Abstract
Study Objectives:
Examine associations of vasomotor and mood symptoms with visually scored and computer-generated measures of EEG sleep.
Design:
Cross-sectional analysis.
Setting:
Community-based in-home polysomnography (PSG).
Participants:
343 African American, Caucasian, and Chinese women; ages 48–58 years; pre-, peri- or post-menopausal; participating in the Study of Women's Health Across the Nation Sleep Study (SWAN Sleep Study).
Interventions:
None.
Measurements and Results:
Measures included PSG-assessed sleep duration, continuity, and architecture, delta sleep ratio (DSR) computed from automated counts of delta wave activity, daily diary-assessed vasomotor symptoms (VMS), questionnaires to collect mood (depression, anxiety) symptoms, medication, and lifestyle information, and menopausal status using bleeding criteria. Sleep outcomes were modeled using linear regression. Nocturnal VMS were associated with longer sleep time. Higher anxiety symptom scores were associated with longer sleep latency and lower sleep efficiency, but only in women reporting nocturnal VMS. Contrary to expectations, VMS and mood symptoms were unrelated to either DSR or REM latency.
Conclusions:
Vasomotor symptoms moderated associations of anxiety with EEG sleep measures of sleep latency and sleep efficiency and was associated with longer sleep duration in this multi-ethnic sample of midlife women.
Citation:
Kravitz HM; Avery E; Sowers MF; Bromberger JT; Owens JF; Matthews KA; Hall M; Zheng H; Gold EB; Buysse DJ. Relationships between menopausal and mood symptoms and Eeg sleep measures in a multi-ethnic sample of middle-aged women: the SWAN Sleep Study. SLEEP 2011;34(9):1221-1232.
Keywords: Anxiety, depressive symptoms, menopausal status, race/ethnicity, delta (slow wave) sleep, delta sleep ratio, REM sleep, REM latency, sleep continuity, vasomotor symptoms
INTRODUCTION
An estimated 85% of women undergoing the menopausal transition report at least one transition-related symptom, typically vasomotor symptoms (VMS), depressed mood, or sleep disruption.1 Recent longitudinal analyses of data from community-based studies have shown that the menopausal transition is associated with increases in depressive2–6 and vasomotor7,8 symptoms. Data from two longitudinal, community-based studies suggest that subjective sleep complaints also worsen during the menopausal transition.9,10
In contrast, objective sleep data, as measured by polysomnography (PSG), tend to be cross-sectional and based on a single night of sleep. Wisconsin Sleep Cohort (WSC) data indicate that, compared with premenopausal women, postmenopausal women had more total sleep time and a lower percentage of wake time; perimenopausal women had a lower percentage of stage 1 sleep; and both peri- and post-menopausal women had higher stage 3+4 (delta or slow wave) sleep percentages.11 Yet, the same peri- and post-menopausal women reported greater sleep dissatisfaction than did premenopausal women.11 Limitations of the WSC study, noted by its investigators, include collection of PSG sleep data on a single night, which may not be representative of habitual sleep, and a sample that was 95% Caucasian, which limits its generalizability in light of racial/ethnic differences in sleep.12,13 The extent to which other symptoms of the menopausal transition, including mood (depressive and anxiety) and VMS, were associated with sleep in this sample is unknown.
Emerging evidence suggests that depressive symptoms and other indicators of negative affect (e.g., anxiety, irritability, hostility) are strong and consistent independent correlates of the association between menopausal transition stage and self-reported sleep complaints.9,14–17 For instance, in the Study of Women's Health Across the Nation (S WAN), perimenopausal women reported more persistent dysphoric moods, including irritability, nervousness, and mood changes, than did premenopausal women, and these symptoms were associated with more subjective sleep complaints.18
VMS, particularly hot flashes, are strong correlates of subjective sleep complaints, but few studies have examined associations among VMS, indices of negative affect, and objective measures of sleep during the menopausal transition. Dennerstein and colleagues9 reported that VMS mediated the association between declining estradiol (E2) levels and subjective symptoms of trouble sleeping during the menopausal transition. Freeman et al.8 observed that anxiety was strongly associated with menopausal hot flashes in a dose-related fashion, even after controlling for depressive symptoms and estradiol levels, and that higher levels of anxiety temporally preceded the development of hot flashes.
Sleep EEG measures may be associated with depressive and anxiety symptoms. Reduced REM latency has been a rather consistent, though not specific, finding in affective but not anxiety disorders.19,20 Delta sleep ratio (DSR) is a computer-generated quantitative index of the temporal distribution of delta sleep, computed as the ratio of average delta wave counts/minute in the first NREM period to average delta wave counts/minute in the second NREM period.21 As such, it is a measure of the time course of delta activity early in the night, i.e., the decline in delta activity from the first to the second NREM period. Higher values indicate relatively greater delta activity in the first NREM period, which is the expected time course of delta activity in human sleep. Lower values have been associated with major depression and increased likelihood of depression recurrence. These indices have not been examined previously in a community cohort of women during the menopausal transition.
In this report, we examined whether vasomotor, depressive, and anxiety symptoms were associated with PSG-assessed measures of sleep in The Study of Women's Health Across the Nation (SWAN), a racially/ethnically diverse cohort of midlife women traversing the menopause. The principal measures of interest were REM latency and DSR. We also examined associations with other PSG measures of sleep duration, continuity, and architecture. Support for examining these associations is based on studies indicating that VMS and depression22–24 and anxiety7,8 are strongly associated, that the increase in depressive symptoms during the menopausal transition are augmented by VMS,4 that depression is linked to shorter REM latency and lower DSR,20 and that depressed compared with nondepressed women are more likely to report poor sleep.25 Depressive and anxiety symptoms were examined separately because we expected they would have different associations with sleep measures.26
METHODS
Study Design and Participants
The Study of Women's Health Across the Nation (SWAN), which was initiated in 1996, is a multi-ethnic, community-based, cohort study of the menopausal transition. A total of 3,302 women were enrolled at 7 sites in the Core SWAN: Boston, MA, Chicago, IL, Detroit area, MI, Los Angeles and Oakland, CA, Newark, NJ, and Pittsburgh, PA. The study design and recruitment of the main cohort have been described in detail.27 Each site recruited Caucasian women and a minority group sample. SWAN cohort eligibility criteria required women to be aged 42–52, premenopausal or early perimenopausal, have an intact uterus and at least 1 ovary, have at least one menstrual period in the previous 3 months, not using any sex steroid hormone in the previous 3 months, and not pregnant.
The SWAN Sleep Study was a cross-sectional study of sleep patterns conducted at 4 of the 7 study sites (Chicago, the Detroit area, Oakland, Pittsburgh) from 2003–2005 and included 370 Caucasian (all sites), African American (Chicago, Detroit area, Pittsburgh), and Chinese (Oakland) women from the SWAN core cohort aged 48–59 years. Each site's institutional review board approved both the Core SWAN study and the Sleep Study protocols, and all women gave written informed consent to participate. Women were paid for participating in the study.
Eligibility for the Sleep Study was determined prior to and during participants' annual Core SWAN assessment and was based primarily on the women's menopausal status. Efforts were focused on recruiting women who were pre- and peri-menopausal as defined by the SWAN Core Study and excluded those women who had surgical menopause (< 1%) or who reported using hormone therapy (approximately 23% of the cohort) at the annual SWAN Core examination preceding Sleep Study recruitment. During the final year of the Sleep Study, eligibility criteria were relaxed to allow inclusion of postmenopausal women in the protocol. Exclusion criteria also included factors known to affect sleep, including active cancer chemotherapy or radiation; regular shift work/night shift employment; oral corticosteroid use; regular consumption of > 4 alcoholic drinks daily; and poor compliance with Core SWAN procedures (e.g., missed > 50% of annual visits). All Core SWAN participants meeting the eligibility criteria at the 4 Sleep Study sites were approached regarding participation. Of these, 30% declined to participate in the Sleep Study; the most frequently cited reasons included “protocol burden,” “too busy,” and “family obligations.” Sleep Study participants did not differ markedly from Core SWAN participants at annual visit 05 with regard to age, self-assessed sleep quality, race/ethnicity, self-reported health status, depressive symptoms (Center for Epidemiologic Studies Depression Scale score), or frequency of reported physician-diagnosed hypertension or diabetes. Mean body mass index (BMI) of the Sleep Study participants was slightly higher than that of non-participants. Sleep Study participants reported having hot flashes slightly less frequently over a 2-week time period preceding Sleep Study participation than did Study non-participants (P < 0.02) but did not differ in the reported frequency of night sweats or cold sweats.
