Abstract
Study Objectives:
Sleep pattern and circadian rhythms are regulated via the retinohypothalamic tract in response to stimulation of a subset of retinal ganglion cells, predominantly by blue light (450–490 nm). With age, the transmission of blue light to the retina is reduced because of the aging process of the human lens, and this may impair the photoentrainment of circadian rhythm leading to sleep disorders. The aim of the study was to examine the association between lens aging and sleep disorders.
Design:
Cross-sectional population based study.
Setting:
The study was performed at the Research Center for Prevention and Health, Glostrup Hospital, Denmark and at the Department of Ophthalmology, Herlev Hospital, Denmark.
Participants:
An age- and sex-stratified sample of 970 persons aged 30 to 60 years of age drawn from a sample randomly selected from the background population.
Interventions:
Not applicable.
Measurements and Results:
Sleep disturbances were evaluated by a combination of questionnaire and the use of prescription sleeping medication. Lens aging (transmission and yellowing) was measured objectively by lens autofluorometry. The risk of sleep disturbances was significantly increased when the transmission of blue light to the retina was low, even after correction for the effect of age and other confounding factors such as smoking habits, diabetes mellitus, gender, and the risk of ischemic heart disease (P < 0.0001).
Conclusions:
Filtration of blue light by the aging lens was significantly associated with an increased risk of sleep disturbances. We propose that this is a result of disturbance of photoentrainment of circadian rhythms.
Citation:
Kessel L; Siganos G; Jørgensen T; Larsen M. Sleep disturbances are related to decreased transmission of blue light to the retina caused by lens yellowing. SLEEP 2011;34(9):1215-1219.
Keywords: Circadian rhythm, cataract, melanopsin, sleep
INTRODUCTION
A good night's sleep is essential for optimum daytime function and well-being. There is an association between sleep disturbances and serious illness such as cancer, and it increases the risk of hypertension and cardiovascular disease.1 Disturbed sleep patterns are more often encountered in the elderly population,2 and it may contribute to increased morbidity and mortality in the old age, because of the association between sleep disturbances and general disease, and also because of the serious side-effects associated with the medication used to treat sleep disturbances, e.g., increased daytime drowsiness that may lead to fall accidents and hip fractures.3 Disturbed sleep patterns are more prevalent in patients with ischemic heart disease,1,4 diabetes mellitus,5 and smokers.6
Sleeping pattern and circadian rhythms are regulated via the retinohypothalamic tract. The photoentrainment of circadian rhythms is initiated by stimulation with blue light (450-490 nm) of a subset of intrinsically photosensitive retinal ganglion cells (ipRCG) containing melanopsin.7 The light stimulus is neurally transmitted via the suprachiasmatic nucleus (SCN) to the pineal gland.8 The pineal gland secretes the chronobiological hormone melatonin in a cyclic pattern with high levels at night and low level during daytime.9 The ipRGCs are responsible for conveying retinal stimuli to the SCN and hence setting the photoentrainment of circadian rhythm,10 but the signal from the ipRGCs may be modulated by signals from the rod and cone photoreceptors.11,12 In other words, melanopsin plays an essential but not exclusive role for photoentrainment.
With time, the natural lens of the eye acquires a yellow-brownish discoloration because of accumulation of chromophores that absorb preferentially in the short wavelength region of visible spectrum.13 Hypothetically, lens yellowing may be a causative factor in the disturbances in sleeping patterns observed in the elderly population because the yellow lens act as a color filter that effectively filters out blue light.13 Consequently, a decreased stimulation of melanopsin is expected with age, and theoretically the aging process of the lens of the eye may be an important causative factor in sleep disorders.
The aim of the present study was to examine the relationship between increased lens aging and sleep disturbances in a cross-sectional population-based study.
