Abstract
We studied the molecular forms of the GPI-anchored urokinase plasminogen activator receptor (uPAR-mEGFP) in the human embryo kidney (HEK293) cell membrane and demonstrated that the binding of the amino-terminal fragment (ATF) of urokinase plasminogen activator is sufficient to induce the dimerization of the receptor. We followed the association kinetics and determined precisely the dimeric stoichiometry of uPAR-mEGFP complexes by applying number and brightness (N&B) image analysis. N&B is a novel fluctuation-based approach for measuring the molecular brightness of fluorophores in an image time sequence in live cells. Because N&B is very sensitive to long-term temporal fluctuations and photobleaching, we have introduced a filtering protocol that corrects for these important sources of error. Critical experimental parameters in N&B analysis are illustrated and analyzed by simulation studies. Control experiments are based on mEGFP-GPI, mEGFP-mEGFP-GPI, and mCherry-GPI, expressed in HEK293. This work provides a first direct demonstration of the dimerization of uPAR in live cells. We also provide the first methodological guide on N&B to discern minor changes in molecular composition such as those due to dimerization events, which are involved in fundamental cell signaling mechanisms.—Hellriegel, C., Caiolfa, V. R., Corti, V., Sidenius, N., Zamai, M. Number and brightness image analysis reveals ATF-induced dimerization kinetics of uPAR in the cell membrane.
Keywords: fluorescence microscopy, GPI-anchored proteins, ligand binding, receptor dimerization
The urokinase-type plasminogen activator receptor (uPAR) is a 45- to 55-kDa protein, heterogeneously glycosylated and inserted into the external leaflet of the plasma membrane through a glycosylphosphatidylinositol (GPI) anchor (1). Compelling evidence (recently reviewed in refs. 2, 3) indicates that uPAR promotes cell motility, invasion, proliferation, and survival through the regulation of pericellular proteolysis, cell-extracellular matrix (ECM) interaction, and intracellular signaling.
uPAR regulates the activity of the plasminogen activation system by binding the serine protease urokinase-type plasminogen activator (uPA) and its zymogen form pro-uPA. A well-known cascade of extracellular proteolytic activation starts as consequence of this interaction (reviewed in refs. 2, 3). uPAR and uPA also act jointly with integrins, FPR-like receptor-1/lipoxin A4 receptor, and the epidermal growth factor receptor (EGFR) to initiate cell signaling (reviewed in refs. 2, 3).
After binding to uPAR, uPA cleaves its receptor between the D1 and D2 domains. The amino-terminal fragment (ATF) of uPA retains the binding affinity (4, 5) for the receptor without activating the proteolytic cascade activated by uPA. ATF is a player in the multifaceted uPAR-mediated signaling. ATF increases the association of uPAR to detergent-resistant membranes (DRMs), independent of the catalytic activity of uPA, and is required for intracellular signaling (6). It has been reported that ATF can trigger the sequential activation of MMP-2, NSMase-2, and ERK1/2 in ECV304 cells that are required for uPA-induced ECV304 proliferation (7).
EGFR has been reported to play a transduction role of uPAR stimuli, mediating uPA-induced proliferation in highly malignant cells that overexpress uPAR (8). It has been reported that uPAR is required for EGF to induce proliferation of murine embryonic fibroblasts (MEFs) and MDA-MB 231 breast cancer cells (9). The results of this study support a model in which uPAR primes the cell to proliferate in response to EGF by facilitating activation of STAT5b. Other studies have observed that uPAR stimulation with ATF transactivates EGFR in mammary MCF-7 cells through a mechanism involving Src and a metalloproteinase (10). Both ATF and EGF stimuli induce an interaction of the EGFR with uPAR and ERK activation but with distinct outcomes. EGFR activation by uPAR stimuli mediates cellular invasion rather than proliferation, while EGFR activation by EGF leads to a proliferative response (10).
Attempting to provide an explanation for the diversity of adaptor proteins shown to facilitate uPAR signaling, Gonias and collaborators (9, 11, 12) have proposed that uPAR coreceptors may be organized dynamically into multiprotein signaling receptor complexes and that expression and assembly of uPAR coreceptors in a specific cell type can determine the response to uPA. Thus, when a protein like EGFR is expressed in cells, it may enter the complex and alter its properties. This model can indeed provide a possible explanation for differences in signaling responses observed in different cell types. Therefore, coreceptor association, oligomerization, and/or dimerization and dynamic segregation of uPAR in membrane microdomains can be regulated by extracellular ligands such as vitronectin, uPA, and ATF, which might act as discriminators of uPAR signaling.
In vitro studies (13–15) have attempted the stoichiometric characterization of uPAR in complex with uPA, ATF, and the inhibitor complex uPA-PAI1; the structure of soluble uPAR bound to ATF also has been solved (16, 17). Besides that, little information is available on the spatial and temporal dynamics and molecular organization of these complexes in live cells (18, 19). As reported in the recent recapitulating work of Smith and Marshall (2) on uPAR signaling, “key molecules involved in signaling downstream of uPAR have been identified, but the relative contributions of the two uPAR ligands uPA and vitronectin to signaling are incompletely understood and many molecular details of uPAR signaling pathways remain unclear.”
Here we introduce a recently described imaging fluorescence fluctuation tool, number and brightness (N&B) analysis (Figs. 1 and 2 and Materials and Methods) for exploring, with unprecedented level of detail, the reorganization of uPAR molecules on interaction with cell surface or extracellular ligands.
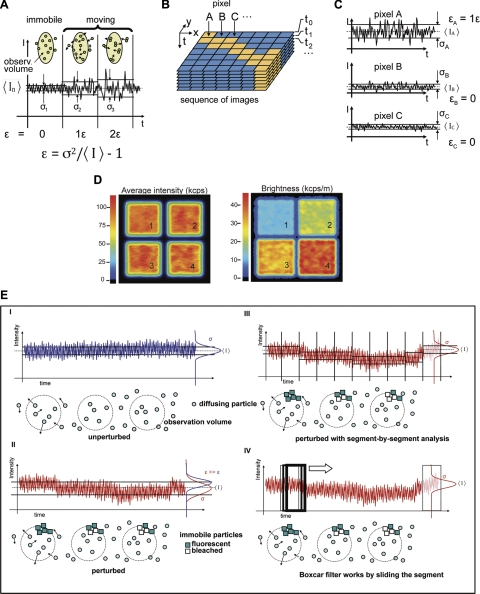
Figure 1.
N&B principle. A) Statistical characterization of fluctuation amplitudes is based on the ratio between variance of the fluctuating signal σ2 and mean intensity value 〈I〉. In the simplest scenario (left panel), when the fluorescence signal does not fluctuate due to fluorophore motion, this ratio describes instrument noise. If the fluorescence signal fluctuates due to mobile fluorophores (middle and right panels) the “extra” amount of variance is directly proportional to the molecular brightness ε of the diffusing species, given in units of detected photon counts per second and per molecule (cps/m) (22, 23, 25, 43). Scheme illustrates sd for an equal number of immobile monomers (σ1) and mobile monomers (σ2) and dimers (σ3). These 3 cases give the same 〈I〉 value, but different ε values (1ε, 2ε); ε = 0 when fluorophores are immobile. B) Schematic illustration of the process of N&B analysis in scanned image sequences. C) Standard deviation collected at the 3 representative pixels A–C at a given pixel dwell time. D) Average fluorescence intensity and molecular brightness images for simulated monomers (240 particles, ε=10 kcps/m; panel 1); dimers (120 particles, ε=20 kcps/m; panel 2); trimers (80 particles, ε=30 kcps/m; panel 3) and tetramers (60 particles, ε=40 kcps/m; panel 4). All particles have the same diffusion coefficient, D = 0.2 μm2/s. E) Schematic illustration of temporal perturbations and boxcar filtering: I) Ideal case in which the 〈I〉 trace is constant and unperturbed during acquisition. Resulting brightness e is determined using variance σ2 and 〈I〉 according to Eq. 8. Illustration below trace depicts fluorescent particles diffusing in and out of the observation volume at different times. II) In the presence of a long-term perturbation (e.g., bleaching), the resulting brightness (ε, in red) is overestimated, because σ2 is larger and 〈I〉 is smaller than in the unperturbed case (ε, in blue). Illustration depicts a population of slowly bleaching, immobile, and dim background particles that contribute to the intensity trace. III) If the perturbed trace is analyzed on a segment-by-segment basis, brightness values in each segment are not affected by the long-term perturbation, and are therefore corrected. This approach was illustrated originally for PCH analysis (21). IV) Boxcar filter works in a similar way, but instead of segmenting the whole trace, it slides a segment (the boxcar) along the data. Compensation for long-term perturbations depends on boxcar size.