Twenty-two pre- and peri-menopausal women who reported hormone therapy (HT) use were excluded from these analyses because HT affects bleeding patterns, resulting in “undetermined” menopausal status in such women. Three postmenopausal women who reported HT use at their last Core SWAN assessment preceding Sleep Study enrollment but not during the Sleep Study were included. Five others were excluded from analyses: 2 did not have a sleep study, and 3 had only a PSG screen for sleep disordered breathing. Thus, 343 women were eligible for inclusion in our analyses.
Procedures and Measures
Data available for SWAN Sleep Study participants included 5 to 7 years of hormonal, behavioral, social, and psychological measures collected during annual Core SWAN visits. Core SWAN data used in this report were collected at the closest visit preceding the Sleep Study.
The SWAN Sleep Study protocol was initiated within 7 days of onset of a menstrual period in women who were still menstruating, and non-cycling women were scheduled at their convenience. Menopausal characteristics were assessed by self-report and vasomotor event monitoring. Measures of sleep included subjective sleep symptoms, daily sleep diaries, and PSG. PSG sleep studies were conducted in participants' homes during the first 3 days of the protocol (Vitaport-3 [TEMEC VP3] ambulatory PSG monitor; TEMEC Instruments B.V., Kerkrade, Netherlands). Depressive and anxiety symptoms were measured by self-report on Day 4 after completing the final PSG night.
SWAN Sleep Study staff visited participants in their homes on each night of sleep studies to apply and calibrate study monitors. Participants slept in their own beds, under their usual circumstances, at their habitual sleep and wake times. The only study restrictions placed on participants were not to sleep in water beds, under electric blankets, or with pets, due to the possible influence of these factors on the monitoring of physiological signals during sleep. In the morning upon awakening from sleep, participants removed study equipment and turned off the VP3 unit. Study data were stored passively on an internal memory card and were uploaded onto laboratory PCs within 24 h of collection. Quality assurance assessments, scoring, and processing of all sleep study records were performed by Neuroscience–Clinical and Translational Research Center (N-CTRC) laboratory staff at the University of Pittsburgh. In the event that data could not be scored, repeat sleep studies were conducted on participants who were willing to do so; 34 repeat night 1 studies (which included a screen for sleep disordered breathing [SDB] and periodic leg movements [PLM]) and 12 repeat nights 2 or 3 were conducted. A PSG scored to screen for sleep disorders preceded PSGs scored only for sleep staging (see below) in 91% of participants.
Signals collected on each study night included bilateral central referential EEG channels (C3 and C4, referenced to A1 + A2), bilateral electro-oculogram (EOG), submentalis electromyogram (EMG), electrocardiogram (ECG; modified V2 lead), and abdominal and thoracic effort as measured by inductance plethysmography. On Night 1, additional signals were collected to assess sleep disordered breathing (nasal pressure cannula; oral-nasal thermistors; inductance plethysmography to measure respiratory effort; fingertip oximetry to measure SpO2 [Nonin X-pod model 312]) and periodic leg movements (bilateral EMG of anterior tibialis). Bilateral EEG leads and multiple study nights were used to minimize the loss of data due to dislodging of electrodes during the night and technical failures, which cannot be remedied during the course of unattended in-home sleep studies. EEG recordings from the C3 electrode were used for scoring unless the signal was lost or contaminated by artifacts, in which case the C4 electrode recordings were used. EEGs and EMGs were sampled at 256 Hz and were scored at these frequencies. Signals for automated analysis were first decimated to 128 Hz. Filter settings were low pass 70 Hz and high pass 0.3 Hz. Summary sleep staging variables were calculated over Nights 2 and 3 for all but sleep disordered breathing and leg movement measures, which were calculated from Night 1 (see above). Data collected on Night 1 were not used in the present analyses (except apnea + hypopnea index [AHI] and periodic leg movements with arousal index [PLMAI]) due to the potentially disruptive effects of breathing and leg movement sensors on sleep, as well as the “first night effect,” which has been observed both in home and laboratory sleep studies.
Visual sleep stage scoring was conducted using Stellate Harmonie software by trained PSG technologists who had established reliability and were blind to participant characteristics (e.g., menopausal status, racial/ethnic group). Raw PSG signals are visually inspected for artifact prior to sleep stage scoring, and unscorable epochs excluded. A low pass filter of 8 Hz was used for EEG signals prior to period-amplitude analysis in the 0.5–2 Hz range to remove high frequency artifacts. The output of EEG period-amplitude analysis was aligned with visual scoring output in the database, to make sure that only scored sleep epochs were included in period-amplitude analyses. Finally, plots displaying period-amplitude analysis aligned with visually scored sleep hypnograms were visually inspected as a final quality control step.28 Intraclass correlation coefficients for wake, NREM, and REM ranged from 0.78 for REM sleep to 0.96 for wakefulness after sleep onset.
Sleep was scored in 20-sec epochs using standard scoring criteria,29 supplemented by apnea-hypopnea criteria derived from American Academy of Sleep Medicine recommendations30 and standard rules for scoring periodic leg movements.31 In regard to sleep cycles, individual NREM and REM periods were defined as follows: a REM period was defined as the amount of time (min) between the first and last epoch of REM sleep, consisting of ≥ 3 min of REM sleep, and followed by ≥ 30 consecutive min of NREM or wakefulness; a NREM period was defined as the amount of time (min) occurring after sleep onset or a REM period (as defined above), and ending with the beginning of a REM period (as defined above) or the end of the recording. We had no formal definition of “skipped” REM periods. REM periods and REM latency were simply defined based on the above criteria.
Dependent Variables
The 2 primary sleep variables of interest were DSR and REM latency. Based on previous research,21,32 delta counts were taken only from NREM sleep (all stages). Delta counts were defined by frequency of 0.5–2 Hz and amplitude ≥ 75 microvolts. Methods for period-amplitude analysis have been described in previous publications.28,33,34 The analysis has an amplitude threshold but does not quantify amplitude. Therefore, we cannot comment on the possibility that mood symptoms are associated with delta amplitude rather than delta abundance. Computer-detected total and average delta wave counts were correlated with visually scored delta percent (Spearman r = 0.75 and 0.77, respectively). A DSR ≥ 1.0 is consistent with homeostatic sleep drive patterns of greater delta activity in the first compared to the second NREM period.
REM latency, defined as the time between sleep onset and the first REM period minus any wakefulness occurring during this interval, was visually scored. Shortened REM latencies (approximately ≤ 65 min) are associated with depression.19,20 Because our sample was not selected for the presence of depression, we used REM latency as a continuous measure rather than a threshold value cutpoint.
Other PSG-assessed measures of interest included sleep duration (time spent asleep), continuity (sleep latency, wakefulness after sleep onset, sleep efficiency), and sleep architecture (percentages of delta and REM sleep). Time in bed was calculated as time from reported lights out (and confirmation of PSG signals consistent with reduced activity) to time of reported awakening from sleep (and confirmation of PSG signals consistent with increased activity). Sleep latency was calculated as time from beginning of the recording period to the first of 10 consecutive min of stage 2 or stage 3–4 sleep interrupted by ≤ 3 min of combined stage 1 or wakefulness. Time spent asleep was the total number of minutes spent in any sleep stage after sleep onset. Wakefulness after sleep onset (WASO) was the total minutes of wakefulness between sleep onset and wake-up time. Sleep efficiency is a percentage computed as the time spent asleep/time in bed × 100. Visually scored NREM and REM sleep percents were calculated as percentage of sleep time spent in NREM sleep stages 1, 2, delta (stages 3+4), and REM sleep.
