Abstract
Study Objectives:
Determine the genetic and environmental contributions to sleep reactivity and insomnia.
Design:
Population-based twin cohort.
Participants:
1782 individual twins (988 monozygotic or MZ; 1,086 dizygotic or DZ), including 744 complete twin pairs (377 MZ and 367 DZ). Mean age was 22.5 ± 2.8 years; gender distribution was 59% women.
Measurements:
Sleep reactivity was measured using the Ford Insomnia Response to Stress Test (FIRST). The criterion for insomnia was having difficulty falling asleep, staying asleep, or nonrefreshing sleep “usually or always” for ≥ 1 month, with at least “somewhat” interference with daily functioning.
Results:
The prevalence of insomnia was 21%. Heritability estimates for sleep reactivity were 29% for females and 43% for males. The environmental variance for sleep reactivity was greater for females and entirely due to nonshared effects. Insomnia was 43% to 55% heritable for males and females, respectively; the sex difference was not significant. The genetic variances in insomnia and FIRST scores were correlated (r = 0.54 in females, r = 0.64 in males), as were the environmental variances (r = 0.32 in females, r = 0.37 in males). In terms of individual insomnia symptoms, difficulty staying asleep (25% to 35%) and nonrefreshing sleep (34% to 35%) showed relatively more genetic influences than difficulty falling asleep (0%).
Conclusions:
Sleep reactivity to stress has a substantial genetic component, as well as an environmental component. The finding that FIRST scores and insomnia symptoms share genetic influences is consistent with the hypothesis that sleep reactivity may be a genetic vulnerability for developing insomnia.
Citation:
Drake CL; Friedman NP; Wright KP; Roth T. Sleep reactivity and insomnia: genetic and environmental influences. SLEEP 2011;34(9):1179-1188.
Keywords: Insomnia, sleep reactivity, heritability, stress, predisposition, FIRST, twins
INTRODUCTION
One of the most influential heuristic models of the evolution of insomnia is the 3P model of insomnia developed by Spielman and colleagues.1 This model provides a useful framework for understanding insomnia and includes predisposing, precipitating, and perpetuating factors that are proposed to play important roles in the initiation and maintenance of the disorder. It has been proposed that stress is a common precipitant of an insomnia disorder and that learned behaviors such as poor sleep hygiene and maladaptive cognitions (e.g., extended bedtime, substance use, catastrophizing) serve to perpetuate the sleep disturbance.1 In contrast, predisposing factors are present before insomnia is manifested and are hypothesized to interact with precipitating factors over time to increase the risk of insomnia in vulnerable individuals. However, for these and other reasons predisposing factors are arguably the least well understood.2
Findings from both retrospective and prospective studies have demonstrated that life stressors are associated with the development of new onset insomnia3–5 and stress exposure correlates with sleep disturbance.6,7 It has also been observed that insomniacs report an increased stress response8 and have increased physiological responses (heart rate reactivity) to stress challenges.9 Investigations attempting to identify potential predisposing factors to insomnia have focused on the response to challenges of the sleep system in non-insomnia subjects. Bonnet and Arand demonstrated that sleep reactivity to diverse challenges appears to be a stable trait characteristic of certain individuals.10 Specifically, certain individuals were found to be particularly vulnerable to experiencing sleep disturbance when exposed to challenges known to disrupt sleep (e.g., caffeine, circadian phase advance).
We have investigated the possibility that this specific characteristic—sleep reactivity—may be a predisposing factor for insomnia.2 Sleep reactivity is a term used to delineate the degree of sleep disruption in response to various challenges. We propose that normal sleeping individuals who show an exaggerated response to stimuli known to disturb sleep are predisposed to subsequently develop an insomnia disorder. We have developed and validated a tool, the Ford Insomnia Response to Stress Test (FIRST), to a priori identify individuals with this exaggerated sleep disruption in response to challenges.11 Based on patient and normal volunteer reports, this 9-item measure has been shown to predict polysomnographic (PSG) responses to diverse challenges, including a disruptive first night effect in the sleep laboratory11 and the sleep effects of low dose caffeine administration.12 Importantly, despite significant sleep disruption in response to a first night challenge in the laboratory, reactive individuals were hyperaroused the following day.11
Numerous studies provide evidence for cognitive and physiological hyperarousal in people with insomnia.13,14 This hyperarousal has been measured with both autonomic (elevated heart rate, increased sympathetic and decreased parasympathetic activation)15,16 and hypothalamic-pituitary-adrenal axis activity (elevated cortisol, increased norepinephrine, increased adrenocorticotrophic hormone).16–18 Importantly, these effects occur during wake as well as sleep.15 Elevated arousal (i.e., high heart rates and sympathetic activation) is also a characteristic of individuals with high sleep reactivity.10 Recently, Fernandez-Mendoza and colleagues showed that elevated somatic and cognitive arousal as well as arousability is related to high FIRST scores, and that despite normal sleep, individuals with high FIRST scores had arousal levels similar to those with insomnia.19 Thus, converging evidence suggests the link between individuals with elevated sleep reactivity and people with insomnia is hyperarousal. While the etiology of sleep reactivity and hyperarousal has yet to be identified, we hypothesize that elevated sleep reactivity predisposes individuals to the subsequent development of insomnia.
In conjunction with research aimed at identifying specific predisposing factors for insomnia, studies have also begun to uncover evidence for a genetic component of insomnia.20–22 A number of family studies have provided evidence for a possible genetic basis for insomnia.23–26 Twin studies have also shown substantial heritability of insomnia and individual symptoms,20–22,27,28 although several studies used single items to define insomnia and/or did not include impairment criteria to define an insomnia disorder. Specific genotypes that account for some phenotypic variance, such as the serotonin transporter, have been identified and suggest that genetic underpinnings might be identified.29 However, a genetic basis for the characteristic of sleep reactivity as assessed by the FIRST and other measures of sleep reactivity has yet to be demonstrated. Due to the genetic underpinnings previously identified in insomnia, we hypothesized a genetic origin for sleep reactivity. Given that sleep reactivity itself may predispose to insomnia, we also hypothesize that the genetic influences on insomnia and sleep reactivity overlap.
