Abstract
BACKGROUND
Despite the increasing use of intravenous immunoglobulin (IVIG) in obstetrics, information on its pharmacokinetics and optimal dosing during each trimester pregnancy is lacking. The aim of this study was to characterize IVIG pharmacokinetics in pregnant women with a history of idiopathic secondary recurrent miscarriage or obstetrical antiphospholipid syndrome and to make dosing recommendations by comparing serum immunoglobulin G (IgG) concentrations in women receiving IVIG to placebo controls, before and during pregnancy.
METHODS
Women enrolled in an IVIG trial for idiopathic secondary recurrent miscarriage (n = 25) or an IVIG study for obstetrical antiphospholipid syndrome (n = 10); 22 received IVIG 0.5–1.0 g/kg and 13 received the equivalent volume of saline, every 4 weeks from pre-pregnancy until 18–20 weeks of gestation, with dosing adjusted for her weight prior to each infusion. Serum IgG concentrations were measured by rate nephelometry before and 0.5 h, and 1, 2, 3 and 4 weeks following an infusion. Sampling was performed pre-pregnancy and in the first and second trimesters.
RESULTS
Area under the curve (AUC) did not differ significantly within the IVIG group between the three sampling periods. Estimated contributions of IVIG [calculated as mean AUC (IVIG group) minus mean AUC (control group)] were 4890.8 g h/l pre-pregnancy, 5591.4 g h/l first trimester and 4755.1 g h/l second trimester (P> 0.05, non-significant). For the IVIG 0.5 and 1.0 g/kg subgroups, the overall estimated contribution of exogenous IVIG was ∼4000 and ∼6400 g h/l, respectively.
CONCLUSIONS
With a weight-adjusted dosage of IVIG, drug exposure, based on AUC calculations, was maintained at the pre-pregnancy level. Therefore, we recommend a weight-adjusted dosage of IVIG during the first and second trimesters.
Keywords: intravenous immunoglobulin, pharmacokinetics, pregnancy, recurrent miscarriage antiphospholipid syndrome
Introduction
Despite the increasing use of intravenous immunoglobulin (IVIG) in obstetrics (Triolo et al., 2003; Mosca et al., 2005; Perricone et al., 2008), information on its pharmacokinetics and optimal dosing during each trimester pregnancy is lacking (Clark and Gall, 1997). This dearth of information and lack of published dosing protocols present a frustrating dilemma to the clinician. Presently, clinicians prescribe IVIG according to empiric dosage regimens which vary from daily, weekly and monthly administration.
Pharmacokinetic parameters of IVIG are largely documented in the non-obstetrical literature. The reported half-life of IVIG varies from hours to weeks amongst cohorts studied (Stephenson and Ensom, 2002). Wide variability of circulating immunoglobulin G (IgG) highlights the importance of measuring IgG blood levels (Pirofsky and Kinzey, 1992). In addition to these variable parameters, the marked physiological changes in pregnancy limit the usefulness of extrapolated pharmacokinetic and pharmacodynamic data from non-obstetrical cohorts. Studying the effectiveness of IVIG in obstetrical cohorts requires optimal dosing protocols, which should be based on pharmacokinetic studies in pregnancy.
To our knowledge, no systematic pharmacokinetic data for IVIG exist for women with poor obstetrical outcomes who are contemplating pregnancy, despite the use of the blood product, associated costs and intermittent worldwide shortages. The aim of this study was to characterize IVIG pharmacokinetics in pregnant women with a history of idiopathic secondary recurrent miscarriage or obstetrical antiphospholipid syndrome, and to make dosing recommendations by comparing serum IgG concentrations in women receiving IVIG to placebo controls, before and during pregnancy.
Materials and Methods
Study population
Recruitment for this prospective cohort study was conducted at two academic medical centers: British Columbia's Recurrent Pregnancy Loss (RPL) Program, Vancouver, Canada, and the University of Chicago Recurrent Pregnancy Loss Program, Chicago, IL, USA. Ethics approval was obtained from the University of British Columbia, Children's and Women's Health Center of British Columbia, and the University of Chicago. Between October 1999 and February 2008, women who met entry criteria were asked to participate; all gave informed written consent.