The identification of bedtime, and therefore sleep latency, is challenging for in-home studies. People fall asleep before they go to bed, take naps, etc. We identified bedtime using a convergence of information from different sources: first, participants' self-reported bedtime from the concurrent sleep diary; second, by the appearance of characteristic “settling” of the PSG signals near the self-reported bedtime; and third, by identifying unambiguous stage 2 NREM sleep and going backwards in the record to a period of persistent and unambiguous wakefulness.
Independent Variables
Vasomotor symptoms
Nocturnal VMS (cold sweats, hot flashes or flushes, night sweats) were assessed for each of the 2 PSG nights using the morning diary, and were coded “yes” if ≥ 1 of the 3 symptoms was recorded or “no” if no symptom was recorded. If data were missing for only one of the 3 symptoms, the VMS score was based on the 2 available symptoms, but if data were missing for 2 symptoms we scored that night's VMS as “missing” unless the one available symptom recorded was present.
Mood symptoms
Depressive symptoms were assessed with the 16-item self-report version of the Inventory of Depressive Symptomatology (IDS),35,36 Sleep domain items (4 items) were omitted to avoid overlap with study outcomes, with a resulting IDS range of scores from 0–24. Anxiety symptoms were assessed with the 10-item version of the self-report State Trait Anxiety Inventory–State scale (STAI–State),37,38 and the score could range from 10–40. Data for IDS and STAI-State were collected on Day 4 of the protocol, which followed the in-home PSG studies, and were scored if ≥ 80% of the IDS domains or STAI-State items were completed; scores were weighted to account for missing items.
Covariates
Variables evaluated as covariates in the present report were those known to influence sleep and/or relationships among sleep, mood and VMS.
Sociodemographics
Race/ethnicity was determined by self-identification as African American, Caucasian, or Chinese. Other sociodemographic variables from the Core SWAN study included age, marital status (single, married/living as if married, separated/widowed/divorced), educational level (high school or less, some college, college degree or beyond), annual household income (< $20,000, $20,000–$50,000, > $50,000), financial strain (not very, somewhat, very difficult paying for basics), and study site. Marital status and income were obtained from the closest available Core SWAN assessment preceding the Sleep Study, and education and financial strain were obtained at the Core SWAN baseline.
Sleep variables
AHI and PLMAI from the screening PSG assessment both were used as continuous variables.
Menopausal transition status
Transition status was determined using bleeding criteria39 assessed at the closest Core SWAN visit preceding the PSG: premenopausal (no change in menstrual bleeding regularity) or early perimenopausal (menses in the preceding 3 months with an increase in bleeding irregularity); late perimenopausal (menses in the previous 12 months, but not the previous 3 months); or postmenopausal (≥ 12 months of amenorrhea).
Overall health- and medication-related variables
Self-assessed overall health (excellent, very good/good, fair/poor)40 and body mass index (BMI; weight in kilograms/height in meters squared) data were obtained from the annual Core SWAN visit preceding the woman's participation in the Sleep Study. Daily medication use (prescription and over-the-counter), recorded at Sleep Study protocol inception and on daily diaries, was coded according to the World Health Organization Anatomical Therapeutic Chemical (ATC) classification.41 For study purposes, medications that could potentially affect sleep parameters were those associated with the following ATC classification codes: opioids (N02A), antiepileptics (N03A), anxiolytics (N05B), hypnotics and sedatives (N05C), antidepressants (N06A), and antihistamines for systemic use (R06A). Use of medication from N05B, N05C, and/or N06A was designated as “psychotropic medication use.”
Health behavior variables
Physical activity was measured at Core SWAN baseline and annual examinations. A composite score, based on the Kaiser Permanente Activity Score42 and a modification of the Baecke scale,43 reflected 3 domains (sports, leisure, and household activities); we used the score from the closest available annual visit preceding the Sleep Study. Daily Sleep Study diaries were the data sources for concurrent smoking and alcohol and caffeine use on PSG sleep study days.
Data Analysis
Analyses were conducted with data from the 343 women having ≥ 1 post-screening PSG sleep study. When both of these nights were available (n = 293), they were consecutive in all but 6 instances. Mean sleep outcomes were computed when both nights were available; otherwise, the result from the single available night was used. For those whose data from Nights 2 and 3 were averaged, there were no overall differences between the 2 nights on any of the sleep variables included in these analyses. If a participant experienced VMS on either PSG night she was coded as having experienced VMS (yes/no). Of the 265 who had VMS data available for both nights, 174 (65.7%) were concordant for not having VMS on both nights, 47 (17.8%) were concordant for having VMS on both nights, 17 (6.4%) had VMS on the first night and not on the second night, and 27 (10.2%) had VMS on the second night and not on the first night. Thus, almost half (n = 44; 48.4%) of the 91 who experienced VMS on either night experienced VMS symptoms on only 1 of the 2 nights.
Univariate descriptors of all sample characteristics and sleep measures were calculated using means (standard deviations [SD]) for normally distributed variables, medians (interquartile range [IQR]) for continuous non-normally distributed variables, and frequencies (%) for categorical variables. Unadjusted differences in sample characteristics and sleep measures between racial/ethnic groups were tested using analysis of variance (ANOVA) for normally distributed variables, Kruskal-Wallis test for continuous non-normally distributed variables, and χ2 test for categorical variables. Unadjusted differences in sleep measures between women with and without VMS were tested using a t-test for normally distributed variables or a Wilcoxon test for continuous non-normally distributed variables. Unadjusted relationships between sleep measures and the IDS or STAI-State scores were tested using Spearman correlations. Two-sided P-values < 0.05 were considered statistically significant.
In preparation for model building, natural log transformations of sleep measures were employed to reduce skewness and satisfy the normality assumption. In the first modeling stage, linear regression models were used to investigate our hypotheses that the DSR would be lower and REM latency would be shorter in women with nocturnal VMS or higher depressive and anxiety symptom scores after adjusting for age, race/ethnicity (referent group was “Caucasian”), and menopausal status (late peri- or post-menopausal vs. pre-/early peri-menopausal). Similarly, linear regression analyses were used to investigate our hypotheses that sleep duration, sleep latency and sleep efficiency, and selected sleep architecture variables (delta and REM percent) would decrease (and WASO would increase) with nocturnal VMS or higher depressive and anxiety symptom scores. These analyses produced 3 models for each sleep outcome.
In the second stage of modeling, VMS was added to the IDS and STAI models as a covariate to test for any changes in the relationship between the sleep outcomes and mood symptoms. In the third stage of modeling, an interaction term between VMS and the mood variable was added to each model.
In the fourth stage, potential confounders were explored in all models that had significant findings in the first, second, or third stage models. Psychotropic medication use was added as a covariate to all of these models. Other confounders were identified by backwards variable selection, and those associated with the sleep measure at P < 0.20 were kept in the these models. A sensitivity analysis, in which women who reported taking psychotopic medication were removed from the analyses, was performed for all significant models.
SAS 9.1 (SAS Institute, Cary, NC) was used to perform the statistical analyses and assumption checks, and Microsoft Excel 2007 (Microsoft Corp., Seattle, WA) was used to graph results.
RESULTS
Sample Characteristics
Almost two-thirds of the women were premenopausal or early perimenopausal, by study design (Table 1). Nocturnal VMS were least prevalent in Caucasians and most prevalent in African Americans. Mean IDS and STAI-State scores were low and did not differ significantly among racial/ethnic groups. Antidepressant use differed significantly among the racial/ethnic groups; it was highest among Caucasian women and lowest among Chinese women.
Table 1.