METHODS
Study Population
Subjects were recruited from the Inter99 Eye Study that is a subset of the Inter99 Study. Methods of recruitment and baseline characteristics of the Inter99 Study and the Inter99 Eye Study population have been described in detail previously.14,15 In short, the Inter99 Study is a population-based epidemiological study comprising an age- and sex- stratified sample of subjects residing in 11 municipalities in the western part of Copenhagen. Every person permanently residing in Denmark is registered by the Civil Registration System by a unique personal identification code. The study population included 14 birth cohorts drawn as a random sample from the Civil Registration System; years of birth 1939/1940 (age group 60 years old), 1944/1945 (age group 55 years old), 1949/1950 (age group 50 years old), 1954/1955 (age group 45 years old), 1959/60 (age group 40 years old), 1964/1965 (age group 35 years old), or 1969/1970 (age group 30 years old). A total of 6784 subjects aged 30 to 60 years underwent the screening program at the Research Center for Prevention and Health at the Glostrup Hospital, and a subgroup of 1437 subjects was invited to participate in Inter99 Eye Study at the Department of Ophthalmology at Herlev Hospital. The Inter99 Eye Study was designed to investigate correlations between ophthalmic parameters and systemic health and disease; the sampling procedure for the eye study resulted in an intentional overrepresentation of subjects with diabetes mellitus, impaired glucose tolerance, and high IHD risk subjects compared to the background population. In short, subjects for the eye study were invited as a control group (n = 502), a group with high risk of ischemic heart disease (n = 142), subjects with newly diagnosed type 2 diabetes mellitus (n = 107), subjects with known diabetes mellitus (n = 11), subjects with impaired glucose tolerance (n = 147), and finally a minor group of subjects who were invited due to a clerical error (n = 61). A total of 970 subjects (67.5% of those invited) underwent the eye examination. Examinations took place in 1999 and 2000. Eye examinations and examinations of general physical health, including oral glucose tolerance testing, took place on separate days. Descriptive characteristics of the study population are shown in Table 1.
Table 1.
Clinical characteristics of the study population
| Number of participants | |
|---|---|
| Males/females | 470/500 |
| Smokers/nonsmokers | 395/552 |
| Normoglycemia/diabetes mellitus | 715/205 |
| Sleeping medication (yes/no) | 89/881 |
| Insomnia (yes/no) | 193/760 |
| Sleep disturbances (yes/no) | 233/720 |
Sleep disturbances were defined as the subject saying “yes” to having troubles sleeping and/or the subject having bought sleeping medication within 1 year of the examination date. The table shows the clinical characteristics of the study population. Values are given in numbers.
The study was approved by the medical ethics committee of Copenhagen County. All participants gave their informed consent, and the study was performed according to the Helsinki Declaration. The Inter99 Study was registered in 2005 at ClinicalTrials.gov (study ID number NCT00289237).
Procedures
As part of the study procedure, all subjects in the Inter99 Eye Study population underwent an eye examination including determination of best corrected visual acuity, lens autofluorometry, dilated photographic fundus examination, as well as a detailed interview on past and present eye disease, visual disturbances, or ocular medication. Examination and interview was performed by a trained nurse. Subjects who were found to have a best corrected visual acuity worse than 0.8, symptoms of eye disease, or a history of eye disease were also seen by a physician.
Lens examination included photographic examination using retroilluminated lens photographs focused on the anterior lens surface. Photographs were taken after dilation of the pupil using one drop of 0.5% tropicamide and one drop of 10% phenylephrine hydrochloride on 35 mm Kodak Ektachrome 64 color slides (Kodak Inc, Rochester, NY, USA) using a Canon 60UVi camera (Canon Inc, Japan). The degree of cortical lens opacities was graded by one of the authors (LK) according to the LOCS III scale.16 Nuclear lens aging was assessed by lens autofluorometry using the Fluorotron (excitation wavelengths 430–490 nm, detection from 530–630 nm, Ocumetrics, San Jose, CA, USA). Lens fluorescence measurements have previously been shown to correlate well to the LOCS III nuclear grading.17 Eyes exceeding 2.5 on the LOCS III scale and pseudophakic eyes were excluded from the study. The autofluorescence measurements yielded two measurements: (1) the concentration of fluorophores (expressed in ng fluorescein equivalents/10 mL) in the lens that can be taken as a physiological measurement of lens aging and represents the degree of lens yellowing, and (2) the transmission of blue light. These two measurements are inversely related (transmission is lower for a more yellow lens). The transmission measurements obtained by the Fluorotron have previously been shown to correlate well with the absorption peak of melanopsin at 480 nm.18
All subjects in the Inter99 Study filled in a detailed questionnaire on education and occupation, lifestyle, and past and present medical history, and a thorough physical examination was performed at the Research Center for Prevention and Health at Glostrup Hospital. The risk of developing ischemic heart disease (IHD) was assessed using the Copenhagen Risk Score.19 The score is based on age, sex, diabetes history, history of cardiac disease, family history of cardiovascular disease, height, smoking status, weight, blood pressure, and total cholesterol. For all subjects, the risk of having an IHD event was projected to occur between the age of 60 years and 70 years to reach a substantial level of risk.
