Abstract
Idiopathic pulmonary fibrosis (IPF) is characterized by exaggerated fibroblast proliferation and accumulation of collagens and fibronectin. The extracellular fibronectin and collagen network is regulated by von Hippel-Lindau protein (pVHL). However, it is unknown whether pVHL contributes to pulmonary fibrosis. We found that lungs from patients with IPF expressed increased levels of pVHL in fibroblastic foci. Bleomycin treatment also induced pVHL in lung fibroblasts, but not in alveolar type II cells. Overexpression of pVHL increased lung fibroblast proliferation, protein abundance of fibronectin and collagen, and extracellular fibronectin. In addition, overexpression of pVHL induced expression of the α5 integrin subunit. Overexpression of pVHL did not alter hypoxia-inducible factor luciferase reporter activity and mRNA expression of vascular endothelial growth factor. Fibroblasts overexpressing pVHL were more sensitive to RGD peptide-mediated reduction in proliferation. Activating α5 and β1 integrin increased proliferation of fibroblasts overexpressing pVHL and those cells were more resistant to the inhibition of α5 integrin. Overexpression of pVHL also increased activation of focal adhesion kinase (FAK). Moreover, suppression of pVHL prevented TGF-β1-induced proliferation of mouse embryonic fibroblasts. Taken together, our results indicate that elevated expression of pVHL results in the aberrant fibronectin expression, activation of integrin/FAK signaling, fibroblast proliferation, and fibrosis.—Zhou, Q., Pardo, A., Königshoff, M., Eickelberg, O., Budinger, G. R. S., Thavarajah, K., Gottardi, C. J., Jones, J., Varga, J., Selman, M., Sznajder, J. I., Raj, J. U., Zhou, G. Role of von Hippel-Lindau protein in fibroblast proliferation and fibrosis.
Keywords: idiopathic pulmonary fibrosis, extracellular matrix, integrin α5β1, focal adhesion kinase
Idiopathic pulmonary fibrosis (IPF) is a devastating disease with a mortality rate exceeding that of many cancers and is currently without effective treatment (1, 2). The mechanisms involved in the pathogenesis of IPF remain unclear (1, 2). A prominent feature of the disease is the proliferation of lung fibroblasts that differentiate to myofibroblasts, forming the so-called fibroblastic/myofibroblastic foci. This is followed by an exaggerated deposition of extracellular matrix (ECM) proteins, such as fibronectin and fibrillar collagens. Fibronectin and collagen are extracellular glycoproteins that interact with the heterodimeric cell surface receptors known as integrins (3, 4). Interaction of ECM proteins and their receptor integrins activates focal adhesion kinase (FAK), leading to cell survival and proliferation (4, 5). Moreover, fibronectin and integrins play a role in the activation of TGF-β1, a potent profibrotic cytokine (5–11). Therefore, it appears that ECM plays a critical role in the progression of pulmonary fibrosis.
Although von Hippel-Lindau protein (pVHL) is best known as a component of an ubiquitin protein ligase complex to target hypoxia-inducible factor (HIF) 1/2α to the proteasome for degradation (12–14), it also regulates the fibronectin and collagen matrix (15, 16). Fibronectin and collagen interact with pVHL in cells and colocalize with a fraction of pVHL in the endoplasmic reticulum (ER; refs. 15, 16). Renal carcinoma cells with loss of pVHL exhibit a defective extracellular fibronectin matrix, suggesting a direct role for pVHL in fibronectin matrix formation (15, 17, 18). In mouse embryonic fibroblasts (MEFs), loss of pVHL results in a decrease in cell proliferation rate, partially through the HIF-mediated up-regulation of cyclin kinase inhibitors p21 and p27 (19–21). However, it is unknown whether pVHL can also regulate cell proliferation by affecting fibronectin and integrin interaction.
In this study, we investigated the expression patterns of pVHL in human normal and IPF lungs as well as in the lungs of experimental fibrotic mice. We also explored the effects of pVHL expression in primary human lung fibroblasts. Our results demonstrate that fibrotic lung tissues have elevated levels of pVHL that are primarily localized in fibroblasts. Overexpression of pVHL increases the expression of fibronectin and integrin α5β1, resulting in the activation of FAK and fibroblast proliferation.
MATERIALS AND METHODS
Materials
RAD (H-Gly-Arg-Ala-Asp-Ser-Pro-OH) and RGD (H-Gly-Arg-Gly-Asp-Asn-Pro-OH) peptides were purchased from Peptides International (Louisville, KY, USA). Bleomycin sulfate was purchased from Sigma-Aldrich (St. Louis, MO, USA). The following antibodies were used in this study: integrin α1, α2 (Millipore, Temecula, CA, USA); integrin α4 (Chemicon,Temecula, CA, USA); integrin α5, αv, and β1 subunits (Santa Cruz Biotechnology, Santa Cruz, CA, USA; pVHL (BD Pharmingen, San Jose, CA, USA); fibronectin (Millipore, Temecula, CA, USA), collagen I (Southern Biotech, Birmingham, AL, USA); hemagglutinin (HA; Sigma-Aldrich), FAK and pFAK (Invitrogen, Carlsbad, CA, USA); α-tubulin, actin, (Sigma-Aldrich); and α-smooth muscle actin (α-SMA; R&D Systems, Minneapolis, MN, USA). Dual luciferase assay kits were purchased from Promega (Madison, WI, USA). TGF-β1 was purchased from Calbiochem (La Jolla, CA, USA). Null (Ad-null) and Cre recombinase (Ad-Cre) adenoviruses were purchased from Vector BioLabs (Philadelphia, PA, USA).
Human tissues
In this study, we used existing deidentified human lung tissue samples from the U.S. National Heart, Lung, and Blood Institute Lung Tissue Research Consortium (LTRC; concept sheet 08-99-0001), and samples from the tissue banks at the University of Giessen School of Medicine (Giessen, Germany) and Instituto Nacional de Enfermedades Respiratorias (Tlalpan, Mexico). Lung parenchyma resected from patients with lung cancer with normal histology were used as controls. Samples were snap-frozen or placed in 4% (w/v) paraformaldehyde immediately after resection. The study protocols have complied with all relevant federal guidelines and were approved by the individual institutional review boards or their equivalent.
Delivery of bleomycin to mouse lungs
Six- to 8-wk-old pathogen-free C57BL/6 mice (Charles River Laboratories, Wilmington, MA, USA) were used throughout this study. All animals were provided with food and water ad libitum, maintained on a 12-h light-dark cycle, and handled according to U.S. National Institutes of Health (NIH) guidelines and the University of Illinois at Chicago Animal Care and Use Committee-approved experimental protocols. Bleomycin sulfate was dissolved in sterile saline solution and applied by intratracheal instillation. Phosphate-buffered saline (PBS, 50 μl) containing 0.05 U bleomycin was instilled in 2 aliquots (25 μl each). Lung tissues were excised and snap-frozen or inflated with 4% (w/v) paraformaldehyde in PBS for histological analysis (22).