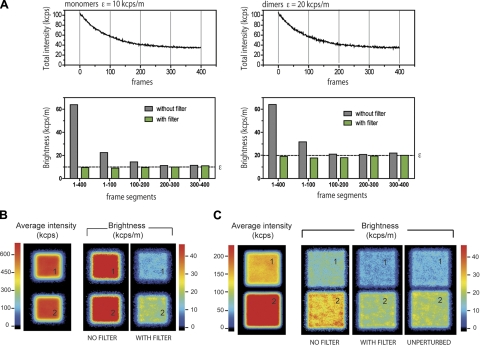
Figure 2.
Effect of long-term perturbations and boxcar filter in N&B analysis. Monomer and dimer particles from Fig. 1E are reanalyzed in the presence of an additional population of particles simulating a static and bleaching background equivalent to 2000 molecules emitting 2.5 kcps/m. A) Top panels: intensity vs. time trace. Bottom panels: comparison between boxcar-filtered and unfiltered N&B analysis of the whole sequence (1–400 frames) and of different segments of 100 frames each. B) Average intensity and brightness images corresponding to the simulated cases in A. Panel 1: monomers. Panel 2: dimers. C) Average fluorescence intensity and molecular brightness images for simulated monomers (120 particles, ε=10 kcps/m; panel 1) and dimers (120 particles, ε=20 kcps/m; panel 2), including moderate background and bleaching (5-fold dimmer than in B; panel 1: monomers; panel 2: dimers]. Boxcar length in all cases was 10 frames.
N&B analysis delivers the molecular brightness (ε) of a fluorophore from the fluorescence fluctuation amplitudes that are, in turn, caused by the fluorescent species diffusing in and out of the observation volume (Fig. 1A–C). If, for example, a protein labeled with a fluorescent dye of brightness 1ε associates as a homodimer, the complex will carry 2 fluorescent labels, and N&B analysis will yield a molecular brightness of 2ε (20). Molecular brightness is therefore a useful marker for monitoring protein association in living cells (21).
N&B has been described as a proof of concept for 2-photon scanning microscopy (22), camera-based total internal reflection fluorescence (TIRF; ref. 23), and confocal microscopy (24) and used for distinguishing monomers from aggregates and immobile proteins (25). More recently, N&B has been applied for investigating the existence of preformed ligand-independent dimers and clusters of ErbB receptor subunits (26). In addition, 2-color N&B analysis has been proposed for detecting large molecular complexes involved in cellular adhesion (27).
Using N&B, we first demonstrate that, in the absence of extracellular ligands, uPAR-mEGFP is sorted to the cell membrane, and there it diffuses as monomer. Then we prove that ATF binding is sufficient for inducing sustained uPAR dimerization in the cell membrane. The results are backed by experiments on control mEGFP-constructs and by simulations and validation studies.
We also show how the current N&B analysis can be optimized (Fig. 2) for revealing minor change of brightness, as in the case of the homophilic dimerization of uPAR, and in perturbed conditions, such as long-term fluctuations and background bleaching. We introduce a simple boxcar filtering data analysis procedure (28) to overcome the major limitations of N&B due to long-term temporal fluctuations. Because N&B is still restricted to a limited number of specialized laboratories, in this work we also provide a comprehensive and practical road map to allow for the application of N&B to a variety of live cell studies by a broader cross-section of the scientific community.
MATERIALS AND METHODS
Theory of N&B analysis
The conceptual formalism behind N&B starts with the measurement of fluorescence intensity values, I, on a pixel-by-pixel basis in a series of frames or sequence of microscopy images (Fig. 1A–C). The temporal separation between frames (frame time) and the number of frames determines the total acquisition time, which poses practical limitations (discussed below). One intensity measurement, at a given pixel, takes place within the pixel dwell time, td, and is repeated frame after frame, for K frames. If td is considerably shorter than the time it takes for a molecule to diffuse through the observation volume, the fluctuations of the number of particles in the pixel can be recorded; otherwise, the fluctuations are lost due to temporal averaging (29, 30). In a biological context, the diffusion coefficient of proteins ranges from ∼0.01 to 30 μm2/s, in this range and in a typical confocal or multiphoton excitation volume diffusion times are of the order of 0.1 to 10 ms. The amplitude of the fluctuations at a given pixel is measured from the variance of the fluorescence signal, σ2. It is defined as:
| (1) |
The average intensity, 〈I〉, and the average squared intensity, 〈I2〉, are computed from the individual intensity values for each pixel in a total of K frames (Fig. 1B):
| (2) |
| (3) |
The variance encompasses all fluctuations, which may have different origins. In a first approximation, we can separate the variance due to detector fluctuations, σd2, from the variance due to molecular fluctuations, σo2. The two variances are independent, so the total variance is given by their sum:
| (4) |
Under the assumption that the detector is being operated below its saturation limit, then the variance due to detector fluctuations is a (usually linear) function of the detected intensity:
| (5) |
For conventional detectors, such as photomultipliers and CCD cameras, one can obtain the coefficients a and c from straightforward calibration measurements. In the case of photon-counting detectors, a = 1 and c = 0; thus:
| (6) |
The second term of Eq. 4, the variance due to molecular fluctuations in and out of the detection volume, is proportional to the intensity via the molecular brightness, εtd4,5:
| (7) |
Using Eqs. 6 and 7 in Eq. 4 and dividing by 〈I〉, one obtains the quantity σ2/〈I〉, which corresponds to the apparent brightness, B (as defined in the works of Gratton and colleagues; ref. 22) and can be used to obtain εtd:
| (8) |
Note that the detected molecular brightness εtd introduced in Eq. 7 is given for a specific pixel dwell time td, in units of detected photon counts per molecule and dwell time. The molecular brightness value in counts per second (cps) is εs = εtd/td (31). Thus, to obtain the molecular parameter εs on each pixel, we divide the total variance by the average intensity, subtract unity, and divide by the pixel dwell time:
| (9) |
The numerical value for εs is smaller than the real brightness value due to instrumental parameters, like detection efficiency and excitation geometry. The real molecular brightness ε is given by:
| (10) |
where ϕ is the detection quantum yield (which can be obtained from calibration; in our case, the value varies from 0.10 and 0.05 depending on the wavelength used), and γ, defined in Eq. 11, accounts for the excitation and detection geometry:
| (11) |
where PSF(r) is the point-spread function of the microscope and varies for different types of microscope (23, 32); it may differ from objective to objective (due to different chromatic and spherical corrections). Also, it may vary from sample to sample as a function of the astigmatism introduced (e.g., due to poor refractive index matching) and if the observed volume on the sample is smaller than the PSF, as in a cell membrane. The γ factor generally remains constant for one specific type of experiment using the same optics. It has been calculated for many different scenarios and can be determined empirically from calibration measurements. (Calculated γ values: confocal γ = 0.3536, ref. 32; 2-photon γ = 0.076 (32); TIRF γ ∼ 0.25, ref. 23; membrane γ < 0.5, ref. 23).
In the described experiments, we have used two different microscope setups. Experiments were acquired under identical conditions for a given setup; hence, we assume that the product γ · ϕ was constant. We refrain from comparing the absolute brightness value directly between the different microscopes. Instead, we use εs in kilocounts per second per molecule (kcps/m), as defined in Eq. 10, throughout and calibrate with the same sample, uPAR-mEGFP on fibronectin, as a reference.
In Fig. 1D, we provide a visible example from a simulated image in which particles with a predetermined brightness are set to diffuse within a predefined square area. In this example, the chosen brightness and concentration parameters of the particles give the same average fluorescence intensity in the images. On one hand, it is impossible to recognize the different particles from their intensity without knowing how many are contained in each area. The molecular brightness image, on the other hand, allows for a straightforward assignment of the fluorescent species to the correct area.
To set up the conditions for N&B analysis in our experiments, we simulated unperturbed scenarios by varying systematically the number of pixels in the analysis, total number of frames in the sequence, molecular brightness, concentration, and diffusion coefficient of the particles (Supplemental Figs. S1–S4). These parameters are interdependent and ultimately are affected by the product of the errors in the retrieved number and brightness values, which in turn depend on the signal-to-noise ratio.
The simulations helped in establishing the limits for the applicability of N&B analysis. A time sequence of 100 frames, with ≥1000 pixels/frame, can be enough for determining the brightness of even a dim molecule (1000 cps/m) that diffuses as slow as membrane proteins (∼0.01 μm2/s), and it is present at low concentration (∼5 nM or ∼0.3 molecules/fl) (Supplemental Figs. S1–S4).