In addition to molecular genetic studies, a powerful technique for studying the genetic basis of sleep characteristics is the use of large twin samples to estimate heritability.30 While this technique cannot be used to identify specific genes associated with a disorder, it does have the advantage of allowing inferences related to the overall presence and strength of genetic variance related to a particular disorder or characteristic. Given that the conceptualization of insomnia in the context of the 3P model suggests the presence of predisposing, precipitating, and perpetuating factors, twin studies are helpful in parsing an important component within this conceptualization (potential genetic predisposition). The twin method also allows for specifying the etiology of the relations between phenotypes (i.e., genetic, shared environmental, nonshared environmental). This method enables one to assess not only whether sleep reactivity and insomnia are heritable, but also to determine to what extent their genetic and environmental influences overlap, which is a crucial step for hypothesis development. There have been a number of twin studies investigating the heritability of insomnia with estimates close to 50%, but these studies used a broad assessment of sleep disturbance and did not specifically investigate the heritability of predisposing factors in individuals prior to developing the disorder.21,22,27,31
The nocturnal aspects of insomnia cover a range of distinct symptoms related to both the initiation and maintenance of sleep as well as sleep reactivity, each of which may be an independent and differentially heritable component of the overall disorder. Although sleep reactivity, an inherent responsivity of the sleep system to stimuli, is thought to reflect a trait sleep response to diverse stimuli, there have been no previous examinations of the potential genetic components of this construct and how they may overlap with sleep disturbance. Thus, the aims of the present study were to (1) estimate the overall heritability of sleep reactivity; (2) estimate the heritability of insomnia per se and its separate sleep disturbance symptoms as delineated in diagnostic nosologies for the disorder; and (3) examine the extent to which genetic and environmental influences on insomnia overlap with those for sleep reactivity.
METHODS
Participants
Participants were 1782 individual twins (723 male; 1059 female) from the ongoing Colorado Longitudinal Twin Study and Community Twin Study32 who had completed the online sleep questionnaire at the time of analyses. The response rate for the study was 65%. The average age was 22.5 years (SD = 2.8; range = 18 to 30). An additional 22 women who reported sleep disturbances due to being pregnant or breastfeeding were excluded from analyses.
Zygosity was determined through tester ratings (repeated for individuals in the longitudinal sample) combined with DNA genotyping. Participants came from 1038 families (196 monozygotic [MZ] male, 298 MZ female, 149 dizygotic [DZ] male, 187 DZ female, 207 DZ opposite-sex twins and one pair of undetermined zygosity (not included in the genetic analyses). In 744 of these families, both twins participated (135 MZ male, 242 MZ female, 91 DZ male, 148 DZ female, 128 DZ opposite-sex pairs); the remaining 294 participants whose co-twins had not completed the survey were included in the genetic analyses because they contributed information about variances and bivariate correlations. Participants were compensated $20. All research protocols were reviewed and approved by the University of Colorado's Investigational Review Board.
Materials, Design, and Procedure
Measures
FIRST:
The validated form of the FIRST11 asked how likely (1 = not likely; 2 = somewhat likely; 3 = moderately likely; 4 = very likely) the participant was to have difficulty sleeping under nine situations: before an important meeting the next day, after a stressful experience during the day, after a stressful experience in the evening, after getting bad news during the day, after watching a frightening movie or TV show, after having a bad day at work, after an argument, before having to speak in public, and before going on vacation the next day. Participants were asked to rate the likelihood even if they had not experienced the situation recently. The dependent measure was the sum of the 9 items.
Insomnia questions
Participants indicated how often in the past month (never, sometimes, usually, or always) they experienced 3 sleep problems: difficulty falling asleep, difficulty staying asleep, and having nonrefreshing sleep. Individuals who did not report “never” for all 3 items were asked a series of follow-up questions, including how long they had had the sleep problem(s) (years and months), and to what extent they considered it to interfere with their “daily functioning (e.g., daytime fatigue, ability to function at work/daily chores, concentration, memory, mood, etc),” with 0 = not at all interfering, 1 = a little, 2 = somewhat, 3 = much, and 4 = very much interfering. Participants were judged to meet DSM-IV-TR criteria for insomnia if they had at least one problem “usually” or “always” for at least a month, with at least “somewhat” interference.33
Procedure
Participants were invited to complete an online survey about individual differences in sleep problems that took approximately 30 to 45 minutes. The questionnaire began with the FIRST and insomnia questions, followed by other questions not analyzed for the current study. Skipped questions were re-presented with instructions to answer or indicate explicitly a preference not to answer. In rare cases, computer errors led to missing data (see below).
Statistical Analyses
Genetic models
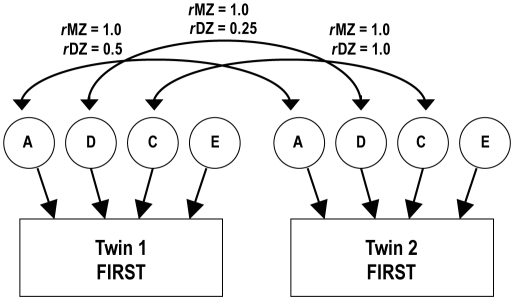
Structural equation models30 provide estimates of the proportions of variance in a measure due to additive genetic (A; heritability), dominant genetic (D), shared family environmental (C), and nonshared environmental (E) influences (Figure 1 illustrates the general form of the ACDE model for a single observed phenotype, measured in MZ and DZ twins). Additive genetic influences for a complex trait are assumed to include the effects of a large number of specific genes that operate together in an additive manner. Dominant genetic variance represents influences that show genetic dominance and can also include epistatic (gene by gene interaction) effects. Shared environmental influences are those that contribute to similarity of twins (e.g., family environment, shared peer groups, mother's nutrition and hormone levels during gestation). In contrast, nonshared environmental influences are those that make twins' behavior/performance uncorrelated (e.g., different experiences, or even the same environment, if the twins respond differently to it for nongenetic reasons; note that, at the level of individual measures, nonshared environment can also include measurement error, because such error will tend to make twins' performances uncorrelated). While it is possible that both C and D influence a phenotype, a limitation of the traditional twin design used in the current study is that C and D cannot both be estimated. Therefore, the pattern of covariances is used to decide whether an ADE model might be more appropriate than an ACE model.
Figure 1.
ACDE twin model. Individual differences in sleep reactivity (depicted with rectangles to denote an observed variable) are modelled as being due to multiple types of influences, represented with circles to denote that they are unobserved: additive genetic (A), dominant genetic (D), shared environmental (C), and nonshared environmental (E). The Twin 1 with Twin 2 A correlation is set to 1.0 for monozygotic (MZ) twins because they share all of their genes, and 0.5 for dizygotic (DZ) twins because they share on average half of their segregating genes by descent. The D correlation is set to 1.0 for MZ twins and 0.25 for DZ twins. The C correlation is set to 1.0 for both types of twins because both are raised together. The E correlations are set to zero because nonshared environment is uncorrelated, by definition. In a traditional twin design, D and C cannot be simultaneously estimated; one is set to zero depending on the pattern of MZ and DZ correlations (C influences tend to make the DZ correlations greater than half the MZ correlations, while D influences tend to make DZ correlations less than half the MZ correlations). Fitting this model to the covariance matrices relating Twin 1's scores to Twin 2's scores in each zygosity group provides estimates of these effects.
