Abstract
Mechanical ventilation (MV) is a life-saving measure in many critically ill patients. However, prolonged MV results in diaphragm dysfunction that contributes to the frequent difficulty in weaning patients from the ventilator. The molecular mechanisms underlying ventilator-induced diaphragm dysfunction (VIDD) remain poorly understood. We report here that MV induces myonuclear DNA fragmentation (3-fold increase; P<0.01) and selective activation of caspase 9 (P<0.05) and Bcl2-interacting mediator of cell death (Bim; 2- to 7-fold increase; P<0.05) in human diaphragm. MV also statistically significantly down-regulates mitochondrial gene expression and induces oxidative stress. In cultured muscle cells, we show that oxidative stress activates each of the catabolic pathways thought to underlie VIDD: apoptotic (P<0.05), proteasomal (P<0.05), and autophagic (P<0.01). Further, silencing Bim expression blocks (P<0.05) oxidative stress-induced apoptosis. Overlapping the gene expression profiles of MV human diaphragm and H2O2-treated muscle cells, we identify Fos, FoxO1, and Stat3 as regulators of Bim expression as well as of expression of the catabolic markers atrogin and LC3. We thus identify a novel Fos/FoxO1/Stat3-Bim intrinsic apoptotic pathway and establish the centrality of oxidative stress in the development of VIDD. This information may help in the design of specific drugs to prevent this condition.—Tang, H., Lee, M., Budak, M. T., Pietras, N., Hittinger, S., Vu, M., Khuong, A., Hoang, C. D., Hussain, S. N. A., Levine, S., Shrager, J. B. Intrinsic apoptosis in mechanically ventilated human diaphragm: linkage to a novel Fos/FoxO1/Stat3-Bim axis.
Keywords: oxidative stress, skeletal muscle, dysfunction, atrophy, respiratory muscle
Although mechanical ventilation (MV) is a critical component of modern intensive care and surgery, increasing attention has been focused recently on the difficulty frequently encountered in weaning patients from MV (1–18). Prolonged MV is associated with the development of ventilator-associated pneumonia and other complications, creating a vicious cycle that ultimately increases mortality and costs (19–22). For this reason, efforts to understand the pathophysiological basis of “failure to wean,” possibly leading to the development of interventions to prevent it, are valuable.
Prolonged MV has been shown to cause both atrophy of diaphragmatic myofibers and diaphragmatic contractile dysfunction [inclusively termed ventilator-induced diaphragm dysfunction (VIDD)]. Both are thought to contribute to weaning difficulties. MV with diaphragm inactivity for periods as short as 18 h has been shown to elicit significant dysfunction and/or atrophy of myofibers in the diaphragm of rats (23–39) and other experimental animals (40, 41). Our group observed severe atrophy (>50% reduction in cross-sectional area) of the diaphragmatic muscle fibers of humans subjected to full ventilator support for as few as 18 h (42). A recent article demonstrated a direct relationship between duration of MV and extent of human diaphragm injury and weakness (43). If MV-induced diaphragm atrophy/weakness can be prevented, the destructive cycle of failure to wean, with all of its attendant problems, might be avoided.
The pathogenetic mechanisms that underlie ventilator-induced diaphragm atrophy (VIDA) appear to be several, but to date attention has been focused on increased proteolysis via up-regulation of caspase 3 (37, 42), calpain (44), and the ubiquitin proteosome system (UPS; refs. 31, 42, 45), as well as the recent finding of increased activity of the lysosomal autophagy system (46). However, caspase 3, in addition to playing a role in dissociating myofibrils from the lattice during proteolysis (47–49), also plays a central role in apoptosis. Although caspase 3 activation correlates with the loss of myonuclei and maintenance of the myonuclear domain in MV rats (37), it remains unknown whether apoptosis is active in MV human diaphragm. Further, caspase 3 is at the bottom of the convergent intrinsic and extrinsic apoptotic pathways, and it is not known which of these pathways leads to the activation of caspase 3 that is seen with MV. The regulatory molecules still further upstream of the caspases, which may determine the activity of one or both of the apoptotic pathways, also remain to be elucidated.
It has been suggested that oxidative stress may be the most proximal upstream event activating several of the previously mentioned processes implicated in VIDD. Evidence for this to date includes the demonstration of increases in biomarkers of oxidative injury (31, 33, 36) and diminished total antioxidant capacity (36, 42) in ventilated diaphragm. Prevention of MV-induced oxidative damage in the diaphragm by antioxidants substantially mitigates the deleterious effects of MV on the diaphragm in rats (26). One article suggested that the source of the reactive oxygen species (ROS) in experimental MV may be mitochondria (50). However, the upstream role that oxidative stress may play in caspase 3 activation or in the activation of the other pathological events currently understood to underlie human VIDD (proteolysis and autophagy) has not been explored in detail.
Given this background, we set out to determine whether apoptosis is active within the human diaphragm during MV and, if so, to identify which of the apoptotic pathways, intrinsic or extrinsic, is responsible for its activation. We identified Bcl2-interacting mediator of cell death (Bim) as a major mediator of the intrinsic apoptosis associated with human MV and further investigated the transcription factors that may be involved in oxidative stress-induced Bim induction. We also report experiments indicating that oxidative stress is able in vitro to activate all of the pathological events that underlie human VIDD. Mitochondrial oxidative stress-FoxO/Fos/Stat3-Bim forms a positive feedback loop that may explain the rapid rate at which ventilator-associated diaphragm atrophy/dysfunction develops relative to disuse atrophy of the limb muscles.
MATERIALS AND METHODS
Human sample collection
Our protocol for case subjects was approved by the Gift of Life Donor Program (http://www.donors1.org), and our protocol for control subjects was approved by the University of Pennsylvania and Stanford University institutional review boards. All biopsy specimens were obtained with written informed consent. The diaphragm biopsy specimens were taken from an identical location in the midportion of the right anterolateral costal diaphragm, away from visible or suspected phrenic nerve branches, and snap-frozen, as described previously (42, 51). Patients were excluded from diaphragm biopsy as control patients if they harbored lung malignancy beyond stage II or a solitary lung metastasis without other disease; had pulmonary function test results >10% below their predicted value; had received prior or current chemotherapy, systemic steroids, or any other medication known to alter muscle structure or function; or had any weight loss noted preoperatively. Other exclusion criteria included heart failure, neuromuscular disease, or chronic metabolic disease.
Control patients received paralyzing doses of a neuromuscular blocking agent, and completeness of diaphragm inactivity was assessed visually by the attending thoracic surgeon as well as by the anesthesiologist who intermittently monitored the systemic electromyographic response in the adductor pollicis after stimulation of the ulnar nerve. Because our control biopsy specimens were obtained 1–2 h after the start of the anesthetic (before reversal of the neuromuscular blockade), it is highly unlikely that any diaphragmatic contractions occurred.