However, we expected to be far from the limits given solely by signal-to-noise considerations because of the presence of strong perturbations, such as cell motion, vesicle trafficking, photobleaching, etc. (Supplemental Movie S1). Such perturbations generally produce large fluorescence fluctuation amplitudes and consequently give a large contribution to the overall variance. In the simplest case, when the source of the “extra” fluctuation is visible as an isolated event in the time sequence (e.g., a filopodium moving into the region of interest) it is possible to remove the frames containing the perturbation. Often, and particularly at the limit of detection, threshold values that describe the amount of extraneous fluctuations are introduced as a cutoff criterion to discard potentially distorted data (26). In more subtle cases, however (for example, when the entire cell membrane moves or bleaches slowly, as in our experiments), we have introduced a short-pass data-filtering algorithm to account for the extra fluctuations.
Validation of boxcar-filtered N&B analysis
One simple form of short-pass data-filtering algorithm is to analyze the data on a segment-by-segment basis as with a boxcar averaging algorithm (as commonly used in analog signal filtering; ref. 28). This process is illustrated in Fig. 1E. The whole intensity vs. time trace is analyzed using a sliding boxcar. The boxcar is a small segment of data (of user-defined length, typically from 2 to 20 frames), which is shifted in a frame-by-frame fashion (Fig. 1E). The brightness value (according to Eq. 9) is calculated in each step, rendering a sequence of brightness values as the boxcar slides along the data set. This sequence is averaged in a final step, effectively yielding a short-pass-filtered brightness value. Note that, in contrast to the boxcar filter, the moving average correction (27) produces the opposite effect, rendering long-pass-filtered data sets, which are then subtracted from the raw data to produce a detrended intensity vs. time trace. Using the boxcar filtering, fluctuations, which persist over a time longer than the boxcar size, are suppressed. In the absence of perturbations, the values retrieved with and without the boxcar filter are the same.
Perturbations that are not on the long-term time scale (i.e., much shorter than the pixel dwell time, such as triplet blinking and intensity fluctuations due to molecular rotation dynamics) are neither affected nor compensated by the boxcar filtering. Fluctuations on similar time scales can almost always be attributed to molecular species codiffusing with a similar diffusion coefficient in the same observation volume. This specific case constitutes a mixed population. Although it is not possible to unmix the population based on temporal filtering, it is possible, under some circumstances, to gauge the composition of the mixture based on the resulting intensity (Supplemental Fig. S5).
To use the boxcar-filtering algorithm on sequences of images, we developed an analysis software (based on LabVIEW; National Instruments, Austin, TX, USA), which imports the raw data from the simulation software as well as the raw data from the acquisition setups used. The filter algorithm was tested on a set of simulated data containing a strong long-term perturbation similar to that produced by a slow photobleaching component or long-term apparatus drift (Fig. 2A). Simulations were carried out using simFCS software [Laboratory for Fluorescence Dynamics (LFD), University of California, Irvine, CA, USA] and our custom-built analysis software. The standard simulation parameters consisted of a total of 400 frames acquired in scanning mode at 32 μs pixel dwell time. We simulated fluorophores diffusing within an 80- × 80-pixel area (6400 pixels total), with 50 nm/pixel. Unless otherwise noted, the molecular brightness is 10 kcps/m, concentration is 10 nM, and the diffusion coefficient D = 2 μm2/s. In the simulations, we stipulated a number of molecules with a specific brightness and a specific diffusion coefficient (which can also be 0, for immobile species). These molecules may switch between predefined states (for example, to simulate bleaching) according to kinetic parameters chosen by the user (forward and backward rates). Further, we defined confinement regions (e.g., square areas, mimicking a membrane section). Each parameter was varied systematically to map different experimental scenarios.
The resulting simulated total intensity vs. time traces of perturbed monomer and dimer populations clearly show the photobleaching as a decay of the total intensity (Fig. 2A, top panels). The intensity traces were subdivided into segments, and the brightness was retrieved for each segment with and without applying the boxcar filter. In the absence of filtering, the retrieved brightness from the whole trace (segment 0–400 in Fig. 2A, bottom panels) is overestimated remarkably, and more important, the 2-fold difference in brightness among the simulated particles (i.e., monomers vs. dimers) is undetectable. When simulated data are analyzed on a segment-by-segment basis, the overestimation is still large in the first 2 segments (0–100, 100–200), as they are still affected strongly by the bleaching background. In the later segments (200–300 and 300–400), after the photobleaching levels off, the unfiltered retrieved brightness corresponds to the actual simulated values. In this type of conventional analysis, the first half of the data would be discarded. In contrast, when the boxcar filter is applied (in this example, the filter width is set to 10 frames), the retrieved values for both the whole trace and for the individual segments remain consistent. The effect of the correction becomes apparent directly in the images shown in Fig. 2B.
In a different simulation (Fig. 2C), we used the same number of monomers and dimers and introduced a bleaching background that is 5-fold dimmer than in the previous case. Although it was possible to detect a difference in particle brightness also without employing the filter, again brightness was considerably overestimated compared to the filtered data and to the unperturbed simulation case (i.e., in the absence of bleaching background, as in Fig. 2A). This case exemplifies how uncorrected, unfiltered N&B can become misleading. The boxcar filter, although very simple, helps in retrieving the correct brightness values.
Construction of expression vectors
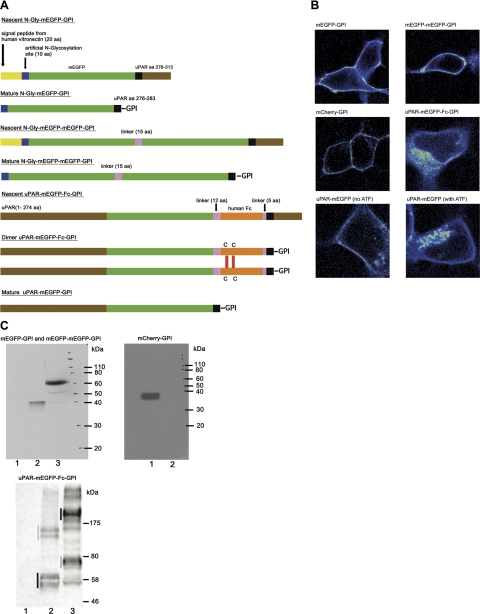
The expression vector for cytosolic monomeric EGFP (pN1-mEGFP) has been described previously (18). To generate vectors encoding GPI-anchored mEGFP and mCherry (33), the vectors pN1-uPAR-mEGFP-GPI and pN1-uPAR-mCherry-GPI were amplified with oligos N-CheF/uPARd and cloned XhoI/NotI in the vector pFRT/TO-Fc (34). Subsequently, the encoded proteins were transferred KpnI/NotI to the pEGFP-N1 expression vector (Clontech Laboratories, Inc., Mountain View, CA, USA). The resulting vectors (pN1-N-mEGFP-GPI and pN1-N-mCherry-GPI) encode proteins composed of the signal peptide of human vitronectin, a short N-glycosylation sequence to favor cell surface sorting, the entire coding region of the respective fluorescent proteins and the GPI-anchoring sequence of uPAR (Fig. 3A). The vector encoding a tandem-arranged, GPI-anchored, mEGFP was generated by amplifying pN1-N-mEGFP-GPI with oligo pairs hVUkpn/FPDWAR and FPAGEF/uPARd and assembling the 2 products, KpnI/Age1 and Age1/NotI2, into KpnI/NotI-digested pEGFP-N1. We generated a uPAR-mEGFP-Fc-GPI construct by insertion of the Fc region (35) of a human IgG1 between the mEGFP coding region and the GPI-anchoring sequence from uPAR. To clone the uPAR-mEGFP-Fc-GPI variant, the following 3 PCR products were assembled in KpnI/NotI-digested pEGFP-N1: the uPAR-GFP hybrid coding region obtained by amplification of pN1-uPAR-mEGFP-GPI with primers URfSK/FPdwAXN (KpnI/Age1 digested); a human IgG1 Fc coding region obtained by amplification of a human Fc sequence (34) with primers FcAXf/FcAr (Age1 digested); and the GPI-anchoring sequence of uPAR obtained by amplification of a uPAR cDNA with primers GPIage/uPARd (Age1/NotI). The resulting vector, pN1-uPAR-mEGFP-Fc-GPI, encodes a chimera composed of the following elements: the ecto-domain of human uPAR (residues 1–274), a full-length mEGFP coding region, a linker (LELEVLFQGPIE), the coding region of human Fc, a linker (TGGAG), and the C-terminal GPI-codifying region of uPAR (residues 278–313). The mEGFP-tagged variant of uPAR (uPAR-mEGFP) was described previously (18). The correct assembly of all expression vectors was confirmed by complete sequencing of the coding regions and by immunoblotting (Fig. 3C).