Enrollment consisted of two groups of women. Group A women were participating in an investigator-initiated, multicentered randomized placebo-controlled trial for idiopathic secondary miscarriage (Stephenson et al., 2010). Briefly, inclusion criteria consisted of women of 18–45 years of age who had a history of idiopathic secondary recurrent miscarriage with their present partner, and the most recent conception took <1 year. Secondary recurrent miscarriage was defined as at least one previous pregnancy of 20 or more weeks of gestation, followed by three or more unexplained miscarriages of <20 weeks, with non-euploid (trisomy, monosomy, polyploidy or unbalanced structural chromosome rearrangement) miscarriages excluded. All couples had a negative screening protocol, consisting of cytogenetic analyses of both partners, maternal thyroid-stimulating hormone, prolactin, anticardiolipin IgG and immunoglobulin M (IgM), lupus anticoagulant, endometrial assessment and an intrauterine cavity evaluation. Subjects were randomized to receive IVIG (Canadian Blood Services IVIG, Gamimune or Gamunex, Talecris Biotherapeutics, Clayton, NC, USA) at a dose of 0.5 g/kg (IVIG group) or the equivalent volume of normal saline (control group). The initial infusion was administered 14–21 days from the projected next menstrual period. The infusion rate was 60 ml/h for the first hour and subsequently increased to a maximum of 180 m1/h. With documentation of pregnancy, the subject received the same infusion every 4 weeks until 18–20 weeks of gestation, with dosing adjusted for her weight prior to each infusion.
Group B women were diagnosed with primary antiphospholipid syndrome (Wilson et al., 1999), clinically based on a history of three or more unexplained miscarriages of <10 weeks of gestation or an unexplained fetal demise of at least 10 weeks of gestation. Laboratory criteria consisted of persistently positive (medium or high titer) anticardiolipin IgG or IgM, or lupus anticoagulant, according to the guidelines of the International Society on Thrombosis and Hemostasis (Shah et al., 1998). Women with concomitant rheumatic disease, kidney disease, chronic hypertension or other medical conditions which could compromise renal function were excluded. All of the Group B women had at least one further unexplained miscarriage or fetal demise following diagnosis, despite treatment with low-dose aspirin and heparin, or maternal morbidity related to heparin use. All subjects in Group B received IVIG (Canadian Blood Services IVIG, Gamimune or Gamunex, Talecris Biotherapeutics). Initially, the dose of IVIG was 0.5 g/kg; with the publication by Yu and Lennon (1999), the dose of IVIG was increased to 1.0 g/kg (IVIG group). The infusion rate and frequency of the infusions were similar to those of Group A women, with dosing adjusted for her weight prior to each infusion.
Serial blood samples were drawn at 0 h (before the infusion) and 0.5 h, and 1, 2, 3 and 4 weeks after the infusion. A maximum of three serial blood sampling periods were planned: prior to pregnancy, in the first trimester (10 ± 2 weeks) and in the second trimester (18 ± 2 weeks).
Sample size calculation
A convenience sample size, consistent with other exploratory pharmacokinetics studies, was used and was determined by the total number of women who volunteered.
Pharmacokinetic analysis
All serum concentrations of IgG were measured by rate nephelometry on the Behring Nephelometer Analyzer in the Complex Chemistry Laboratory, Department of Pathology, Children's and Women's Health Centre of British Columbia, Vancouver, British Columbia, Canada. Specimens were centrifuged at ∼1300g, 3500 rpm for 10 m, serum removed and then frozen (–70°C) for batch analysis. Whenever possible, all of one subjects' samples over the course of the study were analyzed in the same run. Specimens from the University of Chicago were shipped on dry ice. Rate nephelometry measures IgG concentrations by detecting the amount of light scattered by bound haptoglobulin and compares it to a reference range (Vlug et al., 1994). The exact analyzer range varies slightly with reagent lot, but it is from ∼0.40 to 47.0 g/l for IgG. Assay performance is monitored with four serum concentrations of quality control, all with intra- and inter-assay coefficients of variation <5%.
