Abstract
Study Objectives:
There is a lack of experimental evidence to support the hypothesis that sleep may modulate stroke outcome as suggested by clinical observations. We have previously shown that sleep disturbance (SDis) over 3 days aggravates brain damage in a rat model of focal cerebral ischemia. The aim of this study is to further investigate effects of SDis on long-term stroke recovery and neuroplasticity as assessed by axonal sprouting, neurogenesis, and angiogenesis.
Design:
Focal cerebral ischemia was induced by permanent occlusion of the distal branches of middle cerebral artery. Twelve hours after initiation of ischemia, SDis was performed over 3 consecutive days (deprivation of 80% sleep during the 12-h light phase). Weekly assessments on sensorimotor function by the single pellet reaching test (SPR) were performed for 5 weeks after surgery. Axonal sprouting was evaluated by anterograde tracing with biotinylated dextran amine (BDA) and neurogenesis/angiogenesis by bromodeoxyuridine (BrdU) labelling along with cell-type markers. Control groups included ischemia without SDis, sham with SDis, and sham without SDis.
Setting:
Basic sleep research laboratory.
Measurements and Results:
Rats subjected to SDis after ischemia showed significantly less recovery of forearm motor skills during the post-stroke period of 5 weeks. This effect was accompanied by a substantial reduction in axonal sprouting, expression of synaptophysin, and the ischemia-stimulated neural and vascular cell proliferation.
Conclusion:
SDis has detrimental effects on functional and morphological/structural outcomes after stroke, suggesting a role of sleep in the modulation of recovery processes and neuroplasticity.
Citation:
Zunzunegui C; Gao B; Cam E; Hodor A; Bassetti CL. Sleep disturbance impairs stroke recovery in the rat. SLEEP 2011;34(9):1261-1269.
Keywords: Stroke, sleep, sleep deprivation, neuroplasticity, neurogenesis, axonal sprouting, brain repair
INTRODUCTION
After decades of research into the function of sleep, a body of evidence has emerged to support the notion that sleep has multiple functions at different levels of brain organization.1 Animal and human studies have shown that forced wakefulness or sleep deprivation (SDpv), a commonly used paradigm in sleep, alters neurotransmitter and receptor systems,2–4 neuronal activities and related signalling molecules,5,6 as well as cognition and memory consolidation.7 These data suggest a role of sleep in facilitating neuroplasticity at both the synaptic and network (system) levels in the healthy brain.
Clinical observations suggest that sleep may modulate recovery processes and neuroplasticity in the injured brain. For instance, sleep-wake disturbances are frequent after stroke, affecting at least 20% to 40% of stroke patients.8 Clinical studies indicate that stroke patients with sleep-wake disturbances present worse outcomes with more frequent neuropsychiatric (depression and anxiety) and cognitive (memory and attention) disturbances.9,10 More recently, Siccoli et al.11 reported a correlation between electrographic sleep time and cognitive function in both the acute and recovery phase after stroke. However, systematic assessment of the role of sleep in modulating stroke recovery is difficult in humans because of many contributing factors, including heterogeneity of stroke localization and size, and pre-stroke sleep-wake characteristics.
We have recently used rodent models of focal cerebral ischemia to assess the effects of sleep manipulations on stroke outcome. In both mice12 and rats,13 ischemic stroke often induced an increase in slow wave sleep and a decrease in paradoxical sleep. When mice were treated shortly after ischemia with γ-hydroxybutyrate (GHB), a natural metabolite of GABA in the brain and a slow wave sleep enhancing agent in humans,14 they showed a quicker recovery of the grip strength in the paretic forelimb than those injected with the vehicle saline.15 Recently we have demonstrated in a rat stroke model that during the acute phase of stroke, 2 sleep disruption protocols (i.e., sleep deprivation for 12 h [SDpv12h] during the light phase [deprived of 80% sleep defined by EEG/EMG recording] and sleep disturbance [SDis] in which rats were subjected to SDpv12h for 3 consecutive days during the light phase but allowed to sleep during the dark phase) significantly aggravated brain damage.13 Surprisingly, SDis also resulted in a massive increase in expression of neurocan, a gene involved in inhibiting axonal growth.16,17 Taken together, these data not only indicate that sleep disruption modulates stroke pathophysiology, but also suggest that it may have a detrimental effect on stroke outcomes.
The aim of the present study was to test the hypothesis that sleep modulates long-term functional outcomes following stroke. We applied the same SDis procedure in the rat stroke model as described in the previous study13 to assess its effects on functional recovery. In addition, we examined its effects on several endogenous brain repair mechanisms,18–20 including axonal sprouting,21–24 synaptogenesis,25 neurogenesis,26–28 and angiogenesis.29,30
METHODS
Animals
Male Sprague Dawley rats (n = 29), 11–12 weeks old and weighting 323 ± 16g at the time of surgery, were used in this study. They were housed under 12-h light/dark cycle (light on 09:00–21:00) and ambient temperature at 22 ± 0.5°C. Animals were provided ad libitum with food and water but were under food restriction during training and functional testing (below). All experiments were conducted with governmental approval according to local guidelines (Kantonales Veterinäramt Zürich, Switzerland) for the care and use of laboratory animals. Effort was made to minimize the number of animals used.
Experiment Design (Figure 1)
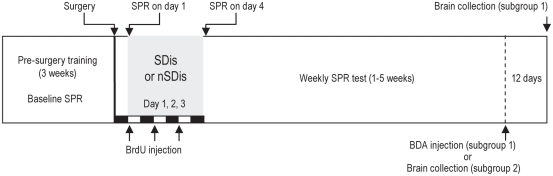
Figure 1.