Figure 3.
Constructs. A) Schematic illustration of mEGFP-tagged controls and uPAR constructs showing nascent and mature proteins that are the result of: ER targeting and signal peptide cleavage, GPI-anchor addition (transamidase catalyzed), N-glycosylation, and membrane sorting. Dimer of uPAR-mEGFP-Fc-GPI is formed through disulfide bonds in the Fc domain. mCherry-GPI was obtained as mEGFP-GPI. B) Image gallery of uPAR-mEGFP and control constructs in HEK293 cells seeded on fibronectin matrices. C) Top left panel: lysates from HEK293 cells transfected with empty vector (lane 1), mEGFP-GPI (lane 2), and mEGFP-mEGFP-GPI (lane 3) were immunoblotted for mEGFP. Top right panel: lysates from HEK293 cells transfected with mCherry-GPI (lane 1) and empty vector (lane 2) were immunoblotted for mCherry. Bottom panel: lysates from HEK293 cells transfected with empty vector (lane 1), wt uPAR-mEGFP (lane 2), and uPAR-mEGFP-Fc-GPI (lane 3) were immunoblotted for mEGFP. In this case, proteins were resolved in nonreducing conditions.
Oligonucelotides
The following nucleotides were used: N-CheF: 5′-ggcctcgagggcgaacttgtatccaatggaactgttactatggtgagcaagggcgaggag-3′; uPARd: 5′-gcgcggccgcttaggtccagaggagag-3′; URfSK: 5′-gcgtcgacggtacccgccaccatgggtcacccgccgctgctg-3′; FPdwAXN: 5′-tgcgcggccgcttactcgagaccggtcttgtacagctcgtccatgcc-3′; FcAXf: 5′-ggaccggtctcgagctggaagttctgttccag-3′; FcAr: 5′-ttaccggtggcacctgcccctttacccggagacaggga-3′; GPIage: 5′-agtggctgtaaccacccaaccggtgtccagtaccgcagtggg-3′; hVUkpn: 5′-cggggtaccatggcacccctgaga-3′; FPDWAR: 5′-ggcgaccggtggcttgtacagctcgtc-3′; FPAGEF: 5′-gatccaccggtcgccaccatggtgagcaaggccgagg-3′.
Cell lines and transfection
All cells were cultured at 37°C and 5% CO2 in DMEM High Glucose GlutaMAX medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin. The HEK293/uPAR-mEGFP cell line was described previously in detail. Transfections were carried out using TransIt-LT1 reagent (MirusBio LLC, Madison, WI, USA) according to the manufacturer's instructions. In particular, the following conditions were used: 100 ng of DNA, DNA-transfection reagent ratio 1:3.
For microscopy, cells were plated in glass-bottom 35-mm wells (MatTek, Ashland, MA, USA) and in full medium containing 25 mM HEPES, and were used at subconfluence. For serum-free experiments, cells were seeded in serum-free DMEM (0.1% BSA and 25 mM HEPES) on fibronectin matrices and were imaged after 6–8 h, at 37°C.
For ATF (gift from Francesco Blasi, IFOM, Milan, Italy) binding, the stock solution was diluted in medium (2 ml/well) at the final concentration of 7 nM, and cells were imaged every 5–10 min, at 37°C in serum-free DMEM (0.1% BSA, 25 mM HEPES). Cells were maintained at 37 ± 0.5°C during image acquisition.
Immunoblotting
Mouse anti-tubulin (B-5-1-2 clone) was from Sigma (St. Louis, MO, USA) Rabbit anti-GFP was produced by the Biochemistry Facility of IFOM (Milan, Italy). Anti-rabbit and anti-mouse HRP-linked secondary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Living Colors mCherry Monoclonal Antibody (mouse) was from Clontech Laboratories.
Cells were harvested at 24 h post-transfection and lysed in RIPA buffer (1% Triton X-100; 150 mM NaCl; 50 mM Tris, pH 7.5; 0.25 mM EDTA; 0.2% NaF; 1% sodium deoxycholate; and Protease Inhibitors Mix). The protein concentration was determined using the Bio-Rad DC Protein Assay (Bio-Rad, Hercules, CA, USA). Proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes (Protran Biosciences, Corston Bath, UK). Proteins of interest were visualized using specific antibodies, followed by peroxidase-conjugated secondary antibodies (Chemilumine, Little Chalfont, UK).
Microscopy
Two different 2-photon, inverted microscope setups were used. The first was described previously (36) and consisted of a microscope stand (IX70; Olympus, Tokyo, Japan), equipped with a ×60/1.2-NA water-immersion objective, combined with a tunable Ti:sapphire laser (Chameleon Ultra; Coherent, Palo Alto, CA, USA) tuned to 890 nm at 1 mW at the sample. Fluorescence was filtered with a combination of dichroic and rejection filters (700dcspxr and et680sp-2p8, respectively; Chroma Technology Corp. Rockingham, VT, USA) and detected with a photomultiplier tube (H7422P-40; Hamamatsu, Tokyo, Japan); the analog PMT signal was converted into digital photon counting signals with a constant fraction discriminator. The pulse train was recorded using a data acquisition card (ISS Inc., Urbana-Champaign, IL, USA). The microscope was controlled with simFCS software (LFD), as described previously (22). With this setup, we acquired 100–200 frames/sequence, at 1.7 frame/s and pixel dwell time of 8 μs/pixel (47 s/sequence).
The second setup was a microscope stand (Ti-U; Nikon Corp., Tokyo, Japan) equipped with a ×60/1.2-NA water-immersion objective, combined with a tunable Ti:sapphire laser (Mai-Tai DS; Spectra Physics, Santa Clara, CA, USA) tuned to 890 nm at 4 mW for EGFP and 940 nm at 4 mW for mCherry (power measured at the sample). Detection and scanning unit was an ALBA-V module (ISS Inc.) using APD detectors (SPCM-AQRH-14; EG&G Perkin Elmer, Waltham, MA, USA). The microscope was controlled with Alba-Vista software (ISS Inc.). With this setup, we acquired sequences up to 200 frames at 0.5 frames/s and td of 32 μs/pixel (3.3–6.6 min/sequence).
Both setups were mounted on active vibration-damped optical tables (Smart Table UT; Newport Corp., Irvine, CA, USA).
RESULTS
N&B reveals that monomer molecules of uPAR are sorted to cell membrane in HEK293
We first studied the molecular forms of uPAR that are present at steady state in the cell membrane when the receptor is not exposed to any ligand. In these experiments, we have used a previously generated HEK293 cell line, which expresses functional chimeras between uPAR and the monomeric EGFP (Fig. 3 and refs. 18, 37). HEK293 cells do not release uPA (38) and do not show constitutive expression of uPAR (38). In these cells, uPAR-mEGFP retains the biological activity of the wild-type receptor (18). These cells express 12 ± 2 × 104 receptors/cell and were shown to be a suitable model for investigating the effect of ligand binding on the dynamics and organization of uPAR molecules in the plasma membrane (18, 19).
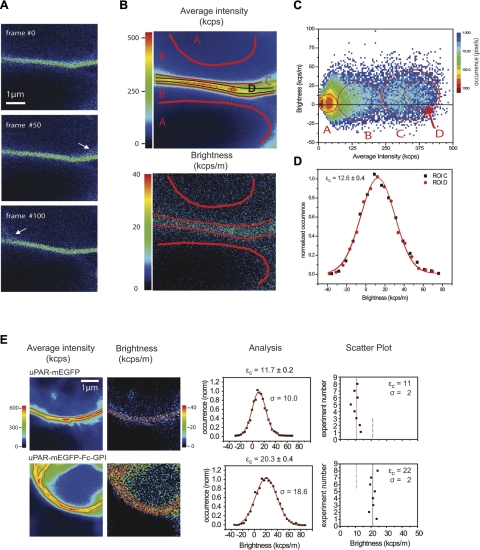
The data analysis procedure is illustrated in a step-by-step manner in Fig. 4. A central section of the cell was imaged (Fig. 4A), and a membrane segment was chosen for the measurement. Three individual frames from the 200-frame sequence are shown in Fig. 4A. Figure 4B illustrates the average intensity and the brightness images recovered from the entire sequence. The average intensity and brightness values calculated at each pixel are represented in the 2-dimensional (2-D) histogram shown in Fig. 4C. The average intensity and brightness images are linked with the 2-D histogram. Regions of interest (ROIs) can be selected on either image (e.g., Fig. 4B, ROIs A–D) and identified in the 2-D histogram (Fig. 4C). Conversely, a subpopulation of pixels can be selected in the 2-D histogram and automatically marked on the image. ROI A includes only pixels that have both low brightness and low mean intensity. In the real-space images (Fig. 4B), this selection from the histogram corresponds to the two dark background intracellular and extracellular areas. Pixels with higher intensity are included in ROIs B–D. These pixels can be recognized in three subsets of the 2-D histogram (Fig. 4C). The intensity and the brightness in ROI B are not negligible, but the image helps to identify this region as the area, which is the most affected by long-term cell fluctuations, background, and out-of-focus distortions. ROI C marks a relatively large portion of the membrane segment. To select only the pixels that are less affected by perturbations, we narrowed ROI C to the central, more intense area, ROI D. The brightness distributions of the subsets C and D, however, are similar (cf. superposition in the analysis graph of Fig. 4D).
