junior high school | 223 | 9.10 | |
| Senior high school | 1258 | 51.33 | |
| Junior college | 745 | 30.40 | |
| University or above | 203 | 8.28 | |
| Contraceptive methods used recently: total n (missing) 1749(817) | |||
| Condom | 1206 | 50.61 | |
| Oral contraception pills | 92 | 3.86 | |
| Emergency contraceptive | 428 | 17.97 | |
| Other methods | 23 | 0.91 |
Efficacy of the emergency contraceptive
A total of 2021 subjects (82.7%) had taken the E-LNG-ECP within 24 h of unprotected intercourse, 361 subjects (14.8%) within 24–48 h and 38 (1.6%) within 48–72 h. The rest (25 women) did not provide the specific time between coitus and drug administration. Only five pregnancies (0.20%, 95% CI 0.07–0.48%) among 2445 women occurred during the study. Among the 2401 women who reported the cycle day on which intercourse occurred, the expected number of pregnancies was 106.8. The efficacy of contraception was 95.3% (95% CI 88.6–98.1%). All five pregnancies ended in abortion (four induced and one spontaneous). No ectopic pregnancy was observed. No pregnancy was deemed incompatible with contraceptive failure according to the estimated date of conception.
Women lost to follow-up in our trial were 76; these were excluded from the denominator in calculations, under the assumption that they would experience failure at the same rate as those with observed outcomes. If all became pregnant, the failure rate would be 3.21%. This is considered as the highest failure rate. On the other hand, if none became pregnant, the failure rate would be 0.20%.
Safety results
No serious adverse events were reported. Among 2521 subjects with safety data, 178 (7.1%; 95% CI 6.1–8.1%) reported at least one adverse event after taking E-LNG-ECP. In total, 4.2% of women reported mild or medium nausea, which occurred usually within 60 min of treatment. The next most frequent adverse event was vaginal bleeding and spotting (2.0%), which usually occurred within 3 days of treatment. Only four subjects (0.2%) reported vomiting, one at 30 min after treatment and three at unspecified times. No one took more E-LNG-ECP because of vomiting. The presence of adverse events is detailed in Table II.
Table II.
Incidence of side effects after treatment with E-LNG-ECP (n = 2521).
| N | % | |
|---|---|---|
| Nausea | 106 | 4.20 |
| Vomiting | 4 | 0.16 |
| Vaginal bleeding | 51 | 2.02 |
| Headache and dizziness | 9 | 0.36 |
| Xerostomia | 8 | 0.32 |
| Transient chest distress | 4 | 0.16 |
| Lower abdominal pain | 4 | 0.16 |
| Anorexia | 2 | 0.08 |
| Exanthema | 2 | 0.08 |
| Fatigue | 2 | 0.08 |
| Dizziness | 1 | 0.04 |
Influence on menstruation
Disturbances of the menstrual cycle were common after the E-LNG-ECP. In total, 20.1% of women reported that their next menses did not occur when expected, including 4.7% whose menses started at least 7 days early and 15.4% whose menses were delayed by >7 days. The average duration of menses was 4.9 days, which was no different than the previous one. The menstrual bleeding volume and the degree of dysmenorrhoea were similar to those in the previous menstrual cycle.
Acceptability of LNG ECPs
Thousand three hundred seventy-two of 2521 women (54.4%) in this study had used LNG ECPs previously and 99.6% obtained them from community pharmacies. The participants' average rating of the ECPs used previously was 7.96 compared with 9.36 for E-LNG-ECP, a difference that is significant (P < 0.05), using Student t-test.
The advantages and disadvantages of E-LNG-ECP were also investigated at the second follow-up. Among 2445 participants, 1363 women (55.7%) listed several advantages of E-LNG-ECP: fewer or no side effects (52.2%), contraceptive efficacy (27.7%), convenient to use (18.0%) and attractive packaging (0.7%). Only 207 women listed some disadvantages, which were: menses disturbance (45.9%), high cost (42.0%), side effects (9.2%), could not frequently use (1.9%), brand name is not well known (0.5%) and contraceptive failure (0.5%).