Smoking habits were assessed by questionnaire and subjects were defined as smokers if they smoked daily or occasionally. Never-smokers and ex-smokers were classified as nonsmokers.
Diagnosis of diabetes mellitus was based on an oral glucose tolerance test (OGTT) administered using 75 g glucose after fasting from midnight. The following criteria were used: fasting p-glucose ≥ 7.0 mmol/L or 2 hour OGTT p-glucose ≥ 11.1 mmol/L.20 Subjects who had a known diagnosis of diabetes mellitus (type 1 or 2) prior to the study were identified by questionnaire.
A subject was defined as having troubles with sleep (termed “sleep disturbances”) if he/she answered “yes” to the question “do you often suffer from insomnia” and/or the subject had been registered to have bought prescription medication for sleeping disorders (benzodiazepines and benzodiazepine receptor agonists [zolpidem, zopiclone, zaleplon] and melatonin [Anatomic Therapeutic Chemical Classification groups NO5B and NO5C]) within 1 year of the examination date. The questionnaire did not allow for grading sleep disorders according to frequency nor did the purchase of prescription medication for sleep disorders since all but 4 subjects had ≥ 2 prescriptions. Information on the use of sleeping medication was obtained from Statistics Denmark21 that is the official statistical census bureau of Denmark, where each purchase of prescription medication is registered by the unique personal identification code.
Statistical analysis was performed using the SAS programme (version 8.2, SAS Institute Inc, Cary, NC, USA). The level of statistical significance was set at P ≤ 0.05.
RESULTS
The overall prevalence of sleep disturbances was 24.4%. Of those who were found to have a sleep disturbance, 82.6% both answered yes to “suffer from insomnia often” and used sleeping medication. The prevalence of sleep disturbances was higher in older age group (32.5% from 55–60 years compared to 15.7% from 30–35 years, P = 0.0002). In the oldest age group (aged 55–60 years), 14.3% used benzodiazepines or benzodiazepine receptor agonists versus 2.2% in the youngest age group (30–35 years of age). Sleep disturbances were significantly more prevalent in females than in males (30.2% versus 18.4%, P < 0.0001), in subjects with diabetes mellitus (30.7% in subjects with diabetes mellitus versus 22.5% in normoglycemic subjects, P = 0.016), and in smokers (28.6%) than in nonsmokers (21.0%, P = 0.007), see also Table 2.
Table 2.
Prevalence of sleep disturbances
| Sleep disturbances (yes/no) | χ2 P-value | |
|---|---|---|
| Males | 85/378 (18.4%) | < 0.0001 |
| Females | 148/342 (30.2%) | |
| Age groups | ||
| 30 + 35 years | 14/75 (15.7%) | 0.0002 |
| 40 + 45 + 50 years | 122/444 (21.6%) | |
| 55 + 60 years | 97/201 (32.5%) | |
| Smokers | 113/282 (28.6%) | 0.007 |
| Nonsmokers | 116/436 (21.0%) | |
| Normoglycemia | 161/554 (22.5%) | 0.016 |
| Diabetes mellitus | 63/142 (30.7%) |
Subjects were recruited in birth cohorts born with 5 year intervals. Subjects were 30 (n = 38), 35 (n = 52), 40 (n = 160), 45 (n = 196), 50 (n = 223), 55 (n = 207), or 60 (n = 94) years old at the date of examination. Smokers were grouped into daily and regular smokers versus never- and ex-smokers. Glucose metabolic status was grouped into subjects with normoglycemia versus subjects with subjects with known or screening detected diabetes mellitus as defined by the WHO 1999 criteria.20
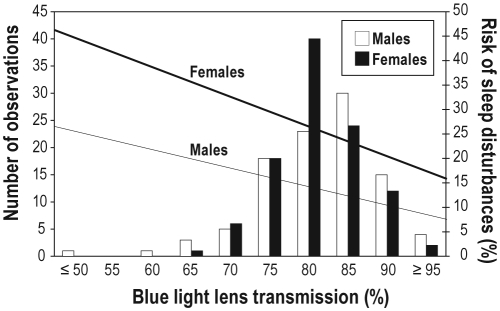
