Abstract
BACKGROUND
The only successful HIV vaccine trial to date is the RV144 trial of the ALVAC/AIDSVAX vaccine in Thailand, which showed an overall incidence reduction of 31%. Most cases were prevented in the first year, suggesting a rapidly waning efficacy. Here, we predict the population level impact and cost-effectiveness of practical implementation of such a vaccine in a setting of a generalised epidemic with high HIV prevalence and incidence.
METHODS
We used STDSIM, an established individual-based microsimulation model, tailored to a rural South African area with a well-functioning HIV treatment and care programme. We estimated the impact of a single round of mass vaccination for everybody aged 15–49, as well as 5-year and 2-year revaccination strategies for young adults (aged 15–29). We calculated proportion of new infections prevented, cost-effectiveness indicators, and budget impact estimates of combined ART and vaccination programmes.
RESULTS
A single round of mass vaccination with a RV144-like vaccine will have a limited impact, preventing only 9% or 5% of new infections after 10 years at 60% and 30% coverage levels, respectively. Revaccination strategies are highly cost-effective if vaccine prices can be kept below 150 US$/vaccine for 2-year revaccination strategies, and below 200 US$/vaccine for 5-year revaccination strategies. Net cost-savings through reduced need for HIV treatment and care occur when vaccine prices are kept below 75 US$/vaccine. These results are sensitive to alternative assumptions on the underlying sexual network, background prevention interventions, and individual’s propensity and consistency to participate in the vaccination campaign.
DISCUSSION
A modestly effective vaccine can be a cost-effective intervention in highly endemic settings. To predict the impact of vaccination strategies in other endemic situations, sufficient knowledge of the underlying sexual network, prevention and treatment interventions, and individual propensity and consistency to participate, is key. These issues are all best addressed in an individual-based microsimulation model.
Keywords: HIV vaccine, Mathematical model, Microsimulation model, South Africa, Cost-effectiveness
1. Introduction
Despite extensive efforts and billions of dollars invested in attempts to curb the spread of HIV [1], HIV prevalence remains disturbingly high in many endemic countries. Although it has been suggested that HIV incidence may be declining among young adults in countries like South Africa, the total number of prevalent infections at a global level increased again in 2009, with now more than 33.3 million people living with HIV, of whom 22.5 million in Sub-Saharan Africa [2]. Only few biomedical prevention interventions have been proven successful in trials and in the field, while other promising interventions remained ineffective in real world situations [3,4]. In 2009, Rerks-Ngarm et al presented the results of the first and only HIV vaccine randomized controlled trial to date that showed a significantly reduced HIV incidence [5]. In their modified intention-to-treat analysis, the ALVAC and AIDSVAX vaccine combination reduced HIV incidence by 31.2% (95% CI: 1.1% to 52.1%). Although the vaccine efficacy was modest and only borderline significant, some argue that the population level impact of such a vaccine might be comparable to that of male circumcision [6]. With numerous HIV prevention trials showing no effect, the RV144 trial result deserves further attention.
Mathematical modelling can explore the potential effects of HIV prevention interventions in a given population. Many mathematical models have been used to this end [7], including compartmental models [8,9,10] and microsimulation (i.e. ‘agent based’) models [11,12]. Compartmental models are generally less complex in structure, work with differential equations, and ignore chance. In contrast, microsimulation models simulate individuals (‘agents’) and therefore allow for more detailed heterogeneity among individuals. These models can also more realistically simulate the many interacting factors that contribute to the spread and control of HIV, including the complexity of sexual networks [13].
Here, we used the established microsimulation model STDSIM to estimate the impact of the ALVAC/AIDSVAX vaccine on the HIV epidemic in a rural South African setting with a high prevalence and incidence with a well-functioning HIV treatment and care programme [14,15]. We present base-case results consisting of a single round of mass vaccination, as well as the impact, cost-effectiveness, and budget impact of repeated vaccination (revaccination) strategies in combination with antiretroviral therapy (ART). In addition, we investigate the impact under alternative assumptions for mechanisms that are often highly simplified in other models: sexual mixing patterns, background prevention interventions, and vaccine delivery.
2. Materials and methods
2.1 Model and vaccine
We used STDSIM, a stochastic microsimulation model for the spread and control of HIV and other STIs [13,16,17]. The model simulates individuals in a dynamic network of sexual contacts, and has been extensively used to evaluate the impact of prevention and treatment interventions on HIV epidemics in Sub-Saharan African settings [11,18,19]. Here, we used a quantification, i.e. parameter settings, for the Hlabisa sub-district in KwaZulu-Natal, South Africa. This area has an HIV prevalence approaching 30% in adults aged 15–49 years in 2010, and a well-developed ART programme [14,20]. In the model, based on results from recent observational studies elsewhere in Africa, ART reduces HIV infectiousness by 92% [21,22] and increases the remaining ART-naive HIV survival by a factor of 3 at time of ART initiation [23]. We assume ART to be given at ≤200 cells/μL from 2004, and at ≤350 cells/μL from mid-2010, according to the new WHO treatment guidelines [24]. The coverage of ART in 2009 as a result of the modelled health seeking behaviour is about 21% of all HIV infected patients, and 75% of those eligible, which corresponds with local data [20]. The modelled circumcision rate is 25% [25], and condom use during casual sexual contacts or commercial sex is 25% from 2003 onwards [25–27]. A detailed description of the model and this quantification can be found in the supplementary material. All modelling results in this paper are averages over 1,000 model runs.