Sample characteristics (N = 343)a
| Characteristic | Total (N = 343) | Caucasian (N = 161) | African American (N = 129) | Chinese (N = 53) | P-valueb |
|---|---|---|---|---|---|
| Age in years, mean (SD) | 51.6 (2.1) | 51.7 (2.1) | 51.4 (2.1) | 52.1 (2.1) | 0.14 |
| Marital status, N (%) | < 0.0001 | ||||
| Married/living as married | 209 (62.6) | 120 (76.0) | 51 (41.1) | 38 (73.1) | |
| Single | 53 (15.9) | 20 (12.7) | 24 (19.4) | 9 (17.3) | |
| Widowed/separated/divorced | 72 (21.6) | 18(11.4) | 49 (39.5) | 5 (9.6) | |
| Education, N (%) | < 0.0001 | ||||
| ≤ High school | 57 (17.1) | 20 (12.7) | 26 (21.1) | 11 (21.2) | |
| Some college | 110 (33.0) | 43 (27.2) | 56 (45.5) | 11 (21.2) | |
| ≥ College degree | 166 (49.8) | 95 (60.1) | 41 (33.3) | 30 (57.7) | |
| Annual household income, N (%) | < 0.0001 | ||||
| < $20,000 | 26 (8.3) | 7 (4.7) | 16 (14.3) | 3 (6.0) | |
| $20,000 – 50,000 | 152 (48.7) | 62 (41.3) | 67 (59.8) | 23 (46.0) | |
| $50,000 | 134 (43.0) | 81 (54.0) | 29 (25.9) | 24 (48.0) | |
| Difficulty paying for basics, N (%) | < 0.0001 | ||||
| Not very | 235 (73.7) | 123 (79.4) | 66 (58.9) | 46 (88.5) | |
| Somewhat | 70 (21.9) | 29 (18.7) | 35 (31.3) | 6 (11.5) | |
| Very | 14 (4.4) | 3 (1.9) | 11 (9.8) | 0 (0.0) | |
| Site, N (%) | < 0.0001 | ||||
| Chicago | 86 (25.1) | 39 (24.2) | 47 (36.4) | 0 (0.0) | |
| Davis/Kaiser | 102 (29.7) | 49 (30.4) | 0 (0.0) | 53 (100.0) | |
| Michigan | 65 (19.0) | 23 (14.3) | 42 (32.6) | 0 (0.0) | |
| Pittsburgh | 90 (26.2) | 50 (31.1) | 40 (31.0) | 0 (0.0) | |
| Menopausal status, N (%) | 0.37 | ||||
| Premenopausal/early perimenopausal | 222 (64.7) | 109 (67.7) | 77 (59.7) | 36 (67.9) | |
| Late perimenopausal | 73 (21.3) | 30 (18.6 ) | 30 (23.3) | 13 (24.5) | |
| Postmenopausal | 48 (14.0) | 22 (13.7) | 22 (17.1) | 4 (7.6) | |
| On hormones | 3 (6.3) | 3 (13.6) | 0 (0.0) | 0 (0.0) | |
| Nocturnal vasomotor symptoms (VMS), N (%) | 0.02 | ||||
| None | 217 (65.2) | 113 (71.5) | 68 (55.7) | 36 (67.9) | |
| VMS | 116 (34.8) | 45 (28.5) | 54 (44.3) | 17 (32.1) | |
| Mood questionnaires, mean (SD) | |||||
| IDS (minus 4 sleep items) | 2.8 (2.6) | 2.6 (2.3) | 3.0 (2.9) | 2.8 (3.1) | 0.50 |
| STAI–State version | 14.9 (5.1) | 14.9 (5.1) | 14.8 (4.8) | 15.5 (5.8) | 0.71 |
| Self-assessed overall health status, N (%) | |||||
| Excellent | 50 (14.9) | 33 (20.9) | 9 (7.1) | 8 (15.4) | 0.0045 |
| Very good/good | 244 (72.6) | 113 (71.5) | 96 (76.2) | 35 (67.3) | |
| Fair/poor | 42 (12.5) | 12 (7.6) | 21 (16.7) | 9 (17.3) | |
| Body mass index, kg/m2, mean (SD) | 30.0 (7.5) | 29.6 (7.0) | 33.4 (7.6) | 23.3 (2.9) | < 0.0001 |
| Medications (ATC codes), N (%) | |||||
| Opioids (N02A) | 8 (2.4) | 6 (3.8) | 1 (0.8) | 1 (1.9) | 0.25 |
| Antiepileptics (N03A) | 13 (3.8) | 7 (4.4) | 3 (2.4) | 3 (5.7) | 0.51 |
| Anxiolytics (N05B) | 8 (2.4) | 3 (1.9) | 5 (3.9) | 0 (0.0) | 0.24 |
| Hypnotics – sedatives (N05C) | 9 (2.7) | 6 (3.8) | 1 (0.8) | 2 (3.8) | 0.27 |
| Antidepressants (N06A) | 46 (13.5) | 30 (18.8) | 13 (10.2) | 3 (5.7) | 0.02 |
| Antihistamines for systemic use (R06A) | 81 (23.8) | 37 (23.1) | 32 (25.2) | 12 (22.6) | 0.90 |
| Total physical activity score (excluding work), mean (SD) | 7.7 (1.7) | 8.2 (1.7) | 7.5 (1.7) | 7.1 (1.5) | 0.0003 |
| Smoking, ≥ 1 mean cigarette per day (sleep diary), N (%) | 35 (10.4) | 10 (6.3) | 25 (20.0) | 0 (0.0) | < 0.0001 |
| Alcohol consumption, > 1 mean drink per day (sleep diary), N (%) | 14 (4.3) | 10 (6.5) | 2 (1.7) | 2 (3.8) | 0.14 |
| Caffeine consumption, > 2 mean drinks per day (sleep diary), N (%) | 75 (22.2) | 55 (34.6) | 13 (10.3) | 7 (13.2) | < 0.0001 |
| Apnea + hypopnea index, per h of sleep, PSG night 1, mean (SD) | 8.4 (9.7) | 9.1 (11.0) | 7.9 (8.6) | 7.6 (8.0) | 0.49 |
| Periodic leg movement index (movements with arousal), per h of sleep, PSG night 1, mean (SD) | 3.9 (5.3) | 4.3 (6.3) | 3.9 (4.7) | 2.7 (3.0) | 0.15 |
N, number; SD, standard deviation; ATC, Anatomical Therapeutic Chemical classification; HT, hormone therapy; IDS, Inventory of Depressive Symptomatology; PSG, polysomnography; STAI, State-Trait Anxiety Inventory–State version; VMS, vasomotor symptoms.
All characteristics do not sum to participant totals for column due to missing data. Percentages are calculated after excluding participants with missing data. Columns may not sum to 100% due to rounding.
To test for difference between ethnicities the following tests were used: for categorical variables a χ2 test, for continuous normally distributed variables an ANOVA, for continuous non-normally distributed variables a Kruskal-Wallis Test.
Previously, we have reported statistically significant racial/ethnic differences for visually scored and quantitative sleep measures.13 In the present report, we also observed significant racial/ethnic differences for DSR, which was not examined previously (Table 2). Delta (stage 3+4) sleep distribution was skewed by race/ethnicity. Nineteen of the 27 women who had 0% delta sleep were African American, and this group also had the lowest mean DSR.
Table 2.