There was an inverse relationship between blue light lens transmission and the risk of having sleep disturbances; the lower the blue light lens transmission, the higher the risk of sleep disturbances (OR 0.95 [95% CI 0.93–0.97] for 1 percentage point change in lens transmission, e.g., from 95% to 96%, P < 0.0001). Results for the largest age group (50-year-old subjects) are shown in Figure 1. Similar results were obtained for the other age groups. The association remained significant even after correction age, sex, diabetes mellitus, smoking, and risk of ischemic heart disease (OR 0.97 [95% CI 0.95–0.99], P = 0.016, see also Table 3). To translate the ORs into a clinical example, a 50-year-old non-smoking, non-diabetic female with a low risk of IHD who was in the 97.5% upper normal range of blue light lens transmission (corresponding to a blue light lens transmission of 94.8%) had a risk of sleep disturbances of 16.4%, whereas a similar (non-smoking, non-diabetic with low risk of IHD) 50-year-old female who was in the 2.5% lower normal range of blue light lens transmission (corresponding to a blue light lens transmission of 70.9%) had a risk of having sleep disturbances of 37.9%.
Figure 1.
Relationship between blue light lens transmission (graphed with the number of observations in bars on the left y-axis) and the risk of having sleep disturbances (graphed on the right y-axis) modeled for a normoglycemic, nonsmoking subject with a low risk of IHD based on the model presented in Table 2. The computed risk for females is shown using the thick line, and for males using the thin line. To account for the effect of age on both blue light lens transmission and the risk of sleep disturbances, the effect of age was avoided in the graph by only presenting the results for the 50-year-old study participants (n = 223). Similar results were found for the other age groups (data not shown).
Table 3.
Odds ratios for sleep disturbances with relation to the optical qualities of the lens
| Effect | Lens transmission |
Lens fluorescence |
||
|---|---|---|---|---|
| OR (95% CI) | χ2 P-value | OR (95% CI) | χ2 P-value | |
| Lens optical quality | 0.97 (0.95 - 0.99) | 0.016 | 1.01 (1.00-1.02) | 0.044 |
| Age groups* | ||||
| 30 + 35 years | 0.54 (0.25-1.15) | 0.108 | 0.54 (0.25-1.16) | 0.114 |
| 40 + 45 + 50 years | 0.66 (0.45-0.97) | 0.036 | 0.64 (0.43-0.94) | 0.024 |
| Females versus males | 2.41 (1.64-3.52) | < 0.0001 | 2.38 (1.63-3.48) | < 0.001 |
| Non-smokers versus smokers | 0.64 (0.46-0.90) | 0.009 | 0.64 (0.46-0.89) | 0.009 |
| Diabetes versus normoglycemia | 1.13 (0.71-1.81) | 0.606 | 1.13 (0.70-1.80) | 0.620 |
| Risk of IHD$ | 1.02 (0.99-1.04) | 0.239 | 1.02 (0.99-1.04) | 0.256 |
Odds ratio estimates for sleep disturbances using a combined analysis comprising lens optical quality: blue light transmission (middle column) and lens fluorescence (measurement of lens yellowing, right column), age, sex, smoking status, glucose metabolism, and the risk of ischemic heart disease. For both blue light lens transmission and lens fluorescence, the odds ratio is given for one unit change (i.e., 1 percentage point change in transmission or 1 ng f-eq/mL change in lens fluorescence).
Subjects were recruited in birth cohorts with an interval of 5 years between each cohort. Age groups are tested against the age groups of 55 and 60 years old.
The risk (in percentage) of an ischemic heart disease (IHD) event projected to occur between the age of 60 and 70 years.