The vaccine efficacy is based on the modified intention-to-treat efficacy estimate of 31.2% of Rerks-Ngarm et al [5]. However, most infections were prevented in the first year after vaccination, suggesting a rapidly waning immunity. Consequently, we assumed the following vaccine efficacy (VE, varying between 0 and 1, where 1 is full protection and 0 equals no protection): VE=0.78 × exp−0.06t, where t = time since vaccination in months, as described in this issue’s editorial. Although the vaccine in the trial consisted of several different injections, we assume the vaccine to consist of 1 injection and protection to occur immediately in order to avoid undue complexity in our model.
2.2 Vaccination strategies
As in other papers in this special issue the base-case scenario consists of a single round of mass vaccination for which the entire population aged 15–49 years is eligible. Two different coverage levels are defined for the base-case scenario: low uptake (30% coverage), and moderate uptake (60% coverage). The mass vaccination campaign is assumed to take place in 2015, and to take a total of 6 months (January 2015 to June 2015). We examined the impact on HIV incidence and prevalence over the period 2015–2025, as well as the proportion of new infections averted over the same period.
A vaccine with such a quickly waning efficacy is unlikely to be introduced in the form of a one-time mass vaccination programme. Therefore, we also examined the impact of revaccination strategies for young adults (aged 15–29), assuming two different frequencies of revaccinating, every 2 years and every 5 years, again at coverage levels of 30% and 60%. Here, we consider revaccination as repeated vaccination programmes with the same vaccine in the target age-group. We assume that revaccination will boost immunity to the same level and duration of protection as afforded by the initial vaccination, regardless of the remaining level of immunity after a previous vaccination. The propensity to participate in health care and prevention programs varies among individuals, resulting in core groups of participants and non-participants in repeated programmes such as repeated vaccination programmes. Therefore, modelled participation in the revaccination campaign is at random during the first round (when individuals become eligible for the first time based on their age), and individuals participating in the first round are more likely to also participate in subsequent vaccination rounds. Consistency of participation in revaccination rounds in the model can range from 0% (i.e. fully random participation in each successive round) and 100% (i.e. the same individuals participate in successive re-vaccination rounds). We assumed a consistency of participation of 50%. When individuals age out of the target population (i.e. aged 30+ years), they are no longer eligible for subsequent vaccination rounds. We assume that one vaccination round takes 6 months (January to June).
We calculated the 20-year (2015–2035) impact and efficiency for several revaccination strategies: i) population aged 15–29; ii) population aged 15–49; iii) population aged 15–24; iv) population with highest HIV incidence (i.e. women aged 15–24, men aged 25–34); and v) Population aged 15–49 and with multiple recent partners (2+ partners in the last 6 months). In addition, we explored the impact of risk compensation of those who received the vaccine on the proportion of infections prevented and efficiency of the vaccination program. Individuals who are vaccinated might perceive themselves at a lower risk of acquiring an HIV infection and reduce their condom use [28]. In addition, vaccination might delay the time for an HIV infected patient to seek HIV specific care. Asymptomatic vaccinated HIV infected patients might be less likely to attend voluntary counselling and testing services, while symptomatic vaccinated patients might seek care elsewhere before visiting an HIV clinic due to a lower perceived risk of having HIV. Therefore, we assumed two types of risk compensation: sexual risk compensation (reduced condom use rates from 25% to 15% in casual relations for vaccinated individuals) and healthcare seeking risk compensation (doubling the HIV-stage specific time until a vaccinated and infected person seeks HIV-related care, see supplementary material). We calculated the cumulative proportion of new infections prevented over the 20-year period, as well as the number needed to vaccinate (NNV) to prevent one new infection [29].
2.3 Cost-effectiveness
HIV infections prevented through vaccination will lead to reductions in costs for HIV treatment and care. Therefore, we estimated the impact of the vaccination programme on the total costs of the combined vaccination and treatment and care programme. For the annual costs of HIV treatment and care, we used published values from Cape Town [30] (see supplementary material). We calculated the cumulative net cost of delivering HIV treatment and care under different vaccination strategies and price levels of the vaccine compared to the cost of HIV treatment and care in the absence of vaccination. Budget impact results were calculated for the 2-year and 5-year revaccination strategies, targeted at the population aged 15–29, and the population with the highest HIV incidence (women aged 15–24, men aged 24–35). Price levels for the vaccine ranged between 10 US$/vaccine and 200 US$/vaccine. All cost results are per vaccine recipient.