Sleep measures by race/ethnicitya
| Variable | Total sample (N = 343) | Caucasian (N = 161) | African American (N = 129) | Chinese (N = 53) | P-valueb |
|---|---|---|---|---|---|
| Visually scored sleep measures | |||||
| Time in bed, minutes, mean (SD) | 453.9 (63.8) | 458.5 (54.5) | 448.6 (76.8) | 452.6 (54.4) | 0.42 |
| Sleep latency, minutes, median (IQR) | 14.3 (14.2) | 12.8 (12.3) | 16.5 (19.8) | 11.3 (11.3) | 0.0012 |
| Time spent asleep, minutes, mean (SD) | 382.9 (58.9) | 395.1 (49.7) | 362.9 (67.0) | 394.9 (50.4) | < 0.0001 |
| Wake after sleep onset, minutes, median (IQR) | 43.5 (32.2) | 42.5 (28.3) | 46.8 (39.7) | 38.8 (28.2) | 0.0028 |
| Sleep efficiency, %, mean (SD) | 84.5 (8.0) | 86.2 (5.9) | 81.1 (9.7) | 87.3 (6.3) | < 0.0001 |
| Stage, %, median (IQR) | |||||
| 1 | 5.6 (4.0) | 5.2 (3.5) | 6.1 (4.9) | 6.0 (4.8) | 0.02 |
| 2 | 65.0 (8.9) | 64.9 (8.5) | 65.8 (8.9) | 63.9 (7.0) | 0.23 |
| 3 + 4 (delta sleep) | 1.8 (4.4) | 2.9 (4.9) | 1.0 (3.2) | 1.4 (3.8) | < 0.0001 |
| REM | 24.8 (6.1) | 24.8 (5.1) | 23.9 (5.9) | 25.8 (4.3) | 0.09 |
| REM latency, minutes, median (IQR) | 61.7 (26.7) | 64.3 (31.3) | 58.9 (25.5) | 62.7 (18.0) | 0.02 |
| Automated sleep measures | |||||
| Total delta wave counts (0.5-2.0 Hz)–whole night, mean (SD) | 4830.1 (2319.3) | 4043.7 (2347.7) | 5431.7 (2068.9) | 4883.7 (2451.7) | < 0.0001 |
| Average delta wave counts per minute – whole night, mean (SD) | 16.8 (7.7) | 14.8 (8.0) | 18.5 (7.1) | 16.8 (7.9) | 0.0002 |
| Delta Sleep Ratio,c median (IQR) | 1.4 (0.7) | 1.4 (0.6) | 1.3 (0.7) | 1.5 (0.7) | 0.03 |
SD, standard deviation; IQR, interquartile range.
Sleep measures averaged from nights 2 and 3 if two nights available; otherwise, data from one night only. Ns for the table range from 337 to 343, depending on the symptom measure and sleep variable.
To test for difference between race/ethnicity the following tests were used: ANOVA for continuous normally distributed variables and Kruskal-Wallis Test for continuous non-normally distributed variables.
Ratio of average delta EEG wave counts per minute in the first NREM sleep period to the average delta EEG wave counts per minute in the second NREM sleep period.
IDS and STAI-State scores were correlated moderately (Spearman r = 0.49), but both correlated only weakly with VMS (Spearman r = 0.12 and 0.16, respectively). Univariate analyses revealed significant associations among nocturnal VMS and symptoms of depression and anxiety in relation to sleep measures. Women reporting nocturnal VMS had longer sleep duration but had less delta sleep percentage compared to women who did not report VMS (Table 3A). Higher IDS scores correlated significantly with more WASO, and higher STAI-State scores correlated significantly with lower sleep efficiency (Table 3B).
Table 3A.
Sleep measures in women with and without nocturnal vasomotor symptoms (N = 333)a
| Sleep Variable | No VMSb (N = 217) | VMSb (N = 116) | P-valuec |
|---|---|---|---|
| Sleep latency, min, median (IQR) | 14.5 (14.0) | 13.3 (14.7) | 0.47 |
| Time spent asleep, min, mean (SD) | 377.7 (59.4) | 393.0 (58.5) | 0.02 |
| Wake after sleep onset, min, median (IQR) | 42.2 (29.7) | 45.6 (31.8) | 0.20 |
| Sleep efficiency, %, mean (SD) | 84.5 (8.6) | 84.7 (6.9) | 0.84 |
| % Delta sleep, median (IQR) | 1.9 (4.5) | 1.6 (4.2) | 0.04 |
| Delta sleep ratio, median (IQR) | 1.3 (0.7) | 1.4 (0.6) | 0.15 |
| % REM sleep, mean (SD) | 24.7 (5.5) | 24.5 (5.2) | 0.69 |
| REM latency, min, median (IQR) | 65.6 (29.3) | 63.5 (32.3) | 0.16 |
Sleep measures averaged from nights 2 and 3 if two nights available; otherwise from one night only. Ns range from 327 to 333.
VMS, vasomotor symptoms recorded in morning sleep diary.
To test for difference between VMS occurrence the following tests were used: t-test for continuous normally distributed variables and Wilcoxon Test for continuous non-normally distributed variables.
Table 3B.
Correlations between mood symptoms and sleep measures (N = 343)a
| Sleep Variable | IDS (4 sleep questions excluded) |
STAI |
||
|---|---|---|---|---|
| rSb | P-value | rSb | P-value | |
| Sleep latency, min | 0.0579 | 0.29 | 0.0486 | 0.37 |
| Time spent asleep, min | 0.0851 | 0.12 | −0.0432 | 0.43 |
| Wake after sleep onset, min | 0.1188 | 0.03 | 0.0658 | 0.22 |
| Sleep efficiency, % | −0.0752 | 0.17 | −0.1069 | 0.05 |
| % Delta sleep | −0.0638 | 0.24 | 0.0200 | 0.71 |
| Delta sleep ratio | 0.0578 | 0.29 | 0.0565 | 0.30 |
| % REM sleep | −0.0031 | 0.95 | −0.0506 | 0.35 |
| REM latency, min | 0.0910 | 0.09 | 0.0531 | 0.33 |
Symptoms and EEG Sleep Measures
After adjusting for age, race/ethnicity and menopausal status, women with nocturnal VMS slept 22 minutes longer than women who did not report VMS. This effect remained significant after including, in separate models, either IDS or STAI, and their interactions with VMS (models 2 and 3; not shown in Table 4). This effect persisted after controlling for psychotropic medication use (Table 4, model 4). Race/ethnicity was the only other significant covariate in model 4; African American women slept approximately 36 minutes less than Caucasian women.
Table 4.
Summary of main multivariate analysis findings: vasomotor symptoms (VMS), Inventory of Depressive Symptomatology (IDS) and State Trait Anxiety Inventory–State Scale (STAI)
| Sleep Outcome Measures | Analysis | Variables | Model 1a Basic Model |
Model 2b VMS added to IDS or STAI |
Model 3c VMS interaction with IDS or STAI |
Model 4d Fully Adjusted |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P-value | β | P-value | β | P-value | β | P-value | |||
| Time spent asleep (min) | VMS | VMS | 22.29 | 0.001 | 21.58 | 0.002 | ||||
| Psychotropics | 15.89 | 0.058 | ||||||||
| Ln (Sleep latency) | VMS | VMS | −0.11 | 0.208 | ||||||
| IDS | IDS | 0.02 | 0.230 | 0.02 | 0.132 | −0.01 | 0.735 | −0.01 | 0.723 | |
| VMS | −0.14 | 0.111 | −0.40 | 0.002 | −0.30 | 0.035 | ||||
| VMS × IDS | 0.09 | 0.008 | 0.05 | 0.166 | ||||||
| PLMAI | 0.02 | 0.004 | ||||||||
| Pay for basics | 0.31 | 0.004 | ||||||||
| AHI | −0.01 | 0.125 | ||||||||
| Psychotropics | 0.19 | 0.118 | ||||||||
| STAI | STAI | 0.01 | 0.259 | 0.01 | 0.142 | −0.004 | 0.694 | −0.002 | 0.880 | |
| VMS | −0.13 | 0.155 | −0.90 | 0.002 | −0.72 | 0.016 | ||||
| VMS × STAI | 0.05 | 0.004 | 0.04 | 0.041 | ||||||
| PLMAI | 0.02 | 0.003 | ||||||||
| Pay for basics | 0.30 | 0.005 | ||||||||
| AHI | −0.01 | 0.165 | ||||||||
| Psychotropics | 0.16 | 0.171 | ||||||||
| Sleep efficiency | VMS | VMS | 1.02 | 0.263 | ||||||
| IDS | IDS | −0.08 | 0.596 | −0.09 | 0.574 | 0.15 | 0.446 | 0.26 | 0.225 | |
| VMS | 1.24 | 0.175 | 3.28 | 0.015 | 2.82 | 0.047 | ||||
| VMS × IDS | −0.70 | 0.038 | −0.51 | 0.164 | ||||||
| Heath | ||||||||||
| Fair/poor | −2.61 | 0.163 | ||||||||
| Good | −1.84 | 0.154 | ||||||||
| Pay for basics | −2.60 | 0.019 | ||||||||
| Marital status | ||||||||||
| Single | 2.32 | 0.064 | ||||||||
| Sep/Wid/Div | 4.25 | 0.005 | ||||||||
| Psychotropics | −0.45 | 0.713 | ||||||||
| STAI | STAI | −0.13 | 0.122 | −0.13 | 0.111 | 0.003 | 0.978 | 0.02 | 0.812 | |
| VMS | 1.19 | 0.193 | 7.70 | 0.007 | 7.02 | 0.020 | ||||
| VMS × STAI | −0.43 | 0.017 | −0.38 | 0.046 | ||||||
| Pay for basics | −2.56 | 0.017 | ||||||||
| Marital status | ||||||||||
| Single | 2.82 | 0.022 | ||||||||
| Sep/Wid/Div | 4.50 | 0.002 | ||||||||
| Psychotropics | −0.45 | 0.700 | ||||||||
Ln, natural logarithm; β, unstandardized β coefficient.
