Abstract
Objectives:
Event-related potential (ERPs) provide an exquisite means to monitor the extent of processing of external stimulus input during sleep. The processing of relatively high intensity stimuli has been well documented. Sleep normally occurs in much less noisy environments. The present study therefore employed ERPs to examine the extent of processing of very low intensity (near-hearing threshold) stimuli.
Design:
Brief duration 1000 Hz auditory tone bursts varying in intensity at random from −5 to +45 dB from normative hearing level (nHL) in 10 dB steps were presented every 1.5 to 2.5 s when the subject was awake and reading a book and again during all-night sleep.
Subjects:
n = 10 healthy young adults.
Measurements and Results:
In the waking state, the auditory stimuli elicited a negative-going deflection, N1, peaking at about 100 ms, followed by a smaller positivity, P2, peaking at about 180 ms. N1-P2 gradually decreased in amplitude with decreases in stimulus intensity and remained visible at near-hearing threshold levels. During NREM sleep, the amplitude of N1 was at baseline level and was reduced to only 15% to 20% of its waking amplitude during REM sleep. P2 was much larger in sleep than in wakefulness. Importantly, during sleep, P2 could be reliably elicited by the auditory stimuli to within 15 dB of threshold. During NREM, a large amplitude negativity peaking at about 350 ms was elicited by the higher intensity stimuli. This N350 was much reduced in amplitude during REM sleep. A significant N350 was not, however, elicited when stimuli intensity levels were below 25 dB nHL.
Conclusions:
Auditory stimuli that are only slightly above hearing threshold appear to be processed extensively during a 200 to 400 ms interval in both NREM and REM sleep. The nature of this processing is, however, very different compared to the waking state.
Citation:
Campbell K; Muller-Gass A. The extent of processing of near-hearing threshold stimuli during natural sleep. SLEEP 2011;34(9):1243-1249.
Keywords: NREM, REM, sleep, event-related potentials, auditory processing, auditory stimuli, hearing threshold
INTRODUCTION
Sleep is said to be a profoundly unconscious state because the processing of all but the most relevant of stimulus input is presumably gated (or inhibited) prior to entry into consciousness. As a result, the sleeper appears to be almost completely unaware of their external environment. Several studies have now examined the extent to which relatively high intensity stimuli are processed during sleep. Sleep, however, normally occurs in environments in which acoustic input is much reduced. Little is known about the processing of such low intensity auditory stimuli.
The quantification of information processing during unconscious states is facilitated by the recording of event-related potentials (ERPs), the tiny changes in the electrical activity of the brain that are elicited by an external stimulus or internal psychological event. ERPs consist of a series of negative- and positive-going components. These components reflect the extent of processing of the auditory stimulus from the peripheral auditory nerve and brainstem centers to higher, cortical regions. It has long been known that the “short” latency auditory brainstem evoked potentials (ABEPs), occurring within the first 10 ms after presentation of a brief auditory stimulus, are almost completed unaffected by sleep.1,2 The presentation of a longer duration auditory stimulus will elicit “long” latency components that peak between 50 and 300 ms after stimulus onset. The most studied of these components is a negative deflection, N1, peaking at 75–100 ms. There is now substantial evidence that the sources of this N1 component originate in or near the auditory cortex, although there may be contributions from the frontal lobe.3 N1 is followed by a later positive deflection, P2, peaking at about 175-225 ms. Collectively, these long latency ERPs have come to be known as the “vertex” potential because they are prominent over the central (or vertex) region of the scalp. In the waking state, the amplitude of N1-P2 varies directly with the intensity of the auditory stimulus.4,5 Several labs have reported that the N1-P2 deflection remains visible to within 5–20 dB of the subjective hearing threshold.6–10
N1 and P2 are much altered by sleep. The amplitude of N1 declines dramatically during drowsiness and sleep onset.11,12 During definitive NREM sleep, its amplitude does not exceed the pre-stimulus zero voltage baseline level, while during REM sleep its amplitude may only reach 15% to 30% of its waking level. Although the amplitude of N1 is difficult to observe during NREM sleep, the amplitude of P2 is usually larger compared to the waking state.13 The large attenuation of N1 but enhancement of P2 during sleep has been explained in detail by Campbell and Colrain.11,12 In brief, they employ the classic, elaborate Näätänen model14 of auditory processing (see also recent revision of this model15) to describe the changes in processing that occur between the conscious, waking state and the unconscious, sleeping state. This model emphasizes that a large N1-P2 can be elicited passively by auditory stimuli, without the need for active attention. However, when attention is directed to the auditory channel, an additional attention-related negative component, termed the processing negativity (PN), is also elicited. Its amplitude varies with the extent of attention that is directed to the channel. Importantly, this long-lasting attention-related negativity will overlap and summate with the scalp-recorded N1 and P2. Although the generator sources of the PN are not known, in the Näätänen model of auditory processing, the PN and N1 negativities are claimed to be independent components reflecting different aspects of processing. The withdrawal of attention may result in the attenuation of the PN. Nevertheless, even unattended, to-be-ignored stimuli elicit some PN. The waking observer will therefore always be at least partially conscious of stimuli occurring in a to-be-ignored channel. Campbell and Colrain label this as the waking processing negativity (wPN) to distinguish it from the type of processing that occurs during sleep. It is this consciousness-related wPN that must be inhibited for sleep to occur and to prevent awakenings during sleep. The wPN thus dissipates during the sleep onset period. With the removal of the overlapping and summating negativity of the attention-related wPN, the amplitude of the scalp-recorded N1 is reduced (less negative-going) to or near baseline level, while the amplitude of the scalp-recorded P2 is enhanced (i.e., also is less negative-going). During REM sleep, a small N1 and P2 are often elicited. Presumably, this is related to a change in information processing, a partial return of the PN, and with it possible consciousness of the external environment. In support of this notion, Cote et al.16,17 and Macdonald et al.18 have noted that infrequently occurring but very high intensity stimuli may elicit a later positivity, a “P3”-like wave peaking at about 300 ms during REM sleep. The P3-like waves are associated with conscious processing in the waking state.19,20 However, while the P3 component can be only elicited during sleep by very rare and very loud, obtrusive stimuli, the earlier P2 is easily elicited by frequently occurring and much less intense acoustic stimuli. Its amplitude does vary directly with the intensity of the stimulus, at least for moderate and high intensity stimuli.16,17 Crowley and Colrain13 hypothesize that the large P2 observed during sleep reflects an inhibitory process, preventing arousals by external stimuli that might otherwise result in disruption. It is not known whether P2 can be reliably recorded following presentation of the low intensity stimuli that typically occur during normal sleep. Such stimuli are unlikely to result in arousals.