International benchmarks suggest that interventions that cost less than three times a country’s gross domestic product (GDP) per capita per Disability Adjusted Life Year (DALY) averted can be considered cost-effective, while interventions that cost less than one time a country’s GDP per capita per DALY averted can be considered highly cost-effective [31]. Here, we assumed that life-years gained crudely reflect DALYs averted. By ignoring the disability loss due to living with an infection, we only slightly underestimate the total number of DALYs averted through vaccination, as the WHO estimates show that the years lived with disability only accounts for 9% of the total DALY loss due to HIV in the Africa region [32]. South Africa’s GDP per capita was 10,140 US$ in 2009 [33]. We divided the total budget of the vaccination strategies by the associated life years gained, and established the maximum vaccine price level that would still render the vaccination strategy cost-effective (i.e. costing less than 30,420 US$ per life year gained) and highly cost-effective (i.e. costing less than 10,140 US$ per life year gained), respectively. All Life-years gained and future costs were discounted at an annual rate of 3% [34].
2.4 Scenario analysis
We used the 60% coverage, 2-year revaccination strategy for young adults to determine the importance of the assumptions regarding the underlying sexual network, participation with and implementation of the vaccination program, and the background treatment and prevention interventions.
We first tested the impact of the vaccine in sexual networks with higher and lower levels of concurrency by adjusting the duration of relationships. As these alternative scenarios resulted in a different HIV epidemic than observed, we fine-tuned the HIV epidemic to accurately reflect the observed HIV epidemic of the Hlabisa sub-district by adjusting circumcision and condom use rates, and the effectiveness of STI treatment in reducing HIV transmission (see supplementary material for more details). In addition - in order to examine the impact of the vaccine for different levels of endemicity - we made two scenarios in which the overall partner change rates were increased and decreased by 10%, resulting in a proportion of 15–49 year olds with 2+ partners in the last 12 months of 44% and 40% respectively (baseline = 42%). The resulting HIV prevalence in the 10% reduction scenario is comparable to the HIV epidemic in South Africa as a whole [2] (see figure S2D).
Since there is heterogeneity in the risk of acquiring and transmitting sexually transmitted infections, the impact of an HIV vaccine will depend especially on the participation rates of high-risk groups [35]. Therefore, we calculated the impact under a scenario where only individuals with multiple recent partners (defined as those having 2 or more partners in the last 6 months) participate (100% participation of men and women with multiple recent partners), and a scenario where only low-risk individuals participate (0% participation of men with multiple recent partners, and 16% participation of women with multiple recent partners), with total population coverage remaining at 60%. In addition, we examined the effect of different durations of a single vaccination round by giving the vaccine to the target population over the course of two year versus delivering it to all participants at once (i.e. over the course of a day), and we looked at how participation consistency affects the impact of the 2-year revaccination strategy by assuming scenarios of 0% consistency and 100% consistency. Finally, we also examined the impact of declining participation rates by assuming a scenario in which initial coverage is 60% in 2015, but declines by to 40% after 4 revaccination rounds (5% decline every vaccination round).
Furthermore, we analysed the impact of the 2-year revaccination strategy under the following treatment and prevention scenarios: i) no ART, ART at ≤200 cells/μl from 2004 onwards, and ART at ≤500 cells/μl in 2012; ii) circumcision rates of 0% and 100%; iii) no condom use and 50% condom use in casual relationships from 2012 onwards. Although some of these values might be unrealistic, we chose them in order to show the maximum effect these parameters might have on the impact of the vaccine.
3. Results
3.1 Vaccination strategies
Figure 1 shows the 10-year impact of the base-case scenarios on the HIV epidemic in Hlabisa, South Africa. A single round of mass vaccination with the ALVAC/AIDSVAX vaccine in this rural setting of South Africa will initially reduce HIV incidence in mid-2015 by about 40% (1.8/100 person-years to 1.1/100 person-years) under 60% coverage, and about 20% (1.8/100 person-years to 1.4/100 person-years) under 30% coverage. However, due to the short-lived efficacy of the vaccine incidence rates quickly rebound, thus limiting its impact on HIV prevalence (figure 1B). The proportion of new infections prevented under coverage levels of 60% and 30% are 9% and 5%, respectively, within the first 10 years after vaccination (figure 1C).
Figure 1. Impact of base-case vaccination scenarios on HIV epidemic in Hlabisa over the period 2015–2025.