Schematic of the experiment design. Sleep disturbance (SDis) was carried out 12 h after surgery by sleep deprivation for 12 h during the light phase for 3 consecutive days. Single pellet reaching (SPR) was assessed after surgery on days 1, (12 h after surgery before SDis was performed), 4, and once per week for 5 weeks. BrdU was injected at the beginning of the light phase, i.e., the beginning of sleep deprivation. The white and black bars indicate the light (12h) and dark (12h) period, respectively. Five weeks after surgery, animals in subgroup 1 were injected with the BDA tracer and survived for additional 12 days, and animals in subgroup 2 were sacrificed at this time point and brains collected (See Method). nSDis, without SDis; BrdU, bromodeoxyuridine; BDA, biotinylated dextran amine.
Animals at the beginning of the study were randomly assigned to 4 groups: (1) ischemia (ischm) with sleep disturbance (SDis) (ischm/SDis, n = 9); (2) ischm without SDis (ischm/nSDis, n = 8); (3) sham with SDis (sham/SDis, n = 6); and (4) sham without SDis (sham/nSDis, n = 6). All animals were included for weekly assessments on sensorimotor function, evaluated by single pellet reaching (SPR), for 5 weeks after surgery. They were injected after surgery with bromodeoxyuridine (BrdU), the S-phase marker that is incorporated into the newly generated cell during DNA synthesis, to assess ischemia-induced cell proliferation that leads to neurogenesis and angiogenesis.26,29 At the end of 5 weeks after surgery, animals in each group were divided randomly into 2 subgroups. Rats in subgroup 1 continued for the axon tracing experiment (ischm/SDis, n = 4; ischm/nSDis, n = 4; sham/SDis n = 2; sham/nSDis n = 4) by injection of the neuroanatomical tracer biotinylated dextran amine (BDA)31 into the motor cortex contralateral to ischemia and survived additional 12 days to allow for the transport of the tracer. Rats in subgroup 2 (ischemia/SDis, n = 5; ischemia/nSDis, n = 4; sham/SDis, n = 4; sham/nSDis, n = 4) were sacrificed at the end of the 5 weeks and brains collected immediately (below) for analyzing gene expression, cell proliferation, and differentiation (neurogenesis/angiogenesis).
Induction of Focal Cerebral Ischemia
Ischemia was induced by permanent occlusion of the distal middle cerebral artery (MCA) and the ipsilateral common carotid artery (CCA), superimposed by temporary occlusion of the contralateral CCA.32 Briefly, rats were anesthetized with 2% isoflurane (30% O2, remainder N2O) and a small piece (5×5 mm) of the skull overlying the MCA was removed and the dura retracted. The MCA and its 3 main branches dorsal to the rhinal fissure were occluded by bipolar electrocoagulation. The ipsilateral CCA to the occluded MCA was permanently ligated with a 4-0 silk suture and the contralateral CCA occluded temporarily with an aneurysm clip for 60 min. Rectal temperature was maintained at 36.5 ± 0.5°C by a warm lamp during the surgery. Sham-control animals were subjected to the same procedure except for removing the piece of the skull and occlusion of the MCA and CCA. Both ischemia and sham surgeries were performed on the contralateral hemisphere to the preferred forelimb for pellet reaching (below).
SPR Training and Testing
SPR, a task of reaching for food with a forelimb (Figure 3A), was used for assessing sensorimotor functions.33 We had carried out a pilot study to evaluate in the ischemia model a battery of motor function tests, including SPR, tape removal, beam walking, and cylinder test, in which SPR showed most consistent results, therefore was chosen for further investigation. For SPR training and testing, animals were placed in a clear Plexiglas box (41×27×37 cm) with a vertical slit open (1×15 cm) in the middle of the front wall and 1 cm above the floor. A shelf (2 cm in wide) with indentations was mounted in the front of the slit and outside the box wall. Animals were trained to reach through the slit a food pellet (45 mg dustless precision pellet, Bio-Serv, Frenchtown, NJ, USA) placed in the indentation on the shelf (Figure 3A). Initially, food pellets were placed in indentations on both the right and left side of the shelf. Once rats displayed a paw preference after several days of training, a pellet was placed only on the side of the preferred-reaching limb to enforce the use of the paw. Animals received a daily training session with 50 pellets for 3 weeks before surgery. Post-surgical test sessions were conducted on days 1 (12 h after surgery and before SDis, Figure 1), 4, 7, 14, 21, 28, and 35 after surgery, with 50 pellets per session per day. The reaching success rate was calculated as the percentage of 50 pellets being retrieved. Reaches were only considered successful if the pellet was eaten. The baseline was averaged by the final 3 days of pre-surgery training and expressed as 100% to the post-surgery score.
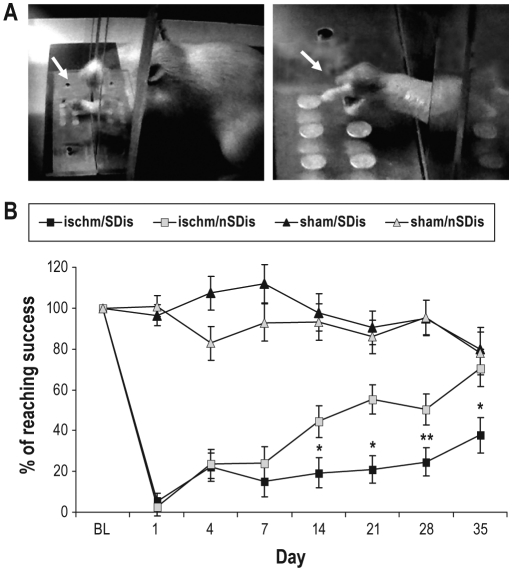
Figure 3.
