Abstract
BACKGROUND
Ovarian tissue cryopreservation is an emerging fertility preservation option, and culturing follicles isolated from this tissue to obtain mature gametes may ultimately be the best solution for patients for whom transplantation is contraindicated. It is unclear, however, how patient-specific variables (including age, weight and menstrual cycle stage) impact follicle growth and quality during three-dimensional culture.
METHODS
We used a mouse model to systematically determine how these variables impact in vitro follicle growth. We characterized metabolic and hormonal profiles of mice at specific ages, weights and cycle stages and secondary follicles from these cohorts were isolated and cultured. We then assessed follicle survival, growth and function, as well as meiotic competence and spindle morphology of the resulting oocytes.
RESULTS
We found that older mice and mice with increased body weight had higher serum cholesterol, abnormal glucose tolerance and lower levels of circulating Anti-Müllerian hormone compared with younger and leaner controls. Secondary follicles isolated from different cohorts and grown in vitro had indistinguishable growth trajectories. However, the follicles isolated from older and heavier mice and those in diestrus had altered hormone profiles. These follicles contained oocytes with reduced meiotic competence and produced oocytes with greater spindle defects.
CONCLUSIONS
These results suggest that the original physical environment of the follicle within the ovary can impact its function when isolated and cultured. These findings are valuable as we begin to use in vitro follicle growth technology for a heterogeneous fertility preservation patient population.
Keywords: follicle culture, oocyte, obesity, age
Introduction
Cancer treatments, while life-preserving, can threaten fertility (Jeruss and Woodruff, 2009; Dohle, 2010; Schmidt et al., 2010). For many patients, the ability to preserve their fertility prior to initiating treatment is essential for their future quality of life (Gorman et al., 2010). For females, fertility preservation is a challenge because of the rarity of gametes and the difficulty in obtaining them. To date, several fertility preservation options are available to female patients ranging from oocyte and embryo cryopreservation to ovarian tissue harvesting (Ata et al., 2010; Donnez et al., 2010; Smitz et al., 2010).
The use of harvested ovarian tissue for fertility preservation is emerging as a promising new option, especially for patients for whom hyperstimulation is contraindicated. For these patients, ovarian tissue can be harvested and used in several ways. For example, the ovarian tissue can be cryopreserved and used for future transplantation to temporarily restore hormonal function and fertility (Donnez et al., 2010). This technology has resulted in several live human births (Donnez et al., 2011). However, transplantation in patients with blood borne malignancies carries the risk of reintroducing cancer cells (Meirow et al., 2008). To avoid this risk, mammalian follicles can be isolated from fresh or cryopreserved ovarian tissue and grown in vitro using various culture systems (O'Brien et al., 2003; Telfer et al., 2008; Xu et al., 2009b, c; Segers et al., 2010; Smitz et al., 2010).
We have engineered a hydrogel encapsulation culture system that, unlike other systems, preserves follicular architecture (Xu et al., 2006a; West et al., 2007). In the mouse, this system supports the growth of pre-antral follicles into antral follicles containing oocytes capable of resuming meiosis and producing metaphase-II arrested (MII) oocytes (Xu et al., 2006a, 2009a). These MII oocytes are fertilization-competent and can give rise to offspring (Xu et al., 2006a). Second-generation biomaterials, which incorporate an interpenetrating fibrin-alginate network, have enhanced oocyte quality as measured by morphology in prepubertal mice (Shikanov et al., 2009). The interpenetrating network creates a mechanical rigidity that decreases with time, allowing the follicle to regulate its own microenvironment (Shikanov et al., 2009). The hydrogel encapsulation culture system has been extended to non-human primates and humans and has resulted in MII oocytes and oocytes with stage IV germinal vesicles (Gvs) indicative of nuclear competence, respectively (Xu et al., 2009b, c; Xu et al., 2011). It is not well understood, however, how patient-specific factors including age, weight, metabolic status or menstrual cycle stage, affect in vitro follicle growth and gamete development in such a system.
First, fertility and fecundity have been well established to sharply decrease in women aged 35 and older (Broekmans et al., 2009). The capacity of assisted reproduction technologies (ARTs) to overcome the maternal age factor is limited in patients older than 40 years (Malizia et al., 2009). At the same time, a concurrent increase in spontaneous miscarriage is observed in human studies (Hassold and Hunt, 2001; Broekmans et al., 2009). The observed decrease in fertility has been largely attributed to decreased oocyte quality and increased incidence of aneuploidy (Hassold and Hunt, 2001). This phenomenon has also been shown to occur in mouse models where the mechanisms of aneuploidy are currently being investigated (Pan et al., 2008; Duncan et al., 2009).
Second, obesity impacts at least 30% of reproductive aged women (Nelson and Fleming, 2007). Obesity is associated with an increased risk of early loss in pregnancies achieved from natural conception and ART (Lintsen et al., 2005; Nelson and Fleming, 2007). Even when confounding factors such as polycystic ovarian syndrome (PCOS) are excluded from analysis and anovulation is corrected for, studies indicate that higher BMI is still associated with worse IVF pregnancy outcomes (Lintsen et al., 2005; Styne-Gross et al., 2005; Minge et al., 2008; Robker, 2008; Watkins et al., 2008). The exact mechanisms by which obesity impacts pregnancy outcomes are unclear. Some studies indicate that obesity negatively impacts oocyte and embryo quality, whereas others suggest that the effect is at the level of uterine implantation (Eckert et al., 2007; Robker, 2008). In parallel to findings in human beings, diet-induced obese mouse studies have shown a wide range of negative reproductive phenotypes in addition to poor outcomes in the offspring from these mice (Igosheva et al., 2010; Jungheim et al., 2010; Robker, 2008).
Finally, it is speculated that the specific hormonal environment in which a follicle develops can impact its outcome in in vitro manipulations. In vitro maturation studies of oocytes aspirated at the time of tubal surgery have suggested a difference in maturation rates based on the stage of the patient's menstrual cycle at time of aspiration (Cha KY, 1992). Primate work has also suggested a role of the menstrual cycle, as cultured secondary follicles obtained in the follicular phase grew and survived better than follicles isolated at the luteal phase (Xu et al., 2009c). However, the initial follicle diameter, which can impact in vitro follicle growth, was larger in follicles isolated from the follicular phase.
Studies investigating how these patient-specific factors affect in vitro follicle growth dynamics are essential to translating this technology into a clinical setting, and significant progress in this area has been achieved recently in non-human primates (Xu et al., 2010). In the present study, we used a mouse model to systematically investigate how mammalian follicles isolated from animals of different ages, weights and stages of the estrous cycle behave in culture using hydrogel encapsulation. We studied follicle growth, follicle functionality, survival, oocyte meiotic competence and MII oocyte spindle morphology in these cohorts. We chose the mouse model system because it is tightly controlled, highly tunable and to date, it is the only species in which a live birth has been reported using this hydrogel encapsulation culture system (Xu et al., 2006a). Such mouse studies will be instrumental in informing future studies in non-human primates and women. Our findings indicate that while follicle growth parameters are similar, follicle function and potentially oocyte health are impacted differentially by these factors.
Materials and Methods
Reagents
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO), immunocytochemical stains and antibodies from Molecular Probes (Eugene, OR) and media formulations from Invitrogen (Carlsbad, CA). Sodium alginate (55–65% guluronic acid) was purchased from FMC BioPolymers (Philadelphia, PA) and fibrin gels were obtained from Tisseel Healthcare (BioScience Division, Westlake Village, CA).
The fibrinogen-containing component of Tisseel was reconstituted in aprotinin (3000 kIU/ml) solution and the thrombin component was reconstituted in 40 mM CaCl2, according to the manufacturer's instructions (Baxter, Deerfield, IL). Both solutions were diluted to the appropriate concentrations in Tris-buffered saline solution and alginate aliquots were prepared as previously described (Shikanov et al., 2009) and diluted to 0.75% (v/v). Interpenetrating networks (IPNs) were prepared by mixing fibrinogen solution (50 mg/ml) with alginate solution 0.75% (v/v) in a 1:1 ratio, and then adding thrombin solution 50 IU/ml to the mixture in a 1:1 ratio, as previously described (Shikanov et al., 2009).