Transplant donor (MV group) biopsy specimens were taken from transplant organ donors before circulatory arrest and organ harvest. Criteria for brain death that must be met before a patient can become a donor include lack of spontaneous respiratory muscle activity during normocapnia and normoxic hypercapnia. Thus, it is clear that transplant donors all had zero diaphragmatic activity from the time that brain death was declared. Donors undergo an additional rigorous selection process, which excludes potential donors with active infection or major organ dysfunction. Thus, our MV subjects were rigorously screened and excluded if they had any of these potentially confounding conditions.
Although we had no nonrespiratory (or locomotor) muscle biopsy tissue from our own control and MV patient cohort on which to perform apoptotic studies, we were kindly given locomotor (quadriceps) biopsy specimens taken for previously published work from another institution (43). The criteria for selection of subjects who underwent those biopsies can be found in that publication and were quite similar to our criteria.
RNA and DNA isolation and quantitative PCR
RNA samples from muscle tissues or cultured C2C12 muscle cells were extracted by TRIzol (Invitrogen, Carlsbad, CA, USA). Tissue samples were homogenized with Omni TH tissue homogenizer (Omni International, Kennesaw, GA, USA). RNA concentration and purity were measured with a NanoDrop spectrophotometer (Invitrogen). RT of the RNA samples was performed using a SuperScript II Reverse Transcriptase kit (Invitrogen) according to the manufacturer's instructions. DNA was isolated by the phenol-chloroform method. In brief, tissue was homogenized and treated with protease K (20 mg/ml) overnight, followed by phenol and chloroform extraction. DNA was then precipitated with 2 vol of ethanol and 0.1 vol of 3 M sodium acetate (pH 4.6). After washing with 75% ethanol, DNA was dissolved in nuclease-free water. mRNA and DNA levels were measured by real-time PCR using ABsolute Blue SYBR Green ROX Mix (Thermo Fisher Scientific, Waltham, MA, USA) on a 7900HT fast real-time PCR system (Applied Biosystems, Carlsbad, CA, USA). γ-Actin and GAPDH were used as controls to calculate the relative ΔCt values. Fold changes of gene expression was calculated as ΔΔCt between the experimental and control groups. All reactions were performed in triplicate. PCR cycles were 94°C for 30 s, 58°C for 40 s, and 72°C for 40 s. Primers used are listed in Table 1.
Table 1.
Primers used in PCR
| Primer | Sequence |
|
|---|---|---|
| Forward | Reverse | |
| hu DNA-β-actin | ACCCACACTGTGCCCATCTAC | TCGGTGAGGATCTTCATGAGGTA |
| hu BCL2 | ACGGGGTGAACTGGGGGAGG | GCATGCTGGGGCCGTACAGT |
| hu Bcl-XL | CGGCTCTCGGCTGCTGCATT | CGGGGCACTGTGCGTGGAAA |
| hu mtCOXI | TTCGCCGACCGTTGACTATTCTCT | AAGATTATTACAAATGCATGGGC |
| hu mtCOXII | CCCCACATTAGGCTTAAAAACAGAT | TATACCCCCGGTCGTGTAGC |
| hu Actin | GACAGGATGCAGAAGGAGATTACT | TGATCCACATCTGCTGGAAGGT |
| hu BAX | GGCCGGGTTGTCGCCCTTTT | CCGCTCCCGGAGGAAGTCCA |
| hu Bim | CACATGAGCACATTTCCCTCT | AAGGCACAAAACCTGCAGTAA |
| hu Bim V1 | TGCCAGCCCTGGCCCTTTTG | CGCCGCAACTCTTGGGCGAT |
| hu Bim V6 | AGAGCCACAAGACAGGAGCCCA | CGCCGCAACTCTTGGGCGAT |
| hu Bim V9 | GGCGCCCTTTCTTGGCCCTT | GCCTCTCCGCAGGCTGCAAT |
| hu Cox 1 | GTTGTAGCCCACTTCCAC | CATCGGGGTAGTCCGAGTAA |
| hu Cox 2 | TTCATGATCACGCCCTCATA | TAAAGGATGCGTAGGGATGG |
| hu Cox 3 | AAAGCACATACCAAGGCCAC | CTTCTAGGGGATTTAGCGGG |
| hu Cox 4 | AGGTGGCCCATGTCAAGCAC | CATGATAACGAGCGCGGTGA |
| hu Fas | TGAAGGACATGGCTTAGAAGTG | GGTGCAAGGGTCACAGTGTT |
| hu FasL | GCAGCCCTTCAATTACCCAT | CAGAGGTTGGACAGGGAAGAA |
| hu Fos | GATAGCCTCTCTTACTACCAC | TGAGGGGCTCTGGTCTGCG |
| hu FOXO1 | GGGTGACAGCAACAGCTCGG | GTGATCCAGGGCTGTCCCCA |
| hu FOXO3 | GGCCCGAGCGCTTCTCCTTC | CGTACAGGATCGCGGACGGC |
| hu FOXO4 | AGGATGGAAGAACTCGATCC | GCTCGTTGGAGTGGCACCTT |
| hu GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
| hu Murf1 | GTATGCCAATTTGGTGCT | GTTACCACCCCGTATGT |
| hu SOD1 | GTGTGCGTGCTGAAGGGCGA | ACCTGCACTGGTACAGCCTGC |
| hu SOD2 | AGGGGGAGTTGCTGGAAGCC | CGTGCTCCCACACATCAATCCCC |
| hu STAT3 | AGCCGAGGGAACAAGCCCCA | GGGCCATCCTGCTAAAATCAGGGG |
| mo bim V1 | AATCCCGACGGCGAAGGGGA | CGCCGCAGCTCCTGTGCAAT |
| mo bim V2 | CAGAACCGCAAGACAGGAGCCC | CGCCGCAGCTCCTGTGCAAT |
| mo bim V3 | AGAACCGCAAGCTTCCATACGACA | GAGGGACATGACGCGTGGGG |
| mo Actin | CCAGCCTTCCTTCTTGGGTAT | TGCTGGAAGGTGGACAGTGAG |
| mo atrogin | CAACATTAACATGTGGGTGTAT | GTCACTCAGCCTCTGCATG |
| mo BAX | ACTTCAACTGGGGCCGCGTG | GAGGCCTTCCCAGCCACCCT |
| mo Bcl2V1 | GCTCAGCCCTGTGCCACCTG | CAGAGGTCGCATGCTGGGGC |
| mo Bcl2v2 | CTGACGCCCTTCACCGCGAG | ACAGCCAGCGGGCAGGAGTT |
| mo BCL-XL | CGGGGCACTGTGCGTGGAAA | AGTTGTGGTGGGGGCAGGGT |
| mo Bim | CGACAGTCTCAGGAGGAACC | CCTTCTCCATACCAGACGGA |
| mo FasL | TGGGTAGACAGCAGTGCCAC | GCCCACAAGATGGACAGGG |
| mo FOS | GATAGCCTCTCTTACTACCAC | TGAGGGGCTCTGGTCTGCG |
| mo FOXO1 | AAGAGGCTCACCCTGTCGCA | ACTGTTGTTGTCCATGGACG |
| mo FOXO3 | GCAACCAAGGAAATGCTCCT | CATCGGGGTTGATGATCCAC |
| mo FOXO4 | CAGATCTACGAATGGATGGTC | ATCGGGGTTCAGCATCCACCA |
| mo LC3b | TCGTTGTGCCTTTATTAGTGCATC | CACTGCTCTGTCTTGTGTAGGTTG |
| mo STAT3 | GCCGACCCAGGTAGTGCTGC | AGCACGGGGCAGGTGTCTCA |
hu, human; mo, mouse; mt, mitochondrial.