Effects of SDis on recovery of single pellet reaching (SPR). (A) Photographs of a rat reaching (arrows) though a slot opened in the front of a training box for a pellet located in an indentation on the shelf. (B) Reaching success is presented as percentage of the baseline (BL, Ishm/SDis 51% ± 12%, Ischm/nSDis 54% ± 6%) value (100%); n = 9 and 8 for the ischem/SDis and ischem/nSDis, respectively. N = 6 per sham group. Repeated-measures ANOVA reveals a significant effect of group* day interaction (F12,101 = 11, P = 0.001), followed by independent group t-test between the ischemia/SDis and ischemia/nSDis. *P < 0.05, **P < 0.01.
In order to enhance the motivation to perform the reaching task, during the 3 training weeks and at one day prior to the testing session (days 4, 7, 14, 21, 28, and 35 after surgery, Figures 1 and 3), all animals underwent a food restriction schedule in which 20 grams of chow were given per day. Under the food restriction schedule, rats were maintained at 95% of their normal body weight.
SDis Protocol
As in the previous study,13 SDis was carried out 12 h after ischemia by sleep deprivation for 12 h (SDpv12h) during the light phase for 3 consecutive days, in which sleep was undisturbed during the dark phase. Gentle handling, such as knocking at the cage or providing new playing materials, was used to perform SDpv12h. The reason for choosing the light phase, the normal sleep period in rodents, to perform SDpv12h was to maximize the effect on reducing sleep. We have previously described in detail the changes induced by the SDis procedure in EEG/EMG-defined vigilance states.13 Briefly, rats subjected to ischemia showed an increase in propensity for slow wave sleep. Thus, SDpv12h by gentle handling was less effective in reducing sleep in ischemia rats than in sham-operated rats; the amount of sleep during SDpv12h accounted for 15% to 22% in ischemia rats vs. 3% to 6% in sham controls. During the following dark phase in which sleep was undisturbed, the amount of sleep was increased (sleep rebound) to a similar extent for both groups. When the total amount of sleep was summed on daily basis (including the light and dark phase), there was no significant change to the baseline. These data indicate that the SDis protocol induced considerable sleep disruption in ischemia rats.
Injection of BrdU, Tissue Collection, and Immunofluorescence Staining
BrdU (5-bromo-2′-deoxyuridine, Sigma, St Louis, MO, USA) diluted in 0.9% of saline was intraperitoneally (i.p.) injected once per day at a concentration of 50 mg/kg. In order to assess the effect of SDis on ischemia-induced endogenous cell proliferation, injections were performed once per day for 3 consecutive days at 09:00, i.e., at the beginning of each SDpv12h (Figure 1).
To evaluate the results, fresh frozen brains from subgroup 2 were harvested at the end of 5 weeks after surgery (Figure 1). Brief isoflurane anaesthesia was used before the animals were decapitated; brains were dissected and frozen immediately on dry ice. Coronal sections at 20 μm were cut on a cryostat at 6 predefined levels at 1-mm intervals, i.e., 2.7 (L1), 1.7 (L2), 0.7 (L3), −0.3 (L4), −1.3 (L5), and −2.3 (L6) mm to bregma.34 Twenty sections at each level were mounted on SuperFrostPlus slides (Menzel GmbH, Braunschweig, Germany) (cryosection) for histology and immunostaining, the remaining sections were dissected into the ipsilateral and contralateral hemisphere to ischemic injury and collected separately in RNase-free tubes for gene expression assay.
Single immunofluorescence staining with an antibody against BrdU (raised in the rat, 1:200, Abcam, Cambridge, MA, USA) was used for detecting cell proliferation; double staining with the anti-BrdU combined with one of various antibodies against specific cell-type markers was for assessing differentiated cell types of BrdU-labelled newborn cells. The staining procedure was performed as follows: 20-μm cryosections were fixed with 4% paraformaldehyde in phosphate saline buffer (PBS, 0.1 M, pH 7.4) for 20 min at room temperature (RT); treated with 2M HCl at 37°C for 1 h to denature DNA, and rinsed well with PBS. Sections were then incubated overnight at 4°C with primary antibodies (the anti-BrdU alone in the single staining; the anti-BrdU with an antibody for a cell-type marker in double staining) diluted in PBS containing 2% normal goat serum (Jackson immunoResearch, West Grove, PA, USA) and 0.3% Triton (Sigma, St. Louis, MO, USA). They were washed with PBS and incubated for 1 h at RT with cyanine dye-conjugated (Cy2 and Cy3) secondary antibodies (Jackson immunoResearch) against the appropriate host species of the primary antibodies (below). The secondary antibodies were diluted in the same incubation buffer as for primary antibodies at 1:100 for Cy2 and 1:300 for Cy3. Finally, sections were washed with PBS and cover-slipped with fluorescence mounting medium (Dako, Carpinteria, CA, USA). The primary antibodies against specific cell-type marker included the neuroblast marker doublecortin (DCX) (goat, 1:200, Santo Cruz Biotechnology, Santa Cruz, CA, USA), the neuronal marker NeuN (mouse, 1:400, Millipore, Billerica, MA, USA), the astrocyte marker glial fibrillary acidic protein (GFAP) (mouse, 1:200, Dako, Carpinteria, CA, USA) and the endothelium marker von Willebrand factor (vWF) (rabbit, 1:400, Sigma). Leica DM 6000B microscope (Leica Microsystems, Wetzlar, Germany) was used to analyze and photograph stained sections.