Animals
CD1 mice (Harlan Laboratories, Indianapolis, IN) were housed in a temperature- and light-controlled environment (12L:12D) and were provided with food and water ad libitum. From the time of weaning, animals were fed Teklad Global irradiated 2916 maintenance chow for 49 days (D49), 100 days (D100) and 270 days (D270). This chow does not contain soybean or alfalfa meal and contains minimal phytoestrogens. It provides 20% of calories from protein, 11% from fat and 69% from carbohydrates. To obtain D100 mice with the weight similar to the D270 mice (D100*), mice were fed Teklad Global irradiated 2919 breeding chow from the time of weaning. This breeding chow has 20% of calories from protein, 23% from fats and 57% from carbohydrates.
Animals were housed 2 per cage, and 2 weeks prior to use, all animals underwent daily vaginal smears to evaluate cycle stage based on cytology (Caligioni, 2009). Although 30% of D270 mice did not cycle regularly, only mice that had completed two consecutive estrous cycles were used for the following studies. The D49 cohort was divided into three groups of at least six animals per cycle stage (estrus, metestrus and diestrus). Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the established Institutional Animal Care and Use Committee protocol at Northwestern University.
Metabolic assays
These assays were performed on D49, D100, D270 and D100* mice during the estrus stage with six mice analyzed per age group. Mice used for these metabolic assays were not used for subsequent follicle studies. Intraperitoneal glucose tolerance testing (IPGTT) was performed following a 6 h fast, and 2g/kg of 50% dextrose (Abbott Labs, North Chicago, IL) was injected intraperitoneally. Blood glucose levels were measured using tail blood at baseline (before injection) as well as 15, 30, 60 and 120 min after glucose injection. The blood was placed on Accu-chek Aviva test strips (Roche Diagnostics, Indianapolis, IN), and levels were measured using an Accu-check Aviva automatic glucose meter. Area under the curve (AUC) for each animal was calculated using the trapezoidal rule (Cheung et al., 2005). Cholesterol was measured in mouse serum using the commercially available fluorometric assay (Molecular Probes, Invitrogen, Carlsbad, CA), which has a sensitivity of 200 nM (80 ng/ml).
Hormone and peptide assays
These assays were performed using D49, D100, D270 and D100* mice. For studies in which age and weight variables were examined, we used an equal distribution of mice at each stage of the estrous cycle. In the D49 cohort, we specifically performed assays at distinct stages of the estrous cycle. To detect serum levels of hormones and peptides, whole blood was obtained using cardiac puncture following ovary harvesting for follicle isolation, and serum was obtained by centrifugation. To assay hormones secreted by cultured follicles, spent culture media from viable follicles isolated from the same mouse were pooled at specific days of culture. All assays were run in triplicate using samples from between 6 and 15 mice per experimental cohort.
Androstenedione, 17β-estradiol (E2) and progesterone were measured from animal serum as well as spent media using commercially available radioimmunoassay kits according to the manufacturer's protocol (androstenedione and 17β-E2: Diagnostic Systems Laboratories, Inc., Webster, TX; progesterone: Diagnostic Products Corporation, Los Angeles, CA). The sensitivities for the androstenedione, E2 and progesterone assays are 0.1, 0.01 and 0.1 ng/ml, respectively. Anti-Müllerian hormone (AMH) and inhibin B were measured in collected serum from individual animals using commercially available ELISA kits (Diagnostic Systems Laboratories, Inc.). Testosterone was determined by extraction radioimmunoassay. The sensitivities for the AMH, inhibin-B and testosterone assays are 0.05, 10 and 0.15 ng/ml, respectively. The mouse FSH radioimmunoassay was performed using reagents obtained from Dr Albert Parlow at the Pituitary Hormones and Antisera Center (Torrance, CA), and the sensitivity was between 1.56 and 3.13 ng/ml. All assays were performed by the Endocrine Technology and Support Core at the Oregon National Primate Research Center (http://www.ohsu.edu/xd/research/centers-institutes/onprc/research-serveices/research-support/endocrine-technology.cfm).
Follicle isolation, encapsulation and culture
Secondary follicles, ranging in average diameter from 150 to 163 µm, were mechanically isolated and encapsulated in Fibrinogen-alginate (FA)-IPNs described previously (Pangas et al., 2003; Kreeger et al., 2006; Xu et al., 2006a; West et al., 2007; Shikanov et al., 2009). Briefly, FA solution was pipetted onto a parafilm-covered glass slide and individual follicles were pipetted into the droplets. Thrombin solution was then pipetted onto each follicle-containing FA droplet. Droplets were covered with a second parafilm-covered glass slide and transferred to a 37°C, 5% CO2 incubator for 5 min. The FA-IPN beads containing follicles were washed in maintenance media [α\xEF\x80 minimum essential medium (MEM) supplemented with 1 mg/ml bovine serum albumin (BSA, MP Biomedicals, Inc., Solon, OH) and penicillin–streptomycin] and individually transferred to 96-well plates in 100 µl of growth media [αMEM supplemented with 3 mg/ml BSA, 10 mIU/ml recombinant follicle-stimulating hormone (rFSH; A.F. Parlow, National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases), 1 mg/ml bovine fetuin, 5 µg/ml insulin, 5 µg/ml transferrin and 5 ng/ml selenium].
Encapsulated follicles were cultured at 37oC in 5% CO2 for 9 days. For each cohort, a total of 3–4 independent experiments were performed each containing 12–42 follicles. Every other day, half of the media (50 µl) was exchanged and stored at −20°C for steroid hormone assays. Follicle survival and diameter were assessed using an inverted Leica DM IRB microscope with transmitted light (Leica, Bannockburn). Live follicles were defined as those with intact morphology and the absence of fragmentation in the oocyte or granulosa cells. The average of two follicle diameter measurements were made from the outer layer of theca cells using ImageJ 1.33 U (NIH, http://rsb.info.nih.gov/ij/), based on a calibrated ocular micrometer. Detailed protocols outlining these methods can be found at http://oncofertility.northwestern.edu/researchers.
Oocyte meiotic competence
In vitro maturation was performed after 9 days of culture, as previously described (Xu et al., 2006b; Shikanov et al., 2009). Briefly, follicles were removed from the FA-IPN beads by a 10-min incubation in a 10 IU/ml solution of alginate lyase in prewarmed L-15 media, transferred to the in vitro maturation media (containing 5 ng/ml epidermal growth factor, 0.2 IU/ml FSH and 1.5 IU/ml hCG) and then incubated at 37°C in 5% CO2 for 14–16 h. Following in vitro maturation, oocytes were denuded from the surrounding cumulus cells by treatment with 0.3% hyaluronidase and their morphology was assessed by light microscopy. Oocytes that did not resume meiosis and instead remained arrested in prophase of meiosis I as evidenced by an intact GV were classified as GV-intact oocytes. Oocytes that resumed meiosis and reached metaphase of meiosis II (MII) as evidenced by polar body extrusion were classified as MII-arrested oocytes (Fig. 2C; inset). Those oocytes that resumed meiosis but did not reach MII, as evidenced by lack of a both a GV and a polar body, were referred to as oocytes that had undergone GV breakdown. Finally, degenerate oocytes were also documented. We report the distribution of meiotic progression as a fraction of the number of cells in each meiotic stage over the total number of follicles that were in vitro matured.