Protein extraction and Western blotting
Protein samples were prepared by lysing diaphragm muscle tissues or cultured C2C12 muscle cells in modified lysis buffer (0.6 mM HEPES, 1 M MgCl2, and 1.7 M KCl) with phosphatase inhibitors (phosphatase cocktails 1 and 2; Sigma-Aldrich, St. Louis, MO, USA) and protease inhibitors (Complete Mini; Roche, San Francisco, CA, USA) with Omni TH homogenizer. Protein concentrations were determined by a DC Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA) following the manufacturer's protocol. Protein (15–30 μg) was separated by SDS-PAGE in 4–12% bis-Tris and transferred to a nitrocellulose membrane (NuPAGE system; Invitrogen) for Western blot analysis. Membranes were incubated with antibodies (1:500 or 1:1000 as in the manufacturer's instructions) in 5% milk in Tris-buffered saline-Tween 20, against cleaved caspase 9, cleaved caspase 3, LC3B, actin, and FoxO1 (c29H4), Stat3. Antibodies were purchased from Cell Signaling Technology (Carlsbad, CA, USA). After overnight incubation with primary antibody at 4°C, the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG for 1 h at room temperature. Membranes were then treated with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) for 5 min, and the resulting images were captured by the ChemiDoc XRS System (Bio-Rad Laboratories) for analysis. Between each step, the membranes were washed 3 times for 5–10 min with 0.1% Tween 20 in 1× Tris-buffered saline.
Caspase activity assay, TUNEL staining, and detection of DNA fragmentation
Caspase 3, 8, and 9 activities were measured with the Caspase-Glo assay system (Promega, Madison, WI, USA). In brief, total protein (2 μg) from cell lysate or muscle lysate was used for the analysis. MG132 was added as instructed by the manufacturer to eliminate potential interference from cellular proteasomal activity. The bioluminescence intensity was measured by plate reader and normalized to the total protein input. Each sample was run in triplicate, and the mean value was used.
TUNEL assays were performed to identify apoptotic myonuclei using the Cell Death Detection Kit (Roche). The myonuclei were counterstained with DAPI. TUNEL-positive nuclei (green) were counted under a fluorescence microscope and expressed as a percentage of the total number of myofiber nuclei (blue).
For DNA laddering, genomic DNA from C2C12 cells and human diaphragm samples were extracted by protease K (100 μg/ml) digestion in 50 mM Tris-HCl (pH 8.0), 50 mM EDTA, 1% SDS, and 10 mM NaCl overnight at 55°C. RNase A (100 μg) was then added, and the solution was incubated for 40 min at 37°C, followed by phenol/chloroform extraction and precipitation with 2.5 vol of ethanol with 0.1 vol of 3 M sodium acetate (pH 4.8). The DNA pellet was resuspended in H2O and quantitated with NanoDrop technology. Extracted genomic DNA from H2O2-treated cells was visualized by electrophoresis on a 2% agarose gel with ethidium bromide staining. In addition, a semiquantitative PCR-based DNA laddering kit from Maxim Biotech, Inc. (San Francisco, CA, USA) was used to detect the level of DNA fragmentation in human diaphragm. In brief, extracted genomic DNA was added to a ligation mix with adapter primer. The adapter primer will only be ligated to fragmented DNA, and the latter was then detected by adapter primer-dependent PCR amplification. The intensity of the PCR bands was quantitated with ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA) and normalized to the total input DNA.
Protein carbonyl assay
Protein oxidation was measured through quantification of protein carbonyls using an Oxiselect Protein Carbonyl ELISA Kit (Cell Biolabs, San Diego, CA, USA). Protein samples and BSA standards were prepared in hypotonic lysis buffer at a concentration of 2 μg/ml and incubated overnight at 4°C on a 96-well protein-binding plate. Dinitrophenylhydrazine was added to each well, followed by blocking with 5% milk and detection with anti-dinitrophenyl antibody and HRP-conjugated secondary antibody. Between each step, the plate was washed with either 1× PBS or 1× wash buffer 3 times. Finally, substrate solution was added to each well, and the reaction was stopped with the stop solution when the half-point of the BSA standard started to change color. The plate was then read on a plate reader at 450 nm. The measured absorbance values were compared with the known values of the BSA standards to interpolate the concentration values. Each sample was run in triplicate, and the mean value was used.
Proteasomal activity assay
Proteasomal activity was measured by a Proteasome-Glo chymotrypsin-like cell-based assay system (Promega). The luminescence was generated while the cellular chymotrypsin-like proteasomal activity cleaves the specific luminogenic proteasome substrate of succinyl-leucine-leucine-valine-tyrosine-aminoluciferin. In brief, C2C12 cells were cultured in a 96-well plate and treated with H2O2 at 40 and 200 μM, respectively, for 24 h. Proteasome-Glo Cell-Based Reagent (100 μl) was added and mixed, and the mixture was incubated at room temperature for 10 min. Luminescence intensity was measured by luminometer (Roche).
Cell culture, plasmid, and siRNA transfections and reporter gene assay
C2C12 cell lines were maintained in 10% FBS-DMEM with 1% antibiotic-antimycotic solution (CellGro; Mediatech, Inc., Manassas, VA, USA). C2C12 cells were transfected at ∼90% confluence. siRNAs against Bim, FoxO1, Fos, and Stat3, as well as the negative control siRNA, were purchased from Ambion (Austin, TX, USA). siRNAs (40 nM) were cotransfected with 0.5 μg of Bim promoter construct (Bim promoter driving luciferase reporter; a kind gift from Dr. Subhas Biswas, Columbia University, New York, NY, USA) with Lipofectamine 2000 (Invitrogen), together with 0.5 μg of pCS2bGal as control. The plasmids and siRNA mix were transfected into C2C12 cells in the absence of antibiotics, and the cells were then allowed differentiation for 4 d in DMEM with 5% horse serum. The cells were lysed in lysis buffer (2.5 ml of 1 M Tris, 7.5 ml of H2O, and 0.1 ml of Triton X-100), and cell lysate was collected for reporter gene assay with a Bright-Glo Luciferase Assay Kit (Promega) according to the manufacturer's protocol. The luminescent intensity was measured by plate reader (Roche), and the light unit was normalized to the activity of β-galactosidase (β-Gal).