Cell Counting
Cell counting was conducted with 40X objective lens in the peri-infarct region through the middle part of infarction, i.e., at L2 and L3 levels, and averaged on 4 sections per rat (n = 4 per group). Double stained sections were examined by 2-channel illumination with simultaneous image recording for 2 different fluorochromes. The number of cells containing a cell-type marker was counted on 100 BrdU positive cells and expressed as a percentage of the cell type in newborn cells.
Injection of BDA, Tissue Collection, Staining, and Analysis
Anterograde tracing with the neuroanatomical tracer biotinylated dextran amine (BDA)31 was used for assessing axonal sprouting of the intercortical and corticostriatal pathways.21,22 Animals in subgroup 1 were anesthetized again after 5 weeks of post-stroke recovery; 2 small injections of 10% of BDA (MW, 10,000 Da; Molecular Probes, Eugene, OR, USA) diluted in 0.01M phosphate buffer were placed through a Hamilton syringe with a volume of 0.3 μL for each injection into the motor cortex (Figures 4A and B) over 20 min. The stereotactic coordinates were A, +1 and –1 mm to bregma; L, 1 mm to midline and 1.5 mm depth from the surface of the cortex.34 Animals were sacrificed 12 days later. For analyzing BDA axon tracing, brains were harvested by perfusion. Under sodium pentobarbital (100 mg/kg, i.p.), animals were perfused with 4% paraformaldehyde in PBS through the ascending aorta. Brains were dissected and postfixed at 4°C for 2 h in the same fixative and cryoprotected with 10% and 30% of sucrose in an ascending manner. Brains were cut at 40 μm, and sections were collected in PBS as floating sections for processing BDA staining.
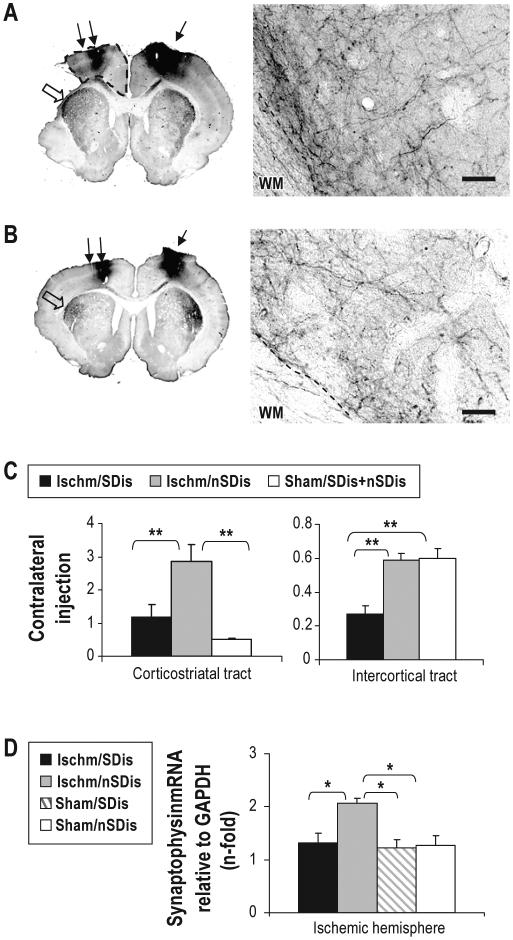
Figure 4.
Effects of SDis on axonal sprouting of corticostriatal and corticocortical projection neurons in the contralateral hemisphere. (A and B) Photomicrographs of anterograde tracing in a rat subjected to ischemia (A) or sham surgery (B). The BDA injection site is indicated by a solid arrow on the right motor cortex, and the area pointed by a blank arrow is shown on the right panel with high-power magnification. The area surrounded by the dashed line in A is measured as the remaining motor cortex, of which volume is compared between 2 ischemia groups (see Results). WM, white matter. Scale bars, 50 μm. (C) Quantification of BDA labeled corticostriatal (left panel, shown by a blank arrow in A and B) and intercortical (right panel, shown by double arrows in A and B) axons. One-way ANOVA (corticostriatal, F 2,47 = 11.25, P < 0.001, intercortical, F 2,37 = 13.01, P < 0.001) followed by post hoc comparisons (n = 4 per group; note that the sham group data are pooled from SDis and nSDis groups since there is no significant difference between the 2 groups). (D) Expression of synaptophysin mRNA. One-way ANOVA (F3,10 = 6.69, P = 0.009, n = 4 per ischemia group and n = 3 per sham group) followed by post hoc comparisons. *P < 0.05, **P < 0.01.
Sections were processed with the ABC immunoperoxidase staining method using Vectastain Elite kits (Vector Laboratories, Burlingame, CA, USA) and diaminobenzidine (DAB, Sigma) as the chromogen. Effort was made to standardize the staining procedure for quantitative analysis, such as processing sections at the same time for all experiment groups with the same developing period in DAB solution.
Stained sections were digitized, and the surface area of BDA labelled axons was quantified with the gray-scale threshold method (NIH imageJ software) in the primary motor cortex and the striatum near the ischemic site to determine cortico-cortical and cortico-striatal axons projected from contralateral cortex (Figures 4A and B). The BDA labelled area was also quantified on the injection side of the primary motor cortex and striatum to serve as an internal control. The cortico-cortical projection was measured at L1 and L2, and the cortico-striatal projection at L2-L4 levels, where the labelling was most abundant. For each rat, 2 to 3 adjacent sections were averaged on each level. Quantification was performed by 2 individuals blinded to experiment groups. Finally, the ratio of contralateral to injection was calculated as a measure for axon sprouting.22 Since there was no difference in the BDA labelled areas between 2 sham groups (sham/SDis and sham/nSDis), the data were pooled together to serve as the sham control group (Figure 4C, sham/SDis+nSDis, n = 4).