Mouse lung fibroblast isolation and culture
Lungs from bleomycin- or PBS-treated mice were harvested, minced, and incubated with dispase (2 mg/ml; Sigma-Aldrich) for 45 min. Tissue slices were plated into cell culture flasks. After fibroblasts had grown out from the tissues, the slices were removed, and the cells were allowed to reach confluence. Confluent fibroblasts were then passaged by trypsin treatment and used for the experiments between passages 3 and 5. Identification of fibroblasts was based on the morphology and expression of vimentin and collagen (23, 24).
Isolation of mouse primary type II alveolar epithelial cells (ATII)
ATII cells were isolated from the lungs of C57BL/6 mice as described previously (24). Briefly, lungs were perfused until they were free of blood, lavaged to remove contaminating macrophages, and treated with intratracheal instillation of dispase. Crude lung cell suspensions were prepared by mincing tissues, filtering through 70-μm Falcon cell strainers (BD Biosciences, San Jose, CA, USA), and centrifuging the filtrate at 1500 rpm for 10 min. Crude cell suspension was purified by negative selection by incubation on CD16/32- and CD45-coated Petri dishes for 30 min at 37°C and adherence for 45 min on cell culture dishes. Cell viability was checked by trypan blue exclusion assay; cell purity was checked by morphology and confirmed by the positive staining of cytokeratin and surfactant protein C and the absence of α-SMA and CD45 staining. Purity and viability of ATII cell preparations were consistently greater than 90 and 95%, respectively.
Histological analysis
The mouse lungs were removed en bloc, fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) and Masson's trichrome. Human lung samples were fixed, embedded in paraffin, sectioned, and stained with H&E, Masson's trichrome, and pVHL antibody (BD Pharmingen, San Jose, CA, USA).
Cell culture
N12 primary human lung fibroblast cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cell cultures were maintained in a humidified atmosphere with 5% CO2-95% air at 37°C and were passaged when they reached 85–90% confluency. MEFs from VHL conditional knockout mice (vhlh 2-lox) were provided by Dr. Volker H. Haase (Vanderbilt University, Nashville, TN, USA) and maintained as described above.
Transient transfection of plasmids
Plasmids were transfected into N12 primary human lung fibroblast cells with Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's recommendations.
Western blotting
Cells cultured on 60-mm dishes were washed 3 times with ice-cold PBS and lysed in 150–250 μl of mRIPA buffer (50 mM Tris at pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and protease inhibitors). The cell lysates were centrifuged at 13,000 g for 5 min, and protein concentrations of the supernatants were determined with a Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Protein (25–50 μg) was then separated by SDS-polyacrylamide gel electrophoresis. The gel was transferred using a Novex Semi-Dry Blotter (Invitrogen) to BA-S 85 nitrocellulose membrane (Optitran; Whatman, Brentford, UK). Proteins were detected with Supersignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA). Gray density of Western blots was measured using ImageJ software (NIH, Bethesda, MD).
Quantitative real-time RT-PCR
Total RNA was isolated using the RNeasy micro kit (Qiagen, Valencia, CA, USA). Complementary DNA (cDNA) was synthesized from 0.5 μg of total RNA using the ABI High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc., Foster City, CA, USA) with a mixture of oligo(dT) and random hexamer primers. PCRs were carried out with ABI SYBR Green PCR master mix (Applied Biosystems) on the ABI StepOnePlus real-time PCR system (Applied Biosystems). Cycle threshold values were normalized to amplification of the mitochondrial ribosomal protein L19 (RPL19). Sequences of primers used for quantitative real-time RT-PCR (qRT-PCR) were as follows: human pVHL, CGTAGCGGTTGGTGACTTG (sense), CCCTGGTTTGTTCCTCTGAC (antisense); human RPL19, ATCATCCGCAAGCCTGTG (sense), TGACCTTCTCTGGCATTCG (antisense); mouse pVHL, GCTCCTGCTGTAGTCCTG (sense), CTTCTCTGCTGTAACTGTCTG (antisense); mouse RPL19, AGCCTGTGACTGTCCATTC (sense), ATCCTCATCCTTCTCATCCAG (antisense); human integrin α5, GACACTAAGAAAACCATCCAGTTTGA (sense), ACGGAGAGCCGAAAGGAAA (antisense); human integrin αv, GTGGACAGTCCTGCCGAGTAC (sense), GAGCTCCCACGAGAAGAAACA (antisense); human integrin α4, CGAACCGATGGCTCCTAGTG (sense), CACGTCTGGCCGGGATT (antisense); human integrin β1, TGCAGTTTGTGGATCACTGATTG (sense), CCCATCTCCAGCAAAGTGAAA (antisense); human fibronectin 1, CCTTCATGGCAGCGGTTT (sense), AGCGTCCTAAAGACTCCATGATCT (antisense); human collagen type 1A1, GGGCAAGACAGTGATTGAATACAA (sense), ACGTCGAAGCCGAATTCCT(antisense); human vascular endothelial growth factor (VEGF), GGAGGCGCAGCGGTTAG (sense), AACCCGGATCAATGAATATCAAA (antisense).
Construct of pAd-VHL-HA
Adenoviruses encoding HA-tagged pVHL (pAd-VHL-HA) were constructed into pAd/CMV/V5-DEST Gateway Vector (Invitrogen). The coding sequence of pVHL-HA was amplified from pcDNA3-VHL-HA (a gift from Dr. Volker H. Haase, Vanderbilt University), cloned into pEnter4 vector on KpnI/XhoI sites, and then recombined into pAd/CMV/V5-DEST.
Assays with treatment of RAD, RGD peptides, and integrin antibodies
N12 cells were infected with either pAd-null or pAd-VHL-HA viruses and incubated overnight. Cells were trypsinized, counted, and replated in the presence of RAD, RGD, or integrin antibodies at the indicated concentrations. Cells were incubated for another 32 h. followed with bromodeoxyuridine (BrdU) labeling. Inhibiting integrin α5 antibody was purchased from Santa Cruz Biotechnology; activating integrin α5 and integrin β1 antibodies were purchased from Chemicon.
Cell proliferation assay
Cell proliferation rates were determined by two methods: counting cell numbers and BrdU incorporation assay (Calbiochem). BrdU assay was carried out according to the manufacturer's protocol. Briefly, BrdU was added to wells of the plate and incubated for 16 h. The cells were fixed and permeablized. The DNA was denatured before the addition of anti-BrdU monoclonal antibody. Incorporated BrdU was detected by horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody and HRP substrate tetramethylbenzidine (TMB). For the BrdU incorporation assay in vhlh 2-lox MEF cells, these cells were infected with Ad-Cre at 50 pfu/cell, followed with incubation for 1 d to achieve the knockdown of pVHL. In the control group, vhlh 2-lox MEF cells were infected with Ad-null. After viral infection, these cells were treated with TGF-β1 (2 or 5 ng/ml), followed with BrdU labeling and incubation.