A = HIV prevalence, B = HIV incidence; C = Cumulative proportion of infections prevented;
Revaccination strategies in young adults (aged 15–29) will have a more profound impact on the HIV epidemic (figure 2). Revaccinating this population every 2 years at coverage levels of 60% will reduce incidence by 37% (from 0.67% to 0.43%, figure 2A) and HIV prevalence by 23% (11.1% to 8.6%, figure 2B) by 2035. The cumulative proportion of new infections prevented by this strategy is 23% over a 20-year period (figure 2C). Revaccinating every 5 years has a smaller but still substantial impact, reducing incidence by 16% (from 0.67% to 0.57%) and HIV prevalence by 12% (11.1% to 9.8%) by 2035.
Figure 2. Impact of different revaccination strategies on HIV epidemic and proportion of infections prevented in Hlabisa over the period 2015–2040.
A = HIV prevalence, B = HIV incidence; C = Cumulative proportion of infections prevented;
3.2 Cost-effectiveness
Table 1 gives an overview of (cost-) effectiveness indicators for different revaccination strategies. Both 2-year and 5-year revaccination strategies are highly likely to be cost-effective, since the lowest maximum price of delivering a vaccine in order to remain highly cost-effective is 104 US$/vaccine (2-year revaccination for population aged 15–49, 60% coverage). Revaccinating every 5 years is more cost-effective compared to revaccinating every 2 years. The most cost-effective strategy is targeting the age-groups with the highest HIV incidence (NNV = 52 for 2-year revaccination, and 45 for 5-year revaccination), while the biggest impact is achieved by vaccinating the population aged 15–49 (proportion of new infections prevented = 32% for 2-year revaccination, and 18% for 5-year revaccination). Risk compensation both regarding healthcare seeking behaviour and sexual behaviour can easily nullify the impact of vaccination. The only strategy that still shows a clear positive effect under high levels of risk compensation is the 2-year revaccination strategy, provided that coverage levels are kept at around 60%.
Table 1. (Cost-) effectiveness of different revaccination strategies with the RV 144 vaccine in the Hlabisa sub-district of KwaZulu-Natal, South Africa.
Results are cumulative over a period of 20 years. NNV=Number Needed to Vaccinate to prevent one new infection. The maximum costs of a single vaccine in order to be cost-effective is based on a cost-effectiveness threshold of 30,420 US$/life-year gained (3 times South Africa’s per capita GDP of 2009 (10,140 US$), cost-effectiveness threshold for highly cost-effective interventions is 10,140 US$/life-year gained. M=Male, F=Female. n/a = not available because no life-years were gained and/or infections prevented
| 2-year revaccination
|
5-year revaccination
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Infections prevented | NNV | Max costs to be cost-effective (US$) | Max costs to be highly cost- effective (US$) | Infections prevented | NNV | Max costs to be cost-effective (US$) | Max costs to be highly cost- effective (US$) | |
| 15–29 years | ||||||||
| 30% coverage | 13% | 64 | 641 $/vaccine | 214 $/vaccine | 7% | 57 | 476 $/vaccine | 158 $/vaccine |
| 60% coverage | 23% | 70 | 505 $/vaccine | 168 $/vaccine | 12% | 60 | 856 $/vaccine | 285 $/vaccine |
| 15–49 years | ||||||||
| 30% coverage | 18% | 80 | 598 $/vaccine | 199 $/vaccine | 9% | 73 | 540 $/vaccine | 180 $/vaccine |
| 60% coverage | 32% | 89 | 312 $/vaccine | 104 $/vaccine | 18% | 72 | 977 $/vaccine | 326 $/vaccine |
| 15–24 years | ||||||||
| 30% coverage | 8% | 64 | 423 $/vaccine | 141 $/vaccine | 8% | 65 | 1,518 $/vaccine | 506 $/vaccine |
| 60% coverage | 15% | 72 | 478 $/vaccine | 159 $/vaccine | 4% | 61 | 861 $/vaccine | 287 $/vaccine |
| M 25–34 years, F 15–24 years | ||||||||
| 30% coverage | 11% | 47 | 1,381 $/vaccine | 461 $/vaccine | 5% | 44 | 1,886 $/vaccine | 629 $/vaccine |
| 60% coverage | 20% | 52 | 976 $/vaccine | 325 $/vaccine | 10% | 45 | 1,352 $/vaccine | 451 $/vaccine |
| 15–29 years (sexual risk compensation) | ||||||||
| 30% coverage | 1% | 1,507 | 184 $/vaccine | 61 $/vaccine | −7% | n/a | n/a | n/a |
| 60% coverage | 12% | 82 | 218 $/vaccine | 73 $/vaccine | 0% | n/a | n/a | n/a |
| 15–29 years (healthcare seeking risk compensation) | ||||||||
| 30% coverage | 6% | 126 | n/a | n/a | −1% | n/a | n/a | n/a |