Pharmacokinetic parameters were calculated via non-compartmental analysis using WinNonlin Professional 5.2 (Pharsight, Mountain View, CA, USA). The parameters determined were the maximum concentration obtained during the dosing interval (Cmax), the trough or minimum concentration obtained before the next dose (Cmin) and the area under the curve for the dosing interval (AUC0−τ). One-way repeated-measures analysis of variance followed by the Student–Newman–Keuls test, if appropriate, was used to determine statistical significance within the IVIG group (divided into two subgroups: 0.5 and 1.0 g/kg) and within the placebo group between the three sampling periods. When only two sampling periods were available, a paired Student's t-test was used. Statistical significance was defined a priori as P< 0.05. Roughly estimated contributions of exogenously administered IVIG to total AUC0−τ was calculated as mean AUC0−τ (IVIG group) minus mean AUC0−τ (control group). This estimated contribution was also calculated separately for the two IVIG subgroups (0.5 and 1.0 g/kg).
Results
Altogether, 40 women gave written consent for this pharmacokinetic study, of whom 35 women completed at least one sampling period (IVIG group, n = 22; control group, n = 13). In the IVIG group, 16 women were enrolled from British Columbia's RPL Program (6 from Group A and 10 from Group B) and 6 from the University of Chicago RPL Program. Two of the 10 women in Group B received IVIG at a dose of 0.5 g/kg and eight received 1.0 g/kg. In the control group, six women were enrolled from British Columbia's RPL Program and seven from the University of Chicago RPL Program. Seventeen (IVIG group, n = 10; control group, n = 7) completed all three sampling periods.
Of the 22 women in the IVIG group, 10 women had a live birth at term, five women did not conceive, one dropped out after the pre-pregnancy visit, one had an ectopic pregnancy, four had a first-trimester miscarriage and one had a second-trimester miscarriage. Of the 13 women in the control group, 6 had a live birth at term, 1 woman did not conceive, 1 dropped out after the pre-pregnancy study period and 5 had a first-trimester miscarriage. Results of the multicentered randomized placebo-controlled trial for idiopathic secondary recurrent miscarriage have recently been published; no treatment benefit was found in the trial or the meta-analysis (Stephenson et al., 2010).
Pharmacokinetic data from all 35 subjects with at least one complete sampling period data set were included in the results. The distribution of demographic and pregnancy history variables is shown in Table I. Amount and dosages of IVIG did not differ significantly within the two subgroups of women receiving IVIG between the three sampling periods, also shown in Table I.
Table I.
Characteristics of the women.
| Characteristic | IVIG group (n = 22 women) |
Control group (n = 13 women) | |
|---|---|---|---|
| Mean maternal age at enrollment (±SD) | 34.4 ± 4.3 years | 36.0 ± 5.4 years | |
| Median number of prior miscarriages/fetal demise (range) | 5 (1–8) | 4 (3–9) | |
| Median number of prior term/preterm (range) | 2 (1–3) | 2 (1–3) | |
| Smoking | |||
| Yes | 2 | 3 | |
| No | 20 | 10 | |
| Amount of IVIG | 0.5 g/kg | 1.0 g/kg | |
| Pre-pregnancy | 35.6 ± 6.3 g | 54.7 ± 2.3 g | Saline placebo |
| First trimester | 43.5 ± 21.6 g | 60.3 ± 7.9 g | Saline placebo |
| Second trimester | 38.6 ± 9.0 g | 64.2 ± 8.1 g | Saline placebo |
All parameters are non-significant (P> 0.05).
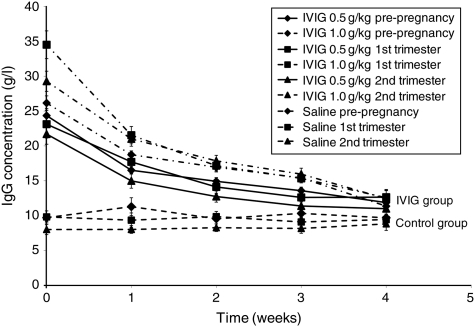
Figure 1 depicts the mean concentration versus time profiles for the three sampling periods for the two subgroups of women receiving IVIG and the control group. Table II shows the pharmacokinetic parameters (mean ± SD) of Cmax, Cmin and AUC0−τ for the two subgroups of women receiving IVIG and the control group. There was no significant difference in the pharmacokinetic parameters within the two subgroups of women receiving IVIG or the control group for the three sampling periods. Estimated contributions of exogenously administered IVIG to total AUC0−τ [AUC0−τ (IVIG) minus AUC0−τ (placebo)] were 4890.8 g h/l pre-pregnancy, 5591.4 g h/l in the first trimester and 4755.1 g h/l in the second trimester, P> 0.05, non-significant. Therefore, the overall estimated contribution of exogenous IVIG was ∼5100 g h/l above the estimated contribution of endogenous IgG (∼5800 g h/l), yielding a total exposure to IgG of ∼10 900 g h/l over 4 weeks.