Gene Expression Assay
Taqman real time polymerase chain reaction (PCR) assay was used for quantitatively detecting changes in expression of synaptophysin, a presynpatic vesicle molecule and a marker for synaptogenesis. Only the brain tissue ipsilateral to the ischemia lesion was used for the assay (Methods). Total RNA was extracted by the Trizol method (Life Technologies, Rockville, MD, USA) for individual animals and treated with RQ1 DNase (Promega, Madison, WI, USA) to digest genomic DNA. Oligo(dT)15 primed first-strand cDNA synthesis was synthesized by the avian myeloblastosis virus (AMV) reverse transcriptase (Promega). The 5′-FAM labelled probes used for the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH, endogenous control, Assay ID: Rn99999916_s1) and synaptophysin (Assay ID:Rn00561986_m1) were purchased from Applied Biosystems (Forster City, CA, USA). Reactions were performed in triplicates for each rat on AB 7900HT fast real time PCR system (Applied Biosystems). The relative level of mRNA expression was calculated as follows: mRNA = 2–(ΔC T experiment rat – ΔCT sham/nSDis rat), where ΔCT = (CT, target – CT, Gapdh)
Lesion Size Determination
Brains from all rats subjected to ischemia were used for determining the lesion volume. Sections were stained with cresyl violet, digitized and measured with the NIH imageJ software (NIH, Bethesda, MD, USA). The damaged area was first calculated by subtraction of the undamaged area in the stroke hemisphere from that in the intact hemisphere and then converted to the volume with the known distance between each level.
Statistics
Data were presented as mean ± standard deviation (SD). The significance of differences in means was assessed by independent t-test, one-way ANOVA, and repeated-measures ANOVA (SPSS, 12.01 for Windows), where appropriate. ANOVA was followed by post hoc comparisons to determine group differences. The significance level was set at P values < 0.05.
RESULTS
Effects of SDis on Brain Damage (Figure 2)
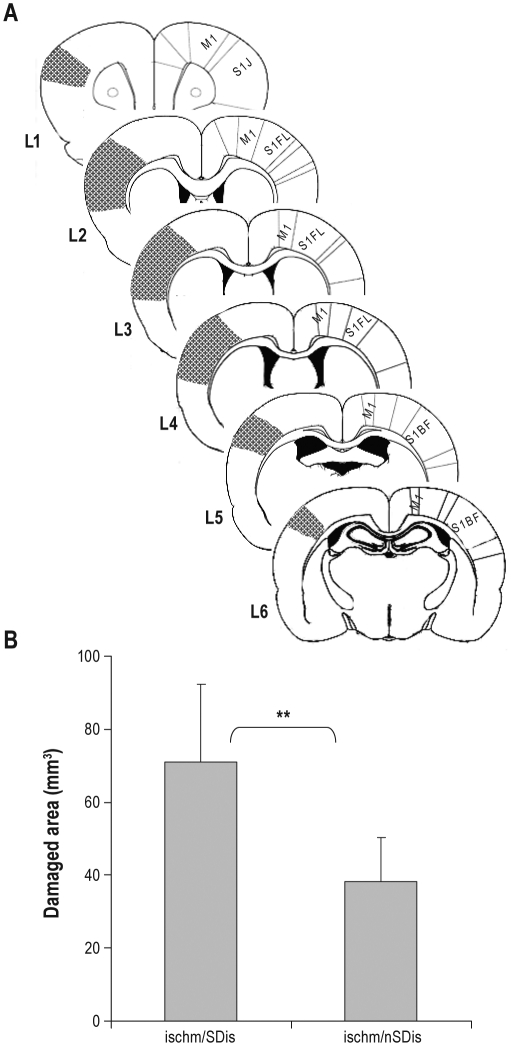
Figure 2.
Effects of SDis on brain damage. (A) Schematic of coronal brain sections illustrating infarction (shadow area) at 6 predefined levels (Methods). M1, primary motor cortex; S1FL, primary somatosensory cortex of forelimb area; S1J, primary somatosensory cortex of jaw area; S1BF, primary somatosensory cortex of barrel field. (B) Ischm, ischemia, n = 9 and 8 for ischem/SDis and ischem/nSDis, respectively. *P = 0.015.
The MCAo-induced damage was mainly located in the primary somatosensory cortex (Figure 2A) and increased by 71% in the ischemia/Sdis group (71.1 ± 21 mm3) compared with the group of ischemia/nSDis (41.5 ± 17 mm3), a result similar to that reported before.13
Effects of SDis on Single Pellet Reaching Recovery (Figure 3)
Twelve hours after surgery (SPR on day 1, Figure 1), the SPR success rate in both ischemia groups dropped to 5% of the baseline level, but showed a small improvement (about 20% of the baseline) on day 4. There was a continuous progress in rats not subjected to SDis in the following 5 weeks, and on day 35 the success rate reached the level of sham-operated rats. In contrast, in rats subjected to SDis, the SPR stayed at the same level as that on day 4 until the end of 4 weeks (day 28) and improved slightly during the final week. Repeated-measures ANOVA revealed a significant effect of group* day interaction (F12,101 = 11, P = 0.001). Following post hoc independent tests indicated a significance difference between the 2 ischemia groups on days 14, 21, 28, and 35.
Effects of SDis on Axonal Sprouting and Synaptogenesis (Figure 4)
Similar to previously reported information,22 axonal sprouting of the corticostriatal projection neurons increased significantly after ischemia, as seen in the ischemia/nSDis group when compared with that in the sham group. There was a significant decrease, however, in the ischemia/SDis group in both the striatum and primary motor cortex. To rule out the possibility that the decreased axonal sprouting induced by SDis in the primary motor cortex was due to a reduction of the surface area, we measured the area of the remaining cortex from the injury boarder to the midline (Figure 4A, the area surrounded by the dash line). The results showed no significant change between the 2 ischemia groups (ischemia/SDis 13.6 ± 1.2 mm3 vs. ischemia/nSDis 12.2 ± 1.5 mm3, P = 0.22).