HIF response element (HRE) luciferase reporter assay
Cells were cotransfected with a luciferase reporter construct driven by phosphoglyceric kinase promoter containing HREs and Renilla luciferase reporter plasmid. Then cells were infected with pAd-Null or pAd-VHL-HA and incubated for 24 h. Another group of cells was exposed to hypoxia (1.5% O2) after cotransfection of HREs and Renilla reporter plasmids as positive control. Dual luciferase assay (Promega) was carried out following the manufacturer's protocol. HRE luciferase activity was normalized to Renilla luciferase activity.
TGF-β assay
N12 cells were infected with either pAd-null or pAd-VHL-HA viruses and incubated for 48 h. The culture medium was collected and cleared by centrifugation, and the supernatants were added to the cultured Mink lung epithelial cells (MLEC-clone 32, provided by Dr. H. William Schnaper, Northwestern University). MLEC-clone 32 is a cell line stably transfected with a luciferase reporter plasmid driven by a fragment of the 5′ end of human plasminogen activator inhibitor-1 gene, which contains TGF-β binding sites (25). After overnight incubation, MLECs were rinsed with PBS and lysed in luciferase buffer (Promega) and activated at 80°C for 5 min. Activated culture medium was immediately cooled on ice. Luciferase activity in the culture medium was measured by luciferase assay and normalized to the amount of protein.
Measurement of secreted fibronectin in culture medium
N12 cells were infected with either pAd-null or pAd-VHL-HA viruses and incubated for 48 h. The conditioned medium was collected and centrifuged at 13,000 g for 5 min to remove the cell debris. Aliquots with the same volume of supernatant were used to measure the secreted fibronectin by Western blot analysis.
Statistical analysis
Results are expressed as means ± se. Data were analyzed using 1-way analysis of variance (ANOVA). When a significant difference was detected by the ANOVA, the Student's t test using Bonferroni correction was used for further analysis. Statistical significance was set at the 0.05 and 0.01 levels.
RESULTS
Lungs of patients with IPF and bleomycin-treated mice overexpress pVHL
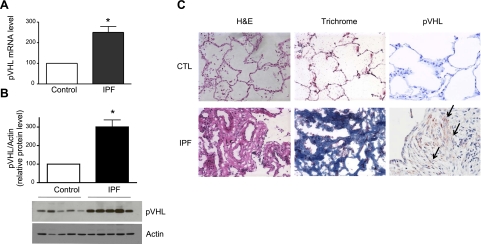
To examine the pVHL expression levels in human lungs, we prepared whole-cell lysates, extracted RNA from lung tissue samples of control patients and patients with IPF patients, and carried out Western blot analysis and qRT-PCR. As shown in Fig. 1A, B, lungs from patients with IPF expressed higher levels of pVHL mRNA, and protein than control lungs. We also performed immunohistochemistry analysis to determine the distribution of pVHL expression. As shown in Fig. 1C, control lungs barely exhibited pVHL expression, whereas IPF lungs exhibited strong staining of pVHL, which was primarily localized in fibroblasts from fibroblast foci. We used Masson's trichrome staining to detect collagen deposition to confirm the presence of fibrosis (Fig. 1C).
Figure 1.
pVHL expression levels are elevated in lungs of patients with IPF. A) pVHL mRNA levels in lung tissues of control patients and patients with IPF were measured by qRT-PCR. RPL19 gene was used as internal control for qRT-PCR. Average pVHL/RPL19 ratio of control samples (n=9) was set to 100, and pVHL/RPL19 ratio of individual IPF samples (n=12) was normalized to the average ratio of control samples. B) Lung samples were homogenized in mRIPA and subjected to Western blot analysis for detection of pVHL and actin (used as loading control). Average pVHL/actin ratio of control samples (n=7) was set to 100, and pVHL/actin ratio of individual IPF samples (n=8) was normalized to the average ratio of control samples. C) Paraffin-embedded lung samples were stained with H&E, trichrome, and pVHL antibody. Arrows indicate elevated staining of pVHL. Graphs represent means + se. *P < 0.05.
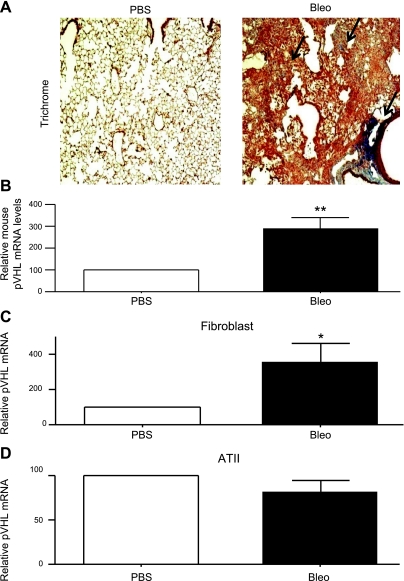
Bleomycin-induced lung injury in mice is a commonly used model to study fibrosis. Mice developed fibrosis 21 d after administration of bleomycin, as confirmed by the exaggerated collagen deposition (Fig. 2A). We extracted RNA from whole lungs of mice that received either PBS or bleomycin and then quantified pVHL mRNA expression by qRT-PCR. As shown in Fig. 2B, lungs of bleomycin-treated mice expressed higher levels of pVHL mRNA than lungs of PBS-treated mice. To further characterize the location of increased pVHL expression, we isolated lung fibroblasts and type II alveolar (ATII) epithelial cells from mice 14 d after the administration of PBS or bleomycin. RNA was then extracted for pVHL mRNA measurement. As shown in Fig. 2C, D, lung fibroblasts isolated from bleomycin-treated mice expressed elevated levels of pVHL mRNA compared to fibroblasts from PBS-treated mice, whereas in ATII cells, no changes were found.
Figure 2.
Lungs from bleomycin-treated mice express elevated pVHL levels. A) Paraffin-embedded lung samples from PBS- and bleomycin-treated mice were subjected to trichrome staining. Arrows indicate collagen staining. B) At 21 d after administration of PBS or bleomycin, mouse lungs were collected and used for RNA extraction. Mouse pVHL mRNA levels were determined by qRT-PCR. RPL19 gene was used as internal control for qRT-PCR. pVHL/RPL19 ratio of PBS-treated mice (n=3) was set to 100, and pVHL/RPL19 ratio of bleomycin-treated mice (n=4) was normalized to that of PBS-treated mice. C, D) At 14 d after administration with PBS or bleomycin, mouse lungs were used to culture mouse lung fibroblasts (C) and ATII cells (D). Mouse pVHL mRNA levels were determined by qRT-PCR. RPL19 gene was used as internal control for qRT-PCR. pVHL/RPL19 ratio of PBS-treated mice (C, n=4; D, n=9) was set to 100, and pVHL/RPL19 ratio of bleomycin-treated mice (C, n=4; D, n=9) was normalized to that of PBS-treated mice. Graphs represent means + se. *P < 0.05; **P < 0.01.
