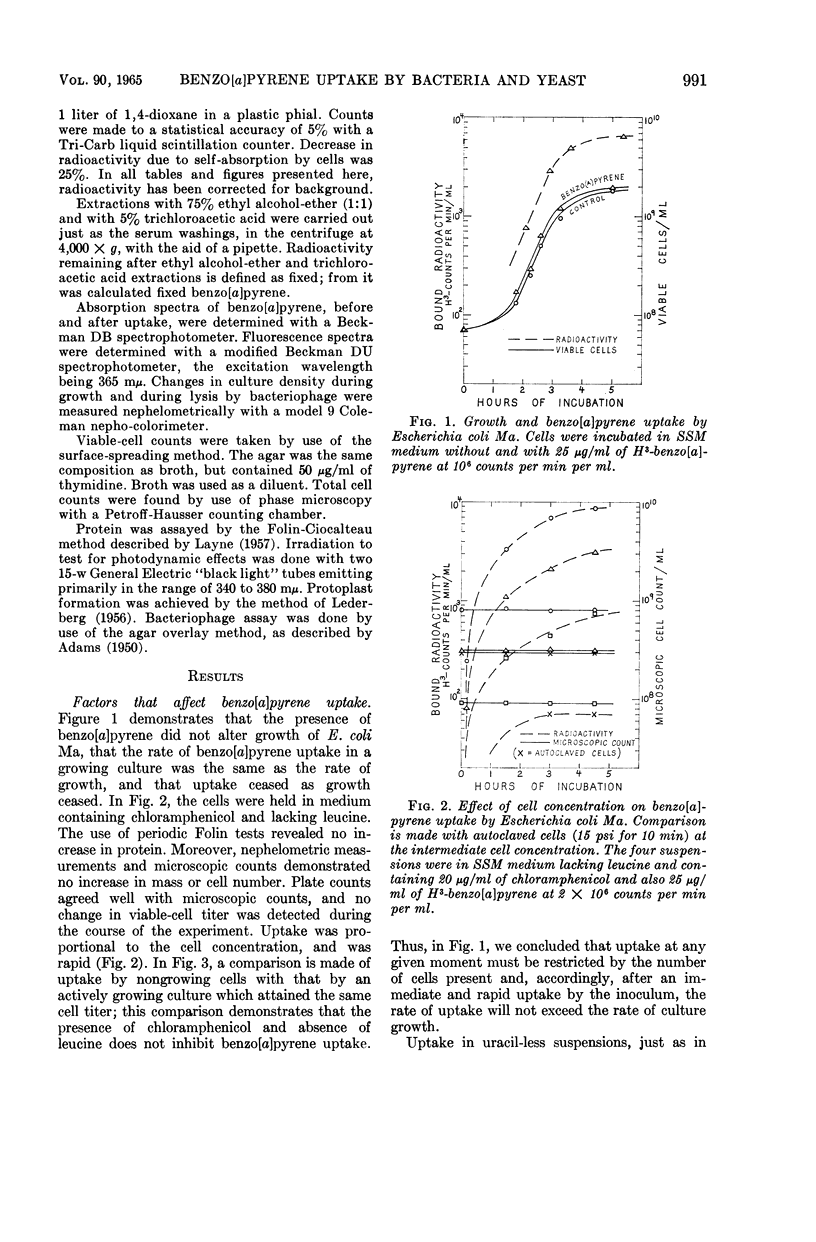
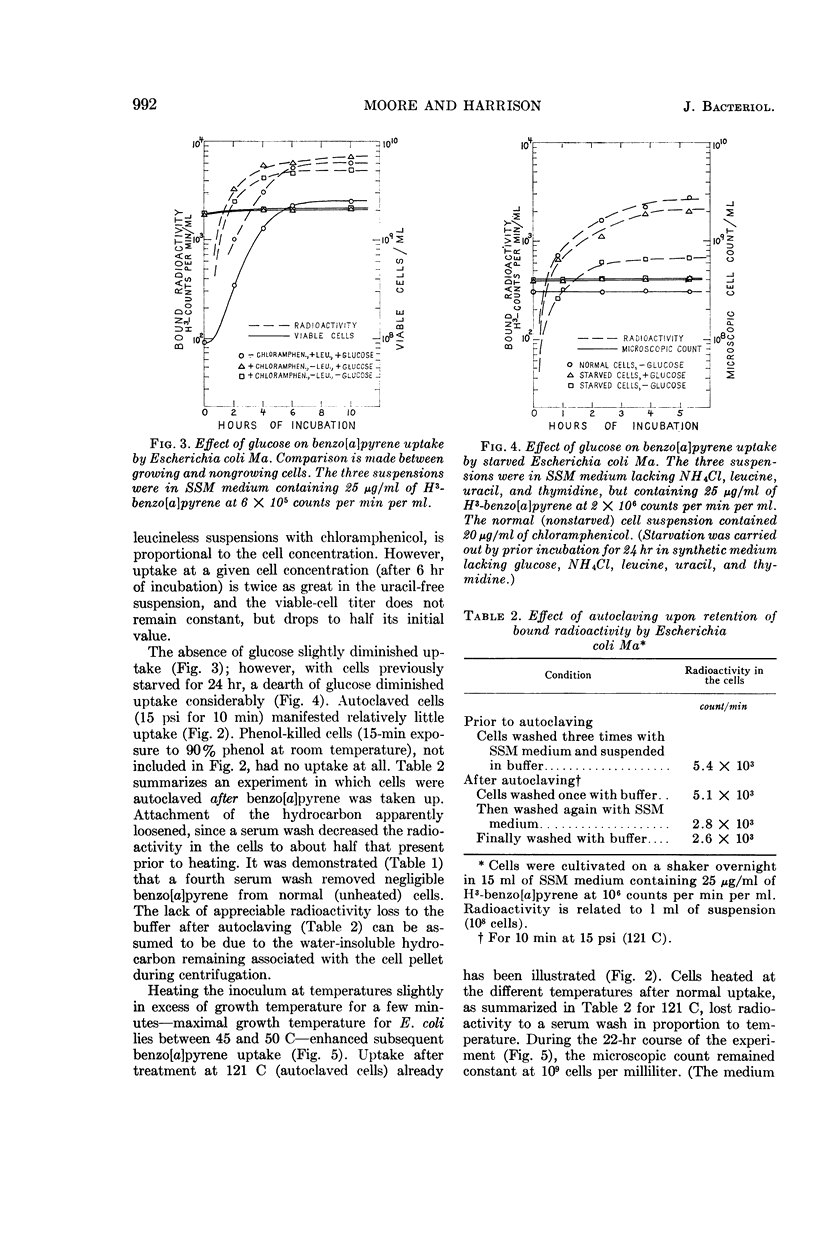
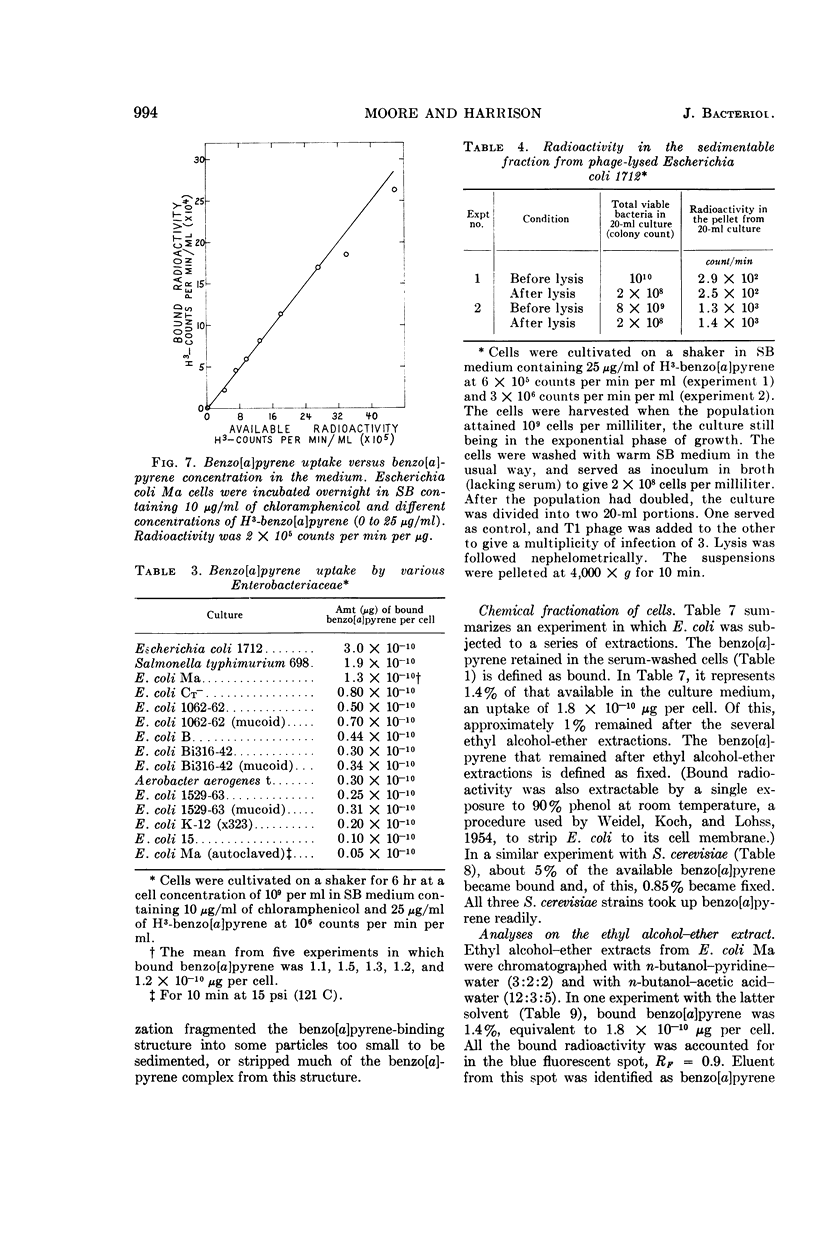
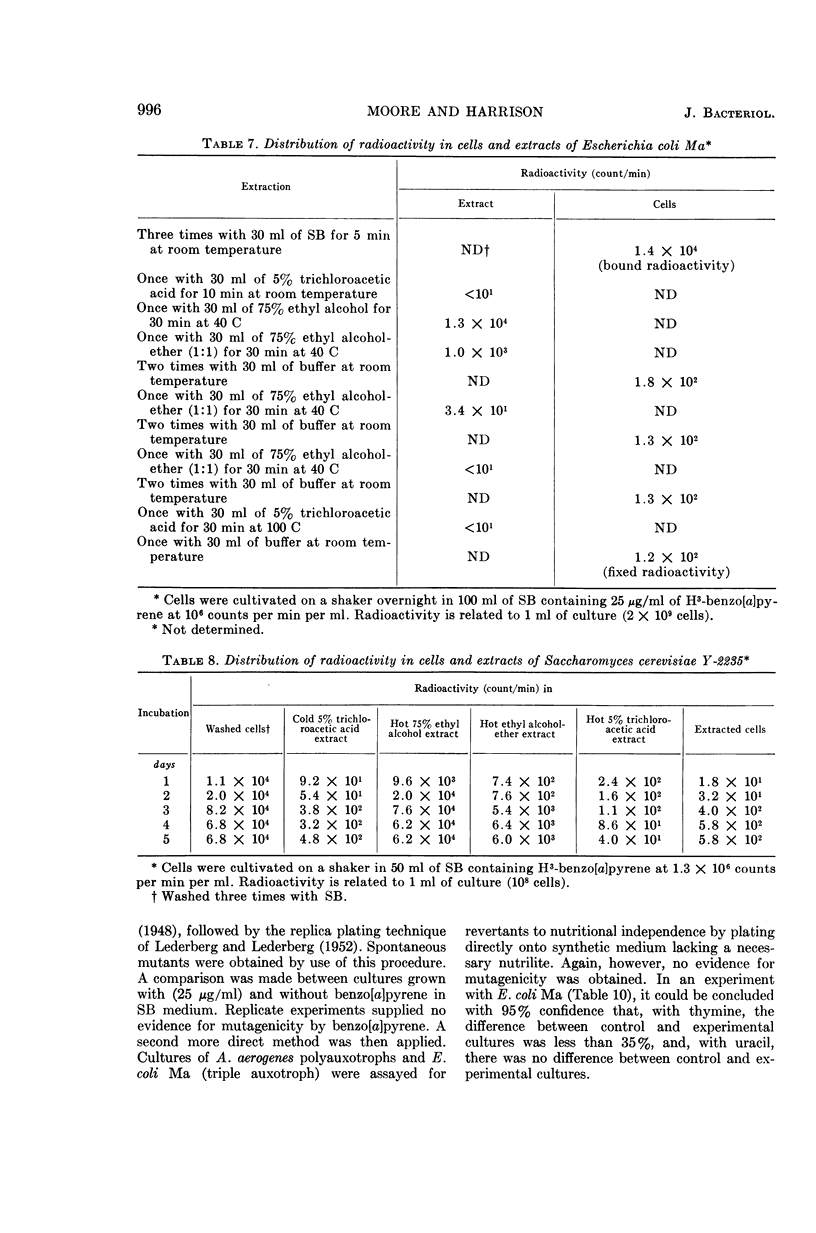
Abstract
Moore, B. G. (Oak Ridge National Laboratory, Oak Ridge, Tenn.), and Arthur P. Harrison, Jr. Benzo[a]pyrene uptake by bacteria and yeast. J. Bacteriol. 90:989–1000. 1965.—Various Enterobacteriaceae and yeast incubated in a medium containing 25 μg/ml of H3-benzo[a]pyrene (30% serum in the medium dissolves the hydrocarbon) retain radioactivity after washings with fresh 30% serum-medium. This radioactivity is defined as bound and represents intact benzo[a]pyrene. Factors relating to the binding of benzo[a]pyrene (benzo[a]pyrene uptake) have been studied in detail with Escherichia coli Ma, a triple auxotroph requiring l-leucine, uracil, and thymine. In defined medium, benzo[a]pyrene uptake by normally growing cells is 10−10 to 2 × 10−10 μg per cell. Uptake is the same in suspensions lacking leucine and containing chloramphenicol where there is neither measurable protein synthesis nor cell division. Uptake is diminished, but not eliminated, by autoclaving the cells; thus, some uptake occurs in the absence of enzymatic