To further confirm the adverse effect of SDis on the ischemia-stimulated axonal sprouting, we determined the expression of synaptophysin, a presynaptic vesicle molecule and a marker for synaptogenesis, with samples collected from the ischemia hemisphere. Matched well to the data described above, there was a significant increase in expression of synaptophysin mRNA in the ischemia/nSDis compared with the sham group, but this increase diminished in the ischemia/SDis group (Figure 4D). Parenthetically, we did not find changes in the expression of neuroplasticity-associated genes GAP43, neurocan, ephrinA5, and ephrinB1 (not shown), with the samples collected almost 7 weeks after ischemia (Figure 1).
Effects of SDis on Neurogenesis and Angiogenesis (Figures 5 and 6)
Figure 5.
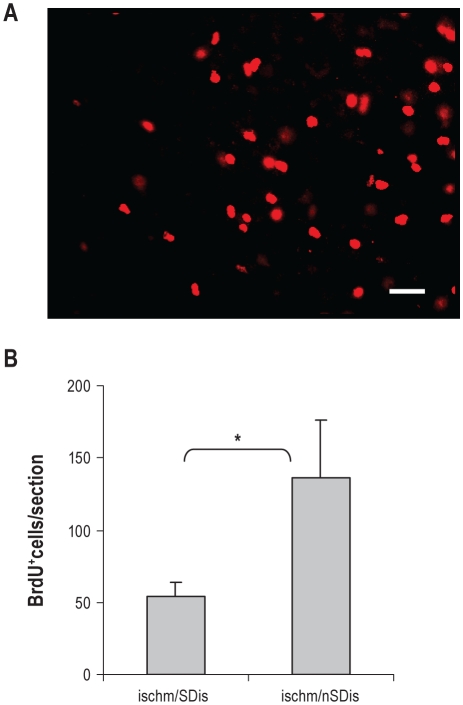
Effects of SDis on cell proliferation. (A) Numerous newborn cells labeled with BrdU in the peri-infarct area, visualized by single immunofluorescence staining. Scale bar, 50 μm. (B) n = 4 per group. **P < 0.01.
Figure 6.
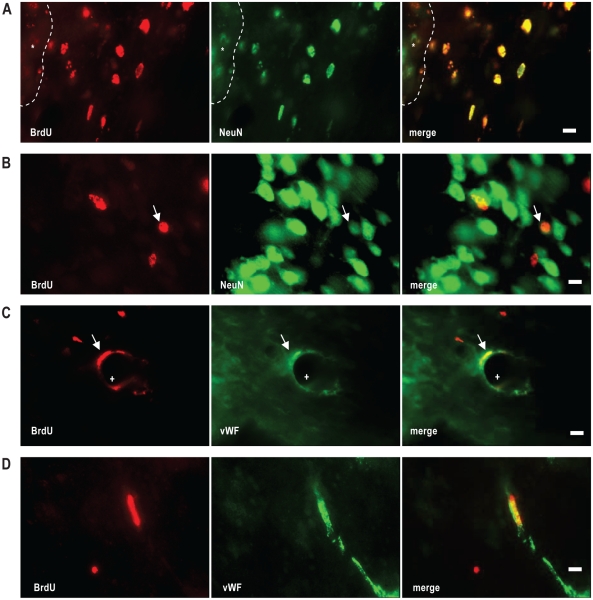
Differentiation of newborn BrdU positive cells, visualized by double immunofluorescence staining. (A and B) BrdU positive cells (red) are colocalized with the neuronal marker NeuN (green). Note in the cortex close to the damage site (*), numerous BrdU positive cells are NeuN immunoreactive (A), but few in the distant cortex (B). (C and D) some BrdU positive cells are costained with the endothelial marker vWF (green). Note that most NeuN/BrdU cells are small in size and short of cytoplasmic NeuN staining, resembling these of GABAergic interneurons (see text). Scale bars, 10 μm.
We first examined the effect of SDis on the number of BrdU positive cells that survived for 6 weeks. Similar to the results observed in a long-term study by Shin et al.,30 BrdU positive cells were very abundant in the peri-infarct zone (Figure 5A), but less in the distant cortex, subventricular zone, contralateral cortex, and the dentate gyrus region. To evaluate the SDis effect, we counted only those located in the peri-infarct region. The results showed a significant decrease of the number of BrdU-positive cells in the ischemia/SDis group compared with that in the ischemia/nSDis (Figure 5B).
We then performed double staining with various cell-type markers to identify the fate of BrdU positive cells and found that they were mainly co-stained either with the neuronal marker NeuN (∼70%, Figures 6A and B) or the endothelium marker vWF (∼ 40%, Figures 6C and D), with only a small fraction (∼ 10%) co-stained with the astrocyte marker GFAP. There was no significant difference between the 2 ischemia groups in the proportion of the double labelled cells. Thus, NeuN/BrdU cells were 70% ± 10% and 78% ± 7%, and vWF/BrdU were 41 ± 7% and 44 ± 6%, for the ischemia/SDis and ischemia/nSDis (n = 4 per group), respectively. Most majority of NeuN/BrdU double stained cells were small in size (< 10 μm in diameter) and short of cytoplasmic NeuN staining (Figures 6A and B), resembling these of GABAergic interneurons described by Dayer et al.35 Only a few cells in the peri-infarct zone were labelled with DCX, a protein transiently expressed in proliferating progenitor cells and newly generated neuroblasts; thus, no further double staining was performed.