Figure 4.
Step-by-step N&B analysis of uPAR-mEGFP in HEK293 cell membranes. A) Two-photon cross-section images through a uPAR-mEGFP/HEK293 cell from a time sequence (160 frames, 256×256 pixels/frame at 8 μs/pixel, 0.6 s/frame, 96 s total acquisition time; λ=890 nm, and ∼1 mW power at sample). Arrows indicate off-plane filopodia. B) Average intensity image (top) and brightness image (bottom). ROIs A–D define different areas in the images. C) Two-dimensional histogram of the average intensity and brightness at each pixel. Logarithmic color scale represents the occurrence at each pair value. Red open circles mark distributions of intensity vs. brightness in selected ROIs A–D. D) Brightness distribution histograms of subsets C, D. Black squares correspond to ROI D (4249 pixels); red circles to ROI C (1638 pixels); solid lines are gaussian fits (using y(ε) = A · (2σ)−1 · (0.5π)−0.5 · exp[−2 · (ε − εc)2/(2σ)2]. εc is the central value obtained from fit to ROI D; the error is the fit error (adjusted R2 = 0.98); and the sd, σ, is 8.9 kcps. E) Representative average fluorescence intensity and molecular brightness images, brightness histogram analysis of selected ROIs, and scatter plots of εc values from replicate experiments on uPAR-mEGFP and uPAR-mEGFP-Fc-GPI in HEK293 cells seeded on fibronectin matrices. Central brightness value εc and fit error (±) in the analysis were obtained as in D. Scatter plots show mean ± sd central brightness values from replicate experiments.
This result shows how the filtering correction is efficacious, especially since the border of the membrane in the image is particularly prone to be affected by small membrane movements and the presence of off-plane perturbations. By using the correction, we can include in the analysis regions that would otherwise have been excluded, without introducing any appreciable error, but improving statistical accuracy. The width of the distribution is large (Fig. 4D), reflecting the pixel-to-pixel variability of the brightness value, which is expected from the limited statistics collected on each pixel.
It is worth noting that we do not observe, and indeed do not expect, the presence of immobile molecules in these membrane segments. Immobile molecules (the so-called immobile fraction) can be observed, for example, in the basal membrane or in segments that are in contact with an immobile extracellular matrix. These (often bright) fractions can reduce the apparent brightness, as they do not contribute to the variance due to fluctuation. However, small fluctuations in the immobile fraction (photobleaching, drift) rapidly lead to a significant overestimation of brightness. The effect of the immobile fraction on the N&B measurement is complex and, when possible, avoided.
Using this analytical procedure, we have determined the brightness of uPAR-mEGFP in cross-sections of the plasma membrane in HEK293 cells seeded on fibronectin matrices. One representative example is given in Fig. 4E. The brightness distribution (Fig. 4E, analysis) in the example was centered at 11.7 ± 0.2 kcps/m, and it was reproduced in 8 independent experiments (Fig. 4E, scatter plot).
For comparison, we produced a uPAR-mEGFP-Fc-GPI construct and transfected in HEK293 (Fig. 3). Fc-fused proteins have been shown to dimerize through cysteine bonds in the Fc domain (Fig. 3A). uPAR-mEGFP-Fc-GPI was exported to the plasma membrane. However, we also observed relevant intracellular fluorescence (Fig. 3B), and detected heterogeneous uPAR-mEGFP-Fc-GPI forms by immunoblot analysis of total cell lysates (Fig. 3C). The N&B analysis restricted to cell membrane segments of uPAR-mEGFP-Fc-GPI/HEK293 cells seeded on fibronectin matrices revealed higher brightness than uPAR-mEGFP (Fig. 4E). The mean brightness, resulting from 8 independent experiments, was equal to 22 ± 2 kcps/m, a value that is 2-fold that of uPAR-mEGFP. The results of these experiments strongly indicate that in the cell membrane, uPAR-mEGFP is exposed as monomer, while the brightness of uPAR-mEGFP-Fc-GPI in the cell membrane is compatible with dimers. They also exclude self-oligomerization of uPAR-mEFGP in the absence of extracellular ligands, such as vitronectin. These results are in agreement with our previously published work in which we could not detect any self-association of uPAR in HEK293 when the cells were seeded on fibronectin matrices and in the absence of serum (18). In our previous work, we used a Förster resonance energy transfer–fluorescence lifetime imaging microscopy (FRET-FLIM) approach, for which we need to coexpress uPAR-mEGFP and uPAR-mRFP1 as a FRET pair. Here, we reach the same conclusion using a single-color experiment and acquiring a comparatively simple time-lapse image sequences.
ATF is sufficient to promote dimerization of uPAR in the cell membrane, in the absence of vitronectin
We have proven previously that, at least in HEK293 cells, dimerization of uPAR is not constitutive; rather it is elicited by the presence of the extracellular matrix protein vitronectin, removed by the action of the uPA-PAI1 ligand and subsequent internalization of the uPAR-:uPA-PAI1 complex (18). In that study, however, we did not determine the effect of uPA or its ATF-binding domain on uPAR dimerization in the absence of vitronectin.
Here, we follow the reorganization of uPAR-mEGFP molecules on binding ATF by monitoring the brightness of the tagged receptor as a function of time, after addition of the ligand to the medium. Starting from resting conditions on cells seeded on fibronectin, the effect of ATF was monitored up to 45 min, and at 37°C. ATF and pro-uPA have the same subnanomolar affinity for uPAR, which was verified also for uPAR-mEGFP in the selected HEK293 cell clones. Thus, ATF was added to the medium at the saturating concentration of 7 nM in the cell dish, and not removed during the acquisition time.
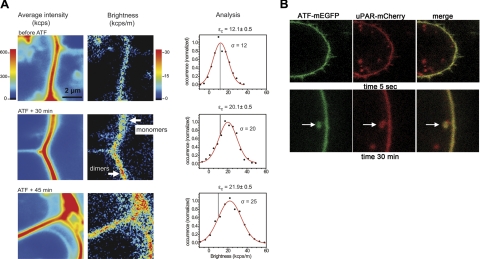
Figure 5A shows the results obtained from 3 different cells imaged at different times after ATF addition. Because of the unavoidable bleaching during the acquisition of the time sequences, a different cell was chosen for imaging at each time point. The addition of ATF to the medium did not induce any macroscopic change in the cells. Throughout the whole kinetics, the overall fluorescence intensity of the membranes was homogeneous, stable, and comparable to that of the untreated controls (Figs. 3B and 5A, right panels). In contrast, the brightness images reveal a progressive change in the organization of uPAR-mEGFP molecules in the course of 45 min. The shift to higher brightness values is clearly visible in the images. The brightness distributions measured in the selected membrane segments is reported in Fig. 5A (graphs). The distributions in the presence of ATF are wider. They overlap with that of the cell before treatment; nevertheless, the central values are distinct and almost 2-fold larger.
Figure 5.
Kinetics of binding, dimerization, and internalization of uPAR:ATF. A) Sequence of 3 representative membrane sections imaged before and after addition of ATF on uPAR-mEGFP/HEK293 cells seeded on fibronectin matrices. ATF was added in excess (7 nM) and let into the medium. From left to right: average fluorescence intensity, brightness images, and corresponding N&B analysis of εc values. The εc values and fit error were obtained as in Fig. 4. σ = sd. For each time point, 100–200 frames/sequence were acquired, at 1.7 frames/s and pixel dwell time of 8 μs/pixel. In the replicate experiment, εc increased from 12 to 20 kcps/m from 0 to 45 min after ATF addition. B) Representative frames taken from time-lapse sequences run on uPAR-mCherry/HEK293 cells in the presence of ATF-mEGFP. Cells expressing uPAR-mCherry were seeded on fibronectin matrices. ATF-mEGFP (7 nM) was added after 4 h. Cells were imaged at 37°C in serum-free medium. Excitation wavelength was 840 nm. Simultaneous 2-channel emission was collected at 0.48 frames/s (2.1 s/frame) (see Supplemental Movie S1).