| 60% coverage | 17% | 95 | 264 $/vaccine | 88 $/vaccine | 6% | 123 | n/a | n/a |
| 15–49 years (targeting high-risk groups) | ||||||||
| 30% coverage | 9% | 50 | 234 $/vaccine | 2,111 $/vaccine | 5% | 47 | 312 $/vaccine | 2,807 $/vaccine |
| 60% coverage | 18% | 53 | 203 $/vaccine | 1,831 $/vaccine | 8% | 51 | 177 $/vaccine | 1,589 $/vaccine |
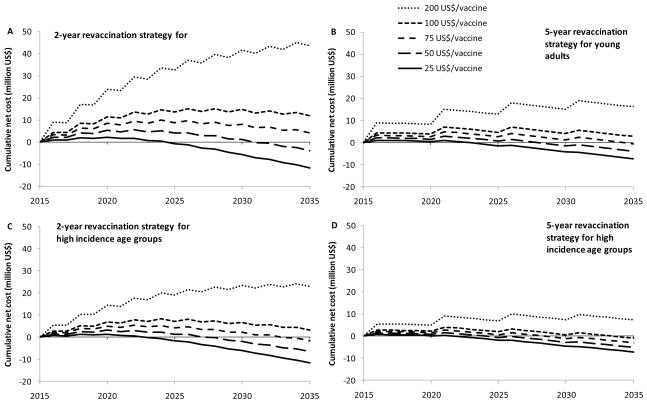
At a price of 75 US$/vaccine, the initial investment of a 2-year revaccination strategy for young adults or high incidence age groups is fully recovered from reductions in HIV treatment and care needs over the 20 years period; and net cost savings will occur (figure 3A and 3C). For the 5-year revaccination strategy, vaccine prices below 100 US$/vaccine will produce similar effects (figure 3B and 3D).
Figure 3. Cumulative net cost of combined ART and vaccination program in Hlabisa.
Cumulative net costs of the vaccination and ART program compared to the total ART costs in the absence of a vaccine are shown for different vaccine price levels (25 US$, 50 US$, 75 US$, 100 US$, and 200 US$ per vaccine). A = 2-year revaccination for the population aged 15–29 (60% coverage); B = 5-year revaccination for the population aged 15–29 (60% coverage); C = 2-year revaccination for the age groups with highest HIV incidence (women aged 15–24; men aged 25–34, 60% coverage). D = 5-year revaccination for the age groups with highest HIV incidence (women aged 15–24; men aged 25–34, 60 % coverage).
3.3 Scenario analysis
Table 2 gives an overview of the effect of different assumptions concerning background sexual networks, background prevention interventions, and vaccine delivery issues on the proportion of infections prevented and NNV after 20 years of a 2-year revaccination strategy for young adults at 60% coverage. The level of the epidemic clearly affects cost-effectiveness. A 10% reduction in partner change rates, reflecting an HIV epidemic comparable to that of South Africa as a whole, would result in a 40% increase in the NNV. In addition, the prevalence of background prevention interventions already in place substantially affects the impact and cost-effectiveness of vaccination, with high levels of circumcision and condom use lowering cost-effectiveness (both more than doubling the NNV). In addition, we show that underlying sexual networks affects the impact of vaccination. Higher levels of concurrency imply more overlapping relationships, and thus a relatively higher impact of the vaccine given its short lasting efficacy. On the other hand, the impact of the vaccine is slightly less in more serial monogamous populations.
Table 2. Impact of alternative assumptions regarding background sexual network, background prevention and treatment interventions, and vaccine programmatic issues on proportion of new infections prevented and number needed to vaccinate (NNV) over the period 2015–2035.
Revaccination strategy is 2-year revaccination for young adults (aged 15–29), at coverage levels of 60%.
| Parameter | Proportion of infections prevented (Baseline = 23%) | NNV (Baseline = 70) |
|---|---|---|
| Different sexual networks a | ||
| More serial monogamy | 21% | 78 |
| More concurrency | 26% | 62 |
| Overall partner change rates b | ||
| +10% (adult HIV prevalence in 2009 = 33%) | 23% | 63 |
| −10% (adult HIV prevalence in 2009 = 20%) | 23% | 97 |
| ART use | ||
| No ART | 17% | 35 |
| ≤ 200 cells/μl | 23% | 43 |
| ≤ 500 cells/μl | 20% | 130 |
| Circumcision rates | ||
| 0% | 24% | 63 |
| 100% c | 21% | 157 |
| Condom use | ||
| No condoms | 24% | 37 |
| 50% by 2012 | 17% | 152 |
| Heterogeneity of participation | ||
| Individuals with multiple recent partners more likely to participate | 25% | 59 |
| Individuals with multiple recent partners less likely to participate | 19% | 90 |
| Duration of vaccination round | ||
| 2 years | 21% | 75 |
| 1 day | 24% | 67 |
| Consistency of participation | ||
| No consistency | 24% | 63 |
| Full consistency | 19% | 86 |
| Declining participation rates d | 19% | 89 |
Sexual networks were adjusted by reducing or increasing duration of relationships and propensity to form new relationships. For comparison purposes, circumcision and condom use rates were used to fine-tune the HIV epidemic in order to replicate the baseline prevalence levels.