Certain ERPs can only be elicited in the sleep state. During NREM sleep, a later, very large amplitude (about 25 μV) central maximum negativity peaking between 300–400 ms may be elicited by moderate to high intensity stimuli.21–23 This “N350” is unique to sleep and cannot be elicited in the waking state. It probably corresponds to the “sleep N2” described by Picton et al.24 that was elicited by an auditory stimulus as the subject spontaneously fell asleep, and appears to be equivalent to the much-reported vertex sharp wave.23 A smaller amplitude N350 can also be elicited in REM sleep. Colrain and Campbell12 postulate that the N350 also reflects an inhibitory process, protecting sleep from loud and obtrusive stimulus input that would otherwise disrupt it. In support of this claim, the amplitude of N350 does vary directly with the intensity of loud auditory stimuli.17,25 It is possible that the protective role of the P2 process may be inadequate for higher intensity stimuli, thus the need for a later N350 process. On the other hand, the P2 may suffice to protect sleep from the effects of lower intensity stimuli, and thus, N350 would not be elicited. Again however, it is not known whether the N350 can be elicited by much lower intensity stimuli.
The purpose of the present study is therefore to determine the extent of processing of very low intensity auditory stimuli during definitive NREM and REM sleep. The intensity of these stimuli varied from 5 dB below to 45 dB above the normative hearing threshold. ERPs were recorded while the subject was awake, prior to sleep and again during the various stages of sleep.
METHODS
Subjects
Ten self-reported good sleepers (5 females) between the ages of 19 and 26 years spent a single night in the sleep lab. All subjects had previously participated in sleep studies. None had a history of neurological, psychiatric, or sleep disorders. All reported normal hearing. Hearing level was subsequently verified during the actual testing session. Subjects were instructed to refrain from naps during the day of the recording. They were also asked to refrain from caffeine and alcohol use in the 24 h prior to testing and this was verified prior to testing. Subjects signed a consent form and received an honorarium for participation in this study. The study was conducted according to the guidelines of the Canadian Tri-Council (Health, Natural and Social Sciences) on ethical conduct involving humans.
Stimuli
Auditory stimuli were synthesized by a SoundBlaster 16-bit waveform generator card. A 1000 Hz pure tone having a total duration of 55 ms and a 5 ms rise-and-fall time was presented to the right ear using EAR 3A insert earphones. This assured constancy of stimulus input in spite of head movements during sleep. Stimuli were presented every 1.5–2.5 s (on average every 2 s). The random rates of stimulus presentation prevented subjects from predicting the onset of the stimulus. The intensity level of the tones varied in 10 dB steps: −5, +5, +15, +25, +35, and +45 dB from the normative hearing level (nHL) of young adults established previously in our lab.9 Within each block, the lower intensity level stimuli were randomly presented 100 times each, and the moderate intensity 35 and 45 dB nHL stimuli, 50 times each. The duration of a block was thus approximately 16.7 min.
Procedure
Subjects arrived at the laboratory at approximately 20:00 in order to allow time for electrode application procedures, hearing threshold assessment, and waking data collection. Following placement of electrodes, subjects were taken to a separate double-walled, sound-attenuated testing chamber. Noise levels in the testing chamber measured approximately 35–40 dB SPL. Prior to the start of the experiment, the hearing threshold for each of the participants was assessed. All subjects were able to reliably detect (detection rate of 0.70 or greater) the +5 dB nHL auditory stimulus. The detection rate for the −5 dB nHL stimulus did not exceed 0.20 for any subject.
The waking physiological data were collected between 22:00 and 23:00. Subjects were asked to read a book or magazine of their choice and ignore the auditory stimuli. Horizontal eye movements were monitored to verify compliance with these instructions. Two blocks of stimuli were presented, with a short break between blocks.
Subjects were permitted to sleep at 23:30. Ten minutes after entering definitive stage N2 sleep (marked by delta activity in the EEG, spindles, and K complexes), stimuli were again presented in separate 16.7 min blocks. Time between blocks was approximately 10–15 min. If there was evidence of arousal or movement, stimulus presentation was paused and only resumed again when the subject returned to the same stage of sleep. This was however relatively rare (on average, fewer than 8 occurrences per subject during the entire stimulus presentation periods). Only blocks in which the subject did not change sleep stage (i.e., the entire block consisted of a homogenous stage of sleep) were retained for further analysis. At least 2 blocks of stimuli were recorded within each stage of sleep.