Extending these models to include other measures (multivariate models) allows for the examination of genetic and environmental correlations. For example, the extent to which the genetic variance in one measure (i.e., the A component for FIRST) correlates with the genetic variance in another (e.g., the A component for insomnia) represents the genetic correlation between the two constructs. Hence, in this study, we used multivariate genetic modeling to estimate the genetic and environmental contributions to sleep reactivity to stress and insomnia.
Treatment of missing data
FIRST:
Three participants did not have FIRST scores due to computer errors. Eight participants were missing data for one FIRST question (either because they refused to answer it or they skipped it). In these cases, the answer was imputed as the mean of their answers for the remaining 8 items (rounded to the nearest whole number).
Insomnia questions:
Eight individuals were missing an insomnia classification because there was not enough information (frequency, duration, or interference) to determine their statuses. Three individuals were missing answers for the sleep problem frequency questions (2 were missing all 3 questions; one was missing only the falling asleep question).
Model Estimation
We used Mplus 634 for the analyses, including participants with missing data. Genetic model fits including categorical sleep problem data were assessed with WLSMV χ2 (weighted least squares, mean- and variance-adjusted χ2) statistic. Because the χ2 is sensitive to sample size, we also used confirmatory fit index (CFI) > 0.95 and root-mean-square error of approximation (RMSEA) < 0.06 as indicators of good fit.35 Statistical significance of parameters of interest was tested with χ2 difference (χ2diff) tests. To correct for the nonindependence of the twin pairs in the phenotypic analyses, we used Mplus's TYPE = COMPLEX option to obtain a scaled χ2 and standard errors robust to nonindependence, and used scaled χ2diff tests36 for nested model comparisons. Binary or multinomial logistic regressions were used to examine predictors of insomnia or the frequency of sleep problems, respectively. The statistical significance of regression variables was determined with z-values obtained by dividing betas by their standard errors.
RESULTS
Phenotypic Analyses
Descriptive statistics
FIRST scores showed a normal distribution (M = 19.67. SD = 5.43, range = 9 to 36, skewness = 0.25, kurtosis = -–0.45), with good internal reliability (Cronbach α = 0.81). Table 1 presents the frequencies of the 3 nocturnal insomnia symptoms. Approximately 21% met criterion for insomnia. Of these 379 individuals, 242 (64%) experienced difficulty falling asleep (usually or always), 193 (51%) had difficulty staying asleep, and 265 (70%) had nonrefreshing sleep. Ninety-nine (26%) of the insomniacs reported usually or always having all 3 sleep problems; 123 (32%) had 2 of the problems; and 157 (41%) reported only one of the problems. As shown in the table, the prevalence of each insomnia symptom (usually or always) was approximately 20%. The prevalence of having ≥ 1 symptom usually or always was 37.2% (no duration criteria). Once the impairment and duration criteria (having any one of these symptoms for > 1 month with impairment) were applied, 37.2% was reduced to 21%.
Table 1.
Frequencies of sleep problems
| Difficulty | Frequency Rating (%) |
|||
|---|---|---|---|---|
| Never | Sometimes | Usually | Always | |
| Falling Asleep | 324 (18%) | 1069 (60%) | 324 (18%) | 62 (4%) |
| Staying Asleep | 673 (38%) | 776 (44%) | 252 (14%) | 79 (4%) |
| Nonrefreshing Sleep | 396 (22%) | 983 (55%) | 327 (18%) | 74 (4%) |
To examine age and sex differences, we regressed each variable on age and sex simultaneously (there were no significant age by sex interactions). There were significant sex differences in all of the measures. Females had higher FIRST scores (M = 20.90, SD = 5.34) than males (M = 17.86, SD = 5.03), z= 11.31, P < 0.001. Females were also more likely to have insomnia (24.8%) than males (16.4%), odds ratio (OR) = 1.70 (95% CI = 1.32 to 2.19), z = 4.08, P < 0.001. Females also experienced more difficulty with falling asleep, OR = 1.56 (95% CI = 1.27 to 1.90), z = 4.34, P < 0.001; staying asleep, OR = 1.83 (95% CI = 1.52 to 2.21), z = 6.32, P < 0.001; and nonrefreshing sleep, OR = 1.59 (95% CI = 1.30 to 1.94), z = 4.54, P < 0.001.
Within this somewhat restricted age range of the sample, age did not significantly influence FIRST scores, insomnia classification, or difficulty falling asleep. Higher age did relate to more difficulty staying asleep, OR = 1.06 (95% CI = 1.03 to 1.10), z = 3.33, P = 0.001; and nonrefreshing sleep, OR = 1.07 (95% CI = 1.03 to 1.11), z = 3.62, P < 0.001.
Relationship of FIRST Scores to Insomnia and Sleep Problems
Individuals with insomnia had higher FIRST scores (M = 23.02, SD = 5.09) than individuals without insomnia (M = 18.75, SD = 5.15). When insomnia was regressed on FIRST and sex (the FIRST by sex interaction was not significant), one standard deviation increase in FIRST scores was associated with a 2.28 higher odds (95% CI = 1.99 to 2.61) of insomnia, z = 11.90, P < 0.001, controlling for sex. Moreover, controlling for FIRST scores, sex differences in insomnia were no longer significant, OR = 1.09 (95% CI = 0.83 to 1.43), z = 0.60, P = 0.552, suggesting that sex differences in sleep reactivity to stress might explain sex differences in the prevalence of sleep problems.
To examine the relation between FIRST scores and the frequencies of the individual problems, we regressed these frequencies on standardized FIRST scores, sex, and their interaction. FIRST scores controlling for sex and the FIRST by sex interaction significantly predicted difficulty falling asleep, OR (associated with one standard deviation change in FIRST) = 2.81 (95% CI = 2.49 to 3.18), z = 16.78, P < 0.001, while sex no longer predicted difficulty falling asleep once FIRST scores were controlled, OR = 0.89 (95% CI = 0.72 to 1.10), z = –1.11, P = 0.267. However, there was a significant FIRST by sex interaction, z = –2.26, P = 0.024: Simple regressions indicated that FIRST scores were related to difficulty falling asleep in females, OR = 2.48 (95% CI = 2.15 to 2.86), z = 12.43, P < 0.001, and in males, OR = 2.87 (95% CI = 2.40 to 3.43), z = 11.54, P < 0.001. A regression predicting the frequency of nonrefreshing sleep showed a similar pattern: FIRST scores controlling for sex and the FIRST by sex interaction significantly predicted the frequency of nonrefreshing sleep, OR = 2.20 (95% CI = 1.96 to 2.47), z = 13.21, P < 0.001, while sex no longer predicted difficulty with nonrefreshing sleep once FIRST scores were controlled, OR = 1.00 (95% CI = 0.81 to 1.23), z = –0.02, P = 0.982. However, there was a significant FIRST by sex interaction, z = –2.20, P = 0.028: Simple regressions showed that, as with difficulty falling asleep, FIRST scores were related to nonrefreshing sleep in females, OR = 1.96 (95% CI = 1.71 to 2.24), z = 9.91, P < 0.001, and in males, OR = 2.26 (95% CI = 1.89 to 2.69), z = 8.94, P < 0.001. Finally, the regression of the frequency of difficulty staying asleep revealed that FIRST scores controlling for sex and the FIRST by sex interaction significantly predicted difficulty staying asleep, OR = 1.91 (95% CI = 1.71 to 2.13), z = 11.32, P < 0.001, and females were still significantly more likely to have difficulty staying asleep controlling for FIRST scores, OR = 1.27 (95% CI = 1.04 to 1.54), z = 2.31, P = 0.021, but the FIRST by sex interaction was not significant, z = –0.94, P = 0.350.