Resulting in HIV prevalence levels as indicated in figure S2D
Scale up from 25% to 100% in 2012
from 60 % coverage in 2015 to 40% coverage in 2023 (after 4 vaccination rounds)
4. Discussion
We show that one-off vaccination with ALVAC/AIDSVAX-like vaccines will have a limited impact in a generalised HIV epidemic with high HIV incidence and prevalence. However, if immune responses can be restored through revaccination, vaccination might become a highly cost-effective intervention. Due to reduced future costs of HIV treatment and care, HIV vaccines with limited and waning efficacy, might still result in net cost savings within about 20 years if prices can be kept below 100 US$/vaccine for 5-year revaccination strategies, and below 75US$/vaccine for 2-year revaccination strategies.
Although these results are promising, countervailing forces should be considered. Risk compensation through reduced condom use or a reduced propensity to seek HIV treatment and care might counteract the initial impact of vaccination. In addition, participation dynamics affect both the impact and cost-effectiveness of the vaccine. Whether individuals with multiple recent partners are more likely to participate can further alter the cost-effectiveness ratio, as well as the level of consistency of participation for revaccination strategies. Also, the question remains as to whether the ALVAC/AIDSVAX vaccine is effective at all. The modified intention-to-treat analysis presented by Rerks-Ngarm et al was only borderline significant (p=0.04), and there were no immunological effects measured [5]. Moreover, the trial took place in Thailand where subtype B and recombinant subtypes of the HIV-1 virus are dominant, while the HIV-1 subtype C is dominant in Southern Africa [36]. It is unknown whether an unmodified ALVAC/AIDSVAX vaccine will display the same, if any, effect on the transmission of subtype C HIV-1. Finally, whether the immune response can be restored through revaccination or not was not tested by Rerks-Ngarm et al. Therefore, confirmation of our results can only follow from subsequent trials incorporating revaccination strategies, preferably carried out in highly endemic areas.
The implementation of an intervention inevitably entails a trade-off between the cost-effectiveness of different, competing, interventions. In addition, it involves non-financial issues such as equity and feasibility [37]. Although a moderately effective vaccine might be cost-effective, interventions need to be prioritized by their marginal cost-effectiveness, which may still be higher for other interventions, such as scaling up male circumcision [38, 39]. Also, while we considered an international benchmark on the basis of GDP per capita to define interventions as (highly) cost-effective, we realize that this benchmark is poorly grounded in economic theory and therefore somewhat arbitrary. In addition, it might also be ethically moot to let the valuation of human life depend on per capita GDP. Nevertheless, we still consider it convenient in the absence of local comparative cost-effectiveness information. Furthermore, in order to avoid unnecessary complexity, we assumed vaccination only for the HIV negative population. There are indications that a large part of the HIV positive population is aware of their status since 21% is on treatment [20], and 60% of those diagnosed with HIV have a CD4 cell count of >200 cells/μL and are thus ineligible to initiate treatment (figure S2G). Since many HIV infected patients are aware of their status and thus unlikely to participate in a vaccination campaign, our assumption that only the HIV negative participates will slightly underestimate the total costs of a vaccination programme. On the other hand, DALYs averted are also slightly underestimated by our assumption that life-years gained reflect DALYs averted, as living with an infection is associated with a somewhat lower quality of life, and accounts for about 9% of the total DALY loss due to HIV in Africa [32]. Finally, we did not consider the broader economic and societal impact of preventing HIV infections through vaccination, which might further improve cost-effectiveness ratios [40].
We show that the population level impact of a vaccine in terms of proportion of new infections prevented does not differ much for different levels of endemicity. Nevertheless, the cost-effectiveness of an ALVAC/AIDSVAX-like vaccine depends on absolute numbers averted per vaccination and will thus be reduced significantly under lower endemicity levels. This implies that population-wide vaccination strategies with such a vaccine may only be cost-effective in highly endemic generalized epidemics. In countries with concentrated epidemics, risk group targeting may be considered.
In our STDSIM model, we were able to explore a wide range of mechanisms such as underlying sexual networks, treatment and prevention interventions, individual based risk compensation, and propensity and consistency of vaccination participation, that could possibly influence the impact of vaccination. We show that simplified assumptions regarding mechanisms might result in conclusions that are not necessarily correct. The complexity of the influence of these different mechanisms therefore merits the use of a model that is capable of simulating the sexual network of a specific settings as well as the interaction between different treatment and prevention interventions that are in place, and an individual based propensity and consistency of participation, i.e. an individual (“agent”) based microsimulation model. On the other hand, our finding that these factors influence model predictions also increases the requirement for quality data to quantify the associated model parameters.