Physiological Recordings
The electroencephalogram (EEG) and electrooculogram (EOG) were recorded using Grass gold-cup electrodes. They were filled with electrolytic paste, and affixed to the scalp with gauze and to the skin with surgical tape. The EEG was recorded from 4 scalp locations placed at midline frontal (Fz), central (Cz), parietal (Pz), and occipital (Oz) sites. The Oz placement was used for the identification of alpha activity, which gradually dissipates during the sleep onset period. The reference electrode was placed on the right mastoid. A vertical EOG was recorded from electrodes placed at the supra- and infra-orbital ridges of the right eye. A horizontal EOG was recorded from electrodes placed at the outer canthus of each eye. Blinks, vertical eye movements, and saccadic eye movements (associated with reading, while the subject was awake) could easily be distinguished from the random slow horizontal eye movements that typically appear in stage N1 sleep. A ground electrode was placed on the forehead. Inter-electrode impedances were kept < 5 kΩ. The filter bandpass of the amplifiers was from 0.16 to 35 Hz. The EEG and EOG data were digitized at a 256 Hz sampling rate and stored continuously to hard disk.
Data Analysis
The different stages (Wake, N2, N3, REM) were reclassified by 2 experienced scorers according to the American Academy of Sleep Medicine (AASM) task force criteria.26 Because of the possible confusion with ERPs labeled “N1” and “N2,” the NREM sleep stages were labeled as s(tage)N2, sN3, and REM. A 16 s epoch was used for sleep staging rather than the usual 30 s in order to increase the precision of the scoring. In cases of scorer disagreement, the entire 16.7 min block of data was excluded from further analysis. Each block retained for analysis, therefore, represented an unambiguous stage of sleep. Most of sN3 occurs in the first half of the night, while most of stage REM occurs in the last half of the night. Possible differences in processing between stages sN3 and REM might thus be explained by time-of-night effects. An approximately equal proportion of sN2 occurs in the 2 halves of the night. The sN2 data were therefore compared in the first (sN2 early) and second (sN2 late) halves of the night, thus permitting the assessment of possible time-of-night differences.
Eye movement and blink artifact occurring in the waking state were corrected in the continuous EEG using an algorithm operating in the time and frequency domain.27 Eye movements during REM sleep are less problematic. This is because they occur at random during this stage of sleep (i.e., are not time-locked to the stimulus) and mainly consist of horizontal rather than vertical eye movements. Horizontal eye movements cause minimal artifact in midline scalp recordings. NREM sleep was examined for possible K complexes occurring 450–650 ms after stimulus onset. These occurred very rarely (< 1% of stimulus presentations). They also occurred as often following the presentation of the below threshold (−5 dB nHL) stimulus compared to the above threshold stimuli. Thus, the rare occurrence of the K complex probably reflects spontaneous rather than stimulus-locked events. The continuous EEG was therefore reconstructed into discrete 500 ms trials beginning 50 ms prior to stimulus onset and continuing for 450 ms following it. Trials were rejected if the EEG exceeded ± 100 μV in the waking state or ± 150 μV during sleep (to permit the inclusion of high amplitude delta waves in the EEG). Within each block, the trials were then sorted and averaged according to electrode site and stimulus intensity level. The data were collapsed over same-stage blocks to improve the signal-to-noise ratio of the ERP. Separate ERPs were thus obtained for each stage (Wake, sN2 early, sN2 late, sN3, REM) for each intensity level (−5, +5, 15, 25, 35, and 45 dB nHL), and for each electrode site (Fz, Cz, Pz). The resulting averages were digitally filtered using an inverted FFT algorithm with a 1–20 Hz bandpass.
The average of all data points in the 50 ms pre-stimulus interval served as a zero voltage baseline from which the ERPs were measured. During wakefulness, both the peaks of N1 and P2 were difficult to measure at the lowest stimulus intensity levels in the individual subject waveforms. N1 was difficult to identify even at higher intensity levels during NREM sleep. Therefore, instead of maximum peak detection methods, the ERP deflections were quantified by computing mean ERP amplitudes.28 Based on the grand averages (average of all individual subjects' averages), the peak latencies of N1 and P2 and N350 were determined for each intensity level, at each stage. Subsequently, all data points within ± 20 ms of the peak latency of N1, P2, and N350 were averaged in the individual subject waveforms. N1, P2, and N350 were quantified at Cz, the site at which these components tend to be largest in amplitude.
The amplitude values obtained in the N1, P2, and N350 latency intervals were subjected to a 2-way ANOVA with repeated measures on stage and intensity level. Greenhouse-Geisser correction factors were applied when appropriate. Simple main effects testing was used to isolate the sources of any significant interaction. It was expected that N1 would be absent in NREM sleep and that the N350 would be absent in the waking state. It was not known if any ERPs would be apparent following the presentation of the near- or below-threshold level stimuli. The ANOVA statistical procedure cannot however be used to determine if an ERP component was actually elicited because the procedure only indicates whether differences among stages or among stimuli are significant. Confidence intervals were therefore computed for each intensity level within the waking and sleeping states to verify the presence of an N1, P2, or N350. This procedure determined if the amplitude of each ERP deflection was significantly different from the zero voltage pre-baseline level. Upper and lower confidence limits were thus computed. In the case of the negative deflections (N1, N350), if the upper limit was greater than 0 (i.e., had a positive polarity), the component was not considered to be present. In the case of the positive deflection, P2, the lower limit could not be less than 0 μV (i.e., had a negative polarity). Because the directionality of each ERP deflection was predicted, a liberal one-tailed test of significance (P < 0.05) was applied to the confidence intervals.