Figure 1.
Mean (±SEM) IgG concentration versus time profiles for women in IVIG or control group; pre-pregnancy, first and second trimesters.
Table II.
Pharmacokinetic parameters (mean ± SD) in the IVIG and control groups.
| Sampling period | IVIG group 0.5 g/kg |
IVIG group 1.0 g/kg |
Control group |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cmax (g/l) | Cmin (g/l) | AUC0−τ (g h/l) | Cmax (g/l) | Cmin (g/l) | AUC0−τ (g h/l) | Cmax (g/l) | Cmin (g/l) | AUC0−τ (g h/l) | |
| Pre-pregnancy (IVIG 0.5 g/kg, n = 15; 1.0 g/kg, n = 5; control n = 13) | 24.4 ± 3.6 | 11.9 ± 1.8 | 10 351.5 ± 1890.8 | 26.2 ± 6.9 | 11.4 ± 3.2 | 11 753.0 ± 2375.0 | 10.7 ± 2.5 | 9.2 ± 2.2 | 5811.1 ± 1440.8 |
| First trimester (IVIG 0.5 g/kg, n = 6; 1.0 g/kg, n = 6; control n = 8) | 23.1 ± 1.9 | 12.7 ± 1.7 | 10 457.7 ± 1187.4 | 34.5 ± 6.2 | 12.6 ± 3.0 | 12 721.6 ± 2608.2 | 10.3 ± 2.6 | 8.8 ± 2.1 | 5998.3 ± 1946.9 |
| Second trimester (IVIG 0.5 g/kg, n = 5; 1.0 g/kg, n = 5; control n = 7) | 21.7 ± 1.1 | 11.0 ± 2.3 | 8566.5 ± 3267.9 | 29.3 ± 2.2 | 12.4 ± 2.5 | 12 008.1 ± 1200.2 | 9.0 ± 2.0 | 7.6 ± 2.1 | 5532.2 ± 1503.7 |
All parameters are non-significant (P> 0.05).
For the IVIG 0.5 g/kg subgroup, the estimated contributions were 4540.4 g h/l pre-pregnancy, 4459.4 g h/l in the first trimester and 3034.3 g h/l in the second trimester, respectively, P > 0.05, non-significant. For the IVIG 1.0 g/kg subgroup, the estimated contributions were 5941.9, 6723.3 and 6475.9 g h/l, respectively, P > 0.05, non-significant. Thus, for the IVIG 0.5 and 1.0 g/kg subgroups, the overall estimated contribution of exogenous IVIG was ∼4000 and ∼6400 g h/l, respectively.
Discussion
To our knowledge, this is the first study which systematically evaluated the pharmacokinetics of IVIG before and during pregnancy in healthy women with poor obstetrical outcomes. We compared their first and secondary trimester IVIG pharmacokinetic parameters to pre-pregnancy values, and also to endogenous IgG levels. We found with a weight-adjusted dosage of exogenously administered IVIG (with either 0.5 or 1.0 g/kg) that drug exposure (based on area-under-the-curve calculations) was maintained at the pre-pregnancy level. Endogenous IgG concentrations did not change significantly during pregnancy, according to the results in the control group.
After IVIG administration, serum IgG concentrations have been reported to demonstrate multicompartmental first-order kinetics, declining rapidly for 1–7 days and followed by a more gradual rate of decline (Waldmann and Strober, 1969; Nosslin, 1973; Morell, 1997). While low-molecular-weight immunoglobulin fragments, such as Fab and Fv, are eliminated through renal filtration, most of the intact IgG is eliminated through concentration-dependent catabolism (Lobo et al., 2004). The initial elimination phase is believed to be due to both immunoglobulin catabolism and distribution to extravascular spaces, while the terminal phase represents further immunoglobulin catabolism (Pirofsky, 1984; Morell, 1997; Lobo et al., 2004).
In pregnancy, complex changes in normal physiologic processes, such as increased plasma volume and increased glomerular filtration rate, can lead to alterations in pharmacokinetic processes of absorption, distribution, metabolism and excretion (Loebstein et al., 1997; Anderson, 2005). In our study, AUC0−τ was evaluated because it is the best indicator of drug exposure during the dosing interval. According to our results, pregnancy did not have a significant effect on exposure to the weight-adjusted dosage of exogenously administered IVIG.