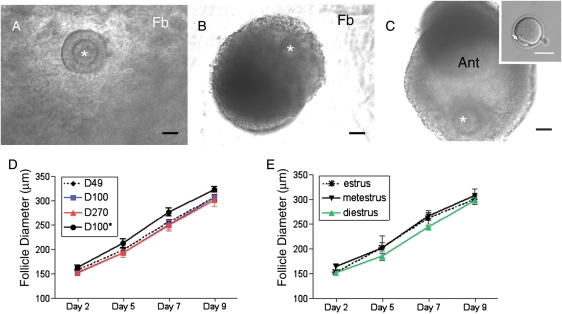
Figure 2.
Follicle growth dynamics during culture. Representative images of follicles grown in the FA-IPN 3-D culture system on (A) Day 2, (B) Day 7 and (C) Day 9 of culture (inset shows an MII-arrested oocyte following in vitro maturation, and the polar body is marked by the arrow). Note that the fibrin (Fb) clears during the culture and is a sign of follicle health. The antral cavity (Ant) is evident at Day 9 of culture. The asterisks highlight the position of the oocyte within the follicle. Follicle growth curves are shown for follicles isolated from (D) mice of different ages and weights or (E) from D49 mice at different stages of the estrous cycle. With the exception of follicles isolated from D100* mice which started at a statistically larger size and grew to a statistically larger terminal size compared with follicles isolated from D100 mice (P<0.05), there were no statistically significant differences in follicle growth trajectories among the cohorts examined.
Immunocytochemistry and confocal microscopy
MII-arrested oocytes were fixed in 2% formaldehyde containing 1% Triton, 0.1 mmol/l Pipes, 5 mmol/l MgCl2 and 2.5 mmol/l EGTA for 1 h at 37°C. Immunocytochemistry was done to detect tubulin, actin and DNA. Oocytes were incubated in primary antibody (α/β tubulin cocktail 1:100; mouse; Sigma) at 4°C for 1 h with gentle agitation, followed by a 1 h incubation in secondary antibody (Alexa 633 goat anti-mouse immunoglobulin G 1:500; Molecular Probes) with rhodamine-phalloidin (1:50; Molecular Probes) and ethidium homodimer (2 μM; Invitrogen) at room temperature. Washes in between antibody incubations were done in phosphate-buffered saline (PBS) containing 0.2% azide, 2% normal goat serum, 1% BSA, 0.1 M glycine and 0.1% Triton X-100. Oocytes were mounted in a 50% glycerol/PBS solution. Samples were analyzed on an inverted Leica SP5 Scanning Confocal Microscope equipped with a 100-W mercury arc lamp and were imaged using 40× and 63× objectives. A 3-D reconstruction was created using Z-stacks of the images. Measurement of spindle length from pole to pole was performed.
Statistical analysis
Statistical comparisons were done between D49, D100 and D270 cohorts to directly assess the impact of age and between D100 and D100* cohorts to directly assess the impact of weight. To directly assess the impact of estrous cycle stage, metestrus, estrus and diestrus D49 cohorts were compared. Results are reported as mean ± SEM. Statistical analysis was performed with one-way analysis of variance followed by post hoc t-tests with corrections for multiple comparisons. For comparisons between two groups, Student's t-test was used. Values of P<0.05 were considered statistically significant. Only statistically significant differences are highlighted. Statistical calculations were performed using the software GraphPad Prism version 4.0.
Results
Baseline characteristics of mouse cohorts
We generated distinct mouse cohorts to investigate the effects of age, weight and estrous cycle stage on in vitro follicle growth and function. For an adult age continuum, we used D49, D100 and D270 CD-1 mice. The D270 mice may represent a population with diminishing reproductive capacity because 30% of the animals in this age group had stopped cycling compared with the other age groups in which all animals had a continuous estrous cycle (Table I). Furthermore, D270 mice have reduced fecundity compared with younger mice (data not shown). These findings are consistent with published data that mice display an age-associated decline in reproductive capacity (Holinka et al., 1979). In generating populations of different age mice, we observed that mouse weight increased significantly with age (Fig. 1A). D49 mice weighed 30.1 ± 0.7 g and D100 mice weighed 32.9 ± 1.0 g, which were both significantly less than D270 mice, which weighed 43.4 ± 1.5 g (P<0.001). Therefore, to distinguish between effects due to increasing age versus weight, we generated a population of younger mice with increased body weight. This was accomplished by feeding a cohort of mice a higher fat diet from the time of weaning. Thus, we were able to obtain D100 mice that were similar in weight to D270 mice (Fig. 1A, D100*; 39.3 ± 0.7 g. To investigate the role of specific estrous cycle stages on in vitro follicle growth dynamics, we used standard vaginal cytology to identify animals in estrus, metestrus and diestrus. To avoid confounding age or weight factors, we only used D49 mice for the estrous cycle studies. Interestingly, diestrus animals weighed an average of 33.9 ± 1.5 g, which was significantly greater than animals in estrus or metestrus, which both weighed an average of 28.9 ± 0.9 g (P<0.05) (Fig. 1B).
Table I.
Serum hormone profiles of mice of different ages and weights.
| D49 | D100 | D270 | D100* | |
|---|---|---|---|---|
| % Cycling | 100 | 100 | 70 | 100 |
| FSH (ng/ml) | 6.5 ± 2.1 | 4.5 ± 0.81 | 5.0 ± 0.95 | 5.6 ± 1.7 |
| Inhibin-B (ng/ml) | 31.2 ± 5.1 | 24.6 ± 4.7 | 31.1 ± 6.9 | 28.2 ± 6.2 |
| AMH (ng/ml) | 10.8 ± 0.6** | 12.0 ± 1.1**,*** | 6.8 ± 0.7 | 8.7 ± 0.8 |
| Progesterone (ng/ml) | 5.4 ± 0.65 | 8.5 ± 2.4 | 10.6 ± 3.2 | 12.1 ± 4.7 |
| Testosterone (ng/ml) | 0.4 ± 0.06 | 0.37 ± 0.8 | 0.42 ± 0.5 | 0.44 ± 0.14 |
| Estradiol (pg/ml) | 11.3 ± 1.20 | 15.6 ± 1.8* | 13.3 ± 1.0 | 22.0 ± 3.2 |
*P=0.05 versus D100*.
**P<0.005 versus D270.
***P<0.005 versus D100*.
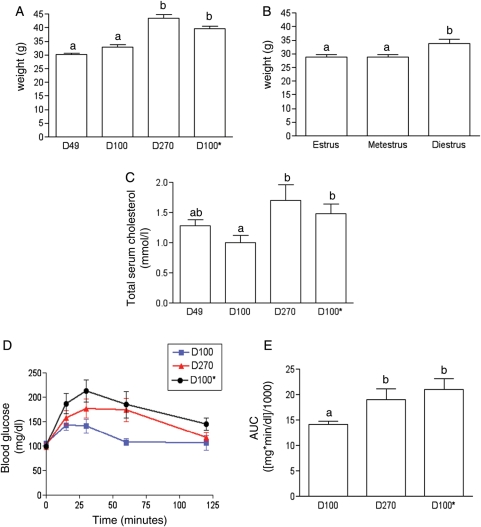
Figure 1.
Weight and metabolic characterization of mouse cohorts. The average weights of (A) different aged mice (D49, D100, D270 and D100*) and (B) D49 mice in different stages of the estrous cycle (estrus, metestrus and diestrus) are shown. To examine the independent effect of animal weight on in vitro follicle growth, a 100 day-old mouse cohort that was similar in weight to D270 mice was generated (D100*) through diet modification. Differences in weight between D49 and D100 compared with D270 and D100* were statistically significant (P<0.001). Differences in weight between mice in estrus and metestrus compared with those in diestrus were also statistically significant (P<0.05). (C) Total serum cholesterol was measured in D49, D100, D270 and D100* mice, and levels in D270 and D100* mice were statistically higher than in D100 mice (P<0.05). (D) IPGTT analysis was performed on D100, D270 and D100* mice following a 6 h fast and blood glucose levels are shown for 2 h post-glucose challenge. (E) Blood glucose levels over the course of the test are shown as the AUC. The difference in glucose tolerance between D100 compared with D270 and D100* mice was statistically significant (P<0.05).