RESULTS
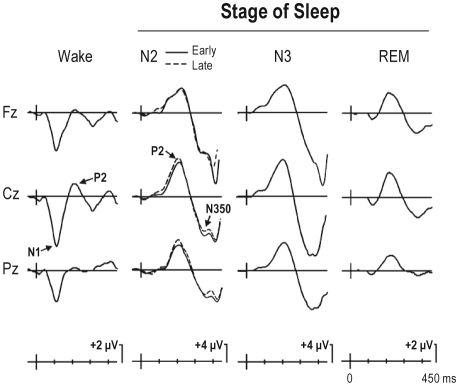
Figure 1 presents the grand averaged ERPs at Fz, Cz, and Pz for the highest intensity level stimulus (45 dB nHL) during the waking and sleeping states. As may be observed, in the waking state (left column), a large amplitude N1 maximal over centro-frontal areas of the scalp was visible at about 100 ms after stimulus onset. The N1 was followed by a smaller amplitude P2, peaking from 180–200 ms. Sleep had a dramatic effect on ERP morphology. During NREM sleep (sN2 early/late and sN3, middle columns), N1 was at or near baseline while P2 increased markedly in amplitude. During REM sleep (right column), N1 was elicited at about 15% of its waking amplitude, and was followed by a more prominent P2 than that elicited during the waking state. A very large (> 15 μV) central maximum negativity, the N350, was apparent during NREM sleep and remained visible, although much attenuated, during REM sleep. The N350 occurred only within the sleep state and was not apparent in the waking state. In the waking state, a small negativity is apparent following P2 but it peaked much earlier (about 280 ms) than the sleep N350.
Figure 1.
Grand averaged (n = 10) ERPs following presentation of the 45 dB nHL intensity level stimulus during the waking (left column) and sleeping states. ERPs elicited during NREM sleep (stages N2 and N3) are traced in the middle columns. Stage N2 is divided into its early (solid line) and late (dashed line) halves, based on time of night. ERPs elicited during REM sleep are traced in the right column. In this and all other figures, negativity at the scalp relative to the mastoid reference is indicated by a downward deflection. Note the differences in the calibration signal in stages Wake and REM compared to stages N2 and N3. This is to allow visualization of large ERP deflections elicited during stages N2 and N3. A large centro-frontal N1 was apparent in the waking state, when subjects were asked to ignore the auditory stimuli. N1 was much reduced during REM sleep and was at pre-stimulus baseline level during NREM sleep. On the other hand, only a small amplitude P2 was elicited during wakefulness but a much larger P2 was apparent during NREM sleep. A very large amplitude central negativity, peaking at about 350 ms was apparent during stages N2 and N3. The amplitude of this N350 was much reduced during stage REM. N350 was not apparent in the waking state.
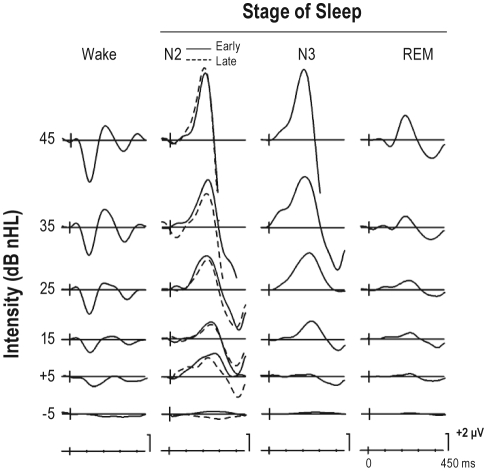
Figure 2 illustrates the effects of lowering the stimulus intensity level on the ERP waveforms elicited during the waking state and each stage of sleep.
Figure 2.
The effects of stimulus intensity level and differences across waking and sleeping states on the late auditory ERPs. These data are from the Cz electrode placement. The ERPs in NREM (stages N2 and N3) are now traced at the same scale as those in stages Wake and REM. The high amplitude N350 is therefore clipped when elicited by the 45 dB nHL stimulus. In the waking state, as stimulus intensity level was lowered, the amplitude of N1 (peaking at about 100 ms) gradually decreased. During NREM sleep, N1 was at baseline level while during REM sleep, a significant, but much reduced N1 was elicited, but only for the 45 dB nHL intensity level stimulus. On the other hand, a large amplitude P2 was apparent during NREM sleep and this deflection became gradually attenuated as stimulus intensity was reduced. A smaller amplitude P2 was apparent during REM sleep, while the P2 was much reduced during wakefulness. A very large amplitude N350 was apparent for the 35 and 45 dB nHL stimuli during NREM sleep. It was markedly attenuated during REM sleep. During all sleep stages, N350 rapidly declined in amplitude as the intensity level of the stimulus was reduced. N350 was not elicited in the waking state.