activity. Uptake is enhanced by heat shock, thymine deprivation, uracil deprivation, and exposure to penicillin. Thus, uptake is affected by the physiological state of the cells. Either the cells play a direct (enzymatic) role in uptake, or they affect uptake indirectly by increasing or altering the benzo[a]pyrene-binding structure. Physical fractionation of cells demonstrates that this structure is associated with the cell wall-membrane complex. All but 1% of the bound radioactivity is extracted with ethyl alcohol-ether. This residual radioactivity is defined as fixed, and may be associated with cell protein. The extracted radioactivity is identified as benzo[a]pyrene. Very little hydrocarbon is metabolized. Adverse photodynamic effects, increase in mutation, and dimunition in bacteriophage replication (in whole cells) have not been observed in the benzo[a]pyrene cultures.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURDETTE W. J. The significance of mutation in relation to the origin of tumors: a review. Cancer Res. 1955 May;15(4):201–226. [PubMed] [Google Scholar]
- DEMAEYER E., DEMAEYER-GUIGNARD J. EFFECTS OF POLYCYCLIC AROMATIC CARCINOGENS ON VIRAL REPLICATION: SIMILARITY TO ACTINOMYCIN D. Science. 1964 Oct 30;146(3644):650–651. doi: 10.1126/science.146.3644.650. [DOI] [PubMed] [Google Scholar]
- EPSTEIN S. S., SMALL M., FALK H. L., MANTEL N. ON THE ASSOCIATION BETWEEN PHOTODYNAMIC AND CARCINOGENIC ACTIVITIES IN POLYCYLIC COMPOUNDS. Cancer Res. 1964 Jun;24:855–862. [PubMed] [Google Scholar]
- HSU W. T., MOOHR J. W., WEISS S. B. THE INFLUENCE OF POLYCYCLIC AROMATIC HYDROCARBONS ON BACTERIOPHAGE DEVELOPMENT. Proc Natl Acad Sci U S A. 1965 Mar;53:517–524. doi: 10.1073/pnas.53.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F. Production de bactériophages par action de la méthyl-bis (chloroéthyl) amine sur des bactéries lysogènes. C R Hebd Seances Acad Sci. 1952 May 26;234(22):2238–2240. [PubMed] [Google Scholar]
- JOHNSON I. S., SIMPSON P. J., CLINE J. C. Comparative studies with chemotherapeutic agents in biologically diverse in vitro cell systems. Cancer Res. 1962 Jun;22:617–626. [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J., ST CLAIR J. Protoplasts and L-type growth of Escherichia coli. J Bacteriol. 1958 Feb;75(2):143–160. doi: 10.1128/jb.75.2.143-160.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIN J., HEINEMANN B., GOUREVITCH A. Induction of lysogenic bacteria as a method of detecting potential antitumour agents. Nature. 1962 Nov 24;196:783–784. doi: 10.1038/196783a0. [DOI] [PubMed] [Google Scholar]
- LUBIN M. Enrichment of auxotrophic mutant populations by recycling. J Bacteriol. 1962 Mar;83:696–697. doi: 10.1128/jb.83.3.696-697.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J. BACTERIAL PROTOPLASTS INDUCED BY PENICILLIN. Proc Natl Acad Sci U S A. 1956 Sep;42(9):574–577. doi: 10.1073/pnas.42.9.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIMURA Y., KOTIN P., FALK H. L. PHOTODYNAMIC TOXICITY OF POLYCYCLIC AROMATIC HYDROCARBONS IN TISSUE CULTURE. Cancer Res. 1964 Aug;24:1249–1259. [PubMed] [Google Scholar]
- OKADA T., HOMMA J., SONOHARA H. Improved method for obtaining thymineless mutants of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1962 Sep;84:602–603. doi: 10.1128/jb.84.3.602-603.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSO P. O., LU P. INTERACTION OF NUCLEIC ACIDS, II. CHEMICAL LINKAGE OF THE CARCINOGEN 3,4-BENZPYRENE TO DNA INDUCED BY PHOTORADIATION. Proc Natl Acad Sci U S A. 1964 Feb;51:272–280. doi: 10.1073/pnas.51.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]