Genetic Analyses
Univariate models
Table 2 shows the MZ and DZ twin correlations, split by sex, for the FIRST, insomnia classification, and sleep problem frequencies. For all variables except the frequency of difficulty falling asleep, the MZ correlations were higher than the DZ correlations, suggesting genetic influences. Moreover, for the FIRST scores and insomnia classification, most of the MZ correlations were greater than twice the DZ correlations, suggesting no shared environmental influences, but possible dominant or epistatic genetic influences. Therefore, we tested ADE models for these 2 phenotypes, and ACE models for the problem frequencies. We also allowed the male and female estimates to differ. Sex differences were examined at the level of unstandardized estimates.
Table 2.
Twin correlations
| Measure | Twin Correlations |
||||
|---|---|---|---|---|---|
| MZ male | DZ male | MZ female | DZ female | DZ OS | |
| FIRST | 0.46* | 0.12 | 0.30* | 0.06 | 0.19* |
| Insomniaa | 0.48* | 0.03 | 0.55* | 0.30* | 0.21 |
| Symptom Frequencyb | |||||
| Falling asleep | 0.19* | 0.36* | 0.19* | –0.03 | 0.28* |
| Staying asleep | 0.35* | 0.11 | 0.24* | 0.09 | 0.27* |
| Nonrefreshing sleep | 0.35* | 0.17 | 0.33* | 0.22* | 0.16 |
MZ, monozygotic; DZ, dizygotic; OS, opposite-sex.
P < 0.05.
Tetrachoric correlations;
polychoric correlations estimated with Mplus.
FIRST:
As shown in Table 3, the ADE model of the FIRST scores, χ2(17) = 16.53, P = 0.487; CFI = 1.00; RMSEA = 0.000, was not significantly better than the more parsimonious AE model, χ2(19) = 18.02, P = 0.521; CFI = 1.00; RMSEA = 0.000; χ2diff(2) = 1.49, P = 0.476. The AE model indicated that for males, sleep reactivity to stress was 43% heritable, with the remaining 57% due to nonshared environmental influences; for females the heritability was 29% with the remaining 71% due to nonshared environmental influences. Model comparisons indicated a significant sex difference in the AE estimates, χ2diff(2) = 7.89, P = 0.019. Specifically, in the unstandardized models, females showed significantly more nonshared environmental variance, χ2diff(1) = 6.59, P = 0.010, but their genetic variance could be equated to that for males, χ2diff(1) = 1.08, P = 0.299. When standardized, this difference translated into a lower heritability for females. The larger nonshared environmental variance for females is not likely due to differential reliability; internal reliability (Cronbach α) was 0.79 for females and 0.80 for males.
Table 3.
Genetic models for FIRST and insomnia
| Measure | Males |
Females |
||||
|---|---|---|---|---|---|---|
| A | D | E | A | D | E | |
| FIRSTa | ||||||
| ADE | 14 | 32 | 55* | 8 | 23 | 69* |
| AE | 43* | — | 57* | 29* | — | 71* |
| Insomniab | ||||||
| ADE | 16 | 30 | 54* | 52 | 3 | 45* |
| AE | 43* | — | 58* | 55* | — | 45* |
ADE models suggested by correlations. Boldface type indicates preferred model. Variance components may not sum to 100% due to rounding error.
P < 0.05, calculated with χ2 difference tests.
Analyzed as continuous variables with maximum likelihood (ML);
Analyzed as binary variables with weighted least squares, means- and variances-adjusted (WLSMV).
Insomnia:
For insomnia classification, the ADE model, χ2(9) = 7.47, P = 0.589; CFI = 1.00; RMSEA = 0.000, did not fit significantly better than the AE model, χ2(11) = 7.82, P = 0.729; CFI = 1.00; RMSEA = 0.000; χ2diff(2) = 0.96, P = 0.620. As shown in Table 3, insomnia was 43% heritable for males and 55% heritable for females. These sex differences were not significant, χ2diff(1) = 0.53, P = 0.467; however, we retained the separate estimates because sex differences in the FIRST variable would require estimating sex differences in the multivariate models with FIRST.
Sleep problem frequencies:
Table 4 presents the ACE models of the frequencies of three sleep problems, analyzed as ordinal variables. As shown in Table 2, the MZ correlations for difficulty falling asleep were actually lower than the DZ correlations, resulting in near-zero estimates of A in the ACE model. Hence, the ACE model fit, χ2(25) = 20.34, P = 0.729; CFI = 1.00; RMSEA = 0.000, was not significantly better than the CE model fit, χ2(27) = 20.47, P = 0.810; CFI = 1.00; RMSEA = 0.000; χ2diff(2) = 0.05, P = 0.978. In this final model then, for males 27% of the variance in difficulty falling asleep was due to shared environmental influences, with the remaining 73% due to nonshared environmental influences; for females, 14% of the variance was due to shared environmental influences and 86% due to nonshared environmental influences. Sex differences in these estimates did not reach statistical significance, χ2diff(1) = 2.38, P = 0.123.
Table 4.
Genetic models for sleep problem frequencies
| Measure | Males |
Females |
||||
|---|---|---|---|---|---|---|
| A | C | E | A | C | E | |
| Falling Asleep | ||||||
| ACE | 1 | 27* | 73* | 3 | 12* | 85* |
| CE | — | 27* | 73* | — | 14* | 86* |
| Staying Asleep | ||||||
| ACE | 31 | 3 | 66* | 21 | 3 | 76* |
| AE | 35* | — | 66* | 25* | — | 76* |
| Nonrefreshing sleep | ||||||
| ACE | 34 | 1 | 65* | 23 | 10 | 67* |
| AE | 35* | — | 65* | 34* | — | 66* |
ACE suggested by correlations. Analyzed as ordinal variables with weighted least squares, means- and variances-adjusted (WLSMV). Boldface type indicates preferred model. Variance components may not sum to 100% due to rounding error.
P < 0.05, calculated with χ2 difference tests.