In addition, the subjects listed their source of ECP information at the first follow-up: community pharmacies (75.4%), papers or magazines (24.3%), relatives or friends (10.6%), counseling by doctors (9.6%), internet (8.3%), broadcasting (6.6%), community education (3.3%) and others (1.3%). It was an open question and the answer was not limited to one choice; so some women used more than one source of information. Higher-educated women were more likely to gain ECP knowledge from papers or magazines, broadcasting and internet, using correlation analysis.
Contraception methods used after E-LNG-ECP treatment
The contraception methods used after E-LNG-ECP treatment were investigated at the first follow-up. A total of 559 women (22.2%) did not use any contraceptive methods after the treatment. Among 1962 women who used contraception, the methods reported were: condoms (66.3%), ECPs (8.45%), oral contraceptive pills (1.55%), intrauterine device (0.28%), fertility awareness methods (0.6%) and spermicides (0.16%).
Discussion
LNG was approved as an OTC emergency contraceptive drug by SFDA in 1998 in China and is mainly sold by community pharmacies, which provides convenient access for women. According to the Good Clinical Practice guidelines of SFDA, the current study was a Phase IV clinical trial of a new tablet, E-LNG-ECP. A total of 2566 women who were seeking ECPs from community pharmacies in five cities were enrolled to assess the contraceptive efficacy, safety and acceptability of E-LNG-ECP. This was the first large-sample post-marketing study of an emergency contraceptive as an OTC drug in China.
With both the LNG and Yuzpe regimens, the earlier the treatment begins the more effective it is. In a World Health Organization (WHO) large randomized controlled trial (RCT) of these methods, delaying the first dose by 12 h increased the odds of pregnancy by almost 50% (Piaggio et al., 1999). In our study, the contraceptive efficacy of E-LNG-ECP was 95.3%, and the failure rate was 0.2%, significantly lower than failure rates in the RCTs of LNG conducted by WHO and other researchers (Ho et al., 1993; Task Force on Post-ovulatory Methods of Fertility Regulation,1998; Piaggio et al., 1999; Wu et al., 1999; Arowojolu et al., 2002; von Hertzen H et al., 2002; Ngai et al., 2005) (see Supplementary data, Table SI) In our study, 82.7% of participants took the drug during the first 24 h after unprotected intercourse, while only 42–46% did so in the WHO RCT (von Hertzen H et al., 2002). Another study showed that the failure rates of ECPs during the first 24 h, 24–48 h and 48–72 h were 0.4, 1.2 and 2.7%, respectively (WHO, 1998). The superior efficacy of E-LNG-ECP found in this study might partially be related to the short interval between unprotected intercourse and taking the drug, and this short interval is good evidence of the easy accessibility of LNG ECP as an OTC drug. Subjects' knowledge about ECP was also helpful in reducing the time required to obtain the ECP. It should be pointed out that the failure rates of ECPs might vary in different studies because of differences in characteristics among populations (such as age, reasons for requesting EC, the time between coitus and drug administration).
In our study, only 165 women (7.1%) reported a side effect, and 13 women reported more than one side effect. The presence of side effects is lower than in previous studies, except for a similar incidence of menstrual bleeding disturbances. One of the highlights in the present study is the significantly lower rate of nausea and vomiting compared with that in clinical trials conducted in different reports (WHO, 1998; Arowojolu et al., 2002; von Hertzen H et al., 2002). Decreased nausea and vomiting are probably primarily related to the enteric-coated tablet, which avoids irritation of the stomach. No pregnancy was observed among subjects who vomited, but very few vomited, precluding further investigation. Also, we could not differentiate method failure from subject failure. The main reason women chose E-LNG-ECP was that they thought it could have fewer gastrointestinal side effects. Therefore, the incidence of side effects, such as nausea and vomiting, may also depend on psychological factors. If women know that the drug is a new and improved preparation, they may feel more reassured and be less apprehensive, which might decrease the incidence of nausea and vomiting.
The influence of LNG on menstruation was previously found to depend on when LNG was taken in the menstrual cycle (Raymond et al., 2006). Taking the drug during the first 3 weeks of one's cycle shortened the menstrual cycle and duration of menses, and this effect increased when it was taken even earlier in the 3-week period. In contrast, taking the LNG EC after ovulation had little influence on menstrual cycle length or duration of menses (Tirelli et al., 2008). In our study, nearly 80% of participants did not experience any menstrual change during the first cycle after treatment, while 20.1% experienced a change in timing of onset of the next menses of >7 days. The duration and volume of menses were not affected.