To measure the effect of siRNA on gene expression and caspase activities, we transfected siRNAs (40 nmol) into C2C12 cells (4×105 cells) at ∼90% confluence with the highly effective siRNA transfection reagent, iRNAMAX (Invitrogen). Transfected cells were allowed to differentiate for 3 d, 200 μM H2O2 was added to the transfected cells for 24 h, and cells were then collected for RNA extraction and RT-PCR analysis.
Dihydroethidium (DHE) staining
Snap-frozen human diaphragm samples were sectioned on a cryostat at 20 μm. DHE was purchased from Invitrogen and reconstituted in anhydrous DMSO (Sigma-Aldrich) in a stock concentration at 10 mM. The staining solution was prepared freshly before use by 1:1000 dilution of the stock DHE solution with 1× PBS. Cryosections were added with freshly prepared 10 μM DHE/PBS solution and incubated for 10 min in a dark chamber. The reaction was stopped by washing 3 times in 1× PBS. Slides were mounted in ProLong Gold antifading reagent (Invitrogen) and imaged by confocal microscopy (Leica, Wetzlar, Germany).
Microarray and data analysis
Gene expression profiles in mechanically ventilated and control samples were analyzed by gene microarray. Total RNA was extracted from both control (n=4) and ventilated samples (n=4) and subjected to 2-color gene microarray analysis (Agilent Technologies, Santa Clara, CA) performed by the Stanford microarray core facility. The raw data from the microarray were analyzed by GeneSpring GX11. The significantly altered entities were determined by choosing a cutoff at P < 0.05 with no multiple test correction. The original microarray data are available in the Stanford Microarray Database (http://smd.stanford.edu/cgi-bin/search/QuerySetup.pl). The altered gene profiles from H2O2-treated C2C12 myotubes were acquired by analyzing the raw data from National Center for Biotechnology Information (NCBI) Gene Ontology (GEO) data profile (accession number GSE 3078) with GeneSpring GX11. Significantly altered genes were chosen by cutoff at P < 0.05.
RESULTS
Characterization of the diaphragm biopsy specimen donors
All analyses were performed on 7 control and 10 MV diaphragm biopsy specimens except where specifically noted. Demographic information, pathological diagnosis leading to thoracotomy (for control group) or organ donation (for the MV group), and medical history for MV and control subjects are summarized in Table 2. Ventilator settings, measurements of arterial blood gases, and vital signs are summarized in Table 3. Similar clinical data for MV and control subjects who donated locomotor (quadriceps) muscle tissue at another institution, which was kindly provided to us for this study, can be found in the original article reporting on these specimens (46). Additional clinical data for both MV and control subjects in whom diaphragm biopsy specimens were taken are presented in Supplemental Tables S2 and S3.
Table 2.
Summary of demographic characteristics, reason for surgery, and medical history for control and MV subjects
| Subject | Age (yr) | Sex | BMI (kg/m2) | Reason for surgery/cause of brain death | Relevant medical history |
|---|---|---|---|---|---|
| Control | |||||
| 1 | 41 | F | 20 | Solitary lung metastasis | Colon carcinoma, nonsmoker, farmer |
| 2 | 67 | F | 30 | Stage 1A adenocarcinoma of lung | HTN, hypothyroid, former smoker |
| 3 | 67 | F | 27 | Stage 2B adenocarcinoma of lung | HTN, former smoker |
| 4 | 52 | M | 23 | Hemoptysis from bronchiectasis | Gout, nephrolithiasis |
| 5 | 44 | M | 23 | Localized mesothelioma | None |
| 6 | 67 | M | 27 | Intramucosal esophageal carcinoma | HTN |
| 7 | 73 | F | 20 | Stage IA adenocarcinoma of lung | HTN |
| MV | |||||
| 1 | 26 | M | 28 | Cardiac arrest with anoxia | Seizure disorder on Dilantin |
| 2 | 53 | F | 45 | CVA | HTN, AF |
| 3 | |||||
| 4 | 17 | F | 24 | MVA, head trauma | None |
| 5 | 49 | M | 24 | MVA, head trauma | HTN, PUD with gastrectomy, smoker |
| 6 | 75 | M | 33 | CVA | HTN, CAD, DM on insulin, smoker |
| 7 | 54 | M | 36 | Cardiac arrest with anoxia | HTN, gout, ethanol abuse, smoker |
| 8 | 55 | M | 31 | CVA | |
| 9 | 37 | M | 21 | Hanging | Cocaine abuse, smoker |
| 10 | 21 | F | 27 | Asphyxiation | HTN, asthma on steroid inhaler |
All control subjects had normal values for spirometry. Clinical data for MV subject 3 was lost. F, female; M, male; BMI, body mass index; MVA, motor vehicle accident; CVA, cerebrovascular accident; HTN, hypertension; AF, atrial fibrillation; PUD, peptic ulcer disease; CAD, coronary artery disease; DM, diabetes mellitus.
Table 3.
Summary of ventilator settings, arterial blood gas measurements, and vital signs for control and case subjects
| Measurement | Control subjects, n = 7 | MV subjects, n = 10 | P |
|---|---|---|---|
| Ventilator settings and related measurements | |||
| Tidal volume (ml/kg body weight) | 7.4 ± 1.3 | 7.0 ± 1.2 | 0.99 |
| Ventilation frequency (breaths/min) | 9.3 ± 1.8 | 15.0 ± 2.6 | <0.001 |
| PEEP (cmH2O) | 0.9 ± 1.6 | 5.3 ± 0.5 | <0.001 |
| FIO2 (%) | 78 ± 24 | 52 ± 8.4 | <0.001 |
| SaO2 (%) | 97.7 ± 3.3 | ||
| PaO2 (mmH2O) | — | 146 ± 60.4 | |
| PetCO2 (mmHg) | 32.4 ± 3.2 | ||
| PaCO2 (mmHg) | 34 ± 6.9 | ||
| Arterial pH (U) | 7.4 ± .04 | ||
| PaO2 at FIO2 1.0 (mmH2O) | 293.0 ± 135.7 | ||
| Vital signs | |||
| Systolic pressure (mmHg) | 110.0 ± 12.6 | 120.8 ± 12.8 | 0.1 |
| Diastolic pressure (mmHg) | 58.6 ± 9.0 | 65.6 ± 8.5 | 0.1 |
| Heart rate (beats/min) | 72.1 ± 5.7 | 95 ± 15.7 | 0.003 |
| Body temperature (°C) | 35.9 ± 0.7 | 35.9 ± 1.3 | 1.0 |
Values are means ± sd. PEEP, positive end-expiratory pressure; FIO2, fractional concentration of inspired oxygen; SaO2, arterial oxygen saturation; PaO2, arterial oxygen pressure; PetCO2, end-tidal carbon dioxide pressure; PaCO2, arterial carbon dioxide pressure.