DISCUSSION
In this study, we have extended our previous findings that sleep disturbances aggravate acute stroke to demonstrate its detrimental impact on long-term functional recovery and on endogenous brain restorative processes, including axonal sprouting, synaptogenesis, neurogenesis, and angiogenesis. With advantages such as homogeneous ischemia and well matched controls in animal stroke model, this study provides for the first time direct evidence that sleep disruption impairs functional and structural outcomes.
Ischemic lesion triggers complex brain restoration and neuronal reorganization at cellular, network, and system levels.18,20,36,37 Two cellular processes, i.e., poststroke axonal sprouting and neurogenesis, have generated great interest in recent years, and assumed to partly underlie functional recovery.37–39 SDis hindered both cellular repair mechanisms (Figures 4–6), which appears to be consistent with SDis-induced impairment of recovery of the skilled SPR (Figure 3). The precise contribution of these cellular events to SPR recovery, however, remains unknown. Post-stroke motor function recovery including the SPR is complex, associated not only with structural changes at the cellular and synaptic level but also with many aspect of cognitive function (such as post-stroke learning) that require higher-level control. The inherent complexity and the problem of relating cellular events to behavioral recovery have recently been discussed in detail by Whishaw et al.40 Whether targeting these cellular events will eventually influence stroke outcome is now under extensive investigation in the research field of neural repair.
The molecular candidates for the SDis-induced decrease of axonal sprouting (Figure 4) remain largely unknown. There are numerous neuroplasticity-related genes that are important for axonal sprouting, including growth-promoting and growth-inhibiting genes, each with a distinct cellular and temporal expression pattern.41 We tested in previous studies the effects of SDis and the sleep promoting drug GHB on expression of a small set of these genes, including promoting genes GAP43 and c-jun, and inhibiting genes neurocan, ephrin A5, and ephrin B1.13,15 It was found that SDis induced a significant increase in the expression of neurocan,13 whereas GHB decreased its expression. We assume that sleep manipulation must have a broad impact on the brain transcriptome. Thus, a large-scale screening of neuroplasticity-related genes and their protein products is indispensable in order to understand the molecular mechanism targeted by sleep alterations.
SDis shortly after stroke also suppressed the stroke-induced cell proliferation (Figure 5), which eventually leaded to decrease in neurogenesis and angiogenesis. The SDis-induced decrease in the number of BrdU labeled cells is not unexpected, since similar results have been observed in healthy rodents that are subjected to sleep deprivation or sleep disruption for several days.42 The suppressed cell proliferation after sleep deprivation was controversially attributed to the increased corticosterone level.43 This does not seem to be the case in our study since SDis showed no increase in the plasma corticosterone level,13 supporting the argument that the inhibited-cell proliferation is independent of the elevated stress hormone.44 It is possible, though, that other molecules such as neurotropic factors and cytokines, which are known to be altered after sleep disruption and also known to influence cell proliferation,45 could contribute to the SDis-induced suppression of cell proliferation.
It is noteworthy that there have been reports that sleep deprivation prior to stroke, either total SDpv for 6 h or selective SDpv (REM sleep) for several days, appears to be neuroprotective.46–48 The authors assumed that pre-stroke SDpv might induce a preconditioning tolerance. Alternatively, in our opinion, an increase in sleep (sleep rebound) directly following SDpv and ischemia surgery12 could account for the protective effects, similar to that induced by a sleep stimulant when administrated after stroke.15 Thus, a careful analysis of sleep under these conditions is essential to interpret the results. On the other hand, it is certainly of particular interest to identify protective pathways associated with pre-stroke SDpv.
In summary, we have demonstrated for the first time the detrimental effects of sleep disturbance on neuroplasticity and functional recovery in an experimental stroke model. If confirmed, these data suggest a potential interest/need in prevention of sleep disturbances and improvement of sleep quality in stroke patients.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Vassalli A, Dijk DJ. Sleep function: current questions and new approaches. Eur J Neurosci. 2009;29:1830–41. doi: 10.1111/j.1460-9568.2009.06767.x. [DOI] [PubMed] [Google Scholar]
- 2.Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–9. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longordo F, Kopp C, Luthi A. Consequences of sleep deprivation on neurotransmitter receptor expression and function. Eur J Neurosci. 2009;29:1810–9. doi: 10.1111/j.1460-9568.2009.06719.x. [DOI] [PubMed] [Google Scholar]
- 5.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–95. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vecsey CG, Baillie GS, Jaganath D, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–5. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–97. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 8.Bassetti CL, Hermann DM. Sleep and stroke. Handb Clin Neurol. 2011;99:1051–72. doi: 10.1016/B978-0-444-52007-4.00021-7. [DOI] [PubMed] [Google Scholar]
- 9.Leppavuori A, Pohjasvaara T, Vataja R, Kaste M, Erkinjuntti T. Insomnia in ischemic stroke patients. Cerebrovasc Dis. 2002;14:90–7. doi: 10.1159/000064737. [DOI] [PubMed] [Google Scholar]
- 10.Hermann DM, Bassetti CL. Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology. 2009;73:1313–22. doi: 10.1212/WNL.0b013e3181bd137c. [DOI] [PubMed] [Google Scholar]
- 11.Siccoli MM, Rolli-Baumeler N, Achermann P, Bassetti CL. Correlation between sleep and cognitive functions after hemispheric ischaemic stroke. Eur J Neurol. 2008;15:565–72. doi: 10.1111/j.1468-1331.2008.02119.x. [DOI] [PubMed] [Google Scholar]