N1 Waveform
The mean N1 data for each sleep stage and stimulus intensity are presented in the upper portion of Table 1. Confidence interval analyses indicated that in the waking state, a significant N1 was elicited for the stimuli ranging from +5 to 45 dB nHL, but not for the −5 dB nHL stimulus. On the other hand, during NREM sleep, none of the stimuli elicited a significant N1, not even the loudest (45 dB nHL) stimulus. During REM sleep, only the 45 dB nHL stimulus elicited a significant N1.
Table 1.
Mean (SDs in parentheses) amplitude (in μV), of N1, P2, and N350, measured at Cz, as a function of intensity of the auditory stimulus and stage of sleep.
| Intensity (dB nHL) | N1 |
||||
|---|---|---|---|---|---|
| Wake | sN2 early | sN3 | sN2 late | REM | |
| 45 | −4.88 (1.95) | +2.40 (2.00) | +1.52 (1.62) | +1.23 (1.70) | −0.82 (1.51) |
| 35 | −3.53 (1.53) | +0.44 (1.71) | +2.30 (2.02) | +0.66 (0.68) | −0.13 (0.89) |
| 25 | −2.92 (1.31) | +0.42 (1.43) | +1.66 (2.01) | +0.74 (1.22) | +0.32 (0.72) |
| 15 | −1.51 (1.27) | +0.86 (1.70) | +0.28 (1.59) | +0.52 (1.02) | +0.25 (0.42) |
| +5 | −1.65 (0.92) | +0.20 (0.89) | +0.12 (1.55) | +0.30 (1.03) | +0.03 (0.50) |
| −5 | +0.14 (0.84) | −0.16 (0.63) | −0.08 (0.93) | −0.24 (0.71) | −0.03 (0.36) |
| Intensity (dB nHL) | P2 |
||||
| Wake | sN2 early | sN3 | sN2 late | REM | |
| 45 | +2.81 (1.88) | +14.88 (5.09) | +14.80 (4.95) | +13.89 (3.55) | +5.58 (3.10) |
| 35 | +3.32 (1.99) | +6.19 (2.52) | +11.83 (4.87) | +6.49 (2.48) | +2.48 (1.86) |
| 25 | +1.10 (1.53) | +3.58 (1.72) | +8.81 (4.18) | +5.39 (1.35) | +2.20 (1.77) |
| 15 | +1.07 (0.81) | +3.54 (1.66) | +3.87 (2.69) | +2.32 (1.01) | +1.11 (1.42) |
| +5 | +0.39 (0.91) | +2.32 (1.06) | +0.16 (1.93) | +0.79 (1.85) | +0.63 (1.32) |
| −5 | +0.14 (0.82) | +0.37 (0.87) | −0.07 (1.52) | −0.28 (0.73) | +0.04 (0.36) |
| Intensity (dB nHL) | N350 |
||||
| Wake | sN2 early | sN3 | sN2 late | REM | |
| 45 | — | −14.61 (12.86) | −25.07 (22.86) | −16.02 (10.61) | −4.25 (1.08) |
| 35 | — | −5.77 (4.23) | −7.15 (9.67) | −2.91 (4.09) | −3.03 (0.74) |
| 25 | — | −2.79 (4.49) | −0.82 (7.73) | −2.33 (3.07) | −1.45 (0.32) |
| 15 | — | −1.21 (2.33) | −1.30 (4.61) | −1.03 (2.93) | −0.79 (0.42) |
| +5 | — | +0.45 (2.87) | −2.16 (4.83) | −0.79 (2.62) | −0.66 (0.36) |
| −5 | — | −0.32 (2.13) | +0.18 (3.80) | −0.28 (2.40) | −0.24 (0.53) |
The repeated-measures ANOVA resulted in a significant Stage × Intensity interaction, F20,180 = 8.16, P < 0.05. Differences across waking and sleeping states were initially isolated for each intensity level. The N1 elicited during the waking state was significantly larger in amplitude than that observed in any of the stages of sleep (NREM and REM) at each of the above- threshold intensity levels (i.e. +5 to 45 dB nHL). N1 amplitude differences between NREM and REM were significant only for the highest intensity level (45 dB nHL): the amplitude of N1 was at baseline level during NREM but returned to about 15% of its waking level during REM sleep. N1 amplitude did not significantly vary among the different NREM stages, (sN2 early, sN2 late, and sN3) at any intensity level.
The effects of intensity level were also isolated within each sleep stage. In the waking state, a significant effect of stimulus intensity level was found. The amplitude of N1 gradually declined in amplitude as intensity level was lowered. The manipulation of intensity level did not have a significant effect on N1 during NREM sleep. During REM sleep, the effect of stimulus intensity was again significant: N1 was significantly larger following presentation for the 45 dB nHL stimulus compared to the lower intensity level (−5 to 25 dB nHL) stimuli.
P2 Waveform
The mean P2 data are presented in the middle portion of Table 1. Confidence interval analyses showed that during wakefulness, a significant P2 was apparent only for the 35 and 45 dB nHL stimuli. During both NREM and REM sleep, a significant P2 was elicited by stimuli ranging from 15 to 45 dB nHL. Furthermore, in stage sN2 early, P2 remained significant for the +5 dB nHL stimulus.
Again, a significant Stage × Intensity interaction was obtained for the amplitude of P2, F20,180 = 6.14, P < 0.05. P2 was significantly larger during NREM sleep than during either the waking state or REM sleep for each intensity level from 15 to 45 dB nHL. Differences among the NREM stages (i.e., sN2 early, sN3, sN2 late) were not significant. The P2 elicited during REM sleep was significantly larger than that observed in the waking state, but only for the loudest 45 dB nHL stimulus. Finally, a significant effect of stimulus intensity level was found during the waking state and during all stages of sleep. The amplitude of P2 gradually decreased from the highest to the lowest intensity stimuli for all stages.