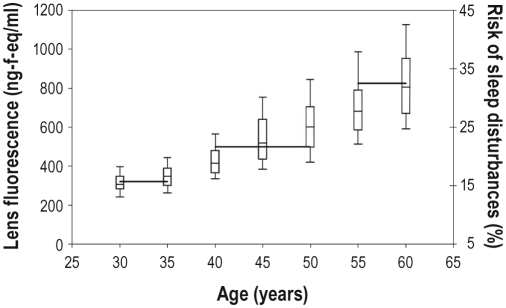
The risk of having sleep disturbances increased significantly with the degree of lens autofluorescence (OR 1.02 [95% CI 1.01–1.02], for a 1 unit change in lens autofluorescence, P < 0.0001; see Figure 2 and Table 3) and this association remained significant even after correction for age, sex, diabetes mellitus, smoking habits, and ischemic heart disease (OR 1.01 [95% CI 1.00–1.02], P = 0.04). Using the same clinical example as above, i.e. a non-smoking, non-diabetic, and low risk of IHD 50-year-old female in the upper 97.5% normal range of lens autofluorescence (corresponding to a lens autofluorescence of 963.1 ng fluorescein eq/mL) had a risk of having sleep disturbances of 37.2%, whereas a similar 50-year-old female in the lower 2.5% normal range for lens autofluorescence (corresponding to a lens autofluorescence of 306.2 ng fluorescein eq/mL) had a 16.8% risk of having sleep disturbances.
Figure 2.
The association between degree of lens yellowing (measured by lens fluorescence), (left y-axis), age, and sleep disturbances (right y-axis). Lens fluorescence values are presented as box and whiskers diagram showing the 10, 25, 50, 75, and 90th percentiles for each age group. The prevalence of sleep disturbances is shown by the horizontal lines
DISCUSSION
We found that the risk of having sleep disturbances was associated to decreasing transmission of blue light to the retina, possibly because of reduced stimulation of the melanopsin containing retinal ganglion cells and hence impaired photoentrainment of circadian rhythm. Melanopsin is stimulated by predominantly blue light (450–490 nm) with a maximum absorption around 480 nm in humans,22 the very part of the visible spectrum that is mostly affected by the aging process of the human lens.13 Our results are supported by reports of a reduced ability of short wavelength light to suppress melatonin secretion23 and to change subjective alertness, sleepiness, and mood24 with age, supposedly because of increased filtering effects of the lens. The strong link between lens yellowing and age could help explain why sleep disorders become more frequent with increasing age. Importantly, sleep quality has been shown to improve after cataract surgery.25 The present study is unique in that it was population based, and that we assessed blue light lens transmission individually using an autofluorescence based technique that has been shown to be a valid measurement of the transmission of the wavelengths relevant for melanopsin stimulation.18
The results of the present study indicate that the spectral characteristics of the light reaching the retina, and specifically the total amount of blue light that penetrates the refractive media of the eye may have a profound impact on sleep quality, most likely mediated via reduced ocular photic regulation of melatonin secretion.26 Our results support that choosing the right indoor lightning conditions may have a beneficial effect on sleep27 and that blue light therapy may be used to modulate circadian sleep disorders.28
We used lens autofluorometry to objectively quantify lens aging. The rate of lens aging is accelerated in patients with diabetes mellitus,29 in subjects with a high risk of ischemic heart disease,30 and in smokers.31 These factors are also related to the prevalence of sleep disturbances,1,27,32 and consequently, they are experimental confounders; correcting for their effects in the statistical analyses reflects a robust, independent association between lens aging and sleep disturbances.
Our study has limitations. We used the question “do you often suffer from insomnia” in combination with registered purchases of sleeping medication to assess if a subject had sleep disturbances. There is a risk that we misclassified subjects since sleeping medication may be used for other conditions, e.g., benzodiazepines may be used to treat anxiety. Overall there was a good agreement between the two measures, with 82.6% of subjects both giving a positive answer to the question and using sleeping medication. The way sleep disturbances was assessed did not allow us to grade the severity of sleep disturbance, and hence we do not know if there is a dose-response relation between the degree of lens yellowing and severity of sleep disturbances. Furthermore, it should be noted that, although population-based, the study population was intentionally sampled to give an overrepresentation of subjects with diabetes mellitus and high risk of ischemic heart disease. The effects of diabetes and the risk of ischemic heart disease were corrected for in the statistical analyses, and the overrepresentation of these subjects should not influence the overall results and conclusions of the study: that the reduced transmission of blue light to the retina because of lens yellowing may have an impact on sleep.