Overexpression of pVHL regulates ECM protein expression in lung fibroblasts
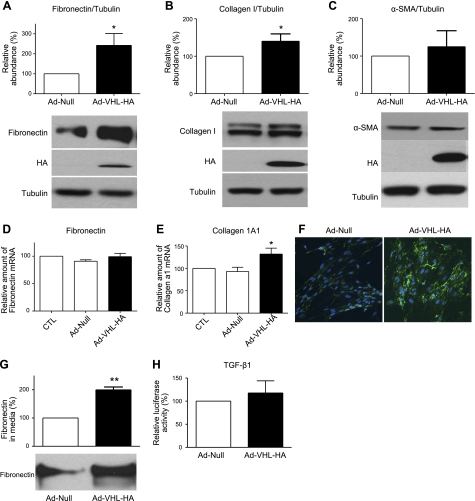
To investigate whether overexpression of pVHL alters matrix proteins, we constructed adenoviruses containing pVHL-HA (pAd-VHL-HA) and then infected N12 primary human lung fibroblasts. As shown in Fig. 3A–C, infection of pAd-VHL-HA adenoviruses increased the amount of fibronectin and collagen protein without affecting α-SMA protein expression. This suggests that the overexpression of pVHL does not affect the differentiation of fibroblasts to myofibroblasts. We also measured mRNA levels of fibronectin and collagen 1A1. As shown in Fig. 3D, E, overexpression of pVHL did not alter fibronectin gene expression, but induced collagen gene expression, suggesting that pVHL regulates fibronectin and collagen through different pathways. In addition, we examined extracellular fibronectin using immunofluorescence staining with fibronectin antibody after overexpression of pVHL. As shown in Fig. 3F, overexpression of pVHL increased the extracellular fibronectin. Furthermore, we measured the secreted fibronectin in the N12 cell culture medium after infection with pAd-Null or pAd-VHL-HA, and we found that overexpression of pVHL increased the secretion of fibronectin (Fig. 3G). We also determined the total TGF-β in N12 cell culture medium after overexpression of pVHL, and our results showed that overexpression of pVHL did not alter levels of total TGF-β (Fig. 3H).
Figure 3.
Overexpression of pVHL increases fibronectin and collagen expression in N12 primary human lung fibroblasts. A–C) N12 human primary lung fibroblasts were infected with pAd-Null or pAd-VHL-HA for 2 d, and the amount of fibronectin (A), collagen (B), and α-smooth muscle actin (C) was determined by Western blot analysis and normalized to the expression levels of tubulin. Overexpression of exogenous pVHL-HA was confirmed by the Western blot analysis with HA antibody. D, E) mRNA expression levels of fibronectin (D) and collagen 1A1 (E) were determined by qRT-PCR. RPL19 gene was used as internal control for qRT-PCR. F) At 2 d after infection with pAd-Null or pAd-VHL-HA, fibronectin (green) was stained with a specific antibody and visualized by fluorescent microscopy. Cell nuclei (blue) were stained with DAPI. G) At 2 d after infection with pAd-Null or pAd-VHL-HA, cell culture medium was collected, and the amount of secreted fibronectin in the same volume of medium was determined by Western blot analysis. Amount of secreted fibronectin in cells infected with pAd-Null was set as 100%. H) N12 cells were infected with either pAd-null or pAd-VHL-HA viruses and incubated for 48 h. Culture medium was collected, activated at 80°C for 5 min, and cooled on ice immediately. Activated culture medium was added to cultured MLEC-clone 32 cells and incubated overnight. MLEC-clone 32 cells were lysed in luciferase buffer; TGF-β-luciferase activity in the culture medium was measured by luciferase assay and normalized to the amount of protein. Relative luciferase activity in cells infected with pAd-Null was set as 100%. Measurement of pAd-Null-infected group was set to 100; graphs represent means + se of ≥3 independent experiments. n = 4 (A–E); 3 (G–H). *P < 0.05, **P < 0.01.
Up-regulation of pVHL increases α5 integrin expression in lung fibroblasts
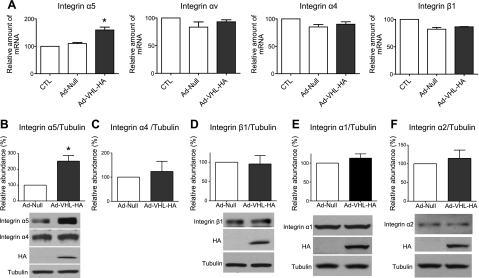
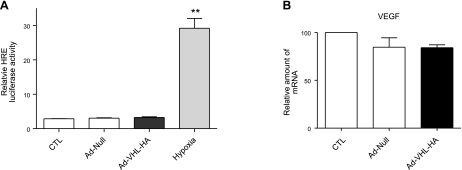
Matrix proteins interact with membrane receptor integrins and contribute to the regulation of cell behavior. Previous reports have shown that integrins are critical for fibroblast proliferation (4, 5, 15, 17, 18). To determine whether overexpression of pVHL alters expression profiles of integrins, we measured the mRNA levels of the α5, αv, α4, and β1 integrin subunits. As shown in Fig. 4A, overexpression of pVHL induced α5 integrin mRNA expression but did not change the mRNA expression levels of αv, α4, and β1 integrins. Furthermore, we measured the protein levels of integrin α5, α4, and β1 after overexpression of pVHL. As shown in Fig. 4B–D, pVHL overexpression increased protein levels of α5, but not those of α4 and β1 integrin. Moreover, pVHL overexpression did not change protein expression levels of integrin α1 and α2, two collagen integrins (Fig. 4E, F). These results suggest that overexpression of pVHL specifically up-regulates the expression of the α5 integrin subunit. To determine whether overexpression of pVHL affects HIF activity, we measured the HIF luciferase reporter activity after infection of pAd-Null or pAd-VHL-HA. As shown in Fig. 5A, overexpression of pVHL had no effects on the HIF reporter activity. In addition, we determined the mRNA expression levels of VEGF, a well established downstream target of HIF. As shown in Fig. 5B, overexpression of pVHL did not alter VEGF expression, suggesting that gain of function of pVHL is independent of HIF.
Figure 4.
Overexpression of pVHL increases integrin α5 expression in N12 primary human lung fibroblasts. A) N12 human primary lung fibroblasts were infected with pAd-Null or pAd-VHL-HA for 2 d, and mRNA expression levels of integrin α5, αv, α4, and β1 were determined by qRT-PCR; RPL19 gene was used as internal control. B–F) Protein expression levels of integrin α5 (B), α4 (C), β1 (D), α1 (E), and α2 (F) were determined by Western blot analysis. Tubulin was used as control for equal loading. Overexpression of exogenous pVHL-HA was confirmed by Western blot analysis. Measurement of uninfected (A) or pAd-Null-infected group (B–F) was set to 100; graphs represent means + se of ≥3 independent experiments. n = 4 (A, C, D); 6 (B); 3 (E–F). *P < 0.05.
Figure 5.