Metabolic and hormonal profiles of mouse cohorts
We next evaluated the metabolic profile, including serum cholesterol and glucose tolerance tests, of animals of different ages and weights. Absolute total serum cholesterol levels were higher in D270 mice compared with the younger cohorts (Fig. 1C). This difference was statistically significant between D100 and D270 mice, in which serum cholesterol levels were 1.0 ± 0.12 and 1.7 ± 0.26 mmol/l (P<0.05), respectively. D100* mice, as would be predicted by their increased weight, had elevated total serum cholesterol levels compared with D100 animals (Fig. 1C, 1.5 ± 0.17 versus 1.0 ± 0.12 mmol/l, P<0.05). These D100* levels were not statistically different from D270 animals. In addition to total serum cholesterol, we also measured blood glucose clearance in response to an intraperitoneal bolus of glucose. In contrast to D100 mice, D100* and D270 mice both had higher peak glucose levels, in response to glucose challenge, that took longer to return to baseline levels (Fig. 1D and E). Although none of these animals were overtly diabetic, as their glucose levels were <200 mg/dl after 2h, D100* and D270 mice did have abnormal glucose tolerance responses that were statistically different from D100 mice (P<0.05).
Similar to the metabolic analysis, serum hormone profiling studies also demonstrated distinct differences between the mouse cohorts. FSH, Inhibin-B, progesterone and testosterone levels did not vary significantly with age (Table I). There was, however, a significant decrease in AMH levels that occurred with age (Table I). AMH levels in D49 and D100 mice were 10.8 ± 0.6 and 12.0 ± 1.1 ng/ml, respectively, compared with 6.8 ± 0.7 ng/ml in D270 mice (P<0.005). When controlling for age, animals with different weights also had characteristic hormone profiles. For example, D100* mice had significantly higher E2 levels compared with D100 mice (Table I, 22.0 ± 3.2 pg/ml, P<0.05). This increase in D100* animals could be due to increased peripheral aromatization of androgen into estrone. Cross-reactivity of estrone and other estrogen forms in this particular assay has been reported (Korenman et al., 1974). We also observed an inverse relationship between AMH levels and weight (Table I). AMH levels were 12.0 ± 1.1 ng/ml in D100 mice, but only 8.7 ± 0.8 ng/ml in D100* mice (P<0.005). FSH, Inhibin-B, progesterone and testosterone levels did not vary significantly with weight (Table I). As expected, there were no significant differences in serum hormone levels on any day of the estrous cycle (data not shown).
Follicle isolation and in vitro follicle growth parameters
To determine how age, weight and estrous cycle stage affect in vitro follicle growth dynamics, we isolated secondary follicles from the various mouse cohorts described earlier. We observed a significant decrease with age in the number of follicles isolated per mouse. Approximately, a total of 14 follicles were isolated from the ovaries of a D49 or D100 mouse compared with only 6 total follicles per D270 mouse ovaries (Table II, P<0.05). Although not statistically significant, on average fewer follicles were isolated from D100* mice compared with D100 mice, suggesting that increased weight may impact follicle dynamics (Table II, 11.8 ± 1.3 versus 13.8 ± 0.8). There were no significant differences in the number of follicles isolated per mouse when the data were stratified according to estrous cycle stage (Table II).
Table II.
In vitro follicle growth characteristics of follicles isolated from mice of different ages, weights or in different stages of the estrous cycle.
| A | D49 | D100 | D270 | D100* |
|---|---|---|---|---|
| # of mice | 21 | 19 | 20 | 15 |
| # Follicles isolated | 298 | 260 | 120 | 180 |
| # Follicles/mouse | 14.2 ± 1.1* | 13.8 ± 0.8* | 6.2 ± 0.6 | 11.8 ± 1.3 |
| Terminal follicle diameter (µm) | 306.1 ± 6.2 | 306.0 ± 0.8** | 301.6 ± 12.5 | 323.3 ± 5.4 |
| Survival in culture (%) | 84.2 ± 1.7*** | 83.0 ± 2.4*** | 72.1 ± 5.0 | 77.5 ± 3.5 |
| B | Estrus | Metestrus | Diestrus | |
| # of mice | 6 | 8 | 7 | |
| # Follicles isolated | 92 | 104 | 102 | |
| # Follicles/mouse | 15.3 ± 1.1 | 13.0 ± 2.3 | 14.6 ± 1.5 | |
| Terminal follicle diameter (µm) | 314.9 ± 9.5 | 308 ± 13.14 | 298.1 ± 5.9 | |
| Survival in culture (%) | 83.5 ± 2.6 | 88.5 ± 3.3**** | 78.2 ± 1.4 | |
*P<0.05 versus D270.
**P=0.05 versus D100*.
***P<0.001 versus D270.
****P<0.05 versus diestrus.
We assessed follicle survival following 9 days of culture in the FA-IPN. Similar to previously reported survival data for follicles isolated from prepubertal mice and cultured in the FA-IPN, more than 80% of follicles isolated from D49 and D100 mice survived (Xu et al., 2006b, c; Shikanov et al., 2009). In contrast, only 72.1 ± 5.0% of follicles isolated from D270 mice (P<0.001 compared with D49 and D100) and 77.5 ± 3.5% from D100* mice survived (Table II). These data suggest that increased age and weight negatively impact follicle survival. Follicles isolated at diestrus had a lower survival rate compared with those isolated at estrus, and this trend was significant when compared with those isolated at metestrus (Table II, P<0.05).
The follicles that survived in culture maintained their spherical 3-D structure during in vitro follicle growth (Fig. 2A–C). In general, secondary follicles grew to large multilayer follicles by Day 7 of culture, and antral cavities were observed on Day 9 of culture (Fig. 2A–C). Follicle diameters increased 100% from ∼150 to 310 µm during the culture period (Fig. 2D and E and Table II). There were no statistically significant differences in the growth rates or terminal diameters in follicles isolated from D49, D100 or D270 mice (Fig. 2D and Table II). Although follicles isolated from D100* animals grew to a statistically larger terminal diameter compared with those isolated from the other cohorts, this may be explained by the observation that the initial follicle diameter in this group was also larger (P<0.05, Fig. 2D). The stage of the estrous cycle when follicles were isolated also did not appear to influence follicle growth and terminal diameter. Follicles isolated in estrus, metestrus and diestrus all followed a similar growth trajectory (Fig. 2E).
Follicle functional assessment
Although in vitro follicle growth parameters were similar regardless of the mouse age, weight or estrous cycle stage, we wanted to determine if follicle function was also equivalent. To examine follicle function, we measured levels of E2, androstenedione and progesterone secreted into the culture medium as a way to assess steroid biosynthesis. Follicles from mice of different ages, weights and estrous cycle stages produced increasing levels of these three steroid hormones during the culture period (Fig. 3A–C). These results indicate that our follicle culture system supports functional follicle development. However, there were key differences in the levels of these hormones produced by follicles isolated from the various mouse cohorts. For example, E2, which is produced by growing follicles and is typically considered a marker of health, was significantly higher in follicles isolated from D49 mice compared with those isolated from D100 and D270 mice on Day 9 of culture. Follicles from D49 mice secreted 9.8 ± 1.4 ng/ml of E2 compared with 5.0 ± 0.7 and 3.5 ± 0.4 ng/ml secreted by follicles from D100 and D270 mice, respectively (Fig. 3A, P<0.001). Follicles from D49 mice also produced significantly higher terminal levels of androstenedione and progesterone compared with follicles isolated from the older mouse cohorts (Fig. 3B and C, P<0.05). These results indicate that follicles isolated from younger mice produce more steroid hormones compared with equivalently sized follicles isolated from older mice. The androstenedione to E2 ratio and the progesterone to E2 ratio calculated from terminal hormone values, however, were not statistically different with respect to animal age (Fig. 3D and data not shown). These ratios ranged from 0.06 to 0.08 and 0.2 to 0.4, respectively.