In a recent systematic review, seven publications that reported IVIG parameters in pregnant women were identified (Koleba and Ensom, 2006). In a randomized placebo-controlled trial, Christiansen et al. (2002) administered IVIG at a dose of 0.8–1.0 g/kg, based on weight at first infusion, weekly until Week 10, then every 2 weeks until Week 26. When the IVIG group was compared with the control group, IgG levels were identical prior to the first infusion (11.4 g/l) and higher at 8 weeks (23.3 versus 10.7 g/l) and 12 weeks (21.7 versus 10.4 g/l).
In a prospective cohort trial, Christiansen, et al. (1992) administered IVIG 10–15 g every 1–2 weeks from gestational week 5–9 until delivery, in 11 women with unexplained recurrent miscarriage. The median serum IgG level was increased by 2.2 g/l (14.7 g/l at mean gestational week 9.8 versus 12.5 g/l before treatment). In another prospective cohort study, Kwak et al. (1995) administered IVIG 0.4 g/kg/day for three consecutive days every month up to 34 weeks of gestation, in six women with recurrent miscarriage ‘of immune etiologies’. The mean serum IgG level was significantly increased by 6.9 g/l (20.8 versus 13.9 mg/l). Lastly, in a prospective cohort study, Bussel et al. (1988) administered IVIG 1 g/kg weekly from 6 to 17 weeks, in seven women who previously delivered a baby with severe neonatal alloimmune thrombocytopenia. The mean serum IgG level was significantly increased by 9.5 g/l (16.9 versus 7.4 g/l). The remaining three publications that reported IVIG parameters in pregnant women were case reports or series (Sorensen et al., 1984; Nicolini et al., 1990; Marzusch et al., 1992).
However, these seven publications reported only single one-point measurements, specifically, baseline and post-infusion serum IgG levels; they did not report on clearance or AUC0−τ (overall drug exposure), which only can be accurately calculated with serial concentrations. Thus, until this study was completed, there were no published AUC0−τ data to guide dosing of IVIG in pregnancy. Based on the results of this study, a weight-adjusted dosage of IVIG during the first and second trimesters was able to maintain drug exposure at the pre-pregnancy level. Therefore, we recommend a weight-adjusted dosage of IVIG during the first and second trimesters.
IVIG continues to be used in pregnancy for concomitant immunological diseases such as systemic lupus erythematosis, dermatomyositis, antiphospholipid syndrome (Triolo et al., 2003; Mosca et al., 2005; Perricone et al., 2008) and fetal alloimmune thrombocytopenia; therefore, pharmacokinetic studies are needed to optimize antenatal dosing. IVIG appears to be an effective and convenient option for diseases mediated by pathogenic autoantibodies or immune complexes, with several mechanisms of actions proposed (Geha and Rosen, 1996).
In a randomized controlled trial, Triolo et al. (2003) found that low-molecular-weight heparin and aspirin had a higher live birth rate than IVIG in women with recurrent fetal loss associated with antiphospholipid antibodies, 84% (16/19) versus 57% (12/21), respectively. But, for patients who failed standard treatment of heparin and aspirin, Triolo et al. (2004) reported in a subsequent study a successful pregnancy outcome in 8 of 10 women treated with IVIG; our Group B had similar entry criteria. Researchers continue to evaluate the effectiveness of IVIG in pregnancy for women with recurrent miscarriage, resistant antiphospholipid syndrome, recurrent IVF failure as well as autoimmune diseases with low prevalence. Without pharmacokinetic studies, a negative study could be due to suboptimal dosing of IVIG.
Limitations of our study deserve mention. Despite recruitment over an 8-year period, there were a limited number of subjects. In addition, the expansion in blood volume that occurs during pregnancy typically is not uniform for all obstetrical patients and, therefore, adjustments in the proposed dosing regimen may be required. Also, some patients may develop intravascular volume depletion due to complications such as gestational hypertension, which could result in increased IgG concentrations. Lastly, we did not evaluate the pharmacokinetics of IVIG in the third trimester or post-partum.