Overexpression of pVHL does not change HIF activity. A) N12 cells were cotransfected with a luciferase reporter construct driven by phosphoglyceric kinase promoter containing HREs and Renilla luciferase reporter plasmid. Then cells were infected with Ad-Null or Ad-VHL-HA and incubated for 24 h. Another group of cells was exposed to hypoxia (1.5% O2) after cotransfection of HREs and Renilla reporter plasmids. Dual luciferase assay (Promega) was carried out following the manufacturer's protocol. HRE luciferase activity was normalized to Renilla luciferase activity. Relative luciferase activity of uninfected group was set to 1; 3 independent experiments. B) N12 human primary lung fibroblasts were infected with pAd-Null or pAd-VHL-HA for 2 d, and mRNA expression levels of VEGF were determined by qRT-PCR; RPL19 gene was used as internal control. Relative mRNA level of VEGF of uninfected group was set to 100; 4 independent experiments. Graphs represent means + se. **P < 0.01.
Overexpression of pVHL promotes lung fibroblast proliferation
Since the interaction of matrix proteins and integrins contributes to cell proliferation, we sought to examine the effects of pVHL on lung fibroblast proliferation. We transfected N12 primary human lung fibroblasts with a plasmid containing HA-tagged pVHL and measured the rate of cell proliferation. The expression of exogenous pVHL was confirmed by Western blot analysis with HA antibody (Fig. 6A). Overexpression of pVHL increased cell proliferation and incorporation of BrdU in human lung fibroblasts (Fig. 6). Similar results were observed in freshly isolated rat lung fibroblasts (data not shown), suggesting that pVHL overexpression causes lung fibroblast proliferation.
Figure 6.
Overexpression of pVHL increases lung fibroblast proliferation. A) N12 primary human lung fibroblasts were transfected with empty vector or a plasmid encoding pVHL-HA. At 2 d after transfection, rates of DNA synthesis were determined by BrdU incorporation assay. BrdU incorporation was normalized to empty-vector group; n = 3. Overexpression of exogenous pVHL-HA was confirmed by Western blot analysis. B) N12 human lung fibroblasts were transfected with empty vector or pVHL-HA plasmids and incubated overnight. N12 cells were then trypsinized and seeded onto new dishes at equal densities. After incubation for indicated time periods, total number of cells was counted with hemacytometer; n = 4. Data are expressed as means+ se. *P < 0.05; **P < 0.01.
Interaction of fibronectin and integrin is required for pVHL-mediated cell proliferation
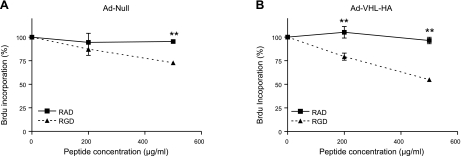
To investigate whether pVHL-mediated fibroproliferation requires the interaction between fibronectin and integrin, we overexpressed pVHL in N12 cells and incubated them with increasing doses of RGD peptide, which blocks the interaction between integrin and fibronectin. RAD peptide was used as control. As shown in Fig. 7, RAD peptide had little effect on the basal cell growth rate. By contrast, in cells overexpressing pVHL, RGD peptide inhibited cell growth even at a lower dose (200 μg/ml) and decreased cell growth by 45% at a dose of 500 μg/ml (Fig. 7B), whereas in null virus-infected N12 cells, a high dose (500 μg/ml) of RGD peptide modestly decreased cell growth (25% inhibition). This result suggests that the matrix-integrin interaction is critical for pVHL-mediated cell proliferation.
Figure 7.
Interaction between fibronectin and integrin is required for pVHL-mediated cell proliferation in N12 primary human lung fibroblasts. N12 fibroblasts were infected with pAd-Null (A) or pAd-VHL-HA (B) for 2 d. After trypsinization, cells were plated in 96-well plates in presence of RAD or RGD peptide at indicated concentrations. Rates of DNA synthesis were determined by BrdU incorporation assay. Rate of BrdU incorporation was normalized to control group. Data are expressed as means + se. n = 3. **P < 0.01.
Integrin α5β1 is essential for pVHL-mediated cell proliferation
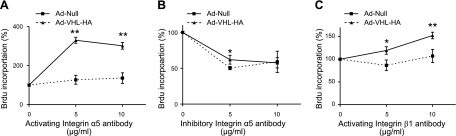
Since pVHL induced α5 integrin expression and α5 forms a dimer with β1, we determined whether α5β1 integrin participates in pVHL-mediated fibroblast proliferation by employing specific antibodies to activate or neutralize integrin α5β1 function. As shown in Fig. 8A, activating α5 integrin antibody increased cell proliferation in both null virus- and pAd-VHL-HA-infected cells, but the growth of the latter cells was significantly enhanced. Inhibitory α5 antibody inhibited proliferation in both null virus- and pAd-VHL-HA-infected cells (Fig. 8B). We also treated cells with activating β1 antibody. As shown in Fig. 8C, while activation of β1 slightly increased the growth of null virus-infected cells, it greatly stimulated proliferation of pAd-VHL-HA-infected cells. These results suggest that pVHL mediates lung fibroblast proliferation via α5 and β1 integrin.
Figure 8.
Integrin α5β1is critical for pVHL-mediated cell proliferation in N12 primary human lung fibroblasts. N12 fibroblasts were infected with pAd-Null or pAd-VHL-HA for 2 d. After trypsinization, cells were plated in 96-well plates in presence of integrin α5 activating antibody (A), integrin α5 inhibitory antibody (B), or integrin β1 activating antibody (C) at indicated concentration. Rates of DNA synthesis were determined by BrdU incorporation assay. Rate of BrdU incorporation was normalized to the group incubated with IgG. Data are expressed as means + se. n = 3 (A); 4 (B, C). *P < 0.05; **P < 0.01.
pVHL mediates fibroblast proliferation via activation of FAK
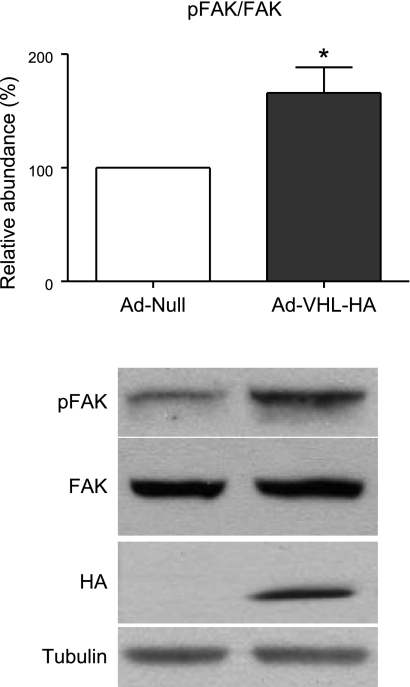
Fibronectin-integrin interaction leads to FAK phosphorylation, contributing to cell adhesion-mediated proliferation and survival (5). To determine whether pVHL mediates fibroblast proliferation through FAK activation, we measured the phosphorylation status of FAK after overexpressing pVHL in N12 fibroblasts. As shown in Fig. 9, overexpression of pVHL increased FAK phosphorylation at Tyr397 without altering the total FAK protein expression, indicating that FAK was activated.