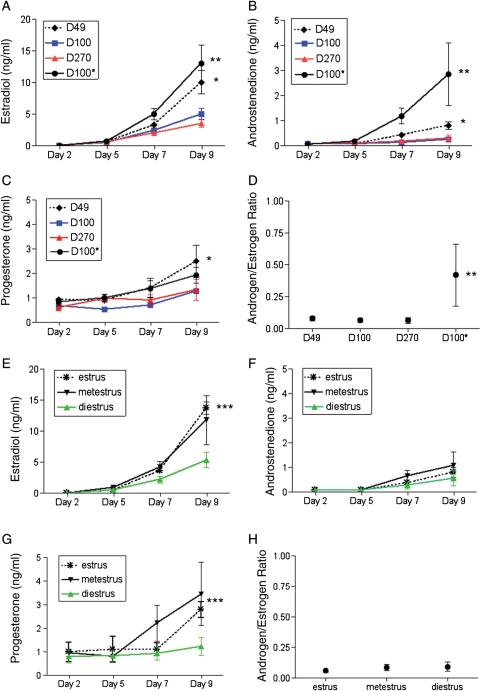
Figure 3.
Steroid production by cultured two-layered secondary follicles. Levels of steroids secreted by follicles isolated from (A–D) mice of different ages and weights and from (E–H) D49 mice at different stages of the estrous cycle are shown. The steroid hormone profiles include E2 (A and E), androstenedione (B and F) and progesterone (C and G) levels. In addition, the androgen/estrogen ratio is shown (D and H). Terminal E2, androstenedione and progesterone levels were significantly higher in follicles isolated from D49 compared with those isolated from D100 and D270 mice (P<0.001, P<0.05, P<0.05; denoted by *). Follicles from D100* mice also secreted significantly higher E2 and androstenedione levels compared with those isolated from D100 mice (P<0.0001, P<0.05; denoted by **). The androgen/estrogen ratio was significantly increased in follicles isolated from D100* mice (P<0.0001; denoted by **). Follicles isolated during estrus produced significantly higher E2 levels compared with those isolated at diestrus (P<0.05; denoted by ***). Progesterone levels were also higher in follicles isolated in metestrus and estrus compared with those isolated in diestrus (P<0.05 comparing metestrus and estrus with diestrus; denoted by***).
Follicles isolated from animals of different weights also varied in hormone production. Follicles isolated from D100* mice produced statistically higher terminal levels of E2 and androstenedione compared with those isolated from D100 mice (Fig. 3A and B, P<0.0001 and P<0.005, respectively) Of note, follicles from D100* mice produced the highest amount of E2 compared with follicles isolated from all of the other mouse cohorts with levels reaching 12.9 ± 2.0 ng/ml at the end of culture (Fig. 3A). These high E2 levels could be a result of aromatization of the significantly high levels of androgens produced by these follicles during the culture period (Fig. 3B, 2.8 ± 0.9 ng/ml). The terminal androstenedione to E2 ratio in follicles from D100* mice was 0.4 ± 0.2, and this value was statistically higher compared with follicles isolated from the other cohorts (Fig. 3D, P<0.0001). These results suggest that follicles from D100* mice produce excess androgens in culture.
We also investigated steroid biosynthesis in follicles isolated from mice at different stages of the estrous cycle. E2, androstenedione and progesterone secretion increased during the culture period regardless of the specific estrous cycle stage (Fig. 3E–G). There was, however, a trend that follicles isolated from animals in diestrus produced lower terminal levels of all three steroid hormones (Fig. 3E–G). The androstenedione to E2 ratio and the progesterone to E2 ratio calculated from terminal Day 9 hormone values, however, were not statistically different between the groups (Fig. 3H and data not shown).
Meiotic competence of oocytes derived from in vitro follicle growth
To determine how animal age, weight and estrous cycle stage impact the quality of the oocytes derived from in vitro follicle growth, we examined the ability of these oocytes to resume meiosis in response to hormonal stimulation. Following in vitro maturation, we noted a degree of oocyte degeneration in all cohorts (Fig. 4A and B). Because oocytes were not removed from the follicle prior to in vitro maturation, this degeneration may have occurred during follicular growth and not during in vitro maturation. Therefore, degeneration may be representative of poor follicle quality. We also noted that the percent of oocytes that reached MII never exceeded 50%, which is less than what we reported previously in studies using follicles from prepubertal mice (Xu et al., 2006b, 2009c; Shikanov et al., 2009). When we cultured follicles and in vitro matured oocytes from prepubertal mice in parallel to our current studies, we obtained similar results to those published previously (data not shown). Thus, these findings indicate that, in general, follicles from adult mice grown in vitro produce oocytes with a decreased ability to resume meiosis and reach MII compared with those isolated from prepubertal mice.
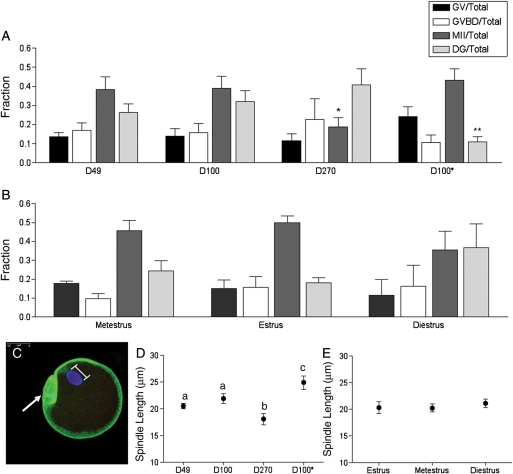
Figure 4.
In vitro maturation results and spindle morphology in the oocyte following in vitro follicle growth. On Day 9 of culture, oocytes were in vitro matured by hormonal stimulation, and light microscopy was used to assess meiotic stage (GV, germinal vesicle intact; GVBD, germinal vesicle breakdown; MII, metaphase II-arrested; DG, degenerate). Meiotic progression is shown for oocytes obtained from (A) follicles isolated from mice of different ages (D49, D100 and D270) and weights (D100 and D100*) as well as (B) those isolated from D49 mice at different stages of the estrus cycle. Meiotic progression was independently compared among follicles isolated from mice of different ages, weights or estrous cycle stages. The fraction of oocytes that reached MII was significantly lower in the D270 cohort compared with the D49 and D100 cohorts (denoted by*). The fraction of degeneration observed in the D100* cohort was significantly less than that observed in the D100 cohort (denoted by**). Following in vitro maturation, MII-arrested oocytes were fixed and immunostained to visualize cortical actin (green), the spindle (blue) and chromosomes (red) by confocal microscopy. A minimum of 15 oocytes per cohort was analyzed, and a representative oocyte is shown in (C) (arrow marks the polar body). The spindle length for each oocyte was determined by a pole-pole measurement as illustrated in (C). The spindle length in oocytes from follicles isolated from (D) mice of different ages and weights and from (E) D49 mice at different stages of the estrous cycle are shown. The spindle length was greater in D49 and D100 cohorts compared with the D270 cohort (P<0.05), as well as in the D100* cohort compared with the D100 cohort. There were no statistically significant differences in spindle length based on cycle stage.