Control subjects had diaphragm inactivity before biopsy for 1.0–2.0 h (mean 1.28±0.48 h), whereas MV (donor) subjects had diaphragm inactivity for far longer before biopsy: range 18–75 h (mean 39.5±18.6 h; control vs. MV, P=0.00006). Patients from whom control and MV human diaphragm samples were taken did not differ significantly in age (59±13 vs. 43±19 yr, respectively; P=0.08). Their body mass index differed slightly, with that of the MV patients being higher (24±3.8 vs. 30±7.4 kg/m2; P=0.04). Usual laboratory values (measured before to surgery in the control group and before organ harvest in the MV group) differed significantly only with respect to hemoglobin and serum glucose levels (Supplemental Table S1). Ventilator settings, vital signs, and arterial blood gases differed significantly with respect to set respiratory rate, positive end-expiratory pressure level, inspired oxygen concentration, and heart rate (Supplemental Table S2), as one might expect, given the differing clinical situations of organ donors and patients undergoing elective lung surgery.
MV activates caspase 9 and induces DNA fragmentation in human diaphragm
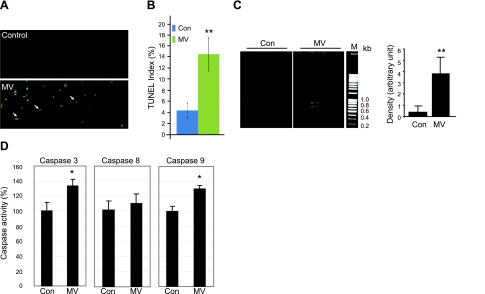
It has been reported that caspase 3 is activated by MV in both rat and human models (33, 38), and it is well accepted that activation of caspase 3 is required for the UPS-mediated myofibril degradation (47). However, activated caspase 3 also results in the activation of double-stranded nuclease. Here we investigated the downstream apoptotic cascade after caspase 3 activation in human diaphragm. First, we performed TUNEL staining on transverse sections of human diaphragm to investigate the level of positive nuclei. There are clearly more positive staining signals seen in MV samples than in control samples (Fig. 1A), and quantitatively there is a 3-fold increase in the TUNEL signal index (positive nuclei per total nuclei) in MV samples over control samples (Fig. 1B). This result represents evidence of increased apoptotic activity in MV vs. control human diaphragm.
Figure 1.
MV results in DNA fragmentation and activates caspase 3 and 9 in human diaphragm. A) TUNEL staining was performed on cryosections of control and ventilated human diaphragm. Fragmented genomic DNA was labeled with FITC-conjugated dUTP; positive signals appear green. Myonuclei are visualized by DAPI staining (blue). Note that the positive TUNEL staining signals (green) are localized in nuclei stained by DAPI (blue). B) TUNEL-positive nuclei were counted and normalized to total myonuclei; ratio is shown as the TUNEL index. Control (Con), n = 9; MV, n = 10. C) DNA fragmentation was measured by PCR-based detection and visualized by electrophoresis in 2% agarose gel. Density of the PCR products was quantitated with ImageJ and normalized to the total DNA input. M, DNA markers. Control, n = 5; MV, n = 8. D) Caspase 8 and 9 enzymatic activities from control and MV human diaphragm lysate were measured by fluorometric assay. Results are presented as relative fluorescence units after normalization to total protein amount. Control, n = 7; MV, n = 9. *P < 0.05; **P < 0.01.
To further confirm the presence of increased apoptotic changes in MV human diaphragm, we determined whether genomic DNA was broken down into DNA fragments, creating a DNA ladder. We used PCR-based detection of DNA fragmentation in control and MV diaphragm. The amount of small DNA fragments is also significantly increased in MV human diaphragm vs. control diaphragm (Fig. 1C).
Because caspase 3 is at a convergence point for different upstream apoptotic pathways, we next examined the activities of caspase 8 and 9 to distinguish which upstream apoptotic pathway, the intrinsic or extrinsic, is activated. The intrinsic apoptotic marker, caspase 9, is significantly activated in MV samples, whereas the extrinsic apoptotic pathway marker caspase 8 is not significantly induced. Downstream caspase 3 activity is also consistently induced (Fig. 1D).
MV selectively induces the intrinsic apoptotic marker gene Bim in human diaphragm
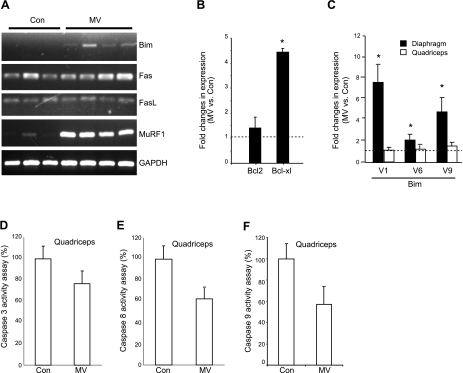
The above biochemical assays revealed that the intrinsic apoptotic pathway is preferentially induced in MV human diaphragm. To validate this observation, we further investigated the molecular changes after MV. RT-PCR revealed that Bim, the intrinsic proapoptotic marker gene in the Bcl2 family, is induced, whereas the extrinsic apoptotic markers Fas and Fas ligand (FasL) do not change (Fig. 2A). Consistent with our previous observation, gene expression of the E3 ubiquitin ligase, MuRF1, is also induced (Fig. 2A). We used quantitative PCR to measure the fold change of the antiapoptotic genes in the Bcl2 family. The antiapoptotic gene bcl2 did not change, although Bcl2-xl is induced (Fig. 2B). Because the Bim gene transcribes 3 different alternative splice variants in humans (V1, V6, and V9), which encode 3 different isoforms of Bim (Bim-EL, Bim-L, and Bim-S), we used specific primers to investigate the expression of each individual splice variant. We found that all three splice variants in human are induced to some degree (7-, 2-, and 5-fold, respectively; Fig. 2C), indicating that all of the Bim isoforms may contribute to the induction of apoptosis in MV diaphragm.
Figure 2.