Figure 9.
pVHL-mediated proliferation in N12 human lung fibroblasts is FAK dependent. N12 human primary lung fibroblasts were infected with pAd-Null or pAd-VHL-HA for 2 d, and amount of phospho-FAK (pY397) and total FAK were determined by Western blot analysis. pFAK/FAK ratio is normalized to Ad-Null group. Data are expressed as means + se. n = 5. *P < 0.05.
pVHL is required for TGF-β1-mediated fibroblast proliferation
TGF-β1 is a potent cytokine inducing pulmonary fibrosis (26). To determine whether pVHL plays a role in TGF-β1-mediated fibroblast proliferation, we measured the BrdU incorporation in TGF-β1-treated vhlh 2-lox MEFs infected with Ad-null or Ad-Cre viruses. As shown in Fig. 10A, infection of Ad-Cre suppressed pVHL expression in vhlh 2-lox MEFs and decreased the phosphorylation of FAK. In addition, knockdown of pVHL decreased MEF proliferation in unstimulated conditions, and TGF-β1 induced MEF proliferation. Moreover, suppression of pVHL prevented the TGF-β1-induced fibroblast proliferation (Fig. 10B), indicating that pVHL is critical to fibrotic cytokine-mediated fibroblast proliferation.
Figure 10.
Suppression of pVHL prevents TGF-β1-mediated fibroblast proliferation. A) vhlh 2-lox MEFs were infected with pAd-Null or pAd-Cre at 50 pfu/cell for 1 d, and amounts of pVHL and Cre were determined by Western blot analysis. Actin was used as loading control. B) After adenoviral infection, vhlh 2-lox MEFs were treated with TGF-β1 (2 or 5 ng/ml) for 8 h and labeled with BrdU for another 16 h. Rate of BrdU incorporation was normalized to the control group. Data are expressed as means + se. n = 3. *P < 0.05; **P < 0.01.
DISCUSSION
It is well known that ECM proteins play fundamental roles in the pathogenesis of pulmonary fibrosis, yet the underlying mechanisms remain unclear. We have provided evidence that pVHL, a fibronectin- and collagen-interacting protein, plays a critical role in the fibrotic response. Fibrotic lungs of humans and mice contain elevated levels of pVHL, especially in lung fibroblasts. Overexpression of pVHL increases protein abundance of fibronectin and collagen, induces integrin α5 protein and gene expression, and stimulates lung fibroblast proliferation. Inhibition of the fibronectin/integrin α5β1/FAK signaling pathway prevents pVHL-mediated cell proliferation. These results suggest that elevated pVHL levels increase expression of matrix proteins and the integrin α5 subunit and initiate FAK activation, leading to fibroblast proliferation, a hallmark of fibrotic diseases.
The increased expression of pVHL in IPF lungs is consistent with microarray studies showing that lungs of patients with IPF expressed higher levels of pVHL mRNA than lungs of control individuals (27). Moreover, our results provide evidence that the elevated pVHL protein is localized mostly in fibroblastic foci, where fibroblasts proliferate and differentiate to myofibroblasts (Fig. 1). This suggests that pVHL mainly affects fibroblasts during the development of IPF.
In the bleomycin lung fibrosis model, we found that lungs of bleomycin-treated mice also expressed elevated pVHL mRNA compared to lungs of mice treated with PBS. In addition, fibroblasts isolated from bleomycin-treated mice expressed higher levels of pVHL mRNA compared with fibroblasts isolated from control mice. By contrast, ATII cells isolated from bleomycin- or PBS-treated mice expressed similar levels of pVHL mRNA. This finding indicates that overexpression of pVHL is limited to fibroblasts in the experimental fibrosis model, and is consistent with results obtained in the IPF lungs. Our results also show that bleomycin instillation caused induction of pVHL mRNA as early as 14 d post-treatment, prior to the onset of maximal fibrosis (21 d after bleomycin treatment; Fig. 2C), suggesting that induction of pVHL may precede fibrosis.
A hallmark of fibrotic diseases is the excessive deposition of ECM proteins, such as fibronectin and collagen. Given the evidence that pVHL-deficient cells lack proper extracellular fibronectin and collagen network (15, 17, 18), we examined whether pVHL overexpression altered the expression of fibronectin and collagen. In human lung fibroblasts, we found that overexpression of pVHL increased collagen at the gene and protein levels. However, overexpression of pVHL increased fibronectin protein levels, but not mRNA levels. This result suggests that pVHL may up-regulate fibronectin and collagen via different mechanisms. Overexpression of pVHL may increase fibronectin expression post-transcriptionally, while regulating collagen transcriptionally. Furthermore, overexpression of pVHL increased the secretion of extracullar fibronectin (Fig. 3G). Interestingly, pVHL overexpression did not change expression levels of α smooth muscle actin, a marker of myofibroblasts, indicating that pVHL may not contribute to fibroblast differentiation. Accordingly, overexpression of pVHL does not affect the amount of total TGF-β (Fig. 3H). Thus, pVHL may participate in earlier events in the formation of the fibroblastic foci, such as fibroblast proliferation, which is consistent with the evidence described above.
Extracellular proteins interact with cell membrane integrin to regulate cellular behavior (5, 28). Previously, it has been shown that pVHL regulates fibronectin matrix through the formation of β1 integrin fibrillar adhesions, particularly αvβ1 integrin (15, 17, 18, 29) and that the αv and β1 intergrin subunits are lost in pVHL-deficient cells (29). However, we found that overexpression of pVHL induced expression of α5 integrin without changes to α1, α2, αv, α4, and β1 integrins (Fig. 4). These results suggest that the loss or gain of pVHL can regulate the complex of fibronectin and integrin in a distinct pattern, which is consistent with their different biological functions of regulating invasion or proliferation. Furthermore, pVHL overexpression induces α5 integrin (fibronectin receptor) but not α1β1, α2β1, and α4β1 (collagen receptor), and pVHL up-regulates collagen to a lesser extent than fibronectin (Fig. 4), suggesting that pVHL-mediated up-regulation of fibronectin may be more important than up-regulation of collagen. Understanding the role of pVHL overexpression may require further analysis of integrin expression profile in lung fibroblasts.
It has been well established that the interaction between ECM proteins and integrins regulates diverse cellular events, including cell proliferation, and that α5 integrin maintains cells in the proliferative phase, instead of differentiation (30, 31). We investigated whether pVHL overexpression changed the proliferation rate of lung fibroblasts. Our data show that indeed overexpression of pVHL increases fibroblast proliferation, as measured by BrdU incorporation and cell numbers (Fig. 6). These results are consistent with a previous report in which the loss of pVHL led to slower proliferation of MEFs and smaller fibrosarcoma in vivo (19). Thus, pVHL expression levels appear to correlate with cell proliferation rates.