When we directly compared meiotic progression among follicles isolated from mice of different ages (D49, D100 and D270), we observed a statistically significant age-dependent decline in the percent of oocytes that reached MII (Fig. 4A); 38.2 ± 6.7% of oocytes from follicles from D49 mice and 39 ± 6.3% of oocytes from follicles from D100 mice reached MII compared with only 20 ± 5.1% of oocytes from D270 counterparts (P<0.05). A higher, but not statistically significant, percentage with degeneration was also noted in the D270 cohort compared with the D49 and D100 counterparts (Fig. 4A). These results are consistent with the well-documented observation that there is a maternal age-associated decline in oocyte quality (Hassold and Hunt, 2001; Pellestor et al., 2005; Hunt and Hassold, 2008; Broekmans et al., 2009). Similar to age, weight also appeared to impact meiotic maturation. When we directly compared meiotic progression of oocytes from D100 and D100* follicles, we noted that there was significantly less degeneration in the D100* cohort (Fig. 4A). However, there was not a corresponding increase in the percent of oocytes that reached MII (Fig. 4A).
We did not observe any statistically significant differences in meiotic progression in oocytes obtained from mice at different stages of the estrous cycle. However, diestrus mice tended to have follicles that, when grown in vitro, produced oocytes with a reduced ability to reach MII (Fig. 4B). Only 35.6 ± 9.9% of oocytes from follicles isolated at disestrus reached MII compared with 45.7 ± 5.4 % and 49.7 ± 3.6% in metestrus and estrus. In addition, more degeneration was observed in oocytes derived from follicles isolated at diestrus compared with those at the other estrous cycle stages (Fig. 4B).
Quality of MII-arrested eggs derived from in vitro follicle growth
To gain a better understanding of the quality of the MII-arrested eggs that resulted from our in vitro maturation studies, we probed spindle morphology and chromosome alignment along the metaphase plate by immunocytochemistry and confocal microscopy (Fig. 4C). We found that the average pole-to-pole length of MII spindles in oocytes from D270 follicles was 18.1 ± 1.0 µm. This length was statistically smaller than that measured in oocytes from either D49 or D100 follicles (Fig. 4D, P<0.05). Similar to increasing mouse age, increasing mouse weight also appeared to correlate with altered spindle morphology. In contrast to age, however, increased weight was correlated with an increase in maximum spindle length (Fig. 4D). Spindle length in oocytes from D100* follicles was 24.9 ± 1.2 µm compared with that in oocytes from D100 counterparts that were 21.9 ± 0.9 µm long (Fig. 4D). There were no significant differences in the MII spindle length when comparing oocytes isolated from follicles from mice at different stages of the estrous cycle (Fig. 4E).
We next examined chromosome alignment along the metaphase plate because an aligned configuration is necessary for proper chromosome segregation that occurs at fertilization with the completion of meiosis II. If chromosomes are not properly aligned on the meiotic spindle, aneuploidy may result, thus compromising the developmental capacity of the oocyte. Spindles with unaligned chromosomes were scored as those containing one or more chromosomes that deviated from the metaphase plate (data not shown). We found that whereas more than 60% of MII oocytes from D49 (n=12) and D100 (n=14) follicles had aligned chromosomes, only 25% (n=16) of MII oocytes from D270 follicles did. This phenotype was even more severe in MII oocytes from D100* follicles where only 12.5% (n=8) of the spindles contained aligned chromosomes. Greater than 50% of the MII oocytes from follicles isolated at the different stages of the estrous cycle had aligned chromosomes.
Discussion
In this study, we determined the effects of animal age, weight and cycle stage on the ability of follicles to grow in a hydrogel encapsulation culture system. Using CD-1 mice of different ages, we investigated whether increasing maternal age impacts in vitro follicle growth and oocyte health. In generating mice of distinct ages, we observed that older mice weighed significantly more than younger ones. Our results are consistent with a recent study that demonstrated that standard control laboratory rodents, which are fed ad libitum and lead a sedentary lifestyle, ultimately become obese and glucose intolerant and are at increased risk of premature death (Martin et al., 2010). Studies purporting to investigate age effects are, thus, likely confounded by the variable of increased weight. Therefore in the current study, we decided to also examine the independent effect of weight on follicle culture dynamics by generating a younger cohort of mice with increased body weight.
Age and weight have profound impacts on both the metabolic and hormonal profiles of the animal. For example, both D100* and D270 mice had increased total serum cholesterol and reduced glucose tolerance compared with controls. AMH levels also differed significantly with age and weight. AMH is a hormone produced by the granulosa cells of growing follicles, and AMH levels are often used clinically as an indirect readout of the ovarian follicle reserve (La Marca and Volpe, 2006). In humans, AMH levels decrease with age. This drop usually precedes both a fall in inhibin B and rise in FSH but is related to the onset of menopause (de Vet et al., 2002; van Disseldorp et al., 2008; Broekmans et al., 2009). AMH levels were significantly lower in D270 animals compared with younger controls, and these reduced levels are consistent with fewer secondary follicles obtained at the time of isolation. Thus, similar to the human, the observed decrease in AMH in the D270 mice likely corresponds to a decrease in the number of growing follicles. Inhibin B and FSH levels were similar in the three age categories indicating the negative feedback control of FSH is still intact. Interestingly, a decrease in circulating AMH levels has been measured in obese patients irrespective of age (Freeman et al., 2007; Piouka et al., 2009). We found that AMH levels in D100* mice were statistically less than in D100 controls. There was also a trend that a fewer number of follicles were isolated from D100* compared with D100 mice. The initial diameter of these follicles was also larger, which is intriguing relative to a recent human study, which demonstrated larger follicles and premature activation of the secondary cohort in PCOS ovary specimens (Stubbs et al., 2007). Thus, the metabolic and hormonal changes that we report in the mice are similar to those that can occur in older and obese human patients.
Follicles isolated from D100* and D270 mice and grown in vitro were of distinctly poorer quality compared with those isolated from leaner and younger control animals, respectively. Fewer follicles were isolated from D100* and D270 mice compared with controls, and of those follicles that were isolated, fewer survived in culture. When grown in vitro, follicles from these mouse cohorts secreted aberrant hormone levels, contained oocytes with decreased meiotic competence and ultimately produced MII-arrested oocytes with spindle abnormalities and chromosome alignment defects. The decreased ability of follicles isolated from D270 mice to grow in vitro and produce a high-quality gamete is not necessarily surprising given the well-documented maternal-age-associated decline in fertility (Hassold and Hunt, 2001; Hunt and Hassold, 2008; Broekmans et al., 2009). In addition, similar to our findings in the mouse, in the non-human primate model of in vitro follicle growth, parameters including follicle survival, hormone production and oocyte quality are reduced with increasing animal age (Xu et al., 2010). In contrast to mouse follicles, however, non-human primate secondary follicles can be categorized into three groups according to their growth rate during a 40-day culture period: no-grow, slow grow and fast grow. The majority of follicles isolated from older non-human primates are in the no-grow category (Xu et al., 2010). Our results suggest that the altered global metabolic and hormonal environment of the animal may translate to poor follicle function and poor gamete quality when follicles from such animal are used for in vitro follicle growth.
Although there are many similarities between follicles isolated from D100* and D270 mice when they are grown in vitro, there are also key differences. For example, follicles from D100* mice secreted larger amounts of androgen and estrogen with a significant shift to androgen relative to estrogen compared with controls. This has important implications because, in cases such as PCOS, the high androgen environment negatively impacts oocyte quality (Teissier et al., 2000). Oocytes from follicles isolated from D100* and D270 animals differed in their ability to resume meiosis. In addition, the meiotic spindle length in MII-arrested oocytes derived from D100* follicles was larger compared with controls, whereas the spindle length in oocytes derived from D270 follicles was shorter compared with controls. These findings suggest that the mechanisms that lead to reduced follicle quality may be different between aged animals and those with increased body weight. For instance, prolonged meiotic arrest and deterioration of oocyte factors may contribute to the age-associated decline in oocyte quality (Hassold and Hunt, 2001; Hunt and Hassold, 2008). In comparison, obesity has been linked to an increase in free oxygen radicals that may negatively impact oocyte health (Hassold and Hunt, 2001; Broekmans et al., 2009; Igosheva et al., 2010). Oocytes from obese mice have altered mitochondrial function, and this has also been reported in oocytes from diabetic mice (Wang et al., 2009; Wu et al., 2010; Robker et al., 2011). Interestingly, MII-arrested oocytes from diabetic mice also contain spindles that are disorganized and contain unaligned chromosomes (Wang et al., 2009). Thus, mitochondrial dysfunction may contribute to the observed spindle phenotype in MII-arrested oocytes obtained from D100* mice.