Model 1: β estimates for VMS, IDS, or STAI based on linear model adjusted for age, menopausal status, and race/ethnicity.
Model 2: β estimates for explanatory variables (VMS added to either IDS or STAI) based on linear model adjusted for age, menopausal status, and race/ethnicity.
Model 3: β estimates adds interaction term (either VMS × IDS or VMS × STAI) to Model 2.
Model 4: Fully adjusted models include the Model 3 variables plus covariates that met criterion for entry into model, as described in text (No interaction term in VMS model 4 for time spent asleep because it was not significant).
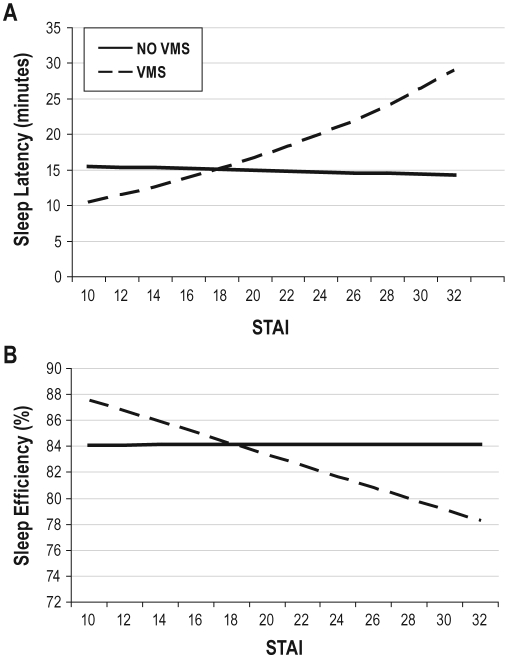
VMS moderated the effect of STAI-State and IDS on sleep latency and on sleep efficiency and VMS also was a significant main effect in the interaction models (model 3). Figure 1 shows that minutes to sleep latency increased 5.5% for each unit increase in STAI-State score in women with VMS compared to those without VMS (β = 0.05, P = 0.004) and sleep efficiency decreased by 0.46 percentage points for each unit increase in STAI-State score in women with VMS compared to those without VMS (β = −0.43, P = 0.017). Table 4 (model 4) shows that the VMS-by-STAI-State interaction effects persisted after covariate adjustment (sleep latency: β = 0.04, P = 0.041; sleep efficiency: β = −0.38, P = 0.046). VMS-by-IDS interactions similarly were significant for sleep latency and efficiency in model 3, but the effects did not persist after full covariate adjustment (model 4), as shown in Table 4.
Figure 1.
Vasomotor symptoms (VMS)-by-State Trait Anxiety Inventory–State scale (STAI) interactions for sleep latency (A) and sleep efficiency (B), adjusted for age, race/ethnicity, and menopausal status. Estimated mean sleep latency (in min) and sleep efficiency (percent) are plotted on the Y-axes and STAI score on both X-axes. After covariate adjustment, the β coefficients remained significant for the VMS-by-STAI interaction terms (β = 0.04, P = 0.04 for the natural log transformed sleep latency and β = −0.38, P < 0.05 for sleep efficiency) and for the VMS main effects (β = −0.72, P = 0.02 for sleep latency and β = 7.02, P = 0.02 for sleep efficiency). See text for details.
In addition to the significant covariates shown in Table 4 (model 4), African American women had significantly longer sleep latency and lower sleep efficiency than did Caucasian women, and the postmenopausal group had significantly shorter sleep latency than did the pre-/early perimenopausal group.
Contrary to our hypotheses, neither the delta (DSR, delta sleep percent) nor REM (latency, REM percent) sleep variables were significantly associated with VMS or mood symptoms in models 1, 2, or 3. WASO also was not significantly associated with any of the 3 symptoms.
Sensitivity Analysis
Significant findings were reanalyzed after removing women taking psychotropic medication. The VMS - sleep duration association remained significant (β = 22.84, P = 0.002). For sleep latency the results were similar, but despite a similar unstandardized β coefficient estimate, the VMS-by-STAI-State interaction for Model 4 showed only a trend effect in this smaller sample (β = 0.04, P = 0.088). For sleep efficiency, the sample size reduction had a similar effect on the significance level for the interaction β estimate in the fully adjusted model (β = −0.33, P = 0.107).
DISCUSSION
Our analyses revealed no evidence to support the primary hypothesis that nocturnal VMS, depressive, or anxiety symptoms are associated with REM latency and DSR. Failure to find associations may be due to our definition of REM latency, which subtracts out wakefulness from the interval between sleep onset and REM (RLmA), and is intended to reduce the “noise” of the definition that does not subtract wakefulness. However, the two do not differ significantly among depressed patients44,45 and in our sample were highly correlated (r = 0.96). More likely, the lack of a relationship between mood symptoms and sleep variables could be related to the low levels of depressive mood/anxiety symptoms in our community sample of middle-aged women, which was not selected for the presence of depression. Perhaps the findings may have differed if the range of symptoms of depression was broader.
There may be limitations regarding the DSR as an indicator of mood changes. Although research has not consistently confirmed DSR to be a correlate of depression or depression outcomes,46,47 it is also worth noting that subsequent studies have sometimes used different methods of measuring delta activity or different definitions of DSR, which could affect findings. We defined DSR using the same analyses as the Kupfer studies, i.e., period-amplitude analysis with a 0.5 to 2.0 Hz frequency band range and an amplitude threshold of 75 μV. As noted by Buysse et al.,32 human studies have shown that delta waveform amplitude as well as incidence decrease with age, and that the mean delta frequency increases.48 Studies which have not found a reduction in overall delta activity associated with depression have sampled a broad range of delta EEG amplitude,49,50 whereas we have limited our explorations to the frequency range of 0.5–2 Hz. High-amplitude delta in particular may be decreased in depressed patients as well as in the elderly32; the latter may or may not be relevant to our middle-aged sample,which was not selected for the presence of depression, in that the effects of mood and aging may be confounded. These differences in samples and methods could explain some of the discrepant findings between the literature and our results.
In contrast to the results for REM latency and DSR, significant differences were found for other sleep measures, specifically sleep latency, sleep efficiency, and sleep duration. VMS moderated the association between mood symptoms, particularly anxiety, and sleep latency and efficiency. VMS also were directly associated with longer sleep duration. Although seemingly counterintuitive, in fact, women with VMS also spent more time in bed (longer total recording period), consistent with its moderating effect on sleep efficiency.
Our findings suggest that worse mood is associated with poorer sleep, but only in those with concurrent VMS, and do not support our prediction that mood symptoms would be associated with shorter REM latency and lower DSR.20,32 Other studies have indicated that in perimenopausal women, sleep disturbances may be related to psychological distress rather than an inevitable consequence of the menopausal transition per se.51,52 Our data suggest that there may be important interplay between psychological and physiological symptoms.