MV transcriptionally up-regulates molecular markers of intrinsic apoptosis in human diaphragm but not in quadriceps muscle. A) Total RNA was extracted from human diaphragm, and RT-PCR was performed and visualized on agarose gel to show the expression changes in apoptotic markers and the ubiquitin ligase MuRF1. B) Quantitative real-time PCR was performed on the ventilated and control human diaphragm. Transcriptional levels of the Bcl2 family members were examined. Control (Con), n = 7; MV, n = 9. C) Quantitative real-time PCR was performed on the ventilated and control human diaphragm, as well as the nonrespiratory quadriceps muscle. Transcriptional expression levels of the Bim splicing variants were examined. Note that changes in expression of the Bim splice variants with ventilation occur in diaphragm but not in nonrespiratory quadriceps muscle. Control, n = 8; MV, n = 7. D–F) Enzymatic activities of caspases were measured in nonrespiratory quadriceps muscles from ventilated and control subjects. Note that MV did not induce the activities of caspase 3 (D), caspase 8 (E), or caspase 9 (F) in quadriceps muscle. Control, n = 8; MV, n = 7. *P < 0.05.
To establish that Bim gene induction in human diaphragm is due to MV and not to some other potentially confounding systemic process, we examined Bim expression in quadriceps muscle, a nonventilatory human skeletal muscle, from both control and MV patients. The expression levels of all three Bim splice variants remain unchanged or show only subtle changes between MV and control quadriceps specimens (Fig. 2C), suggesting that Bim gene induction is confined to the respiratory muscle. We also measured caspase 3, 8, and 9 activities in the quadriceps muscles and observed no induction of these activities in MV vs. control subjects (Fig. 2D–F). MV-induced intrinsic apoptosis within the diaphragm is thus highly unlikely to be occurring as a result of systemic confounding factors apart from MV itself.
MV induces oxidative stress and impairs mitochondrial function in human diaphragm
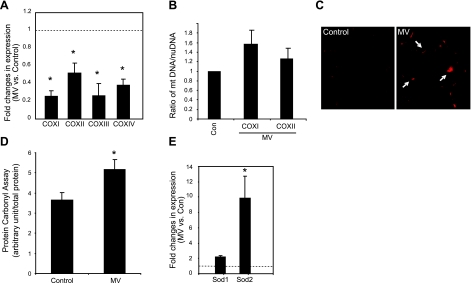
The alterations at the molecular level described above further validate the fact that the intrinsic apoptotic pathway is activated within the diaphragm by MV. Because the intrinsic apoptotic pathway is associated with mitochondria, we investigated the effect of MV on mitochondrial function in human diaphragm. Using molecular markers, we quantitated the expression levels of cytochrome c oxidases (COX I, II, III, and IV), which reside on the mitochondrial inner membrane and serve as the major components of the electron transport chain. We found that the mRNA levels of both nuclear-encoded COX IV and mitochondria-encoded COX I/II/III are significantly reduced vs. control levels (Fig. 3A), indicating a functional impairment of the coupling between electron donor and acceptor as well as mitochondrial energy production. These gene expression changes are not caused by an alteration in mitochondrial content, because the mitochondrial DNA content, represented as the ratio of mitochondrial DNA to nuclear genomic DNA, does not change in MV diaphragm (Fig. 3B).
Figure 3.
MV induces oxidative stress and impairs mitochondrial function in human diaphragm. A) Total RNA was extracted from ventilated and control human diaphragm, and quantitative real-time PCR was performed. Fold changes of COX gene expression levels were calculated as MV over control after normalization to γ-actin levels. Note that expression levels of COX I, II, III, and IV genes in ventilated human diaphragm are reduced. Control (Con), n = 7; MV, n = 9. B) DNA from ventilated and control human diaphragm was extracted, and real-time PCR was performed to examine the levels of the genomic DNA of the β-actin gene as well as the mitochondrial DNA levels of the COX I and II genes. Ratio of the COX I or II DNA levels vs. β-actin nuclear DNA levels was used to indicate relative mitochondrial content. Note that mitochondrial DNA content does not change with MV. Control, n = 5; MV, n = 5. P = 0.15. C) DHE staining was performed on cryosections of ventilated and control human diaphragm. Positive DHE staining shows as red (arrows). D) Carbonyl assay was performed on protein extracts from ventilated and control human diaphragm. Light unit of the carbonyl assay result was normalized to total protein input. Control, n = 7; MV, n = 9. E) Real-time PCR was used to quantitate the expression levels of the cytosolic and mitochondrial antioxidant genes, SOD1 and SOD2, respectively. Note that only SOD2 is significantly elevated with MV. Control, n = 7; MV, n = 9. *P < 0.05.
To determine whether this mitochondrial dysfunction may result from (or cause) MV-induced oxidative stress, we measured the level of free radicals by DHE staining. Indeed, we found that the level of superoxides is elevated in MV samples (Fig. 3C). The accumulation of these superoxides in skeletal muscle may alter protein function by direct oxidation, and, indeed, in the MV samples the total amount of carbonylated protein increased to 150% of the control value (Fig. 3D). Consistent with a mitochondrial role in this oxidative stress, the mitochondria-resident antioxidant gene SOD2 is more dramatically induced than the cytosolic antioxidant SOD1 (Fig. 3E).
Oxidative stress is sufficient to activate proteasomal, autophagic, and intrinsic apoptotic activity in muscle cells in vitro
In previous studies using both human and rat models, MV has been shown to induce E3 ubiquitin ligase proteasomal activity and autophagy lysosomal activity in the diaphragm. In the current study, we provide evidence that MV activates intrinsic apoptosis in human diaphragmatic muscle. Because oxidative stress is induced in MV human diaphragm, we hypothesized that MV-induced oxidative stress is a key coordinator that might activate all three of these pathological subevents (UPS activity, autophagy, and apoptosis) known to occur in the diaphragm during MV.
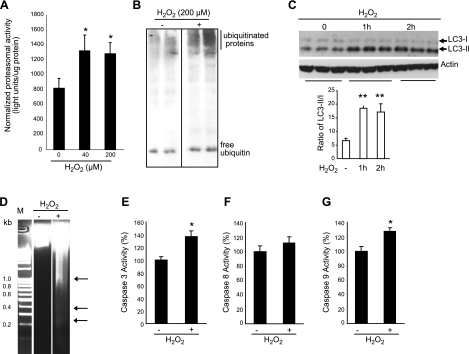
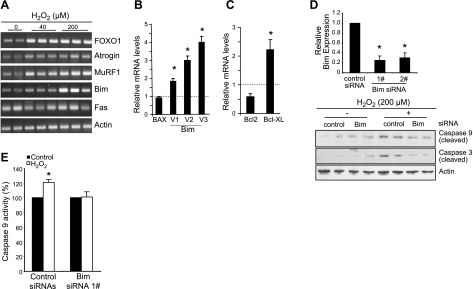
We tested this hypothesis by treating cultured, differentiated C2C12 muscle cells with H2O2 to induce oxidative stress. When subjected to oxidative stress in vitro, the proteasomal activity of the muscle cells is significantly induced (Fig. 4A), and the level of ubiquitinated proteins is dramatically increased (Fig. 4B), similar to the observation in human MV diaphragm (45). Expression levels of the E3 ubiquitin ligases MuRF and atrogin, as well as their upstream inducer gene FoxO1, are increased as well (Fig. 5A).