The E-LNG-ECP was found to be acceptable by women in our study. Advantages described by participants included few side effects, satisfactory efficacy and ease of use, while disadvantages included gastrointestinal side effects (nausea), menstrual disturbance and high cost. An estimated 19% to 22% of women were not using any contraceptive method after ECP treatment, while another 7.5% were relying on ECPs, which are not as effective as any ongoing method of contraception.
The present study also investigated the accessibility of ECPs: 99.6% of participants who had used ECPs in the past had obtained them from community pharmacies rather than clinics. Moreover, participants' knowledge about EC mainly came from community pharmacies (75.4%), and only a small proportion of participants received counseling from clinicians (9.6%). The community pharmacy was not only a place for dispensing EC pills but also an important source of information about using the ECP correctly.
In summary, this study suggested that an LNG enteric-coated tablet was safe and effective for use as an OTC EC drug. The E-LNG-ECP remarkably reduced the gastrointestinal side effects, such as nausea and vomiting. E-LNG-ECP should be available as an OTC drug in community pharmacies, while instructions on using ECPs that are dispensed by pharmacists or healthcare practitioners should be strengthened, particularly the importance of timing. Our findings provide convincing evidence for the safety, efficacy and accessibility of ECPs in an OTC setting; however, an RCT should be performed to compare results with the standard LNG treatment.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
L.C. supervised the whole study procedure, including conception, design and completion. Q.C., W.X., D.Z., R W., Y L. and J.K. were responsible for the collection of data. Q.C. and W.X. contributed to data analysis and drafting the manuscript. All seven authors participated in the ultimate interpretation of the study data and revision of the manuscript.
Conflict of interest
L.C. has received consulting fees from Regenex Corporation.
Funding
The study was funded by Regenex Corporation. Funding to pay the Open Access publication charges for this article was provided by Regenex Corporation.
Supplementary Material
Acknowledgements
We are grateful to Prof Naiqing Zhao for the statistical analysis of the study. We acknowledge Drs Chengliang Xiong, Shan Chen, Hongling Huang, Yujuan Jiang, and Ms. Huiying Li for their contributions to this study. Finally, we thank Prof James Trussell for his insightful comments and revisions of the manuscript.
References
- Arowojolu AO, Okewole IA, Adekunle AO. Comparative evaluation of the effectiveness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians. Contraception. 2002;66:269–273. doi: 10.1016/s0010-7824(02)00337-2. doi:10.1016/S0010-7824(02)00337-2. [DOI] [PubMed] [Google Scholar]
- Cheng L, Gülmezoglu AM, Oel CJ, Piaggio G, Ezcurra E, Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2004;3 doi: 10.1002/14651858.CD001324.pub2. CD001324. [DOI] [PubMed] [Google Scholar]
- Gemzell-Danielsson K. Mechanism of action of emergency contraception. Contraception. 2010;82:404–409. doi: 10.1016/j.contraception.2010.05.004. doi:10.1016/j.contraception.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Ho PC, Kwan MSK. A prospective randomized comparison of levonorgestrel with the Yuzpe regimen in post-coital contraception. Hum Reprod. 1993;8:389–392. doi: 10.1093/oxfordjournals.humrep.a138057. [DOI] [PubMed] [Google Scholar]
- Leung VW, Levine M, Soon JA. Mechanisms of action of hormonal emergency contraceptives. Pharmacotherapy. 2010;30:158–168. doi: 10.1592/phco.30.2.158. doi:10.1592/phco.30.2.158. [DOI] [PubMed] [Google Scholar]
- Liao XM. Guideline for Reporting, Monitoring and Management of Drug Adverse Events. Changchun, China: Changchun Yinsheng Press; 2004. [Google Scholar]