Because SWAN is an observational study, we were unable to discontinue medications prior to PSG studies. Antidepressants are known from previous studies to affect sleep parameters, particularly REM latency and DSR. Moreover, VMS and anxiety symptoms even within non-pathological ranges are associated with depressive symptoms, and more severe VMS during peri- and post-menopause have been associated with higher depression and anxiety scores.8,53 Because VMS and mood symptoms can improve with antidepressant use,54,55 we first adjusted for psychotropic medication use and then reanalyzed our significant findings after excluding women who reported using them. In the sensitivity analyses, the results for both sleep latency and efficiency became statistically nonsignificant despite little change in the parameter estimates, likely due to reduced power after excluding 57 women in each of these two analyses.
As we observed previously in our cohort, there were significant differences between African American and Caucasian women on these PSG indices of sleep continuity.13 Durrence and Lichstein12 found relatively consistent results in a review of the four available PSG studies. Compared with Caucasians' sleep, the sleep of African Americans is lighter (increased stages 1 and 2, decreased stages 3 and 4) and more fragmented (longer sleep latencies, greater wakefulness after sleep onset). Consistent with these findings, Mezick et al.56 found that a group of African American men and women had longer sleep latency, shorter sleep duration, less continuous sleep, and a smaller percentage of delta sleep, compared with a combined Caucasian and Asian group, which persisted after controlling for socioeconomic status. Their results were consistent for both PSG and actigraphic sleep measures.
These results are consistent, to some extent, with observations that post-menopausal women as compared with pre-/early perimenopausal women tend to self-report worse sleep but demonstrate better sleep quality as measured by PSG indices.11 In our sample, the post-menopausal group had the largest proportion of women reporting nocturnal VMS, but compared with the pre-/early perimenopausal and late perimenopausal groups, the shortest mean sleep latency.
Limitations of our study include the cross-sectional nature of the data as well as use of self-report menopausal status and nocturnal VMS. We defined menopausal transition status according to bleeding criteria rather than hormone levels. Using SWAN's definition, Gracia et al.57 found significantly different hormone levels among the menopausal stages. Studies, including SWAN (e.g., Sowers et al.58) and the Seattle Midlife Women's Health Study (Woods et al.59), have shown that hormone levels and changes in these levels can differ in their associations with objective and subjective sleep outcomes. Thus, varied findings among studies may be related to populations studied, whether objective or subjective sleep measures were used, and whether the tested associations were between bleeding indicators of status or ovarian or gonadatropin hormones and sleep. As well, the method of analysis and design, cross-sectional or longitudinal, should be considered. Longitudinal analyses of individual symptom clusters and their interrelation over time are needed.
Regarding VMS, we could not examine more specifically the independent effects of frequency and severity of these events because too few women (4%) reported nocturnal VMS that were not bothersome. Thurston et al.60 observed that VMS frequency predicted bothersomeness, but that the two terms were not interchangeable as other factors also contributed to VMS severity. Moreover, because retrospective self-reports of previous night's VMS occurrence can be inaccurate, concurrent measures of temperature or skin conductance could strengthen the evidence for associations between VMS and specific sleep parameters.61 We should also note that, by design, 65% of our sample were either pre- or early perimenopausal, and 65% of the sample, including 73% of the pre- and early perimenopausal women, reported no VMS.
This study also has a number of strengths. Most importantly, sleep measures were obtained from repeated in-home polysomnographic studies conducted in a large sample of women from three racial/ethnic groups.
In conclusion, we found evidence for EEG sleep correlates of VMS and mood symptoms, particularly anxiety, in this multi-ethnic sample of midlife women. VMS moderated associations of anxiety with EEG sleep measures of sleep latency and sleep efficiency and was associated with longer sleep duration. These findings provide further support for examining the role of VMS in the sleep of midlife women, and its moderating effects on mood, which is commonly associated with sleep symptoms, in observational, mechanistic, and treatment studies of sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Buysse serves as a consultant for the following: Actelion, Cephalon, Eisai, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Philips, Sanofi-Aventis, Sepracor/Sunovion, Servier, Somnus Therapeutics, Takeda, and Transcept Pharmaceuticals, Inc. He has helped to produce CME materials and has given paid CME lectures indirectly supported by industry sponsors. Ms. Avery currently is working on a behavioral clinical trial involving low income heart failure patients that is funded by Novartis and is on the Board of Directors for the Illinois Gas Company. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). Funding for the SWAN Sleep Study is from the National Institute on Aging (Grants AG019360, AG019361, AG019362, AG019363). Sleep data were processed with the support of RR024153. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Institutions where this study was performed:Rush University Medical Center, Chicago, IL; University of California-Davis, Davis, CA; University of Michigan, Ann Arbor, MI; University of Pittsburgh, Pittsburgh, PA.
Clinical Centers: University of Michigan, Ann Arbor – MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Rachel Wildman, PI 2010–present; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Sherry Sherman 1994–present; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory:University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001–present; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995–2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair
The authors thank the study staff at each site and all the women who participated in SWAN. The authors also thank Dr. Charles Spielberger for permission to use the STAI questionnaire.
REFERENCES
- 1.McKinlay SM, Brambilla DJ, Posner J. The normal menopause transition. Maturitas. 1992;14:103–15. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 2.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: The Study of Women's Health Across the Nation (SWAN) J Affect Disord. 2007;103:267–72. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bromberger JT, Kravitz HM, Matthews K, et al. Predictors of first lifetime episodes of major depression in midlife women. Psychol Med. 2009;39:55–64. doi: 10.1017/S0033291708003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: The Harvard Study of Moods and Cycles. Arch Gen Psychiatry. 2006;63:385–90. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 5.Freeman EW, Sammel MD, Liu L, et al. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 6.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63:375–82. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 7.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women's Health Across the Nation (SWAN) Am J Public Health. 2006;96:1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman EW, Sammel MD, Lin H, et al. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause. 2005;12:258–66. doi: 10.1097/01.gme.0000142440.49698.b7. [DOI] [PubMed] [Google Scholar]
- 9.Dennerstein L, Lehert P, Guthrie JR, et al. Modeling women's health during the menopausal transition: A longitudinal analysis. Menopause. 2007;14:53–62. doi: 10.1097/01.gme.0000229574.67376.ba. [DOI] [PubMed] [Google Scholar]
- 10.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- 11.Young T, Rabago D, Zgierska A, et al. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–72. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 12.Durrence HH, Lichstein KL. The sleep of African Americans: A comparative review. Behav Sleep Med. 2006;4:29–44. doi: 10.1207/s15402010bsm0401_3. [DOI] [PubMed] [Google Scholar]
- 13.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in mid-life women: The SWAN Sleep Study. Sleep. 2009;32:73–82. [PMC free article] [PubMed] [Google Scholar]
- 14.Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: A community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Moline ML, Broch L, Zak R, et al. Sleep in women across the life cycle from adulthood through menopause. Sleep Med Rev. 2003;7:155–77. doi: 10.1053/smrv.2001.0228. [DOI] [PubMed] [Google Scholar]