Figure 4.
Oxidative stress is sufficient to activate the UPS, autophagic, and intrinsic apoptotic pathways. A) Differentiated C2C12 myotubes were treated with H2O2 for 24 h, and proteasomal activity was measured in the cell lysate. Measured light unit was normalized to the total protein level. n = 6. B) C2C12 myotubes were treated with H2O2 for 24 h, and equal amounts of total protein extracts were run with PAGE (4–12%). Polyubiquitinated proteins were visualized by Western blot analysis with anti-ubiquitin antibody. Note that the free ubiquitin level did not change significantly. C) C2C12 cells were treated with H2O2 (1 mM), and cell lysates were subjected to Western blot analysis with anti-LC3 antibody. Total input protein levels were 20 μg, and the α-actin protein level was also detected for normalization. Protein expression levels of LC3-I and II were quantitated by ImageJ and normalized to the actin protein level. Ratio of LC3-II to LC3-I is significantly induced. n = 3. D) Genomic DNA was extracted from H2O2 (1 mM, 24 h)-treated C2C12 myotubes and visualized with 2% agarose gel. Representative gel is shown. M, DNA marker. Arrows indicate DNA fragments. E–G) Caspase 3 (D), 8 (E), and 9 (F) activities were assayed on C2C12 cell lysates, with and without H2O2 treatment (200 μM, 24 h). n = 6. *P < 0.05; **P < 0.01.
Figure 5.
Oxidative stress-induced Bim is required for the activation of intrinsic apoptosis in cultured muscle cells. A) Differentiated C2C12 myotubes were treated with H2O2 for 24 h. Total RNA was extracted from myotubes for RT-PCR reaction, and PCR products were visualized by running with 1.5% agarose gel and stained by ethidium bromide. B, C) Quantitative real-time PCR was performed on H2O2-treated C2C12 muscle cells. γ-Actin was used for normalization. Each sample was run in triplicate; fold change was calculated by normalizing the H2O2-treated to the nontreated myotube samples. n = 3. D) Control and Bim siRNAs were transfected into C2C12 muscle cells, which were then induced to differentiate into myotubes and treated with H2O2 (200 μM) for 24 h. Real-time PCR was used to quantitate the efficiency of siRNAs (top), and Western blot analysis was performed to show the level of cleaved/activated caspase 3 and 9 (bottom) in the presence or absence of Bim siRNA. n = 3. E) C2C12 cells was transfected with control and Bim siRNAs for 3 d, and 200 μM H2O2 was added for 24 h. Cell lysates were collected for caspase 9 activity assay by a Caspase-Glo assay system and normalized to the total protein input. n = 6. *P < 0.05.
To investigate autophagic changes in response to in vitro oxidative stress, we used LC3 as a molecular marker. LC3 protein is first translated and processed as a 18-kDa protein (LC3-I); LC3-I then conjugates with phospholipid to become a membrane-bound mature form (LC3-II). Because LC3-II is formed only when autophagosomes are generated, the LC3-II/LC3-I ratio represents the density of endogenous autophagosomes in cells. The measurement of the conversion of LC3I to LC3-II is a widely accepted autophagic marker. We found that H2O2-induced oxidative stress significantly increases the LC3-II/LC3-I ratio, indicating that oxidative stress is sufficient to induce autophagy in this model (Fig. 4C).
H2O2 treatment is also able to induce apoptotic changes in cultured C2C12 myotubes. DNA fragmentation in C2C12 myotubes is induced in H2O2-treated cells over control (Fig. 4D). H2O2 also demonstrate an ∼40% increase in caspase 3 activity over control (Fig. 4E). Precisely as seen in the in vivo situation, the intrinsic apoptotic marker caspase 9 is significantly induced by H2O2-induced in vitro oxidative stress, whereas the extrinsic marker caspase 8 is not significantly induced (Fig. 4F, G). The intrinsic apoptotic marker gene Bim is induced, whereas the extrinsic apoptotic gene Fas is unaltered (Fig. 5A). The splice variants of Bim transcripts are all induced by oxidative stress in vitro (Fig. 5B). Bcl2-xl gene expression is induced (Fig. 5C), also similar to the expression profile in MV diaphragmatic muscle. Taken together, these results indicate that oxidative stress is sufficient to trigger all of the major pathological events reported in human MV diaphragm. Because the in vitro oxidative stress model recapitulates the pathological events occurring in human MV diaphragm, this in vitro model appears appropriate to use for further characterization of the upstream regulatory mechanisms likely to be involved in oxidative stress-induced in vivo diaphragmatic apoptosis.
Bim is a crucial mediator for oxidative stress-induced muscle apoptosis
Because Bim is induced in both the in vivo (MV human diaphragm) and the in vitro oxidative stress models, we set out to determine whether Bim is a crucial mediator of oxidative stress-induced intrinsic apoptosis. siRNAs against Bim were transfected into C2C12 cells, which were then induced to differentiation for 3 d, followed by 24 h of H2O2 treatment. We find that H2O2-induced activation of caspase 3 and 9 is blocked after siRNA-mediated silencing of Bim gene expression (Fig. 5D). The induction of the enzymatic activity of caspase 9 was consistently suppressed by silencing Bim gene expression (Fig. 5E).
Fos, FoxO1, and Stat3 participate in the regulation of oxidative stress-induced Bim gene expression in vitro
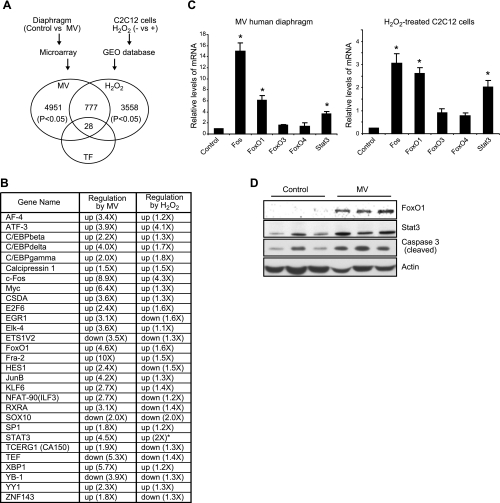
To further understand the molecular mechanisms by which MV activates Bim-associated intrinsic apoptosis, we performed a systemic search for altered transcriptomes by gene microarray using MV human diaphragm and analyzed this database against one generated with H2O2-treated myotubes. Four MV and 4 control diaphragm samples were subjected to a dual-color Agilent microarray gene analysis, and the raw data were analyzed by GeneSpring GX11.0. With the cutoff set at P < 0.05, we acquired 4951 entities with altered expression in the human MV model. To determine how many of these genes are regulated by oxidative stress, we compared them with those that were altered in H2O2-treated C2C12 cells (GEO database). We first analyzed the raw data (accession number GSE3078) from the GEO database of the NCBI GenBank and acquired 3558 entities with P < 0.05. The overlap of these two datasets identified 777 entities that are commonly regulated by the two oxidative stress models. Among them there are 28 transcription factors mined by GeneGo software, which are listed in Fig. 6A, B.