With the administration of 0.5–1.0 g/kg every 4 weeks, the overall estimated contribution of exogenous IVIG was ∼5100 g h/l above the estimated contribution of endogenous IgG (∼5800 g h/l), yielding a total exposure to IgG of ∼10 900 g h/l. Based on our data, we recommend a weight-adjusted dosage of IVIG during the first and second trimesters to maintain similar drug exposure as seen prior to pregnancy. This study provides important information on exposure to exogenous IgG during pregnancy which can be used by clinicians to optimize antenatal dosing of IVIG. Specifically, for either dose (0.5 or 1.0 g/kg of IVIG), weight adjustments in pregnancy yielded optimal dosing.
Authors' roles
Both authors designed the study, obtained funding, interpreted the results and wrote the manuscript. M.H.H.E. conducted the IVIG data analysis. M.D.S. enrolled the women and supervised their care and blood collection from both sites.
Funding
Supported by the Canadian Blood Services Bayer Blood Partnership Fund and the University of Chicago Clinical and Translational Science Award NIH UL1 RR024999.
Acknowledgements
The authors thank Drs Stephanie Ensworth and Susan Purkiss for contributing patients, Pat Schultz and Edwina Houlihan for study coordination and E. Chong, G. Chong, M. Kinnear, M. Al-Khatib and L. Ting for assistance in study conduct and data analysis.
References
- Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- Bussel JB, Berkowitz RL, McFarland JG, Lynch L, Chitkara U. Antenatal treatment of neonatal alloimmune thrombocytopenia. N Engl J Med. 1988;319:1374–1378. doi: 10.1056/NEJM198811243192103. [DOI] [PubMed] [Google Scholar]
- Christiansen OB, Mathiesen O, Lauritsen JG, Grunnet N. Intravenous immunoglobulin treatment of women with multiple miscarriages. Hum Reprod. 1992;7:718–722. doi: 10.1093/oxfordjournals.humrep.a137724. [DOI] [PubMed] [Google Scholar]
- Christiansen OB, Pedersen B, Rosgaard A, Husth M. A randomized, double-blind, placebo-controlled trial of intravenous immunoglobulin in the prevention of recurrent miscarriage: evidence for a therapeutic effect in women with secondary recurrent miscarriage. Hum Reprod. 2002;17:809–816. doi: 10.1093/humrep/17.3.809. [DOI] [PubMed] [Google Scholar]
- Clark AL, Gall SA. Clinical uses of intravenous immunoglobulin in pregnancy. Am J Obstet Gynecol. 1997;1776:241–253. doi: 10.1016/s0002-9378(97)80043-9. [DOI] [PubMed] [Google Scholar]
- Geha RS, Rosen FS. Intravenous immunoglobulin therapy. In: Austen KF, Burakoff SJ, Rosen FS, Strom TB, editors. Therapeutic Immunology. Cambridge, MA: Blackwell Science; 1996. pp. 280–296. [Google Scholar]
- Koleba T, Ensom MH. Pharmacokinetics of intravenous immunoglobulin: a systematic review. Pharmacotherapy. 2006;26:813–827. doi: 10.1592/phco.26.6.813. [DOI] [PubMed] [Google Scholar]
- Kwak JY, Quilty EA, Gilman-Sachs A, Beaman KD, Beer AE. Intravenous immunoglobulin infusion therapy in women with recurrent spontaneous abortions of immune etiologies. J Reprod Immunol. 1995;28:175–188. doi: 10.1016/0165-0378(94)00918-w. [DOI] [PubMed] [Google Scholar]
- Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997;33:328–343. doi: 10.2165/00003088-199733050-00002. [DOI] [PubMed] [Google Scholar]
- Marzusch K, Schnaidt M, Dietl J, Wiest E, Hofstaeeter C, Golz R. High-dose immunoglobulin in the antenatal treatment of neonatal alloimmune thrombocytopenia: case report and review. Br J Obstet Gynaecol. 1992;99:260–262. doi: 10.1111/j.1471-0528.1992.tb14511.x. [DOI] [PubMed] [Google Scholar]
- Morell A. Intravenous Immunoglobulins in Clinical Practice. New York: Marcel Dekker Inc.; 1997. Pharmacokinetics of intravenous immunoglobulin preparations. 1–18. [Google Scholar]