In contrast to the CE pattern for difficulty falling asleep, difficulty staying asleep and nonrefreshing sleep showed relatively more genetic influences and fewer shared environmental influences. Specifically, for difficulty staying asleep, the ACE model fit, χ2(25) = 27.13, P = 0.349; CFI = 0.924; RMSEA = 0.020, was not significantly better than the AE model fit, χ2(27) = 27.35, P = 0.445; CFI = 0.988; RMSEA = 0.008; χ2diff(2) = 0.04, P = 0.978. Difficulty staying asleep was 35% heritable for males and 25% heritable for females, with the remaining variance attributable to nonshared environmental influences. Sex differences were not significant, χ2diff(1) = 0.91, P = 0.341.
Estimates were similar for nonrefreshing sleep. The ACE model fit, χ2(25) = 28.73, P = 0.276; CFI = 0.909; RMSEA = 0.027, was not significantly better than the AE model fit, χ2(27) = 29.03, P = 0.360; CFI = 0.951; RMSEA = 0.019; χ2diff(2) = 0.19, P = 0.909. The frequency of nonrefreshing sleep was 35% heritable for males and 34% heritable for females, with the remaining variance attributable to nonshared environmental influences. Sex differences were not significant, χ2diff(1) = 0.01, P = 0.941.
Multivariate Model of FIRST and Insomnia
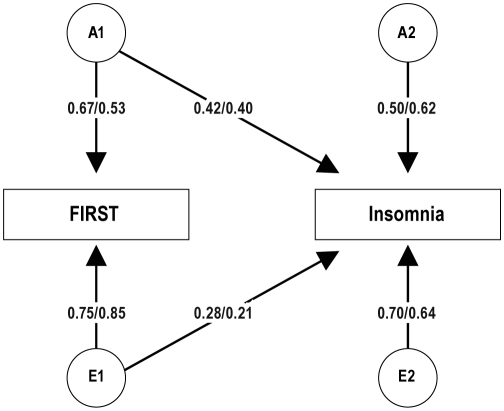
The results presented in the previous section establish that both sleep reactivity to stress, as measured with the FIRST, and insomnia have significant genetic and nonshared environmental influences. In this section we examine to what extent these influences overlap with a bivariate genetic Cholesky decomposition model30: In this model, the A and E estimates for the FIRST (i.e., A1 and E1) also predict insomnia, and insomnia also has its own A and E influences (i.e., A2 and E2). This model, which fits the data well, χ2(46) = 41.65, P = 0.655; CFI = 1.00; RMSEA = 0.000, is shown in Figure 2 (with only one twin depicted). As shown by the cross path estimated for A1 to Insomnia, the genetic influences on FIRST also significantly influenced insomnia, and to a similar extent in males and females (0.42 vs. 0.40, respectively). As indicated by the path from A2 to insomnia, insomnia also had genetic influences that were independent of FIRST, though these influences were only marginally significant for males, χ2diff(1) = 3.05, P = 0.081. The path from E1 to insomnia indicates that the environmental influences on FIRST scores also influenced insomnia, to a higher extent in males than females (0.28 vs. 0.21, respectively). The path from E2 to insomnia indicates that insomnia had nonshared environmental influences that were independent of those for FIRST.
Figure 2.
Bivariate AE Cholesky model of FIRST and insomnia with separate estimates for males and females (depicted as male/female). The model shows standardized path coefficients that can be squared to obtain variance components. The A and E variables for the first phenotype (i.e., A1 and E1) are allowed to predict both FIRST and insomnia (the latter is depicted in the cross path). Insomnia is also allowed to have its own A and E influences (i.e., A2 and E2) that are independent of FIRST scores. All parameters were significant at P < 0.05, except for the path from A2 to insomnia in males, which was marginally significant (P = 0.081). From this model, one can compute the genetic and environmental correlations between the 2 phenotypes, depicted in Figure 3.
Though sex differences were only significant for FIRST scores, we maintained a sex differences model for all the parameters. However, the only parameters that actually showed significant sex differences were E1 to FIRST, χ2diff(1) = 9.51, P = 0.002, and E1 to insomnia, χ2diff(1) = 4.28, P = 0.039. That is, females had more environmental influences than males on FIRST, but these influences overlapped less with insomnia in females than males.
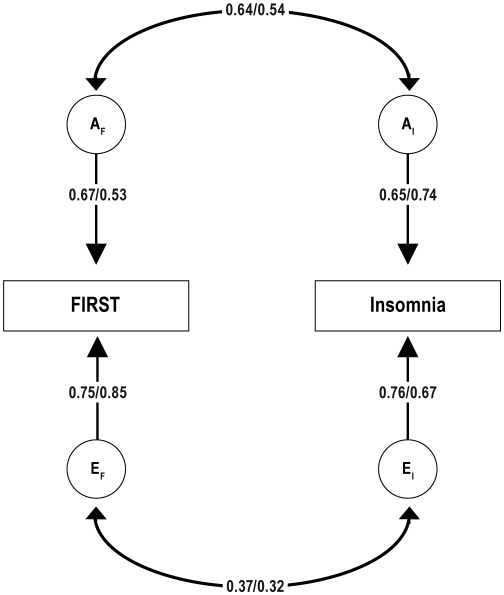
Another way of representing these results is with the genetic and environmental correlational model presented in Figure 3, which is statistically identical to the Cholesky model presented in Figure 2. As shown in this model, the genetic correlation between FIRST and insomnia (represented on the curved double-headed arrows between AF and AI) was 0.64 in males and 0.54 in females, and the environmental correlation (represented on the curved double-headed arrows between EF and EI) was 0.37 in males and 0.32 in females. Using covariance algebra on the parameter estimates in this model, one can compute the phenotypic correlations predicted by the model37 and calculate the percentages of those phenotypic correlations that are due to overlapping genetic and nonshared environmental influences. For males, the predicted phenotypic correlation (0.49) was 56% due to genes and 46% due to nonshared environment. For females, the predicted phenotypic correlation (0.39) was due 53% to genes and 47% to nonshared environment.
Figure 3.
Bivariate AE correlational model of FIRST and insomnia with separate estimates for males and females (depicted as male/female). The model shows standardized path coefficients that can be squared to obtain variance components. This model is derived from the Cholesky model depicted in Figure 2 and is statistically identical. In this model, the AF and EF variables for FIRST are correlated with the AI and EI variables for insomnia. All parameters were statistically significant (P < 0.05).