5. Conclusions
Despite the fact that the trial results of Rerks-Ngarm et al were initially labelled as interesting but not useful for implementation [41], our results suggest that a vaccine with limited and waning efficacy might be a cost-effective intervention in generalized HIV epidemics and can even lead to net cost savings, however provided that the immune response can be restored through revaccination and no risk-compensation takes place. A single round of mass vaccination will indeed only have a limited and short-lived impact. Since the trial results are borderline significant and took place in Thailand, subsequent trials of ALVAC/AIDSVAX-like vaccines in high endemic countries are needed. Furthermore, we present clear advantages of individual-based modelling in evaluating HIV prevention interventions, since the impact of the vaccine is dependent on the background sexual network, combination of prevention interventions, and individuals’ propensity and consistency to participate in vaccination campaigns.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quinn TC, Serwadda D. The future of HIV/AIDS in Africa: a shared responsibility. Lancet. 2010 doi: 10.1016/S0140-6736(10)62183-6. Epub ahead of print: 30 november 2010. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. Report on the Global AIDS epidemic 2010. Geneva: UNAIDS; [Google Scholar]
- 3.Padian NS, McCoy SI, Balkus JE, Wasserheit JN. Weighing the gold in the gold standard: challenges in HIV prevention research. AIDS 2010. 2010;24:621–635. doi: 10.1097/QAD.0b013e328337798a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type 1 infection. Clin Infect Dis. 2010;51:725–731. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2010;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 6.Kaldor JM, Wilson DP. How low can you go: the impact of a modestly effective HIV vaccine compared with male circumcision. AIDS. 2010;24:2573–2578. doi: 10.1097/QAD.0b013e32833ead96. [DOI] [PubMed] [Google Scholar]
- 7.Baggaley RF, Ferguson NM, Garnett GP. The epidemiological impact of antiretroviral use predicted by mathematical models: a review. Emerg Themes Epidemiol. 2005;2:9. doi: 10.1186/1742-7622-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 9.Vissers DC, Voeten HA, Nagelkerke NJ, Habbema JD, de Vlas SJ. The impact of pre-exposure prophylaxis (PrEP) on HIV epidemics in Africa and India: a simulation study. PLoS One. 2008;3:e2077. doi: 10.1371/journal.pone.0002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baggaley RF, Griffin JT, Chapman R, Hollingsworth TD, Nagot N, et al. Estimating the public health impact of the effect of herpes simplex virus suppressive therapy on plasma HIV-1 viral load. AIDS. 2009;23:1005–1013. doi: 10.1097/QAD.0b013e32832aadf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korenromp EL, Bakker R, Gray R, Wawer MJ, Serwadda D, et al. (2002) The effect of HIV, behavioural change, and STD syndromic management on STD epidemiology in sub-Saharan Africa: simulations of Uganda. Sex Transm Infect. 2002;78 (Suppl 1):i55–63. doi: 10.1136/sti.78.suppl_1.i55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korenromp EL, Van Vliet C, Grosskurth H, Gavyole A, Van der Ploeg CP, et al. Model-based evaluation of single-round mass treatment of sexually transmitted diseases for HIV control in a rural African population. AIDS. 2000;14:573–593. doi: 10.1097/00002030-200003310-00013. [DOI] [PubMed] [Google Scholar]
- 13.Korenromp EL, Van Vliet C, Bakker R, De Vlas SJ, Habbema JD. HIV spread and partnership reduction for different patterns of sexual behavior – a study with the microsimulation model STDSIM. Math Pop Studies. 2000;8:135–173. [Google Scholar]
- 14.Houlihan CF, Bland R, Mutevedzi P, Lessells RJ, Ndirangu J, et al. Cohort Profile: Hlabisa HIV treatment and care programme. Int J Epidemiol. 2010 doi: 10.1093/ije/dyp402. Epub ahead of print: Feb 12 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanser F, Hosegood V, Barnighausen T, Herbst K, Nyirenda M, et al. Cohort Profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol. 2008;37:956–962. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orroth KK, Freeman EE, Bakker R, Buve A, Glynn JR, et al. Understanding the differences between contrasting HIV epidemics in east and west Africa: results from a simulation model of the Four Cities Study. Sex Transm Infect. 2007;83 (Suppl 1):i5–16. doi: 10.1136/sti.2006.023531. [DOI] [PubMed] [Google Scholar]
- 17.van der Ploeg CPB, Van Vliet C, De Vlas SJ, Ndinya-Achola JO, Fransen L, et al. STDSIM: A Microsimulation Model for Decision Support in STD Control. Interfaces. 1998;28:84–100. [Google Scholar]
- 18.Freeman EE, White RG, Bakker R, Orroth KK, Weiss HA, et al. Population-level effect of potential HSV2 prophylactic vaccines on HIV incidence in sub-Saharan Africa. Vaccine. 2009;27:940–946. doi: 10.1016/j.vaccine.2008.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vissers DC, DEV SJ, Bakker R, Urassa M, Voeten HA, et al. The impact of mobility on HIV control: a modelling study. Epidemiol Infect. 2011:1–9. doi: 10.1017/S0950268811000069. [DOI] [PubMed] [Google Scholar]
- 20.Cooke GS, Tanser FC, Barnighausen TW, Newell ML. Population uptake of antiretroviral treatment through primary care in rural South Africa. BMC Public Health. 2010;10:585. doi: 10.1186/1471-2458-10-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 22.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walensky RP, Wolf LL, Wood R, Fofana MO, Freedberg KA, et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151:157–166. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. Rapid Advice: Antiretroviral Therapy For HIV Infected In Adults And Adolescents. Geneva: World Health Organization; 2009. [Google Scholar]
- 25.Demographic and Health Survey of South Africa. [accessed April 30, 2010];Pretoria: Department of Health. 2003 at http://www.measuredhs.com/countries/country_main.cfm?ctry_id=55&c=SouthAfrica.