N350 Waveform
The mean N350 data are presented in the lower portion of Table 1. Confidence interval testing revealed a significant N350 for only the loudest (45 and 35 dB nHL) stimuli during early and late portions of stage sN2, and sN3. The higher intensity (25 to 45 dB nHL) stimuli also elicited a significant N350 during REM sleep.
A significant Stage × Intensity interaction was again obtained, F15,135 = 3.99, P < 0.05. N350 was significantly larger in NREM (stages sN2 early, sN2 late, and sN3) than in REM sleep for the 35 and 45 dB nHL stimuli. Within NREM sleep, N350 was significantly larger during stage sN3 than during either sN2 early or sN2 late, but again only for the 35 and 45 dB nHL stimuli. Differences between early and late stage sN2 were not significant for any intensity level. The amplitude of N350 significantly decreased as a function of stimulus intensity across all stages of sleep.
DISCUSSION
As expected, during the waking state, the amplitude of N1 gradually declined as the intensity level of the stimuli decreased. A significant N1 was apparent for intensity levels ranging from +5 to 45 dB nHL. The amplitude of P2 was relatively small even for the highest (but still moderate) intensity level stimulus and thus systematic changes in its amplitude with changing intensity level were less consistent. These results are very similar to those previously reported by our lab9 using an identical rate of stimulus presentation and are consistent with a large number of studies, indicating that the auditory N1-P2 is able to accurately predict hearing threshold to within 5–20 dB of the behavioral threshold.5–8,10
ERPs provide an exquisite means of assessing perceptual and cognitive information processing during unconscious states such as natural sleep. A large series of studies have now indicated that the morphology of the long latency N1-P2 vertex potential, elicited by moderate to high intensity auditory stimuli, undergoes dramatic changes during natural sleep.11 Saremi et al.29 have discussed the features of auditory stimuli that are most likely to disturb sleep. Particularly important is indeed the intensity of the stimulus although other features such as the rise-time of the stimulus, its duration and rate of presentation also play a role. It is unusual however for the sleeper to be bombarded with frequently occurring high intensity stimuli in the normal environment. The present study examined the extent of processing of much lower intensity stimuli. In this and most other ERPs studies examining information processing during sleep, data were retained only if there were no evidence of arousals or changes in the stage of sleep. It would, of course, be appropriate to sort and average trials on the basis of whether a stimulus was associated with an arousal/disturbance or not. Unfortunately, the amplitude of N1-P2 is exceedingly low during sleep. The averaging procedures will allow the small amplitude N1-P2 to emerge from the background EEG, provided a sufficiently large number of trials are presented. As is apparent in the figures, during undisturbed sleep, the number of stimulus presentations was sufficient, and clear ERP components were apparent following the presentation of even very low intensity stimuli despite the high amplitude noise of slow wave sleep. There were far too few actual arousals/disturbances to permit an averaging of ERPs following presentation of stimuli associated with these events. Bonnet and colleagues30 indicate however that ERPs themselves, and particularly the N350, may reflect stimulus-related arousals that other measures (for example, EEG, EMG, EKG) fail to detect.
A novel finding in this study is that the ERPs elicited by both the moderate and the very low intensity level stimuli were also much affected by sleep. During NREM sleep, N1 was difficult to observe even at the highest intensity levels. P2 was however larger during NREM compared to the waking state, at least for higher intensity stimuli. This replicates many other studies using much higher intensity stimuli. The large decrease in N1 and large increase in the amplitude of P2 during sleep is also consistent with the removal of the long-lasting, summating negativity, waking PN. The absence of an N1 (and the overlapping wPN) during the entire NREM period, regardless of the intensity of the auditory stimulus, is consistent with the profound absence of consciousness of the external environment. On the other hand, during REM sleep, N1 was significantly different from the zero voltage baseline level but only for the highest 45 dB nHL intensity level stimulus when it reached about 15% of its waking amplitude; a significant N1 could not be detected when stimulus intensity level was at or below 35 dB nHL. Very high intensity and rarely presented stimuli have been reported to elicit longer latency P3-like deflections peaking between 250 and 350 ms16–18 perhaps reflecting an “intrusion into consciousness.” A P3 was not apparent in the present study either during wakefulness or REM sleep, presumably because stimuli were presented too rapidly and the intensity level of even the highest 45 dB nHL stimulus was much too low to result in forced consciousness.
The absence of an N1 during NREM sleep does not imply that processing of low intensity stimuli ceased altogether. A large P2 and (at times) N350 were apparent. Within sleep, the amplitude of P2 was significantly larger during all NREM sleep stages than during stage REM except for the lowest intensity levels. It has been postulated that P2 might reflect the inhibition of processing of stimulus input that potentially disturbs sleep.13 In this study, its amplitude did vary directly with the intensity of the stimulus; this is in keeping with the fact that higher intensity stimuli pose a greater risk for sleep disturbance. Importantly, during both NREM and REM sleep, P2 remained visible even when the intensity level of the stimulus was as low as 15 dB nHL (or even 5 dB nHL in early stage sN2). It would appear that almost any auditory stimulus, including those that are barely above hearing threshold, might affect the quality of sleep.