The mean increase of uPAR-mEGFP brightness with time indicates that the population of receptors converted from monomes (εc=12.1±0.5 kcps/m) to predominantly dimers (εc=21.9±0.5 kcps/m) as a result of ATF binding. However, brightness was not homogenous through the membrane in the presence of ATF, and local heterogeneities could be observed. After 30 min of incubation with the ligand, the receptors appeared to form clusters of dimers, while in some areas individual molecules remained (see time 30 min in Fig. 5A). At later times (45 min), dimeric clusters predominated. Large brightness values compatible with a small fraction of multimolecular uPAR clusters were also observed (Fig. 5A; see how the distribution at 45 min becomes wider toward high values), but statistically these values cannot be attributed to a separate subpopulation.
The receptor brightness distribution is broad and centered at a value between that of monomers and dimers. Assuming a negligible concentration of aggregates higher than dimers and a significant subpopulation of monomers coexisting with dimers, we can estimate a residual mole fraction of monomers of ∼20% at 45 min ATF exposure. This value was obtained by considering the brightness of the pure dimer to be 24.2 kcps/m, which is double the value measured before ATF addition, and applying the lookup plot in Supplemental Fig. S5. The experimental sequence was repeated twice with similar results (Fig. 5A).
The ATF binding kinetics was replicated in a 2-color experiment in which uPAR-mCherry/HEK293 cells were treated with ATF-mEGFP (Fig. 5B). The localization of ATF-mEGFP to the cell membrane was immediate (Fig. 5B, time 5 s). In contrast, we did not observe unspecific binding of ATF-mEGFP to HEK293 cells that do not express uPAR (data not shown).
At 30 min, the images confirmed the presence of ATF-mEGFP at the membrane. Interestingly, at this time point the images also revealed endocytic vesicles containing uPAR-mCherry and ATF-mEGFP, suggesting that some uPAR:ATF complexes undergo internalization while at the membrane other uPAR molecules dimerize. We did not observe a relevant increase of internalization of ATF at later time (data not shown) or fading of uPAR-mCherry fluorescence at the cell membrane. The result confirms the previously observed stability of the membrane fluorescence of uPAR-mEGFP up to 45 min after treatment with ATF (Fig. 5A).
Controls
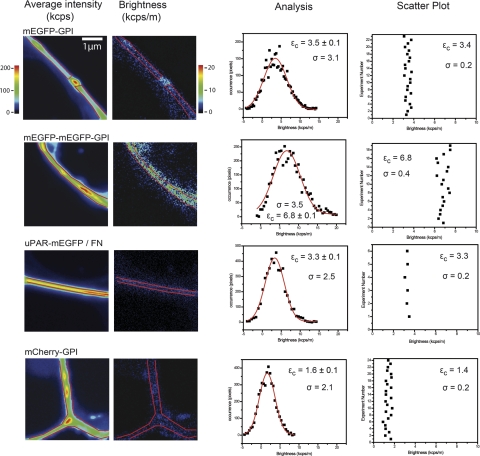
As control for the assignment of mEGFP brightness, we have produced two reference constructs, mEGFP-GPI and tandem mEGFP-mEGFP-GPI, that are sorted to the cell membrane with the same GPI anchor of uPAR (Fig. 3). We have studied these constructs expressed in HEK293 cells and in comparison with uPAR-mEGFP using a distinct microscope setup (Fig. 6). These experiments aimed to prove that the assignment of monomeric mEGFP brightness to uPAR-mEGFP in HEK293 cells seeded on fibronectin was correct independently of the experimental conditions; the boxcar-filtered N&B analysis could correctly retrieve the minimum increase of brightness due to dimerization events; and the method could detect a less bright fluorophore, such as mCherry in the mCherry-GPI transfected cells.
Figure 6.
Control experiments. From left to right: representative average fluorescence intensity and molecular brightness images, brightness histogram analysis, and scatter plots of εc values from replicate experiments on mEGFP-GPI, mEGFP-mEGFP-GPI, uPAR-mEGFP-GPI, and mCherrry-GPI. All constructs were expressed in HEK293 cells, and with the exception of uPAR-mEGFP, cells were imaged in serum-rich medium. uPAR-mEGFP/cells were plated on fibronectin matrices and maintained in serum-free medium during the experiment. Central brightness εc and fit error (±) were obtained as in Fig. 4. Scatter plots show mean ± sd central brightness values from replicate experiments. σ = sd.
We have run these experiments on a commercial setup that has a lower optical transmission, imposing less favorable working conditions (second setup in Materials and Methods). In this setup, we needed to apply a longer pixel dwell time that favors the occurrence of temporal perturbations and higher power at the sample that induces greater bleaching effects. The mean brightness of uPAR-mEGFP under these unfavorable working conditions was 3.5 ± 2.5 kcps/m, and it was reproducibly consistent with that of mEGFP-GPI. Both uPAR-mEGFP and mEGFP-GPI showed a brightness that was half of that of the tandem mEGFP-mEGFP-GPI (Fig. 6). The variability from sample to sample in the monomeric and dimeric mEGFP constructs was ∼6%.
This result supports the conclusion that uPAR-mEGFP dimers or larger multimolecular uPAR clusters are absent in the applied cultured conditions and confirms our previous observations derived from FRET-FLIM (18) experiments. The result also shows how N&B is a simpler and more straightforward approach for studying homophilic protein–protein interactions with stoichiometric details, without the necessity of expressing suitable and biologically active FRET-pair constructs.
Finally, we analyzed in the same cell line and experimental conditions mCherry-GPI (Figs. 3B and 6), which is 2-fold less bright and also less photostable than mEGFP (33). The retrieved molecular brightness value of a fluorophore depends on the excitation power and the optical transmission of the instrument; thus, a direct comparison of absolute brightness values is difficult. However, the molecular brightness ratio of two fluorophores is independent of the experimental setup as long as the acquisition is carried out under the same conditions, and the values that we measured for mCherry-GPI were consistently ≈50% that of mEGFP-GPI, as expected (Fig. 6). The variability from sample to sample in the mCherry-GPI cells was 18% due to the lower brightness of the fluorophore. This result shows that N&B can measure correct brightness ratios and detect dim fluorophores even using a less sensitive setup and conditions at which temporal perturbations might occur heavily.
DISCUSSION
In a previous work, we did not detect any homophilic uPAR interaction in resting (unstimulated) conditions in the cell membrane. Conversely, we proved by FRET that uPAR-uPAR interaction occurred in the presence of vitronectin, and it was counteracted by uPA-PAI1. We could also provide a partial characterization of the stoichiometry of uPAR assemblies, by applying local measurements of the brightness (i.e., PCH analysis; refs. 18, 19), and showing the formation of dimers. However, we could not combine this information in an image of what is happening to uPAR in the cell membrane. In this contribution, we demonstrate that we can now distinguish and image minimal changes in the organization of uPAR molecules in live cells, in real time, providing the first direct evidence that unstimulated uPAR is present in the cell membrane as a mobile monomeric molecule, undistinguishable from an inactive mEGFP-GPI protein. This result supports our previous hypothesis that, at least in HEK293 cells, the receptor is not assembled into distinct homophylic uPAR complexes. Stimulation of the receptor with ATF is sufficient to modify the organization of uPAR molecules in the membrane, inducing a sustained dimerization of the receptor. Following the change of brightness as a function of time, we could detect the dimerization of the receptor starting several minutes after ligand addition, while by 2-color time lapse images, the localization of ATF-mEGFP to the membrane appeared to be immediate. This apparent discrepancy cannot be ascribed to the length of acquisition of the time sequences for N&B analysis, because the sequences could be acquired in ∼100–200 s/time point (in the first setup; see Materials and Methods). Nevertheless, we pushed N&B sensitivity to the limit, focusing away from the basal side of cells with moderate-low expression of uPAR. Under these conditions, early and sparse dimerization events could have escaped.
However, we tend to exclude the possibility that dimerization of uPAR occurred rapidly and extensively after ATF binding, because in such a case the dimeric brightness would predominate in the distributions. Our results suggest that ATF binds instantaneously to uPAR monomers, as reported in literature and predicted by structural data. The late onset of dimers, which we can capture by N&B, might occur through a reorganization of the receptor molecules. Such reorganization, triggered by ATF, might involve in-time recruitment or rearrangement of other signaling molecules and membrane components that interact with and are affected by uPAR and its ligands (i.e., signaling EGFR and integrins). The observation that, even after 30 min, a relevant heterogeneity of monomers and dimers still exists suggests that the two molecular forms of uPAR might participate in different protein assemblies and signaling mechanisms.