- 12.Baumann CR, Kilic E, Petit B, et al. Sleep EEG changes after middle cerebral artery infarcts in mice: different effects of striatal and cortical lesions. Sleep. 2006;29:1339–44. doi: 10.1093/sleep/29.10.1339. [DOI] [PubMed] [Google Scholar]
- 13.Gao B, Cam E, Jaeger H, Zunzunegui C, Sarnthein J, Bassetti CL. Sleep disruption aggravates focal cerebral ischemia in the rat. Sleep. 2010;33:879–87. doi: 10.1093/sleep/33.7.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Cauter E, Plat L, Scharf MB, et al. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men. J Clin Invest. 1997;100:745–53. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao B, Kilic E, Baumann CR, Hermann DM, Bassetti CL. Gamma-hydroxybutyrate accelerates functional recovery after focal cerebral ischemia. Cerebrovasc Dis. 2008;26:413–9. doi: 10.1159/000151683. [DOI] [PubMed] [Google Scholar]
- 16.Shen LH, Li Y, Gao Q, Savant-Bhonsale S, Chopp M. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia. 2008;56:1747–54. doi: 10.1002/glia.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies JE, Tang X, Denning JW, Archibald SJ, Davies SJ. Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur J Neurosci. 2004;19:1226–42. doi: 10.1111/j.1460-9568.2004.03184.x. [DOI] [PubMed] [Google Scholar]
- 18.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008 a;63:272–87. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008 b;63:549–60. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- 21.Napieralski JA, Butler AK, Chesselet MF. Anatomical and functional evidence for lesion-specific sprouting of corticostriatal input in the adult rat. J Comp Neurol. 1996;373:484–97. doi: 10.1002/(SICI)1096-9861(19960930)373:4<484::AID-CNE2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 22.Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22:6062–70. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramic M, Emerick AJ, Bollnow MR, O'Brien TE, Tsai SY, Kartje GL. Axonal plasticity is associated with motor recovery following amphetamine treatment combined with rehabilitation after brain injury in the adult rat. Brain Res. 2006;1111:176–86. doi: 10.1016/j.brainres.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos CM, Tsai SY, Guillen V, Ortega J, Kartje GL, Wolf WA. Motor recovery and axonal plasticity with short-term amphetamine after stroke. Stroke. 2009;40:294–302. doi: 10.1161/STROKEAHA.108.519769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroemer RP, Kent TA, Hulsebosch CE. Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke. 1998;29:2381–93. doi: 10.1161/01.str.29.11.2381. discussion 93–5. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26:1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- 27.Wiltrout C, Lang B, Yan Y, Dempsey RJ, Vemuganti R. Repairing brain after stroke: a review on post-ischemic neurogenesis. Neurochem Int. 2007;50:1028–41. doi: 10.1016/j.neuint.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Burns TC, Verfaillie CM, Low WC. Stem cells for ischemic brain injury: a critical review. J Comp Neurol. 2009;515:125–44. doi: 10.1002/cne.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–96. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 30.Shin HY, Kim JH, Phi JH, et al. Endogenous neurogenesis and neovascularization in the neocortex of the rat after focal cerebral ischemia. J Neurosci Res. 2008;86:356–67. doi: 10.1002/jnr.21494. [DOI] [PubMed] [Google Scholar]
- 31.Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103:23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- 32.Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–43. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- 33.Gharbawie OA, Gonzalez CL, Whishaw IQ. Skilled reaching impairments from the lateral frontal cortex component of middle cerebral artery stroke: a qualitative and quantitative comparison to focal motor cortex lesions in rats. Behav Brain Res. 2005;156:125–37. doi: 10.1016/j.bbr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sixth edition. London, Amsterdam, Burlington: Elsevier; 2007. [Google Scholar]
- 35.Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–27. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–42. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 37.Chopp M, Li Y, Zhang ZG. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40:S143–5. doi: 10.1161/STROKEAHA.108.533141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmichael ST. Themes and strategies for studying the biology of stroke recovery in the poststroke epoch. Stroke. 2008;39:1380–8. doi: 10.1161/STROKEAHA.107.499962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmichael ST. Translating the frontiers of brain repair to treatments: starting not to break the rules. Neurobiol Dis. 2010;37:237–42. doi: 10.1016/j.nbd.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whishaw IQ, Alaverdashvili M, Kolb B. The problem of relating plasticity and skilled reaching after motor cortex stroke in the rat. Behav Brain Res. 2008;192:124–36. doi: 10.1016/j.bbr.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev. 2009;13:187–94. doi: 10.1016/j.smrv.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci U S A. 2006;103:19170–5. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mueller AD, Pollock MS, Lieblich SE, Epp JR, Galea LA, Mistlberger RE. Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1693–703. doi: 10.1152/ajpregu.00858.2007. [DOI] [PubMed] [Google Scholar]
- 45.Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–95. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Hsu JC, Lee YS, Chang CN, Chuang HL, Ling EA, Lan CT. Sleep deprivation inhibits expression of NADPH-d and NOS while activating microglia and astroglia in the rat hippocampus. Cells Tissues Organs. 2003;173:242–54. doi: 10.1159/000070380. [DOI] [PubMed] [Google Scholar]
- 47.Weil ZM, Norman GJ, Karelina K, et al. Sleep deprivation attenuates inflammatory responses and ischemic cell death. Exp Neurol. 2009;218:129–36. doi: 10.1016/j.expneurol.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moldovan M, Constantinescu AO, Balseanu A, Oprescu N, Zagrean L, Popa-Wagner A. Sleep deprivation attenuates experimental stroke severity in rats. Exp Neurol. 2010;222:135–43. doi: 10.1016/j.expneurol.2009.12.023. [DOI] [PubMed] [Google Scholar]