- Meng CX, Marions L, Byström B, Gemzell-Danielsson K. Effects of oral and vaginal administration of levonorgestrel emergency contraception on markers of endometrial receptivity. Hum Reprod. 2010;25:874–83. doi: 10.1093/humrep/deq007. doi:10.1093/humrep/deq007. [DOI] [PubMed] [Google Scholar]
- Ngai SW, Fan S, Li S, Cheng L, Ding J, Jing X, Ng EH, Ho PC. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307–311. doi: 10.1093/humrep/deh583. doi:10.1093/humrep/deh583. [DOI] [PubMed] [Google Scholar]
- Noé G, Croxatto HB, Salvatierra AM, Reyes V, Villarroel C, Muñoz C, Morales G, Retamales A. Contraceptive efficacy of emergency contraception with levonorgestrel given before or after ovulation. Contraception. 2010;81:414–420. doi: 10.1016/j.contraception.2009.12.015. doi:10.1016/j.contraception.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Palomino WA, Kohen P, Devoto L. A single midcycle dose of levonorgestrel similar to emergency contraceptive does not alter the expression of the L-selectin ligand or molecular markers of endometrial receptivity. Fertil Steril. 2010;94:1589–1594. doi: 10.1016/j.fertnstert.2009.09.013. doi:10.1016/j.fertnstert.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Piaggio G, von Hertzen H, Grimes DA, Van Look PFA. on behalf of the Task Force on Postovulatory Methods of Fertility Regulation. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721. doi: 10.1016/s0140-6736(98)05718-3. doi:10.1016/S0140-6736(98)05718-3. [DOI] [PubMed] [Google Scholar]
- Pittrof R, Rubenstein P, Sauer U. LNG may still be the best oral EC option. J Fam Plann Reprod Health Care. 2010;36:105–106. doi: 10.1783/147118910791069394. doi:10.1783/147118910791069394. [DOI] [PubMed] [Google Scholar]
- Raymond EG, Goldberg A, Trussell J, Hays M, Roach E, Taylor D. Bleeding patterns after use of levonorgestrel emergency contraceptive pills. Contraception. 2006;73:376–381. doi: 10.1016/j.contraception.2005.10.006. doi:10.1016/j.contraception.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Task Force on Post-ovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen 0f combined oral contraceptives for emergency contraception. Lancet. 1998;352:428–433. doi:10.1016/S0140-6736(98)05145-9. [PubMed] [Google Scholar]
- Tirelli A, Cagnacci A, Volpe A. Levonorgestrel administration in emergency contraception: bleeding pattern and pituitary-ovarian function. Contraception. 2008;77:328–332. doi: 10.1016/j.contraception.2008.01.013. doi:10.1016/j.contraception.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Trussell J, Raymond EG. Emergency Contraception: a Last Chance to Prevent Unintended Pregnancy. 2011. June http://ec.princeton.edu/questions/ec-review.pdf . [Google Scholar]
- Trussell J, Rodríguez G, Ellertson C. New estimates of the effectiveness of the Yuzpe regimen of emergency contraception. Contraception. 1998;57:363–369. doi: 10.1016/s0010-7824(98)00042-0. doi:10.1016/S0010-7824(98)00042-0. [DOI] [PubMed] [Google Scholar]
- von Hertzen H, Piaggio G, Ding J, Chen J, Song S, Bártfai G, Ng E, Gemzell-Danielsson K, Oyunbileg A, Wu S, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803–1810. doi: 10.1016/S0140-6736(02)11767-3. doi:10.1016/S0140-6736(02)11767-3. [DOI] [PubMed] [Google Scholar]
- Wu SC, Wang CP, Wang Y, Cheng W, Zuo SH, Li H, Xu X, Wang RF, Dong J. Clinical observation of levonorgestrel and low dose of mifepristone for emergency contraception. Chin J Gynecol Obstet. 1999;34:327–330. [Google Scholar]
- Zhao LZ, Zhong GP, Bi HC, Ding L, Deng Y, Guan S, Chen X, Huang ZY, Huang M. Determination of levonorgestrel in human plasma byliquid chromatography–tandem mass spectrometrymethod: application to a bioequivalence study of two formulations in healthy volunteers. Biomed Chromatogr. 2008;22:519–526. doi: 10.1002/bmc.963. doi:10.1002/bmc.963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.