Because we have employed N&B, which is an imaging approach based on fluorescence fluctuation, the detected uPAR dimers and monomers are necessarily diffusing species. It has been suggested that DRM fractions are enriched in uPAR dimers (15). However, it is important to note that, in contrast to FRET, N&B does not impose any restriction on the distance between two uPAR-mEGFP molecules. Two uPAR-mEGFP molecules that belong to the same coreceptor signaling assembly, which diffuses as a whole in the cell membrane, would also give rise to a “dimeric brightness.” In such a circumstance, a direct interaction between uPAR-mEGFP molecules would not necessarily occur.
N&B analysis indeed shows some heterogeneity in an apparent clusterization. The clusters, however, do not indicate that the concentration of the receptor is locally high. These are clusters of brightness, not clusters of molecules. The fluorescence intensity images, which depend on the local receptor concentration, are in fact homogeneous. The resolution of N&B analysis is not such to distinguish whether monomers and dimers of uPAR belong to different membrane microdomains, which might be below the size of few hundred nanometers. N&B, in fact, does not beat the diffraction limit and does not provide super-resolution. The observation volume and, thus, the resolution of our images are those of conventional multiphoton microscopy. Nevertheless, N&B at the level of discriminating monomeric from dimeric species can be seen as a single-molecule technique. It reaches the molecular sensitivity because it allows assessing the average brightness of individual fluorophores that diffuse in and out the observation volume. In principle, under favorable conditions, the brightness distribution can be resolved down to the point at which a few individual molecules are present in the observation volume. In practice, we encountered the limit already at higher concentrations (∼10 molecules/observation volume), because of the low signal-to-noise ratio at the chosen experimental conditions: images of thin membrane sections, far from the basal side of the cells (i.e., few pixels), and low expression level of the already dim uPAR-mEGFP (i.e., few photons).
We also provide direct proof of the internalization of ATF-uPAR complexes, because we could detect intracellular vesicles containing both uPAR-mCherry and ATF-mEGFP close to the cell membrane. Former work excluded the internalization of uPA and ATF bound to uPAR (39). In contrast, several reports have described the internalization of the ternary complex formed by the covalent binding of the specific plasminogen activator inhibitor type-1 (PAI-1) to uPAR-bound uPA. The ternary uPAR:uPA:PAI-1 complex rapidly associates with the LDL receptor-related protein-1 (LRP-1, or other members of the LDL receptor family), and is sequestered in clathrin-coated pits. The ligands are then targeted to the lysosomes for degradation, while the uPAR recycles back to the cell surface (40–42). In this contest, it was also demonstrated that the binding of uPA-PAI1 to vitronectin-engaged uPAR results in the dissociation of uPAR clusters prior to internalization (18).
A more recent report, however, has established the existence of a second constitutive endocytic route for uPAR internalization in HEK293 cells (38). It has been demonstrated that this route is uPA:PAI-1 and lipid raft independent but amiloride sensitive, and it is associated with rapid recycling of uPAR to the cell surface. The researchers (38) concluded that this alternative endocytic mechanism might support the sustained expression of uPAR at its functional sites as well as ensuring the preservation of a constant membrane area. Although we had not studied the fate, exact nature, and number uPAR-mCherry and ATF-mEGFP molecules in the endocytic vesicles, our results are in line with the observations supporting the existence of a uPA-PAI-1-independent internalization pathway. Our data also suggest that this pathway might be active on the dimeric ATF-bound receptor.
Overall, our work provides the first direct evidence that novel imaging approaches, such as N&B, can efficaciously contribute to disclose missing molecular details of uPAR signaling in the contest of live cell studies. We show that the spatiotemporal dynamics of uPAR can be quantitatively and stoichiometrically described in response to stimuli. This information is given directly in the form of live cell imaging, and it is robust against long-term perturbations (strong fluctuating background, low signal, etc.).
N&B can be used to analyze data in the context of conventional confocal or 2-photon microscopy. In contrast to FRET-based studies, N&B does not introduce serious constraints, such as the necessity to use a specific fluorophore pair, where this pair needs to be introduced in the protein sequence, or how distant the interacting biomolecules need to be to produce a signal. In N&B, the labels can be placed on noncritical positions of the protein, and, also, interactions over long distances become detectable.
We have found that the biggest barrier for applying this method in live cell studies comes from the fact that the data are often and sometimes heavily disturbed by extraneous fluctuations, particularly in cell membranes in which the cell or parts of it, like filopodia, move on the time scale of tens of seconds. Extraneous fluctuations have a strong effect on the retrieved brightness, always inducing a major overestimation of the brightness value, with consequences that can be misleading in the interpretation of protein signaling and interaction studies. Because of that, until now the great potential and precision of N&B was never fully demonstrated. Recent studies have restricted the analysis to the more stable basal side of the cells (large protein complexes or aggregates; ref, 25) and/or managed to lower the temperature for decreasing long-term temporal fluctuations (26). In addition, no studies have been published providing a practical guideline using this approach at such a level of detail and usefulness.
We show that the introduction of a simple boxcar correction can largely restore the precision of unperturbed measurements, even for dimerization events in experiments performed at 37°C. This filter is different from subtracting a moving average or from other common forms of detrending, as it does not alter (add to, subtract from, average, etc.) the original data in the process. Furthermore, the data are not subjected to a user-defined criterion, and more data can be handled with better precision. The literature on boxcar filtering is related mainly to analog signal processing and not to fluctuation analysis in images. For this reason, we conducted a series of simulations to verify the functionality of this procedure and define the starting conditions for our experiments.
Combining FRET and N&B approaches in the most recent development, which enables the determination of composition and stoichiometry of heterophylic complexes (cross-N&B; ref. 27), we can definitely start answering some of the debated questions: how, when, and where GPI-anchored uPAR undergoes homophilic interaction; how the receptor dynamics and molecular reorganization regulate uPAR signaling; how the interaction of uPAR with other membrane and extracellular molecules is regulated at the kinetic and stoichiometric level; and how it affects signaling.
Supplementary Material
Acknowledgments
The authors are grateful to Elvira Arza for technical help. The authors thank Enrico Gratton for the valuable discussion.
The authors acknowledge the financial support from the Cariplo Foundation (V.R.C. and N.S.) and the Italian Association for Cancer Research (AIRC; N.S.). CNIC is supported by the Spanish Ministry of Science and Innovation and by the Pro-CNIC Foundation. Raw data in Figs. 4 and 5 were acquired at the Laboratory for Fluorescence Dynamics (LFD) at the University of California, Irvine (UCI; Irvine, CA, USA). The LFD is supported jointly by the National Center for Research Resources of the U.S. National Institutes of Health (PHS 5 P41-RR003155) and UCI.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Ploug M., Ronne E., Behrendt N., Jensen A. L., Blasi F., Dano K. (1991) Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J. Biol. Chem. 266, 1926–1933 [PubMed] [Google Scholar]
- 2. Smith H. W., Marshall C. J. (2010) Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell. Biol. 11, 23–36 [DOI] [PubMed] [Google Scholar]
- 3. Blasi F., Sidenius N. (2010) The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 584, 1923–1930 [DOI] [PubMed] [Google Scholar]
- 4. Appella E., Blasi F. (1987) The growth factor module of urokinase is the binding sequence for its receptor. Ann. N. Y. Acad. Sci. 511, 192–195 [DOI] [PubMed] [Google Scholar]
- 5. Bdeir K., Kuo A., Sachais B. S., Rux A. H., Bdeir Y., Mazar A., Higazi A. A., Cines D. B. (2003) The kringle stabilizes urokinase binding to the urokinase receptor. Blood 102, 3600–3608 [DOI] [PubMed] [Google Scholar]
- 6. Sahores M., Prinetti A., Chiabrando G., Blasi F., Sonnino S. (2008) uPA binding increases UPAR localization to lipid rafts and modifies the receptor microdomain composition. Biochim. Biophys. Acta 1778, 250–259 [DOI] [PubMed] [Google Scholar]