To further validate the significance of induction of fibronectin and α5 integrin after pVHL overexpression, we chose to block the interaction between integrin and fibronectin with RGD peptides, using RAD peptides as the control. RGD peptides were efficient in reduction of cell proliferation in pVHL-infected cells compared with null virus-infected cells (Fig. 7), suggesting that cells with elevated pVHL are more sensitive to the loss of interaction between integrin and fibronectin. We also utilized specific α5 and β1 integrin antibodies to either activate or neutralize fibronectin/α5 and β1 integrin signaling. In control virus-infected cells, activating α5 and β1 integrin slightly increased fibroblast proliferation, while neutralizing α5 integrin inhibited fibroblast proliferation by 50% (Fig. 8), suggesting that α5 integrin is essential for fibroblast proliferation. More important, in fibroblasts overexpressing pVHL, activating α5 and β1 integrin greatly increased fibroblast proliferation, and these cells were more resistant to the inhibitory effects of neutralizing antibody of α5 integrin on cell proliferation. These results indicate that fibronectin and α5β1 integrin are key mediators of pVHL-induced fibroblast proliferation.
Integrins transduce extracellular signals to the downstream effectors including FAK to affect biological processes (5, 32). We investigated whether pVHL mediates signaling through FAK activation. Our results have demonstrated that overexpresssion of pVHL increased FAK phosphorylation at Tyr397, suggesting the activation of FAK (Fig. 9). These results demonstrate that pVHL-mediated fibroblast proliferation requires integrin/FAK signaling.
TGF-β1 is a potent profibrotic cytokine, and forced expression of TGF-β1 is sufficient to induce pulmonary fibrosis in a mouse model (26). To gain further insight of the role of pVHL in fibrogenesis, we knocked down pVHL in MEFs and examined the proliferation in these cells after TGF-β1 treatment. As shown in Fig. 10A, MEFs with suppression of pVHL grow slower than wild-type MEFs, which is consistent with a previous report (19). Furthermore, suppression of pVHL leads to the decreased phosphorylation of FAK (Fig. 10A), and suppression of pVHL prevents TGF-β1-induced fibroblast proliferation (Fig. 10B). A previous report by Horowitz et al. (33) suggests that TGF-β1 activates FAK and promotes the survival of lung myofibroblasts. Thus, we speculate that pVHL promotes fibroblast proliferation via activation of FAK during fibrogenesis.
Together, our findings suggest that pVHL is essential for proliferation of primary lung fibroblasts, and that up-regulation of pVHL correlates with increased levels of fibronectin, collagen, integrin α5β1, phosphorylation of FAK, and fibrosis. To our knowledge, this is the first evidence linking pVHL and FAK activation. FAK phosphorylation is mediated by the interaction of integrin and fibronectin. Therefore, we hypothesize that early induction of pVHL may facilitate fibronectin/integrin α5β1/FAK signaling, leading to lung fibroblast proliferation and fibrosis. Interestingly, integrin/FAK signaling has been implicated in fibrogenesis. For example, profibrotic cytokine TGF-β1 up-regulates fibronectin and integrin α5β1 (34); fibronectin/integrin α5β1 signaling is both necessary and sufficient for IPF fibroblasts to cross basement membranes (35). Moreover, TGF-β1 activates FAK and promotes the survival of lung myofibroblasts (33). Thus, integrin α5β1 and FAK may be novel therapeutic targets for treatment of IPF.
Although pVHL functions have been mostly examined in pVHL-deficient cells, our study suggests that a gain-of-function of pVHL is associated with pulmonary fibrosis. We provide evidence that gain of pVHL does not affect HIF activity, suggesting that the gain-of-function of pVHL is HIF independent (Fig. 5). A recent report also suggests that pVHL expression is elevated in skeletal muscles of patients with chronic obstructive pulmonary disease (COPD; ref. 36). However, it is unclear how elevated pVHL contributes to the pathogenesis of COPD. Notably, Hickey and colleagues (37) have recently reported that mutation of pVHL at codon 200 (R200W) causes the Chuvash disease with pulmonary vascular remodeling and hypertension, and in older mice lung fibrosis. In addition, VHL-null hearts developed fibrosis in a HIF-1-dependent mechanism (38). Thus, the role of pVHL in fibrosis may be organ and cell specific. Another possibility is that during the early stage of fibrosis, overexpression of pVHL increases interstitial fibroblast proliferation. In the later stage, the abnormal architecture of the lung creates a hypoxic microenvironment, which activates HIF pathway and increases cytokine and growth factor expression, resulting in epithelial-mesenchymal transition and fibrosis (39, 40).
Acknowledgments
This work was supported in part by U.S. National Institutes of Health grants HL-071643, HL-093014 (J.I.S.), and AR-042309 (J.V.), and a Parker B. Francis foundation fellowship (G.Z.). The authors greatly appreciate Ms. Christine Y. Kim for her insightful reading of their manuscript.
REFERENCES
- 1. Selman M., King T. E., Pardo A., American Thoracic Society, European Respiratory Society, and American College of Chest Physicians (2001) Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 134, 136–151 [DOI] [PubMed] [Google Scholar]
- 2. Du Bois R. M. (2010) Strategies for treating idiopathic pulmonary fibrosis. Nat. Rev. Drug Disc. 9, 129–140 [DOI] [PubMed] [Google Scholar]
- 3. Geiger B., Bershadsky A., Pankov R., Yamada K. M. (2001) Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev. Mol. Cell. Biol. 2, 793–805 [DOI] [PubMed] [Google Scholar]
- 4. Berrier A. L., Yamada K. M. (2007) Cell-matrix adhesion. J. Cell. Physiol. 213, 565–573 [DOI] [PubMed] [Google Scholar]
- 5. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 6. Fontana L., Chen Y., Prijatelj P., Sakai T., Fassler R., Sakai L. Y., Rifkin D. B. (2005) Fibronectin is required for integrin alphavbeta6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEB J. 19, 1798–1808 [DOI] [PubMed] [Google Scholar]
- 7. Dallas S. L., Sivakumar P., Jones C. J., Chen Q., Peters D. M., Mosher D. F., Humphries M. J., Kielty C. M. (2005) Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J. Biol. Chem. 280, 18871–18880 [DOI] [PubMed] [Google Scholar]
- 8. Munger J. S., Huang X., Kawakatsu H., Griffiths M. J., Dalton S. L., Wu J., Pittet J. F., Kaminski N., Garat C., Matthay M. A., Rifkin D. B., Sheppard D. (1999) The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 [DOI] [PubMed] [Google Scholar]
- 9. Annes J. P., Chen Y., Munger J. S., Rifkin D. B. (2004) Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J. Cell Biol. 165, 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horan G. S., Wood S., Ona V., Li D. J., Lukashev M. E., Weinreb P. H., Simon K. J., Hahm K., Allaire N. E., Rinaldi N. J., Goyal J., Feghali-Bostwick C. A., Matteson E. L., O'Hara C., Lafyatis R., Davis G. S., Huang X., Sheppard D., Violette S. M. (2008) Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 177, 56–65 [DOI] [PubMed] [Google Scholar]