Sleep disturbances are very common in the elderly4 and a major cause of decreased quality of life, and possibly an age-independent risk factor for increased morbidity and mortality.1 Sleep disorders are most commonly treated using benzodiazepines and benzodiazepine receptor agonists. Although these substances are very effective in inducing sleep, they are also associated with severe side effects such as daytime drowsiness, thus increasing the risk of fall accidents and hip fractures and associated morbidity and mortality.3,33 It has been estimated that in the United States alone the costs related to sleep disturbances exceed $15.4 billion.34 Reducing the prevalence of sleep disturbances would not only improve health but also have economic benefits.
During cataract surgery, the natural lens is replaced with an artificial lens that is available with different spectral characteristics, i.e. different colors. Retrospective studies have shown that cataract surgery improves sleep quality,25 but prospective data, especially prospective studies evaluating the effect of the different implant lenses, are still lacking. Nevertheless, it seems prudent at this stage to recommend physicians to reconsider the prescription of sleeping tablets in patients who have undergone cataract surgery.
In conclusion, the results of the present study show that while the age-related lens yellowing is of relatively little importance for visual function, it may be responsible for insomnia in the elderly because of disturbed photoentrainment of circadian rhythms.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The Inter99 study was initiated by Torben Jørgensen (Principal Investigator), Knut Borch-Johnsen (Principal Investigator, diabetes part), Hans Ibsen and Troels Thomsen. The Inter99 steering committee comprises Torben Jørgensen, Knut Borch-Johnsen, and Charlotta Pisinger. The authors thank all the participants who took part in the survey and the staff for their dedicated effort. The study received financial support from the The Danish Medical Research Council, The Danish Centre for Evaluation and Health Technology Assessment, Novo Nordisk, Copenhagen County, The Danish Heart Foundation, The Danish Pharmaceutical Association, Augustinus foundation, Ib Henriksen foundation and Becket foundation, the Danish Diabetes Association, the Danish Organisation for Prevention of Vision (Værn om Synet), the Danish Association of the Blind (Øjenfonden), and through a Patient-Oriented Diabetes Research Career Award from the Juvenile Diabetes Research Foundation to Dr. Larsen (grant no. 8-2002-130).
The study was presented in part at the Nordic Congress of Ophthalmology 2010 in Reykjavik, Iceland.
REFERENCES
- 1.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10(Suppl 1):S7–11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Hazardous benzodiazepine regimens in the elderly: effects of half-life, dosage, and duration on risk of hip fracture. Am J Psychiatry. 2001;158:892–8. doi: 10.1176/appi.ajp.158.6.892. [DOI] [PubMed] [Google Scholar]
- 4.Bonanni E, Tognoni G, Maestri M, et al. Sleep disturbances in elderly subjects: an epidemiological survey in an Italian district. Acta Neurol Scand. 2010;122:389–97. doi: 10.1111/j.1600-0404.2010.01324.x. [DOI] [PubMed] [Google Scholar]
- 5.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Samet J, Caffo B, Punjabi NM. Cigarette smoking and nocturnal sleep architecture. Am J Epidemiol. 2006;164:529–37. doi: 10.1093/aje/kwj231. [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki A, Kardon RH. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol. 2007;27:195–204. doi: 10.1097/WNO.0b013e31814b1df9. [DOI] [PubMed] [Google Scholar]
- 8.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skene DJ, Arendt J. Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem. 2006;43:344–53. doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- 10.Güler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–5. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altimus CM, Guler AD, Alam NM, et al. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13:1107–12. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grünert U, Jusuf PR, Lee SC, Nguyen DT. Vis Neurosci. 2010. Bipolar input to melanopsin containing ganglion cells in primate retina; pp. 1–12. [DOI] [PubMed] [Google Scholar]