- Mosca M, Strigini A, Carmignani A, Genazzana R, Bombardieri S. Pregnant patient with dermatomyositis successfully treated with intravenous immunoglobulin therapy. Arthritis Rheum. 2005;53:119–121. doi: 10.1002/art.20913. [DOI] [PubMed] [Google Scholar]
- Nicolini U, Tannirandorn Y, Gonzalez P, Fisk NM, Beacham J, Letsky EA, Rodeck CH. Continuing controversy in alloimmune thrombocytopenia: fetal hyperimmunoglobulinemia fails to prevent thrombocytopenia. Am J Obstet Gynecol. 1990;163:1144–1146. doi: 10.1016/0002-9378(90)90674-v. [DOI] [PubMed] [Google Scholar]
- Nosslin B. Protein Turnover. Amsterdam, NY: Associated Scientific Publishers; 1973. Analysis of disappearance time-curves after single-injections of labeled proteins. 113–30. [DOI] [PubMed] [Google Scholar]
- Perricone R, DeCarolis C, Krögler B, Greco E, Giacomelli R, Cipriani P, Fontana L, Perricone C. Intravenous immunoglobulin therapy in pregnant patients affected with systemic lupus erythematosus and recurrent spontaneous abortion. Rheumatology (Oxford) 2008;47:646–651. doi: 10.1093/rheumatology/ken046. [DOI] [PubMed] [Google Scholar]
- Pirofsky B. Intravenous immune globulin therapy in hypogamma globulinemia. A review. Am J Med. 1984;76:53–60. doi: 10.1016/0002-9343(84)90320-6. [DOI] [PubMed] [Google Scholar]
- Pirofsky B, Kinzey DM. Intravenous immune globulins. A review of their uses in selected immunodeficiency and autoimmune diseases. Drugs. 1992;43:6–14. doi: 10.2165/00003495-199243010-00002. [DOI] [PubMed] [Google Scholar]
- Shah NM, Khamashta MA, Atsumi T, Hughes GRV. Outcome in patients with anticardiolipin antibodies: a 10 year follow up of 52 patients. Lupus. 1998;7:3–6. doi: 10.1191/096120398678919624. [DOI] [PubMed] [Google Scholar]
- Sorensen RU, Tomford JW, Gyves MT, Judge NE, Polmar SH. Use of intravenous immune globulin in pregnant women with common variable hypogammaglobulinemia. Am J Med. 1984;76:73–77. doi: 10.1016/0002-9343(84)90323-1. [DOI] [PubMed] [Google Scholar]
- Stephenson MD, Ensom MHH. An update on the role of immunotherapy in reproductive failure. Immunol Allergy Clin N Am. 2002;22:623–642. [Google Scholar]
- Stephenson MD, Kutteh WH, Purkiss S, Librach C, Schultz P, Houlihan E, Liao C. Intravenous immunoglobulin and idiopathic secondary recurrent miscarriage: a multicentered randomized placebo-controlled trial. Hum Reprod. 2010;25:2203–2209. doi: 10.1093/humrep/deq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triolo G, Ferrante A, Ciccia F, Accardo-Palumbo A, Perino A, Castelli A, Giarratano A, Licata G. Randomized study of subcutaneous low molecular weight heparin plus aspirin versus intravenous immunoglobulin in the treatment of recurrent fetal loss associated with antiphospholipid antibodies. Arthritis Rheum. 2003;48:728–731. doi: 10.1002/art.10957. [DOI] [PubMed] [Google Scholar]
- Triolo G, Ferrante A, Accardo-Palumbo A, Ciccia F, Cadelo M, Castelli A, Perino A, Licata G. IVIG in APS pregnancy. Lupus. 2004;13:731–735. doi: 10.1191/0961203304lu2011oa. [DOI] [PubMed] [Google Scholar]
- Vlug A, Nieuwenhuys EJ, van Eijk RV, Geertzen HG, van Houte AJ. Nephelometric measurements of human IgG subclasses and their reference ranges. Ann Biol Clin (Paris) 1994;52:561–567. [PubMed] [Google Scholar]
- Waldmann TA, Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Wilson W, Gharavi A, Koike T, Lockshin MD, Branch DW, Piette J, Brey R, Derksen R, Harris EN, Hughes GR, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome. Arthritis Rheum. 1999;42:1309–1311. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. N Engl J Med. 1999;340:227–228. doi: 10.1056/NEJM199901213400311. [DOI] [PubMed] [Google Scholar]