Bastien and Campbell25 have noted that a later N350 can be elicited by slowly presented (> every 5 s) 60 dB SPL stimuli (approximately equivalent to the 45 dB nHL). The more rapidly presented 45 and 35 dB nHL stimuli used in the present study also elicited significant N350s, its amplitude varying directly with the intensity level of the stimuli. The N350 is also thought to reflect an inhibitory process and can only be elicited during sleep.23,25 It does therefore appear that both the P2 and N350 processes serve to prevent sleep disturbance by moderate 35 and 45 dB nHL stimulus intensities. We hypothesized that only the P2 process would be required to inhibit sleep disturbance by lower intensity stimuli. This conclusion may not be valid. A small amplitude N350 was apparent when the subject was presented with the lower intensity +5 to 25 dB nHL stimuli, at least in stage sN2. However, its amplitude did not significantly differ from zero. The amplitude of N350 did show large inter- and intra-subject variability. It is possible that a large N350 was elicited in some subjects but not in others, resulting in a smearing of the overall grand average; this might be a reflection of individual sensitivity in the potential of low intensity level stimuli to disturb sleep. A much smaller amplitude and significantly reduced N350 was also apparent during REM sleep for the 25–45 dB nHL stimuli. It remains to be determined whether this REM-elicited N350 reflects the same processes as the N350 observed during NREM sleep.
In summary, the present results do provide insight into how very low intensity, very unobtrusive stimuli are processed during sleep. During NREM sleep, N1 was not elicited even by the moderate intensity level stimuli. The absence of an N1 probably reflects the loss of a “general consciousness” of the external environment. It does not necessarily indicate that preconscious processing has halted. Stimuli that were just 15 and perhaps only 5 dB above threshold did elicit a later positivity, P2, peaking at about 180 ms. During REM sleep, a significant, small amplitude N1 could only be elicited by the loudest 45 dB nHL stimulus. The removal of N1 and the enhancement of P2 are thought to reflect an inhibitory processing, preventing consciousness of the external environment, and protecting sleep from disturbance. At the higher 35 and 45 dB nHL intensity levels, a negativity peaking at about 350 ms (N350) was elicited. The appearance of a large N350 following such moderate intensity input might be a reflection of the need for additional inhibitory processes because that afforded by the P2 proves to be insufficient to protect sleep. The present study thus demonstrates that even near-threshold stimuli are extensively processed during sleep. These results may have implications for understanding the sensitivity of our perceptual system to external stimulation during sleep.
The subjects who participated in this study had also participated in previous sleep studies. They were also good sleepers. It is thus probable that much higher intensity stimuli would be required to result in frequent sleep disturbance. This may not be the case with poor sleepers or in cases in which sleep fragmentation is common.
In addition, the results have important implications in the applied, audiological setting. Traditional behavioral assessment of auditory threshold requires the active cooperation of the patient. This might not be possible in certain populations (e.g., infants, children, and the senile). ERPs can be elicited in the absence of attention (as was the case in the waking state in the present study). Nevertheless, the recording of ERPs in the waking and alert patient also requires active cooperation in order to reduce movement and other sources of artifact. This may be difficult. The present study indicates that ERPs can be recorded during sleep and can provide objective and highly sensitive measures of auditory threshold. Because hearing loss is often frequency-specific, a wider range of frequencies will need to be employed in future studies. Importantly, long testing periods may be required to identify the very low amplitude ERPs in individual subjects. This does limit the practical utility of ERP methods when testing takes place during wakefulness, but may be less problematic during sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was performed at the University of Ottawa, Canada. This research was funded by research grants from Natural Sciences and Engineering Research Council of Canada (NSERC) to Dr. Campbell. The authors thank the assistance of Parastoo Jamshidi and Margaret Macdonald in the collection of the data and in the staging of the sleep recordings.
REFERENCES
- 1.Campbell KB, Bartoli EA. Human auditory evoked potentials during natural sleep: The early components. Electroencephalogr Clin Neurophysiol. 1986;65:142–9. doi: 10.1016/0168-5597(86)90047-x. [DOI] [PubMed] [Google Scholar]
- 2.Bastuji H, Garcia-Larrea L, Bertrand O, Mauguière F. BAEP latency changes during nocturnal sleep are not correlated with sleep states but with body temperature variations. Electroencephalogr Clin Neurophysiol. 1988;70:9–15. doi: 10.1016/0013-4694(88)90189-7. [DOI] [PubMed] [Google Scholar]
- 3.Näätänen R, Picton TW. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 4.Picton TW, Woods DL, Baribeau-Braun J, Healey TMG. Evoked potential audiometry. J Otolaryngol. 1977;6:90–119. [PubMed] [Google Scholar]
- 5.Hyde M. The N1 response and its applications. Audiol Neurootol. 1997;2:281–307. doi: 10.1159/000259253. [DOI] [PubMed] [Google Scholar]