Heritability of FIRST in Non-Insomniacs
In the previous bivariate models, the genetic and environmental influences on FIRST scores were shown to overlap with those for insomnia. Moreover, the genetic correlation was only moderate (0.64 in males and 0.54 in females), suggesting that the genetic influences on the FIRST were not identical to those for insomnia. If sleep reactivity to stress were indeed a predisposing genetic factor for insomnia, one would expect some overlap, as we found. However, we may also expect that FIRST scores should be heritable even in the absence of insomnia. Hence, we modeled the heritability of the FIRST excluding anyone who met criteria for insomnia. As with the full sample, the ADE model, χ2(17) = 16.30, P = 0.503; CFI = 1.00; RMSEA = 0.000, was not significantly better than the more parsimonious AE model, χ2(19) = 16.37, P = 0.633; CFI = 1.00; RMSEA = 0.000; χ2diff(2) = 0.07, P = 0.966. The AE model indicated that the FIRST was 39% heritable in non-insomniac males and 26% heritable in non-insomniac females, both χ2diff(1) > 14.18, P < 0.001. The sex difference remained significant, χ2diff(2) = 9.62, P = 0.008. Hence, even when individuals who met criteria for insomnia were excluded from the analysis, the FIRST showed a similar pattern of genetic and environmental influences.
Analyses Controlling for Depression/Anxiety
Because insomnia can be comorbid with anxiety and/or depression, we conducted secondary analyses to ascertain the extent to which our results are unique to sleep reactivity to stress vs. depression/anxiety. Participants answered 2 questions about whether they had ever been diagnosed with depression or an anxiety disorder, respectively (14 participants declined to answer one or both of the questions). Approximately 14% and 8% of the participants reported a history of depression and anxiety, respectively. Participants also completed the Center for Epidemiological Studies Depression Scale (CES-D),38 a 20-item self-report questionnaire that asks about the frequency of depressive symptoms within the past week. We used log-transformed summed scores excluding one item, “restless sleep,” to avoid overlap with the sleep questions. The internal reliability estimate (Cronbach α) for the remaining 19 items was 0.90.
Phenotypic analyses:
In a multiple regression predicting FIRST scores with sex, depression history, anxiety history, and CES-D scores (there were no significant interactions with sex), CES-D significantly predicted FIRST scores (standardized β = 0.32, z = 3.38, P < 0.001), as did anxiety history (anxiety diagnosis associated with 0.26 SD increase in FIRST scores; z = 2.54, P = 0.011). Depression diagnosis did not significantly predict FIRST scores controlling for anxiety diagnosis and CES-D (diagnosis associated with 0.10 SD increase in FIRST scores;z = 1.35, P = 0.178), nor did sex. In total, the measures explained 19.6% of the FIRST variance.
In a multiple logistic regression predicting Insomnia with FIRST, sex, and the depression/anxiety measures (again, there were no significant interactions with sex), FIRST scores predicted insomnia over and above the other measures (one SD increase in FIRST associated with 1.80 higher odds of insomnia; z = 7.85, P < 0.001). Both depression diagnosis and CES-D also predicted insomnia controlling for FIRST scores (one SD increase in CES-D associated with 2.40 higher odds of insomnia; z = 11.69, P < 0.001; and depression diagnosis associated with 2.59 higher odds of insomnia; z = 5.04, P < 0.001). Neither anxiety nor sex significantly predicted insomnia when controlling for the other variables. In total, the measures explained 35.2% of the insomnia variance.
Genetic analyses:
To examine the influence of depression and anxiety variation on the genetic results, we regressed FIRST scores on CES-D and depression and anxiety diagnosis history, then ran the genetic models presented earlier on the unstandardized residuals. The resulting models reflect the heritability of FIRST and its correlation with insomnia with the FIRST's variance due to depression and anxiety removed (because insomnia is a dichotomous variable, it was not possible to obtain similar residuals).
The univariate AE model (compare to Table 3) of residualized FIRST scores indicated that they were 39% heritable in males and 25% heritable in females. As with the non-residualized scores, the sex difference came from a significantly larger nonshared environmental variance for females.
In the bivariate model (compare to Figure 3), the genetic correlations between residualized FIRST scores and insomnia were 0.54 for males, χ2diff(1) = 5.58; P = 0.018; and 0.26 for females; χ2diff(1) = 2.44; P = 0.118. The nonshared environmental correlations were 0.13 for males, χ2diff(1) = 1.73; P = 0.188; and 0.19 for females; χ2diff(1) = 4.52; P = 0.034. Hence, for both sexes, the genetic and environmental correlations between FIRST and insomnia were reduced when current and prior depression and anxiety variance was removed from FIRST scores.
DISCUSSION
The goals of this study were to determine the genetic and environmental etiologies of (1) sleep reactivity to stress, (2) insomnia, and (3) their interrelation. Findings using a twin methodology clearly indicated that sleep reactivity as assessed by the FIRST has a substantial heritable component (29% to 43%) demonstrating that genetic factors have a significant influence on this trait consistent with a previous family study of sleep reactivity.26 Insomnia was also found to have significant genetic influences, similar to that found in previous twin studies.20–22,27,28 Environmental influences unique to individuals also appear to contribute to sleep reactivity to stress and insomnia, while shared environmental effects were negligible. The new finding that sleep reactivity and insomnia share genetic influences is consistent with the hypothesis that this trait may represent a genetic vulnerability for developing insomnia.
Although we found that females exhibited more sleep problems and higher sleep reactivity to stress than males, we found few sex differences in the underlying genetic structures of these phenotypes and their interrelations. The primary difference was that females showed more nonshared environmental variance in FIRST scores than males (though similar genetic variance), resulting in lower overall heritability (i.e., the proportion of genetic influences out of the total variance) of this construct in females. The only other sex difference we found was that the higher environmental variance in females' FIRST scores overlapped less with insomnia, though this effect was relatively small (environmental influences on FIRST scores predicted 8% of the variance in insomnia in males but 4% in females). One interpretation of these results is that women may have more environmental influences on sleep reactivity to stress, but these additional influences are unlikely to also lead to insomnia.
Interestingly, in the phenotypic analyses, we found that when both FIRST scores and sex were included as predictors of insomnia, the sex difference in insomnia was no longer significant. This result suggests that the sex differences in sleep reactivity may account for the sex differences in insomnia. Hence, sleep reactivity to stress may be an important component of future research on sex differences in insomnia.
The present findings emphasize the importance of assessing environmental exposures as well as underlying diathesis in future studies aimed at determining the factors contributing to insomnia. Clearly, there are environmental triggers of transient sleep disturbance. The present results suggests that environmental triggers (e.g., stress) also have an impact on sleep reactivity, and more so for females. The present results suggest that some of these environmental effects may influence the vulnerability to developing insomnia. These data emphasize the need for longitudinal studies that investigate how environmental events operate in the early and even prodromal stages of the evolution to an insomnia disorder in dynamic interaction with patients' environmental milieu (i.e., frequency, magnitude, and resulting sleep patterns).