- 7. Maupas-Schwalm F., Bedel A., Auge N., Grazide M. H., Mucher E., Thiers J. C., Salvayre R., Negre-Salvayre A. (2009) Integrin alpha (v) beta(3), metalloproteinases, and sphingomyelinase-2 mediate urokinase mitogenic effect. Cell. Signal. 21, 1925–1934 [DOI] [PubMed] [Google Scholar]
- 8. Liu D., Aguirre Ghiso J., Estrada Y., Ossowski L. (2002) EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell 1, 445–457 [DOI] [PubMed] [Google Scholar]
- 9. Jo M., Thomas K. S., Takimoto S., Gaultier A., Hsieh E. H., Lester R. D., Gonias S. L. (2007) Urokinase receptor primes cells to proliferate in response to epidermal growth factor. Oncogene 26, 2585–2594 [DOI] [PubMed] [Google Scholar]
- 10. Guerrero J., Santibanez J. F., Gonzalez A., Martinez J. (2004) EGF receptor transactivation by urokinase receptor stimulus through a mechanism involving Src and matrix metalloproteinases. Exp. Cell Res. 292, 201–208 [DOI] [PubMed] [Google Scholar]
- 11. Jo M., Thomas K. S., Marozkina N., Amin T. J., Silva C. M., Parsons S. J., Gonias S. L. (2005) Dynamic assembly of the urokinase-type plasminogen activator signaling receptor complex determines the mitogenic activity of urokinase-type plasminogen activator. J. Biol. Chem. 280, 17449–17457 [DOI] [PubMed] [Google Scholar]
- 12. Jo M., Thomas K. S., O'Donnell D. M., Gonias S. L. (2003) Epidermal growth factor receptor-dependent and -independent cell-signaling pathways originating from the urokinase receptor. J. Biol. Chem. 278, 1642–1646 [DOI] [PubMed] [Google Scholar]
- 13. Lijnen H. R., De Cock F., Collen D. (1994) Characterization of the binding of urokinase-type plasminogen activator (u-PA) to plasminogen, to plasminogen-activator inhibitor-1 and to the u-PA receptor. Eur. J. Biochem. 224, 567–574 [DOI] [PubMed] [Google Scholar]
- 14. Shliom O., Huang M., Sachais B., Kuo A., Weisel J. W., Nagaswami C., Nassar T., Bdeir K., Hiss E., Gawlak S., Harris S., Mazar A., Higazi A. A. (2000) Novel interactions between urokinase and its receptor. J. Biol. Chem. 275, 24304–24312 [DOI] [PubMed] [Google Scholar]
- 15. Cunningham O., Andolfo A., Santovito M. L., Iuzzolino L., Blasi F., Sidenius N. (2003) Dimerization controls the lipid raft partitioning of uPAR/CD87 and regulates its biological functions. EMBO J. 22, 5994–6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang M., Mazar A. P., Parry G., Higazi A. A., Kuo A., Cines D. B. (2005) Crystallization of soluble urokinase receptor (suPAR) in complex with urokinase amino-terminal fragment (1–143). Acta Crystallogr. D Biol. Crystallogr. 61, 697–700 [DOI] [PubMed] [Google Scholar]
- 17. Barinka C., Parry G., Callahan J., Shaw D. E., Kuo A., Bdeir K., Cines D. B., Mazar A., Lubkowski J. (2006) Structural basis of interaction between urokinase-type plasminogen activator and its receptor. J. Mol. Biol. 363, 482–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caiolfa V. R., Zamai M., Malengo G., Andolfo A., Madsen C. D., Sutin J., Digman M. A., Gratton E., Blasi F., Sidenius N. (2007) Monomer dimer dynamics and distribution of GPI-anchored uPAR are determined by cell surface protein assemblies. J. Cell Biol. 179, 1067–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malengo G., Andolfo A., Sidenius N., Gratton E., Zamai M., Caiolfa V. R. (2008) Fluorescence correlation spectroscopy and photon counting histogram on membrane proteins: functional dynamics of the glycosylphosphatidylinositol-anchored urokinase plasminogen activator receptor. J. Biomed. Opt. 13, 031215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muller J. D., Chen Y., Gratton E. (2000) Resolving heterogeneity on the single molecular level with the photon-counting histogram. Biophys. J. 78, 474–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Y., Muller J. D., Ruan Q., Gratton E. (2002) Molecular brightness characterization of EGFP in vivo by fluorescence fluctuation spectroscopy. Biophys. J. 82, 133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Digman M. A., Dalal R., Horwitz A. F., Gratton E. (2008) Mapping the number of molecules and brightness in the laser scanning microscope. Biophys. J. 94, 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Unruh J. R., Gratton E. (2008) Analysis of molecular concentration and brightness from fluorescence fluctuation data with an electron multiplied CCD camera. Biophys. J. 95, 5385–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dalal R. B., Digman M. A., Horwitz A. F., Vetri V., Gratton E. (2008) Determination of particle number and brightness using a laser scanning confocal microscope operating in the analog mode. Microsc. Res. Tech. 71, 69–81 [DOI] [PubMed] [Google Scholar]
- 25. Ossato G., Digman M. A., Aiken C., Lukacsovich T., Marsh J. L., Gratton E. (2010) A two-step path to inclusion formation of huntingtin peptides revealed by number and brightness analysis. Biophys. J. 98, 3078–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagy P., Claus J., Jovin T. M., Arndt-Jovin D. J. (2010) Distribution of resting and ligand-bound ErbB1 and ErbB2 receptor tyrosine kinases in living cells using number and brightness analysis. Proc. Natl. Acad. Sci. U. S. A. 107, 16524–16529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Digman M. A., Wiseman P. W., Choi C., Horwitz A. R., Gratton E. (2009) Stoichiometry of molecular complexes at adhesions in living cells. Proc. Natl. Acad. Sci. U. S. A. 106, 2170–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lindemann B. (1969) Economic boxcar integration. Med. Biol. Eng. 7, 239–240 [DOI] [PubMed] [Google Scholar]
- 29. Schwille P., Haupts U., Maiti S., Webb W. W. (1999) Molecular dynamics in living cells observed by fluorescence correlation spectroscopy with one- and two-photon excitation. Biophys. J. 77, 2251–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perroud T. D., Huang B., Zare R. N. (2005) Effect of bin time on the photon counting histogram for one-photon excitation. Chemphyschem 6, 905–912 [DOI] [PubMed] [Google Scholar]
- 31. Sanchez-Andres A., Chen Y., Muller J. D. (2005) Molecular brightness determined from a generalized form of Mandel's Q-parameter. Biophys. J. 89, 3531–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berland K. M., So P. T., Gratton E. (1995) Two-photon fluorescence correlation spectroscopy: method and application to the intracellular environment. Biophys. J. 68, 694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. (2005) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 [DOI] [PubMed] [Google Scholar]
- 34. Madsen C. D., Ferraris G. M., Andolfo A., Cunningham O., Sidenius N. (2007) uPAR-induced cell adhesion and migration: vitronectin provides the key. J. Cell Biol. 177, 927–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schriebl K., Trummer E., Lattenmayer C., Weik R., Kunert R., Muller D., Katinger H., Vorauer-Uhl K. (2006) Biochemical characterization of rhEpo-Fc fusion protein expressed in CHO cells. Protein Expr. Purif. 49, 265–275 [DOI] [PubMed] [Google Scholar]
- 36. Hellriegel C., Gratton E. (2009) Real-time multi-parameter spectroscopy and localization in three-dimensional single-particle tracking. J. R. Soc. Interface 6, S3–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zacharias D. A., Tsien R. Y. (2006) Molecular biology and mutation of green fluorescent protein. Methods Biochem. Anal. 47, 83–120 [PubMed] [Google Scholar]
- 38. Cortese K., Sahores M., Madsen C. D., Tacchetti C., Blasi F. (2008) Clathrin and LRP-1-independent constitutive endocytosis and recycling of uPAR. PLoS One 3, e3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cubellis M. V., Wun T. C., Blasi F. (1990) Receptor-mediated internalization and degradation of urokinase is caused by its specific inhibitor PAI-1. EMBO J. 9, 1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nykjaer A., Petersen C. M., Moller B., Jensen P. H., Moestrup S. K., Holtet T. L., Etzerodt M., Thogersen H. C., Munch M., Andreasen P. A., Gliemann J. (1992) Purified alpha 2-macroglobulin receptor/LDL receptor-related protein binds urokinase.plasminogen activator inhibitor type-1 complex. Evidence that the alpha 2-macroglobulin receptor mediates cellular degradation of urokinase receptor-bound complexes. J. Biol. Chem. 267, 14543–14546 [PubMed] [Google Scholar]
- 41. Conese M., Nykjaer A., Petersen C. M., Cremona O., Pardi R., Andreasen P. A., Gliemann J., Christensen E. I., Blasi F. (1995) alpha-2 Macroglobulin receptor/Ldl receptor-related protein(Lrp)-dependent internalization of the urokinase receptor. J. Cell Biol. 131, 1609–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Czekay R. P., Kuemmel T. A., Orlando R. A., Farquhar M. G. (2001) Direct binding of occupied urokinase receptor (uPAR) to LDL receptor-related protein is required for endocytosis of uPAR and regulation of cell surface urokinase activity. Mol. Biol. Cell 12, 1467–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qian H., Elson E. L. (1990) Distribution of molecular aggregation by analysis of fluctuation moments. Proc. Natl. Acad. Sci. U. S. A. 87, 5479–5483 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.