- 6.Stapells DR. Cortical event-related potentials to auditory stimuli. In: Katz J, editor. Handbook of clinical audiology. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2002. pp. 378–406. [Google Scholar]
- 7.Cone-Wesson B, Wunderlich J. Auditory evoked potentials from the cortex: audiology applications. Curr Opin Otolaryngol Head Neck Surg. 2003;11:372–77. doi: 10.1097/00020840-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Yeung KNK, Wong LLN. Prediction of hearing thresholds: Comparison of cortical evoked response audiometry and auditory steady state response audiometry techniques. Int J Audiol. 2007;46:17–25. doi: 10.1080/14992020601102238. [DOI] [PubMed] [Google Scholar]
- 9.Muller-Gass A, Marcoux A, Jamshidi P, Campbell KB. The effects of very slow rates of stimulus presentation on event-related potential estimates of hearing threshold. Int J Audiol. 2008;47:34–43. doi: 10.1080/14992020701647934. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda K, Hayashi A, Matsuda O, Sekiguchi T. An ignoring task improves validity of cortical evoked response audiometry. Neuroreport. 2010;21:709–15. doi: 10.1097/WNR.0b013e32833b502a. [DOI] [PubMed] [Google Scholar]
- 11.Campbell KB, Colrain IM. The impact of sleep onset on averaged evoked potentials. Int J Psychophysiol. 2002;46:197–214. doi: 10.1016/s0167-8760(02)00112-5. [DOI] [PubMed] [Google Scholar]
- 12.Colrain IM, Campbell KB. The use of evoked potentials in sleep research. Sleep Med Rev. 2007;11:277–93. doi: 10.1016/j.smrv.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowley K, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep, and modality. Clin Neurophysiol. 2004;115:732–44. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav Brain Sci. 1990;13:201–88. [Google Scholar]
- 15.Näätänen R, Kujala T, Winkler I. Auditory processing that leads to conscious perception: A unique window to central auditory processing opened by the mismatch negativity and related responses. Psychophysiology. 2011;48:4–22. doi: 10.1111/j.1469-8986.2010.01114.x. [DOI] [PubMed] [Google Scholar]
- 16.Cote KA, Campbell KB. P300 to high intensity stimuli during REM sleep. Clin Neurophysiol. 1999;110:1345–50. doi: 10.1016/s1388-2457(99)00074-7. [DOI] [PubMed] [Google Scholar]
- 17.Cote KA, Etienne L, Campbell KB. Neurophysiological evidence for the detection of external stimuli during sleep. Sleep. 2001;24:791–803. [PubMed] [Google Scholar]
- 18.Macdonald M, Jamshidi P, Campbell K. Infrequent increases in stimulus intensity may interrupt central executive functioning during rapid eye movement sleep. Neuroreport. 2008;19:309–13. doi: 10.1097/WNR.0b013e3282f4ede8. [DOI] [PubMed] [Google Scholar]
- 19.Escera C, Alho K, Winkler I, Näätänen R. Neural mechanisms of involuntary attention to acoustic novelty and change. J Cogn Neurosci. 1998;10:590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- 20.Muller-Gass A, Macdonald M, Schröger E, Sculthorpe L, Campbell K. Evidence for the auditory P3a reflecting an automatic process: elicitation during highly-focused continuous visual attention. Brain Res. 2007;1170:71–78. doi: 10.1016/j.brainres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Ogilvie RD, Simons IA, Kuderian RH, MacDonald T, Rustenburg J. Behavioral, event-related potential, and EEG/FFT changes at sleep onset. Psychophysiology. 1991;28:54–64. doi: 10.1111/j.1469-8986.1991.tb03386.x. [DOI] [PubMed] [Google Scholar]
- 22.Harsh J, Voss U, Hull J, Schrepfer S, Badia P. ERP and behavioral changes during the wake/sleep transition. Psychophysiology. 1994;31:244–52. doi: 10.1111/j.1469-8986.1994.tb02213.x. [DOI] [PubMed] [Google Scholar]
- 23.Colrain IM, Webster KE, Hirst G, Campbell KB. The roles of vertex sharp waves and K-complexes in the generation of N300 in auditory and respiratory-related evoked potentials during early stage 2 NREM sleep. Sleep. 2000;23:97–106. [PubMed] [Google Scholar]
- 24.Picton TW, Hillyard SA, Krausz HI, Galambos R. Human auditory evoked potentials. I. Evaluation of components. Electroencephalogr Clin Neurophysiol. 1974;36:179–90. doi: 10.1016/0013-4694(74)90155-2. [DOI] [PubMed] [Google Scholar]
- 25.Bastien C, Campbell KB. The evoked K-complex: all-or-none phenomenon? Sleep. 1992;15:236–45. doi: 10.1093/sleep/15.3.236. [DOI] [PubMed] [Google Scholar]
- 26.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–31. [PubMed] [Google Scholar]
- 27.Woestenburg JC, Verbaten MN, Slangen JL. The removal of the eye-movement artifact from the EEG by regression analysis in the frequency domain. Biol Psychol. 1983;16:127–47. doi: 10.1016/0301-0511(83)90059-5. [DOI] [PubMed] [Google Scholar]
- 28.Picton TW, Bentin S, Berg P, et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–52. [PubMed] [Google Scholar]
- 29.Saremi M, Greneche J, Bonnefond A, Rohmer O, Eschenlauer A, Tassi P. Effects of nocturnal railway noise on sleep fragmentation in young and middle-aged subjects as a function of type of train and sound level. Int J Psychophysiology. 2008;70:184–91. doi: 10.1016/j.ijpsycho.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Bonnet MH, Doghramji K, Roehrs T, et al. The scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med. 2007;3:133–45. [PubMed] [Google Scholar]
- 31.Gora J, Colrain IM, Trinder J. Respiratory-related evoked potentials during the transition from alpha to theta EEG activity in stage 1 NREM sleep. J Sleep Res. 1999;8:123–34. doi: 10.1046/j.1365-2869.1999.00144.x. [DOI] [PubMed] [Google Scholar]