Although follicle function and ultimate gamete quality appeared to be negatively impacted by increased animal age and weight, follicular growth and expansion were not. All follicles, irrespective of which mouse cohort they were isolated from, had nearly identical growth trajectories. In fact, the only cohort in which follicles did not grow beyond the pre-antral stage in vitro were those isolated from the subset of D270 mice that had ceased cycling (data not shown). The start size of the follicles obtained from these animals was significantly smaller, potentially explaining the lack of granulosa cell proliferation. Therefore, in general, the ability of somatic cells to proliferate and organize into an antral structure during in vitro follicle growth should not be used as a sole hallmark in the assessment of follicle and gamete health or quality.
The physical environment of the ovary may contribute to the age- and weight-related changes we observed in follicle function and quality during in vitro follicle growth. Specifically, tissues increase in rigidity or decrease in flexibility during the aging process, and the human ovary becomes more fibrotic as women age (Motta et al., 2002; Laszczynska et al., 2008; Woodruff and Shea, 2011). Increased mechanical rigidity negatively impacts follicle quality. For example, follicles cultured in vitro using low-stiffness hydrogels had enhanced follicle growth and coordinated differentiation of the oocyte and somatic cells compared with those follicles cultured using high-stiffness hydrogels (Xu et al., 2006b; West et al., 2007). Qualitative studies in our laboratory have demonstrated that ovaries from both D100* and D270 animals are more rigid than controls (data not shown). Experts blinded to the study condition qualitatively scored the relative rigidity of mouse ovaries based on manual manipulation using a scale of 1–3. The age of the mouse could be predicted 95% of the time by analyzing the gross morphology of the ovary, and D100* animals were identified as the most rigid 93% of the time (data not shown). The mechanisms by which rigidity increases with both animal age and weight and influences follicle quality are currently under investigation.
In general, the impact of estrous cycle stage on in vitro follicle growth and quality was much more subtle than age or weight. Interestingly, the diestrus animals were heavier compared with metestrus and estrus. Diestrus follicles had similar growth patterns but secreted less estrogen and progesterone compared with follicles isolated at metestrus or estrus. In addition, oocyte quality tended to be worse in the diestrus cohort. Others have shown that obtaining unstimulated oocytes at the time of Caesarean Section, tuboplasty or oophorectomy, during a patient's luteal phase had a significantly higher maturation rate than those obtained during the follicular phase (Cha et al., 1992). Furthermore, a meta-analysis of patients undergoing IVF noted that patients stimulated with human menopausal gonadotrophin with traces of LH in addition to FSH have improved pregnancy outcomes compared with those stimulated only with recombinant FSH, suggesting an impact on follicle and oocyte quality from the specific hormonal environment (Coomarasamy et al., 2008). Similar to the human 'luteal phase', in the post LH surge, estrus and metestrus cohorts exposure to both gonadotrophins may be important as the next cohort of follicles are recruited. Our work in non-human primates suggests a role of estrous cycle on follicle growth parameters and survival in culture as secondary follicles obtained at follicular phase had improved growth and survival compared with the luteal phase. Yet, the start size of the follicular phase follicles was significantly greater than in the luteal phase, potentially explaining these conflicting findings (Xu et al., 2009c).
We have demonstrated that follicles isolated from animals with increased weight, of advanced age or in diestrus are not as high quality compared with those from controls when grown in vitro. These follicles have aberrant hormone production and diminished gamete health. Thus, the original physical environment of the follicle within the ovary can impact its function when isolated and cultured. This finding is incredibly important as we translate the in vitro follicle growth technology to a diverse fertility preservation patient population. Patients who seek fertility preservation are of all ages, weight status and menstrual cycle stage and can be smokers, drinkers and taking a variety of medications. Optimizing follicle development in the human, while facing this range of uncontrolled phenotypes is a challenge to the emerging field. Our culture system provides a unique opportunity to modulate the follicular environment, and future studies will be aimed at defining conditions that can potentially correct the metabolic-, age- and cycle-related insults that have occurred in vivo. Taken together, the extension of our success with in vitro follicle growth from the prepubertal mouse to non-human primates to eventually humans, is further enhanced with an understanding of the impact of age, weight and cycle stage on follicle health and oocyte quality in culture.
Authors' roles
J.E.H. played role in conception and design; data collection, analysis and interpretation; manuscript writing. F.E.D. took part in data analysis and interpretation; manuscript writing. M.X. and J.K.J. were involved in conception and design; data collection. L.D.S. played role in conception and design; final approval of manuscript. T.K.W. took part in conception and design; manuscript writing; final approval of manuscript.
Funding
The project described was supported by Award Number U54HD041857 (to T.K.W. and L.D.S.) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Acknowledgements
The authors thank the personnel in the Endocrine Technology and Support Core and Drs Ariella Shikanov and Susan Barrett for experimental help. The authors acknowledge Dr Jessica Hornick for insightful discussions concerning the data and Stacey C. Tobin for editorial assistance on the manuscript.
References
- Ata B, Chian R-C, Tan SL. Cryopreservation of oocytes and embryos for fertility preservation for female cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:101–112. doi: 10.1016/j.bpobgyn.2009.11.007. doi:10.1016/j.bpobgyn.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. doi:10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha KY, Do BR, Chi HJ, Yoon TK, Choi DH, Koo JJ, Ko JJ. Viability of human follicular oocytes collected from unstimulated ovaries and matured and fertilized in vitro reproduction. Fertil Dev. 1992;4:695–701. doi:10.1071/RD9920695. [Google Scholar]
- Cheung BW, Cartier LL, Russlie HQ, Sawchuk RJ. The application of sample pooling methods for determining AUC, AUMC and mean residence times in pharmacokinetic studies. Fundam Clin Pharmacol. 2005;19:347–354. doi: 10.1111/j.1472-8206.2005.00329.x. doi:10.1111/j.1472-8206.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- Coomarasamy A, Afnan M, Cheema D, van der Veen F, Bossuyt PM, van Wely M. Urinary hMG versus recombinant FSH for controlled ovarian hyperstimulation following an agonist long down-regulation protocol in IVF or ICSI treatment: a systematic review and meta-analysis. Hum Reprod. 2008;23:310–315. doi: 10.1093/humrep/dem305. doi:10.1093/humrep/dem305. [DOI] [PubMed] [Google Scholar]
- de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/s0015-0282(01)02993-4. doi:10.1016/S0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- Dohle GR. Male infertility in cancer patients: review of the literature. Int J Urol. 2010;17:327–331. doi: 10.1111/j.1442-2042.2010.02484.x. doi:10.1111/j.1442-2042.2010.02484.x. [DOI] [PubMed] [Google Scholar]