The moderate heritability estimates of individual symptoms of insomnia related sleep disturbance found in the present study have several implications. Over the last several decades, insomnia has increasingly become recognized as a disorder with both characteristic diurnal and nocturnal symptoms. The moderate heritability of these symptoms when assessed separately, but stronger heritability when looked at as a constellation of symptoms and impairment (i.e., insomnia disorder), is consistent with this view. Lower heritability estimates of specific insomnia symptoms (e.g., sleep latency) relative to global measures of sleep quality have also been found in a recent twin study20 and is consistent with findings demonstrating that nocturnal insomnia symptoms frequently change over time in a given patient,39,40 while the disorder itself is a chronic condition.41 The genetic overlap of multiple symptoms of insomnia found in previous studies also suggests that they may be related to a common underlying trait.20
The significant genetic correlations between FIRST scores and insomnia demonstrate that insomnia shares both genetic and environmental influences with sleep reactivity as reflected in the FIRST. However, each measure also had significant unique genetic and environmental influences, indicating that they are not identical constructs. Indeed, high sleep reactivity is present in many individuals who do not meet criteria for insomnia and only a small portion of insomniacs do not report high sleep reactivity to stress.42 One possibility is that sleep reactivity is, at least in part, a genetic predisposing factor for insomnia.2 That is, sleep reactivity may be a necessary but nonsufficient trait that conveys a vulnerability to insomnia that is manifested following exposure to stressful triggers over time or other precipitating events; however, even individuals with high sleep reactivity may require environmental triggers for the disorder to manifest. Consistent with this conceptualization, although measures of arousability are similar between insomniacs and those with high sleep reactivity (i.e., high FIRST scores) non-insomniacs have lower levels of stress exposure.19 Findings from a recent prospective study indicate that a broad measure of arousability is predictive of incident insomnia.5 Thus, it is possible that physiological or cognitive arousability plays a moderating role in the relationship between sleep reactivity to stress and the development of chronic insomnia, as previous investigators have proposed.19 However, prospective studies that assess sleep reactivity in non-insomniacs along with general arousability and stress exposures over time as the disorder of insomnia evolves are needed to address this question.
The present study used the Ford Insomnia Response to Stress Test (FIRST) to assess sleep reactivity.11 Other measures have been developed to assess similar constructs, such as “arousability” which has been related to insomnia,5,43 but the FIRST is the only measure validated using objective sleep assessment.11,12 The findings of the present study add to its validity by demonstrating that this measure has a significant genetic component. Thus, FIRST scores provide valuable information including a generalized estimate related to sleep responses to multiple sleep disruptive stimuli, a readily assessed measure amenable to large-scale epidemiological studies, and a cost effective and practical means for assessing this trait in research and clinical settings.
In terms of the relationship of FIRST scores to insomnia independent of depression and anxiety, several conclusions can be drawn. The phenotypic and genetic results indicate that depression and/or anxiety had significant relations to FIRST scores, a finding that is not surprising given their common relations to stress. However, even with all three covariates in the model, the relation between FIRST and insomnia remained significant, suggesting that sleep reactivity to stress may tap a dimension independent of these mood disorders. The univariate genetic model of residualized FIRST scores indicates that this dimension is significantly heritable in both sexes. Finally, the bivariate genetic model suggested that eliminating variance due to depression and anxiety reduced both the genetic and environmental correlations between FIRST and insomnia; however, significant genetic (male) and environmental (female) correlations remained. Taken together, these results suggest that sleep reactivity to stress constitutes a correlate of insomnia that is related, but not identical, to depression and anxiety.
Despite these findings demonstrating heritability of the FIRST, there are limitations to this approach. For example, the accuracy of self-report of sleep reactivity may be influenced by memory of sleep responses to previous events, perceptual differences between subjects, as well as the habitual sleep pattern of the individual. Other variables such as the type, magnitude, and frequency of sleep disrupting stimuli likely add to the variability of self-report measures of sleep reactivity. However, the FIRST was significantly heritable, despite these sources of increased variability, a finding consistent with the stability and predictive value of the FIRST measure and its underlying construct, sleep reactivity. Future studies may identify useful objective methods for assessing sleep reactivity, particularly given the increased accuracy, availability, and cost effectiveness of objective ambulatory sleep recording techniques.
Future studies should also determine whether objective measures of sleep reactivity will be differentially predictive of insomnia incidence relative to self-report measure of sleep reactivity. A limitation of our study is that we relied on patient reports of insomnia symptoms rather than a clinical interview which may have attenuated the results. In addition, twin studies have traditionally had higher response rates for women,44 which may have contributed to a somewhat higher prevalence for insomnia in our sample compared with some previous studies.45 However, our prevalence estimates for insomnia are consistent with estimates from recent epidemiological studies using similar criteria for insomnia disorder.46 Furthermore, as our heritability estimates for insomnia (∼40%) are similar to those of previous studies,20–22,27,28 there is little reason to believe that a slight selection for sleep problems has biased our results in terms of heritability. Our restricted age range and lack of a population-based sample is also a limitation in terms of generalizability. Nonetheless, given previous studies that show a family history of insomnia is more closely associated with cases of early onset of the disorder24,47 one would not expect greater heritability estimates in an older sample. Similarly, one might speculate that non-shared environmental effects would be larger in an older sample where environmental triggers may be more prominent due to increased exposure to potential triggers of insomnia.
Summary
Our results provide strong evidence for the heritability of sleep reactivity to stress in both insomniacs and non-insomniacs and add to the growing literature suggesting that insomnia has significant genetic determinants. The overlap between genetic and environmental influences on sleep reactivity and insomnia supports the hypothesis that sleep reactivity to stress may be a significant risk factor for the development of the disorder of insomnia. At the same time, the partial independence of this reactivity trait from that of insomnia and its existence in those without the disorder supports the conclusion that sleep reactivity is not the same construct as insomnia and may not be sufficient for developing the disorder. The phenotype of individuals with high sleep reactivity should be studied further in terms of its predictive value for incident insomnia, relationship to objective PSG measures of reactivity, and long-term stability. Additional studies are also needed to determine the heritability of polysomnographically measured sleep responses to stress and their relation to the present findings as well as the future development of insomnia. Our finding that that there are common genetic influences for sleep reactivity and insomnia suggests that uncovering specific genes that influence this trait may be a productive area for future insomnia research.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Drake has received grant support from Merck Inc., Cephalon, Zeo Inc., and Takeda Inc. unrelated to this study and participated in speaking engagements for Cephalon and Asante Communications. Dr. Roth has received grants from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and Xenoport. He has served as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis, AVER, BMS, BTG, Cephalon, Cypress, Dove, élan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Hypnion, Impax, Intec, Intra-Ceullular, Jazz, Johnson and Johnson, King, Lundbeck McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Proctor and Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport. Additionally, Dr. Roth has served as a speaker for Cephalon, Sanofi, and Takeda. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank Sally Ann Rhea for project management and Andy Gross for questionnaire implementation. This study was supported by Henry Ford Hospital and NIH grant MH063207 (NPF).