- 26.Colvin M, Abdool Karim SS, Connolly C, Hoosen AA, Ntuli N. HIV infection and asymptomatic sexually transmitted infections in a rural South African community. Int J STD AIDS. 1998;9:548–550. doi: 10.1258/0956462981922683. [DOI] [PubMed] [Google Scholar]
- 27.Demographic and Health Survey of South Africa. [accessed April 30, 2010];Pretoria: Department of health. 1998 at http://www.doh.gov.za/facts/1998/sadhs98/)
- 28.Newman PA, Roungprakhon S, Tepjan S, Yim S. Preventive HIV vaccine acceptability and behavioral risk compensation among high-risk men who have sex with men and transgenders in Thailand. Vaccine. 2009;28:958–964. doi: 10.1016/j.vaccine.2009.10.142. [DOI] [PubMed] [Google Scholar]
- 29.Kelly H, Attia J, Andrews R, Heller RF. The number needed to vaccinate (NNV) and population extensions of the NNV: comparison of influenza and pneumococcal vaccine programmes for people aged 65 years and over. Vaccine. 2004;22:2192–2198. doi: 10.1016/j.vaccine.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 30.Harling G, Wood R. The evolving cost of HIV in South Africa: changes in health care cost with duration on antiretroviral therapy for public sector patients. J Acquir Immune Defic Syndr. 2007;45:348–354. doi: 10.1097/QAI.0b013e3180691115. [DOI] [PubMed] [Google Scholar]
- 31.Evans DB, Lim SS, Adam T, Edejer TT. Evaluation of current strategies and future priorities for improving health in developing countries. BMJ. 2005;331:1457–1461. doi: 10.1136/bmj.38658.675243.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO. Burden of Disease estimates. Available from http://www.who.int/healthinfo/global_burden_disease/estimates_regional/en/index.html)
- 33.UNDP. International Human Development Indicators. United Nations Development Programme; 2010. [Google Scholar]
- 34.WHO. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Geneva: World Health Organization; 2003. [Google Scholar]
- 35.Garnett GP. Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. J Infect Dis. 2005;191 (Suppl 1):S97–106. doi: 10.1086/425271. [DOI] [PubMed] [Google Scholar]
- 36.Tebit DM, Arts EJ. Tracking a century of global expansion and evolution of HIV to drive understanding and to combat disease. Lancet Infect Dis. 2011;11:45–56. doi: 10.1016/S1473-3099(10)70186-9. [DOI] [PubMed] [Google Scholar]
- 37.Jehu-Appiah C, Baltussen R, Acquah C, Aikins M, d’Almeida SA, et al. Balancing equity and efficiency in health priorities in Ghana: the use of multicriteria decision analysis. Value Health. 2008;11:1081–1087. doi: 10.1111/j.1524-4733.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 38.Binagwaho A, Pegurri E, Muita J, Bertozzi S. Male circumcision at different ages in Rwanda: a cost-effectiveness study. PLoS Med. 2010;7:e1000211. doi: 10.1371/journal.pmed.1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uthman OA, Popoola TA, Uthman MM, Aremu O. Economic evaluations of adult male circumcision for prevention of heterosexual acquisition of HIV in men in sub-Saharan Africa: a systematic review. PLoS One. 2010;5:e9628. doi: 10.1371/journal.pone.0009628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bärnighausen T, Bloom DE, Canning D, et al. Rethinking the benefits and costs of childhood vaccination: The example of the Haemophilus influenzae type B vaccine. Vaccine. 2011;29:2371–2380. doi: 10.1016/j.vaccine.2010.11.090. [DOI] [PubMed] [Google Scholar]
- 41.Dolin R. HIV vaccine trial results--an opening for further research. N Engl J Med. 2009;361:2279–2280. doi: 10.1056/NEJMe0909972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.