- 16.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166:1262–8. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 17.Pien GW, Sammel MD, Freeman EW, et al. Predictors of sleep quality in women in the menopausal transition. Sleep. 2008;31:991–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Bromberger JT, Assman SF, Avis NE, et al. Persistent mood symptoms in a multiethnic community cohort of pre- and perimenopausal women. Am J Epidemiol. 2003;158:347–56. doi: 10.1093/aje/kwg155. [DOI] [PubMed] [Google Scholar]
- 19.Benca RM, Obermeyer WH, Thisted RA, et al. Sleep and psychiatric disorders: A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Germain A, Nofzinger EA, et al. Mood disorders and sleep. In: Stein DJ, Kupfer DJ, Schatzberg AF, editors. The American psychiatric publishing textbook of mood disorders. Washington, DC: American Psychiatric Publishing, Inc.; 2006. pp. 717–37. [Google Scholar]
- 21.Kupfer DJ, Frank E, McEachran AB, et al. Delta sleep ratio: A biological correlate of early recurrence in unipolar affective disorder. Arch Gen Psychiatry. 1990;47:1100–5. doi: 10.1001/archpsyc.1990.01810240020004. [DOI] [PubMed] [Google Scholar]
- 22.Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression. Results from the Massachusetts Women's Health Study. Ann Epidemiol. 1994;4:214–20. doi: 10.1016/1047-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 23.Bromberger JT, Schott LL, Kravitz HM, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: Results from the Study of Women's Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010;67:598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause. 2002;9:392–8. doi: 10.1097/00042192-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Joffe H, Soares CN, Thurston RC, et al. Depression is associated with worse objectively and subjectively measured sleep, but not more frequent awakenings, in women with vasomotor symptoms. Menopause. 2009;16:671–9. doi: 10.1097/gme.0b013e3181957377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadimitriou GN, Linkowski P. Sleep disturbance in anxiety disorders. Int Rev Psychiatry. 2005;17:229–36. doi: 10.1080/09540260500104524. [DOI] [PubMed] [Google Scholar]
- 27.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Marcus R, Kelsey J, editors. Menopause: biology and pathobiology. San Diego, CA: Academic Press; 2000. pp. 175–88. [Google Scholar]
- 28.Doman J, Detka C, Hoffman T, et al. Automating the sleep laboratory: Implementation and validation of digital recording and analysis. Int J Biomed Comput. 1995;38:277–90. doi: 10.1016/s0020-7101(05)80010-8. [DOI] [PubMed] [Google Scholar]
- 29.Rechtschaffen A, Kales A. Los Angeles, CA: Brain Information Service/Brain Research Institute, UCLA; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 30.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 31.The Atlas Task Force. Recording and scoring leg movements. Sleep. 1993;16:749–59. [PubMed] [Google Scholar]
- 32.Buysse DJ, Frank E, Lowe KK, et al. Electroencephalographic sleep correlates of episode and vulnerability to recurrence in depression. Biol Psychiatry. 1997;41:406–18. doi: 10.1016/S0006-3223(96)00041-8. [DOI] [PubMed] [Google Scholar]
- 33.Buysse DJ, Hall M, Tu XM, et al. Latent structure of EEG sleep variables in depressed and controls: Descriptions and clinical correlates. Psychiatry Res. 1998;79:105–22. doi: 10.1016/s0165-1781(98)00028-6. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Kupfer DJ, Frank E, et al. Electroencephalographic sleep studies in depressed outpatients treated with interpersonal psychotherapy. I. Baseline studies in responders and nonresponders. Psychiatry Res. 1992;40:13–26. doi: 10.1016/0165-1781(92)90035-2. [DOI] [PubMed] [Google Scholar]
- 35.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol Med. 1996;26:477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 36.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. Erratum. Biol Psychiatry 2003;54:585. [DOI] [PubMed] [Google Scholar]
- 37.Spielberger CD. Tampa, FL: University of South Tampa; 1979. Preliminary Manual for the State-Trait Personality Inventory (STPI) Unpublished manuscript. [Google Scholar]
- 38.Spielberger CD, Reheiser EC. Measuring anxiety, anger, depression, and curiosity as emotional states and personality traits with the STAI, STAXI, and STPI. In: Hersen M, Hilsenroth MJ, Segal DL, editors. Comprehensive handbook of psychological assessment, volume 2: personality assessment. Hoboken, NJ: John Wiley & Sons, Inc.; 2003. pp. 70–86. [Google Scholar]
- 39.World Health Organization Scientific Group. Geneva: World Health Organization; 1996. Research on the Menopause in the 1990s. Report of a WHO Scientific Group. WHO Tech Serv Rep Ser 866; pp. 1–107. [PubMed] [Google Scholar]
- 40.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 41.World Health Organization. Guidelines for ATC Classification. [Accessed December 10, 2007]. Available at: http://www.whocc.no/atcddd.
- 42.Sternfeld B, Ainsworth BA, Quesenberry CP., Jr. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–23. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 43.Baecke JAH, Burema J, Fritjers JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Amer J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 44.Coble P, Kupfer DJ, Shaw DH. Distribution of REM latency in depression. Biol Psychiatry. 1981;16:453–66. [PubMed] [Google Scholar]
- 45.Reynolds CF, Taska LS, Jarrett DB, et al. REM latency in depression: Is there one best definition? Biol Psychiatry. 1983;18:849–63. [PubMed] [Google Scholar]
- 46.Armitage R, Hoffmann R, Trivedi M, Rush AJ. Slow-wave activity in NREM sleep: Sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000;95:201–3. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 47.Landolt HP, Gillin JC. Similar sleep EEG topography in middle-aged depressed patients and healthy controls. Sleep. 2005;28:239–47. doi: 10.1093/sleep/28.2.239. [DOI] [PubMed] [Google Scholar]
- 48.Feinberg I, Fein G, Floyd TC, et al. Delta (.5-3 Hz) EEG waveforms during sleep in young and elderly normal subjects. In: Chase MH, editor. Sleep disorders: basic and clinical research. New York: Spectrum; 1983. pp. 449–62. [Google Scholar]
- 49.Armitage R, Calhoun JS, Rush AJ, et al. Comparison of the delta EEG in the first and second non-REM periods in depressed adults and normal controls. Psychiatry Res. 1992;41:65–72. doi: 10.1016/0165-1781(92)90019-y. [DOI] [PubMed] [Google Scholar]
- 50.Mendelson WB, Sack DA, James SP, et al. Frequency analysis of the sleep EEG in depression. Psychiatry Res. 1987;21:89–94. doi: 10.1016/0165-1781(87)90067-9. [DOI] [PubMed] [Google Scholar]
- 51.Shaver JL, Paulsen VM. Sleep, psychological distress, and somatic symptoms in perimenopausal women. Fam Pract Res J. 1993;13:373–84. [PubMed] [Google Scholar]
- 52.Baker A, Simpson S, Dawson D. Sleep disruption and mood changes associated with menopause. J Psychosom Res. 1997;43:359–69. doi: 10.1016/s0022-3999(97)00126-8. [DOI] [PubMed] [Google Scholar]
- 53.Juang K-D, Wang S-J, Lu S-R, et al. Hot flashes are associated with psychological symptoms of anxiety and depression in peri- and post- but not premenopausal women. Maturitas. 2005;52:119–26. doi: 10.1016/j.maturitas.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Argyropoulos SV, Wilson SJ. Sleep disturbances in depression and the effects of antidepressants. Int Rev Psychiatr. 2005;17:237–45. doi: 10.1080/09540260500104458. [DOI] [PubMed] [Google Scholar]
- 55.Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes. Systematic review and meta-analysis. JAMA. 2006;295:2057–71. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 56.Mezick EJ, Matthews KA, Hall M, et al. Influence of race and socioeconomic status on sleep: Pittsburgh Sleep SCORE Project. Psychosom Med. 2008;70:410–6. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gracia CR, Sammel MD, Freeman EW, et al. Defining menopause status: Creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12:128–35. doi: 10.1097/00042192-200512020-00005. [DOI] [PubMed] [Google Scholar]
- 58.Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31:1339–49. [PMC free article] [PubMed] [Google Scholar]
- 59.Woods NF, Smith-Dijulio K, Percival DB, et al. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: Observations from the Seattle Midlife Women's Health Study. J Womens Health. 2007;16:667–77. doi: 10.1089/jwh.2006.0138. [DOI] [PubMed] [Google Scholar]
- 60.Thurston RC, Bromberger JT, Joffe H, et al. Beyond frequency: Who is most bothered by vasomotor symptoms? Menopause. 2008;15:841–7. doi: 10.1097/gme.0b013e318168f09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freedman RR. Hormone dynamics and menopausal symptoms: The clinical role of vasomotor symptoms and sleep disturbances. In: Soares CN, Warren M, editors. The menopausal transition: interface between psychiatry and gynecology. Basel, Switzerland: S. Karger AG; 2009. pp. 88–101. [Google Scholar]