REFERENCES
- 1.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541–53. [PubMed] [Google Scholar]
- 2.Drake CL, Roth T. Predisposition in the evolution of insomnia: evidence, potential mechanisms, and future directions. In: Roth T, Lee-Chiong T, editors. Sleep Med Clin. 2006. pp. 333–49. [Google Scholar]
- 3.Gregory AM, Caspi A, Moffitt TE, Poulton R. Family conflict in childhood: a predictor of later insomnia. Sleep. 2006;29:1063–7. doi: 10.1093/sleep/29.8.1063. [DOI] [PubMed] [Google Scholar]
- 4.Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K, Soldatos CR. Onset of insomnia: role of life-stress events. Psychosom Med. 1981;43:439–51. doi: 10.1097/00006842-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 5.LeBlanc M, Merette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32:1027–37. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall M, Buysse DJ, Nofzinger EA, et al. Financial strain is a significant correlate of sleep continuity disturbances in late-life. Biol Psychol. 2008;77:217–22. doi: 10.1016/j.biopsycho.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mezick EJ, Matthews KA, Hall M, et al. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34:1346–54. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 9.Stepanski E, Glinn M, Zorick F, Roehrs T, Roth T. Heart rate changes in chronic insomnia. Stress Med. 1994;10:261–6. [Google Scholar]
- 10.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26:1029–36. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 11.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27:285–91. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 12.Drake CL, Jefferson C, Roehrs T, Roth T. Stress-related sleep disturbance and polysomnographic response to caffeine. Sleep Med. 2006;7:567–72. doi: 10.1016/j.sleep.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–5. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Vgontzas AN, Tsigos C, Bixler EO, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW, Gay TJ, Masterton JP, Bruce DW. Relationship between sleep habits, adrenocortical activity and personality. Psychosom Med. 1971;33:499–508. doi: 10.1097/00006842-197111000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Mendoza J, Vela-Bueno A, Vgontzas AN, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 72:397–403. doi: 10.1097/PSY.0b013e3181d75319. [DOI] [PubMed] [Google Scholar]
- 20.Barclay NL, Eley TC, Buysse DJ, Rijsdijk FV, Gregory AM. Genetic and environmental influences on different components of the Pittsburgh Sleep Quality Index and their overlap. Sleep. 2010;33:659–68. doi: 10.1093/sleep/33.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heath AC, Kendler KS, Eaves LJ, Martin NG. Evidence for genetic influences on sleep disturbance and sleep pattern in twins. Sleep. 1990;13:318–35. doi: 10.1093/sleep/13.4.318. [DOI] [PubMed] [Google Scholar]
- 22.Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29:645–9. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 23.Bastien CH, Morin CM. Familial incidence of insomnia. J Sleep Res. 2000;9:49–54. doi: 10.1046/j.1365-2869.2000.00182.x. [DOI] [PubMed] [Google Scholar]
- 24.Beaulieu-Bonneau S, LeBlanc M, Merette C, Dauvilliers Y, Morin CM. Family history of insomnia in a population-based sample. Sleep. 2007;30:1739–45. doi: 10.1093/sleep/30.12.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dauvilliers Y, Morin C, Cervena K, et al. Family studies in insomnia. J Psychosom Res. 2005;58:271–8. doi: 10.1016/j.jpsychores.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Drake CL, Scofield H, Roth T. Vulnerability to insomnia: the role of familial aggregation. Sleep Med. 2008;9:297–302. doi: 10.1016/j.sleep.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarren M, Goldberg J, Ramakrishnan V, Fabsitz R. Insomnia in Vietnam era veteran twins: influence of genes and combat experience. Sleep. 1994;17:456–61. doi: 10.1093/sleep/17.5.456. [DOI] [PubMed] [Google Scholar]
- 28.Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6:179–85. doi: 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]
- 29.Deuschle M, Schredl M, Schilling C, et al. Association between a serotonin transporter length polymorphism and primary insomnia. Sleep. 33:343–7. doi: 10.1093/sleep/33.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neale MC, Cardon LR. Dordrecht; Boston: Kluwer Academic Publishers; 1992. Methodology for genetic studies of twins and families. [Google Scholar]
- 31.Watson NF, Kapur V, Arguelles LM, et al. Comparison of subjective and objective measures of insomnia in monozygotic twins discordant for chronic fatigue syndrome. Sleep. 2003;26:324–8. doi: 10.1093/sleep/26.3.324. [DOI] [PubMed] [Google Scholar]
- 32.Rhea SA, Gross AA, Haberstick BC, Corley RP. Colorado Twin Registry. Twin Res Hum Genet. 2006;9:941–9. doi: 10.1375/183242706779462895. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. 4th ed. Washington, DC: American Psychiatric Association; 2000. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. [Google Scholar]
- 34.Muthén LK, Muthén BO. sixth ed. Los Angles, CA: 2010. Mplus user's guide. [Google Scholar]
- 35.Hu L, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3:424–53. [Google Scholar]
- 36.Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66:507–14. doi: 10.1007/s11336-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bollen KA. New York, NY: John Wiley and Sons; 1989. Structural equations with latent variables. [Google Scholar]
- 38.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 39.Vallieres A, Ivers H, Bastien CH, Beaulieu-Bonneau S, Morin CM. Variability and predictability in sleep patterns of chronic insomniacs. J Sleep Res. 2005;14:447–53. doi: 10.1111/j.1365-2869.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 40.Hohagen F, Rink K, Kappler C, et al. Prevalence and treatment of insomnia in general practice. A longitudinal study. Eur Arch Psychiatry Clin Neurosci. 1993;242:329–36. doi: 10.1007/BF02190245. [DOI] [PubMed] [Google Scholar]
- 41.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men-a 10-year prospective population based study. Sleep. 2001;24:425–30. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 42.Jefferson C, Roth T, Roehrs T, Drake CL. Sleep reactivity to stress in insomniacs. In: Penzel T, Fietze I, Chokroverty S, editors. Proceedings of the World Association of Sleep Medicine; 2005; Berlin, Germany: Medimond; 2005. pp. 79–82. [Google Scholar]
- 43.Coren S. Prediction of insomnia from arousability predisposition scores: scale development and cross-validation. Behav Res Ther. 1988;26:415–20. doi: 10.1016/0005-7967(88)90076-9. [DOI] [PubMed] [Google Scholar]
- 44.Lykken DT, McGue M, Tellegen A. Recruitment bias in twin research: the rule of two-thirds reconsidered. Behav Genet. 1987;17:343–62. doi: 10.1007/BF01068136. [DOI] [PubMed] [Google Scholar]
- 45.Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res. 1997;31:333–46. doi: 10.1016/s0022-3956(97)00002-2. [DOI] [PubMed] [Google Scholar]
- 46.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR, ICD-10, and RDC/ICSD-2 criteria: Results from the America Insomnia Survey (AIS) Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 47.Hauri P, Olmstead E. Childhood-onset insomnia. Sleep. 1980;3:59–65. doi: 10.1093/sleep/3.1.59. [DOI] [PubMed] [Google Scholar]