- Donnez J, Jadoul P, Squifflet J, Van Langendonckt A, Donnez O, Van Eyck A-S, Marinescu C, Dolmans M-M. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:87–100. doi: 10.1016/j.bpobgyn.2009.09.003. doi:10.1016/j.bpobgyn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, Pellicer A, Dolmans MM. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011 doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- Duncan FE, Chiang T, Schultz RM, Lampson MA. Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol Reprod. 2009;81:768–776. doi: 10.1095/biolreprod.109.077909. doi:10.1095/biolreprod.109.077909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert JJ, Houghton FD, Hawkhead JA, Balen AH, Leese HJ, Picton HM, Cameron IT, Fleming TP. Human embryos developing in vitro are susceptible to impaired epithelial junction biogenesis correlating with abnormal metabolic activity. Hum Reprod. 2007;22:2214–2224. doi: 10.1093/humrep/dem147. doi:10.1093/humrep/dem147. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF., III Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87:101–106. doi: 10.1016/j.fertnstert.2006.05.074. doi:10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- Gorman JR, Malcarne VL, Roesch SC, Madlensky L, Pierce JP. Depressive symptoms among young breast cancer survivors: the importance of reproductive concerns. Breast Cancer Res Treat. 2010;123:477–485. doi: 10.1007/s10549-010-0768-4. doi:10.1007/s10549-010-0768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. doi:10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Holinka CF, Tseng YC, Finch CE. Reproductive aging in C57BL/6J mice: plasma progesterone, viable embryos and resorption frequency throughout pregnancy. Biol Reprod. 1979;20:1201–1211. doi: 10.1095/biolreprod20.5.1201. doi:10.1095/biolreprod20.5.1201. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. doi:10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5:e10074. doi: 10.1371/journal.pone.0010074. doi:10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. doi:10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046. doi: 10.1210/en.2010-0098. doi:10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenman SG, Stevens RH, Carpenter LA, Robb M, Niswender GD, Sherman BM. Estradiol radioimmunoassay without chromatography: procedure, validation and normal values. J Clin Endocrinol Metab. 1974;38:718–720. doi: 10.1210/jcem-38-4-718. doi:10.1210/jcem-38-4-718. [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723. doi: 10.1016/j.biomaterials.2005.06.016. doi:10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Marca A, Volpe A. Anti-Mullerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf) 2006;64:603–610. doi: 10.1111/j.1365-2265.2006.02533.x. doi:10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- Laszczynska M, Brodowska A, Starczewski A, Masiuk M, Brodowski J. Human postmenopausal ovary—hormonally inactive fibrous connective tissue or more? Histol Histopathol. 2008;23:219–226. doi: 10.14670/HH-23.219. [DOI] [PubMed] [Google Scholar]
- Lintsen AM, Pasker-de Jong PC, de Boer EJ, Burger CW, Jansen CA, Braat DD, van Leeuwen FE. Effects of subfertility cause, smoking and body weight on the success rate of IVF. Hum Reprod. 2005;20:1867–1875. doi: 10.1093/humrep/deh898. doi:10.1093/humrep/deh898. [DOI] [PubMed] [Google Scholar]
- Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360:236–243. doi: 10.1056/NEJMoa0803072. doi:10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. ‘Control' laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci USA. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. doi:10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra'anani H, Slyusarevsky E, Amariglio N, Schiff E, Rechavi G, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23:1007–1013. doi: 10.1093/humrep/den055. doi:10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- Minge CE, Bennett BD, Norman RJ, Robker RL. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reverses the adverse effects of diet-induced obesity on oocyte quality. Endocrinology. 2008;149:2646–2656. doi: 10.1210/en.2007-1570. doi:10.1210/en.2007-1570. [DOI] [PubMed] [Google Scholar]
- Motta PM, Heyn R, Makabe S. Three-dimensional microanatomical dynamics of the ovary in postreproductive aged women. Fertil Steril. 2002;78:360–370. doi: 10.1016/s0015-0282(02)03216-8. doi:10.1016/S0015-0282(02)03216-8. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Fleming R. Obesity and reproduction: impact and interventions. Curr Opin Obstet Gynecol. 2007;19:384–389. doi: 10.1097/GCO.0b013e32825e1d70. doi:10.1097/GCO.0b013e32825e1d70. [DOI] [PubMed] [Google Scholar]
- O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. doi:10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol. 2008;316:397–407. doi: 10.1016/j.ydbio.2008.01.048. doi:10.1016/j.ydbio.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. doi:10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- Pellestor F, Anahory T, Hamamah S. Effect of maternal age on the frequency of cytogenetic abnormalities in human oocytes. Cytogenet Genome Res. 2005;111:206–212. doi: 10.1159/000086891. doi:10.1159/000086891. [DOI] [PubMed] [Google Scholar]
- Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296:E238–E243. doi: 10.1152/ajpendo.90684.2008. doi:10.1152/ajpendo.90684.2008. [DOI] [PubMed] [Google Scholar]
- Robker RL. Evidence that obesity alters the quality of oocytes and embryos. Pathophysiology. 2008;15:115–133. doi: 10.1016/j.pathophys.2008.04.004. doi:10.1016/j.pathophys.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Robker RL, Wu LL, Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol. 2011;88:142–148. doi: 10.1016/j.jri.2011.01.008. doi:10.1016/j.jri.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Larsen EC, Andersen CY, Andersen AN. Risk of ovarian failure and fertility preserving methods in girls and adolescents with a malignant disease. BJOG. 2010;117:163–174. doi: 10.1111/j.1471-0528.2009.02408.x. doi:10.1111/j.1471-0528.2009.02408.x. [DOI] [PubMed] [Google Scholar]
- Segers I, Adriaenssens T, Ozturk E, Smitz J. Acquisition and loss of oocyte meiotic and developmental competence during in vitro antral follicle growth in mouse. Fertil Steril. 2010;93:2695–2700. doi: 10.1016/j.fertnstert.2009.11.035. doi:10.1016/j.fertnstert.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. doi:10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. doi:10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs SA, Stark J, Dilworth SM, Franks S, Hardy K. Abnormal preantral folliculogenesis in polycystic ovaries is associated with increased granulosa cell division. J Clin Endocrinol Metab. 2007;92:4418–4426. doi: 10.1210/jc.2007-0729. doi:10.1210/jc.2007-0729. [DOI] [PubMed] [Google Scholar]
- Styne-Gross A, Elkind-Hirsch K, Scott RT., Jr Obesity does not impact implantation rates or pregnancy outcome in women attempting conception through oocyte donation. Fertil Steril. 2005;83:1629–1634. doi: 10.1016/j.fertnstert.2005.01.099. doi:10.1016/j.fertnstert.2005.01.099. [DOI] [PubMed] [Google Scholar]
- Teissier MP, Chable H, Paulhac S, Aubard Y. Comparison of follicle steroidogenesis from normal and polycystic ovaries in women undergoing IVF: relationship between steroid concentrations, follicle size, oocyte quality and fecundability. Hum Reprod. 2000;15:2471–2477. doi: 10.1093/humrep/15.12.2471. doi:10.1093/humrep/15.12.2471. [DOI] [PubMed] [Google Scholar]
- Telfer E, McLaughlin M, Ding C, Thong K. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- van Disseldorp J, Faddy MJ, Themmen AP, de Jong FH, Peeters PH, van der Schouw YT, Broekmans FJ. Relationship of serum antimullerian hormone concentration to age at menopause. J Clin Endocrinol Metab. 2008;93:2129–2134. doi: 10.1210/jc.2007-2093. doi:10.1210/jc.2007-2093. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol. 2009;23:1603–1612. doi: 10.1210/me.2009-0033. doi:10.1210/me.2009-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Papenbrock T, Fleming TP. The preimplantation embryo: handle with care. Semin Reprod Med. 2008;26:175–185. doi: 10.1055/s-2008-1042956. doi:10.1055/s-2008-1042956. [DOI] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. doi:10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28:3–6. doi: 10.1007/s10815-010-9478-4. doi:10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–5445. doi: 10.1210/en.2010-0551. doi:10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009a;103:378–386. doi: 10.1002/bit.22250. doi:10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009b;24:2531–2540. doi: 10.1093/humrep/dep228. doi:10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. doi: 10.1530/REP-10-0284. doi:10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011;26:1061–1072. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006a;12:2739–2746. doi: 10.1089/ten.2006.12.2739. doi:10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006b;75:916–923. doi: 10.1095/biolreprod.106.054833. doi:10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009c;81:587–594. doi: 10.1095/biolreprod.108.074732. doi:10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]