- 13.Kessel L, Lundeman JH, Herbst K, Andersen TV, Larsen M. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg. 2010;36:308–12. doi: 10.1016/j.jcrs.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 14.Kessel L, Hougaard JL, Mortensen C, Jorgensen T, Lund-Andersen H, Larsen M. Visual acuity and refractive errors in a suburban Danish population: Inter99 Eye Study. Acta Ophthalmol Scand. 2004;82:19–24. doi: 10.1111/j.1395-3907.2004.0179.x. [DOI] [PubMed] [Google Scholar]
- 15.Jørgensen T, Borch-Johnsen K, Thomsen TF, Ibsen H, Glümer C, Pisinger C. A randomised non-pharmacological intervention study for prevention of ischemic heart disease. Baseline results. Inter99 (1) Eur J Cardiovasc Prevention Rehab. 2003;10:377–86. doi: 10.1097/01.hjr.0000096541.30533.82. [DOI] [PubMed] [Google Scholar]
- 16.Chylack LT, Jr., Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111:831–6. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 17.Siik S, Chylack LT, Jr., Friend J, et al. Lens autofluorescence and light scatter in relation to the lens opacities classification system, LOCS III. Acta Ophthalmol Scand. 1999;77:509–14. doi: 10.1034/j.1600-0420.1999.770504.x. [DOI] [PubMed] [Google Scholar]
- 18.Broendsted AE, Stormly HM, Lund-Andersen H, Sander B, Kessel L. Human lens transmission of blue light: a comparison of autofluorescence-based and direct spectral transmission determination. Ophthalmic Res. 2011;46:118–24. doi: 10.1159/000323576. [DOI] [PubMed] [Google Scholar]
- 19.Thomsen TF, Davidsen M, Jørgensen T, Ibsen H, Jensen G, Borch-Johnsen K. A new method for CHD prediction and prevention based on regional risk scores and randomized clinical trials; PRECARD and the Copenhagen Risk Score. J Cardiovasc Risk. 2001;8:291–7. doi: 10.1177/174182670100800508. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Geneva: WHO. Department of noncommunicable disease surveillance; 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO Consultation. Part 1: Diagnosis and classification of diabetes mellitus. [DOI] [PubMed] [Google Scholar]
- 21.Denmark: StatBank; 2009. Statistics Denmark. www.statistikbanken.dk. [Google Scholar]
- 22.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–54. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herljevic M, Middleton B, Thapan K, Skene DJ. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol. 2005;40:237–42. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Sletten TL, Revell VL, Middleton B, Lederle KA, Skene DJ. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythms. 2009;24:73–84. doi: 10.1177/0748730408328973. [DOI] [PubMed] [Google Scholar]
- 25.Asplund R, Lindblad BE. Sleep and sleepiness 1 and 9 months after cataract surgery. Arch Gerontol Geriatr. 2004;38:69–75. doi: 10.1016/j.archger.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: ocular and neural signal transduction. J Biol Rhythms. 1997;12:537–46. doi: 10.1177/074873049701200608. [DOI] [PubMed] [Google Scholar]
- 27.Turner PL, Van Someren EJ, Mainster MA. The role of environmental light in sleep and health: effects of ocular aging and cataract surgery. Sleep Med Rev. 2010;14:269–80. doi: 10.1016/j.smrv.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Shirani A, St Louis EK. Illuminating rationale and uses for light therapy. J Clin Sleep Med. 2009;5:155–63. [PMC free article] [PubMed] [Google Scholar]
- 29.Theil PK, Kessel L, Hansen T, Lund-Andersen H, Pedersen O, Larsen M. Lens fluorescence in relation to glucose tolerance and genetic predisposition to type 2 diabetes mellitus in a population-based study. Curr Eye Res. 2006;31:733–8. doi: 10.1080/02713680600850971. [DOI] [PubMed] [Google Scholar]
- 30.Kessel L, Jorgensen T, Glumer C, Larsen M. Early lens aging is accelerated in subjects with a high risk of ischemic heart disease: an epidemiologic study. BMC Ophthalmol. 2006;6:16. doi: 10.1186/1471-2415-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessel L, Hougaard JL, Sander B, Kyvik KO, Sørensen TI, Larsen M. Lens ageing as an indicator of tissue damage associated with smoking and non-enzymatic glycation - a twin study. Diabetologia. 2002;45:1457–62. doi: 10.1007/s00125-002-0925-3. [DOI] [PubMed] [Google Scholar]
- 32.Espiritu JR. Aging-related sleep changes. Clin Geriatr Med. 2008;24:1–14. doi: 10.1016/j.cger.2007.08.007. v. [DOI] [PubMed] [Google Scholar]
- 33.Taylor SR, Weiss JS. Review of insomnia pharmacotherapy options for the elderly: implications for managed care. Popul Health Manag. 2009;12:317–23. doi: 10.1089/pop.2008.0047. [DOI] [PubMed] [Google Scholar]
- 34.Stoller MK. Economic effects of insomnia. Clin Ther. 1994;16:873–97. [PubMed] [Google Scholar]