- 11. Puthawala K., Hadjiangelis N., Jacoby S. C., Bayongan E., Zhao Z., Yang Z., Devitt M. L., Horan G. S., Weinreb P. H., Lukashev M. E., Violette S. M., Grant K. S., Colarossi C., Formenti S. C., Munger J. S. (2008) Inhibition of integrin alpha(v) beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 177, 82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin W. G., Jr. (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. [see comment] Science 292, 464–468 [DOI] [PubMed] [Google Scholar]
- 13. Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. [see comment] Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 14. Masson N., Willam C., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 20, 5197–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohh M., Yauch R. L., Lonergan K. M., Whaley J. M., Stemmer-Rachamimov A. O., Louis D. N., Gavin B. J., Kley N., Kaelin W. G., Jr., Iliopoulos O. (1998) The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol. Cell 1, 959–968 [DOI] [PubMed] [Google Scholar]
- 16. Grosfeld A., Stolze I. P., Cockman M. E., Pugh C. W., Edelmann M., Kessler B., Bullock A. N., Ratcliffe P. J., Masson N. (2007) Interaction of hydroxylated collagen IV with the von Hippel-Lindau tumor suppressor. J. Biol. Chem. 282, 13264–13269 [DOI] [PubMed] [Google Scholar]
- 17. Kurban G., Hudon V., Duplan E., Ohh M., Pause A. (2006) Characterization of a von Hippel Lindau pathway involved in extracellular matrix remodeling, cell invasion, and angiogenesis. Cancer Res. 66, 1313–1319 [DOI] [PubMed] [Google Scholar]
- 18. Tang N., Mack F., Haase V. H., Simon M. C., Johnson R. S. (2006) pVHL function is essential for endothelial extracellular matrix deposition. Mol. Cell. Biol. 26, 2519–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mack F. A., Patel J. H., Biju M. P., Haase V. H., Simon M. C. (2005) Decreased growth of Vhl-/- fibrosarcomas is associated with elevated levels of cyclin kinase inhibitors p21 and p27. Mol. Cell. Biol. 25, 4565–4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goda N., Ryan H. E., Khadivi B., McNulty W., Rickert R. C., Johnson R. S. (2003) Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol. Cell. Biol. 23, 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frew I. J., Krek W. (2007) Multitasking by pVHL in tumour suppression. Curr. Opin. Cell Biol. 19, 685–690 [DOI] [PubMed] [Google Scholar]
- 22. Budinger G. R., Mutlu G. M., Eisenbart J., Fuller A. C., Bellmeyer A. A., Baker C. M., Wilson M., Ridge K., Barrett T. A., Lee V. Y., Chandel N. S. (2006) Proapoptotic Bid is required for pulmonary fibrosis. Proc. Natl. Acad. Sci. U. S. A. 103, 4604–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eickelberg O., Kohler E., Reichenberger F., Bertschin S., Woodtli T., Erne P., Perruchoud A. P., Roth M. (1999) Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-beta1 and TGF-beta3. Am. J. Physiol. 276, L814–L824 [DOI] [PubMed] [Google Scholar]
- 24. Konigshoff M., Kramer M., Balsara N., Wilhelm J., Amarie O. V., Jahn A., Rose F., Fink L., Seeger W., Schaefer L., Gunther A., Eickelberg O. (2009) WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J. Clin. Invest. 119, 772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abe M., Harpel J. G., Metz C. N., Nunes I., Loskutoff D. J., Rifkin D. B. (1994) An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216, 276–284 [DOI] [PubMed] [Google Scholar]
- 26. Kim K. K., Kugler M. C., Wolters P. J., Robillard L., Galvez M. G., Brumwell A. N., Sheppard D., Chapman H. A. (2006) Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. U. S. A. 103, 13180–13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pardo A., Gibson K., Cisneros J., Richards T. J., Yang Y., Becerril C., Yousem S., Herrera I., Ruiz V., Selman M., Kaminski N. (2005) Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2, e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meighan C. M., Schwarzbauer J. E. (2008) Temporal and spatial regulation of integrins during development. Curr. Opin. Cell Biol. 20, 520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Esteban-Barragan M. A., Avila P., Alvarez-Tejado M., Gutierrez M. D., Garcia-Pardo A., Sanchez-Madrid F., Landazuri M. O. (2002) Role of the von Hippel-Lindau tumor suppressor gene in the formation of beta1-integrin fibrillar adhesions. Cancer Res. 62, 2929–2936 [PubMed] [Google Scholar]
- 30. Sastry S. K., Lakonishok M., Thomas D. A., Muschler J., Horwitz A. F. (1996) Integrin alpha subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J. Cell Biol. 133, 169–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu J., DeYoung S. M., Zhang M., Cheng A., Saltiel A. R. (2005) Changes in integrin expression during adipocyte differentiation. Cell Metab. 2, 165–177 [DOI] [PubMed] [Google Scholar]
- 32. Mitra S. K., Schlaepfer D. D. (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 33. Horowitz J. C., Rogers D. S., Sharma V., Vittal R., White E. S., Cui Z., Thannickal V. J. (2007) Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell. Signal. 19, 761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weston B. S., Wahab N. A., Mason R. M. (2003) CTGF mediates TGF-beta-induced fibronectin matrix deposition by upregulating active alpha5beta1 integrin in human mesangial cells. J. Am. Soc. Nephrol. 14, 601–610 [DOI] [PubMed] [Google Scholar]
- 35. White E. S., Thannickal V. J., Carskadon S. L., Dickie E. G., Livant D. L., Markwart S., Toews G. B., Arenberg D. A. (2003) Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am. J. Resp. Crit. Care Med. 168, 436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jatta K., Eliason G., Portela-Gomes G. M., Grimelius L., Caro O., Nilholm L., Sirjso A., Piehl-Aulin K., Abdel-Halim S. M. (2009) Overexpression of von Hippel-Lindau protein in skeletal muscles of patients with chronic obstructive pulmonary disease. J. Clin. Pathol. 62, 70–76 [DOI] [PubMed] [Google Scholar]
- 37. Hickey M. M., Richardson T., Wang T., Mosqueira M., Arguiri E., Yu H., Yu Q. C., Solomides C. C., Morrisey E. E., Khurana T. S., Christofidou-Solomidou M., Simon M. C. (2010) The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J. Clin. Invest. 120, 827–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lei L., Mason S., Liu D., Huang Y., Marks C., Hickey R., Jovin I. S., Pypaert M., Johnson R. S., Giordano F. J. (2008) Hypoxia-inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol. Cell. Biol. 28, 3790–3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Higgins D. F., Kimura K., Bernhardt W. M., Shrimanker N., Akai Y., Hohenstein B., Saito Y., Johnson R. S., Kretzler M., Cohen C. D., Eckardt K. U., Iwano M., Haase V. H. (2007) Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 117, 3810–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou G., Dada L. A., Wu M., Kelly A., Trejo H., Zhou Q., Varga J., Sznajder J. I. (2009) Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L1120–L1130 [DOI] [PMC free article] [PubMed] [Google Scholar]