Figure 6.
Systemic search for transcription factors regulated in common by oxidative stress within the in vitro and in vivo models. A) Microarray analysis was performed on RNA samples from control and human MV diaphragm, and data were analyzed by GeneSpring GX11. Raw data on H2O2-treated C2C12 cells from the GEO database in the NCBI GenBank were analyzed by GeneSpring GX11. With cutoff set at P < 0.05, commonly regulated entities were mined as shown in Venn diagram. GeneGo software was then used to identify known transcription factors for these commonly regulated genes. Note that 28 nonredundant transcription factors were identified. B) Transcription factors regulated in common in both MV human diaphragm and H2O2-treated muscle cells. *Gene was not found on microarray but was confirmed by real-time PCR. C) Real-time PCR was performed on RNA from MV human diaphragm and H2O2-treated muscle cells; fold changes were calculated by normalizing to their respective controls (diaphragm control, n=7; MV, n=9; C2C12 cells, n=3). *P < 0.05. D) Total proteins from human diaphragm (control and ventilated, n=3) were extracted; Western blot analysis was performed. Actin protein was detected as the loading control.
To determine whether any of these altered transcription factors might serve as upstream regulators of Bim gene expression, we analyzed the human and mouse Bim promoter sequence and found that the cis-DNA elements of the transcription factors Fos, FoxO1, and Stat3 are present in the 5′-flanking DNA sequence of the Bim promoter (see Supplemental Data). We then confirmed the regulatory patterns of Fos, FoxO1, and Stat3 in both the MV human diaphragm and H2O2-treated muscle cell models by quantitative PCR (Fig. 6C, D). At the protein level, FoxO1 and Stat3 are also induced in ventilated human diaphragm (Fig. 6D), but Fos protein is undetectable (not shown).
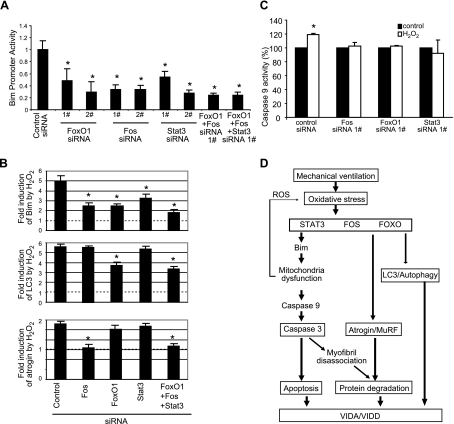
To examine whether these oxidative stress-induced transcription factors might regulate Bim gene expression, we cotransfected siRNAs targeting each of these transcription factors into C2C12 cells with a Bim promoter-driven luciferase reporter. These experiments show that each of the siRNAs reduces the promoter activity (Fig. 7A), indicating that all of these 3 transcription factors are involved in constitutive Bim gene expression.
Figure 7.
Fos, FoxO1, and Stat3-mediated regulation of autophagy, atrophy, and apoptosis in response to oxidative stress. A) C2C12 cells were transfected with siRNAs, together with a Bim promoter reporter construct and β-Gal reporter construct. Reporter gene assay was performed 3 d later; luciferase light unit was normalized to the cotransfected β-Gal activity. Note that two different siRNAs (1# and 2#) against different regions of each gene were used to minimize the potential off-target effect. n = 3. B) Regulatory effect of Fos, FoxO1, and Stat3 on atrophic, autophagic, and apoptotic markers in response to oxidative stress. C2C12 cells were transfected with siRNAs for 3 d and treated with H2O2 (200 μM) for 24 h on d 4. Total RNA was collected, and real-time PCR was performed to examine the gene expression levels. Note that all three transcription factors inhibit Bim gene expression in response to oxidative stress, but Fos also regulates atrogin, and FoxO1 regulates LC3. n = 4. C) Fos, FoxO1, and Stat3 are required for H2O2-dependent activation of apoptosis. siRNAs against Fos, FoxO1, and Stat3 were transfected into C2C12 cells for 3 d and treated with H2O2 (200 mM) for 24 h. Caspase 9 activity was measured and normalized to the total protein input. n = 3. D) Schematic diagram of the proposed molecular mechanisms underlying MV-induced diaphragm atrophy and dysfunction. See detailed description in text. *P < 0.05.
To test whether these transcription factors regulate Bim gene expression in response to H2O2-induced oxidative stress, we transfected siRNAs against each transcription factor into C2C12 cells and treated the cells with H2O2. Each of the siRNAs dramatically reduced the induction of Bim gene expression by H2O2 treatment (Fig. 7B). Cells transfected by all three of the siRNAs had still further suppression of Bim induction (Fig. 7B). Of interest, the siRNAs against Fos, but not against FoxO1 and Stat3, also block the oxidative stress-induced atrogin gene expression (Fig. 7C). FoxO1 appears to regulate the autophagy marker gene LC3, but Fos and Stat3 do not appear to have a role in the regulation of autophagy (Fig. 7B).
We further tested whether these transcription factors regulate the activation of the intrinsic apoptotic pathway by examining the enzymatic activity of caspase 9. Silencing of the expression of Fos, FoxO1, and Stat3 eliminates oxidative stress-induced activation of caspase 9 consistently (Fig. 7C).
DISCUSSION
MV is used in hundreds of thousands of patients yearly to support the respiratory system during surgical procedures and as a life-saving intervention in critically ill patients with respiratory failure. However, weaning patients from the ventilator to independent breathing can be difficult, sometimes resulting in prolonged ventilator dependence, which may cause a cascading series of complications that substantially increases morbidity, mortality, and costs of care.
It is well established that MV is associated with dysfunction and atrophy of the muscle fibers within the major inspiratory muscle, the diaphragm (52), which has been demonstrated in both experimental animals (e.g., ref. 31) and humans (42, 43). It is logical to believe that VIDD is a major underlying cause of weaning difficulties, because the diaphragm is responsible for most ventilation in normal adults and twitch transdiaphragmatic pressures (representing maximal diaphragmatic force production) are markedly lower in patients who require ventilation than those who do not (53–55). Further, the atrophy component of VIDD (VIDA) seems to be more critical than the dysfunction component, because modeling indicates that the degree of diaphragm fiber atrophy recently identified clinically is sufficient to account for essentially all of the diaphragmatic weakness that has been measured in physiological studies of ventilated patients performed over the past 2 decades (42).
Previous work suggested that MV-associated diaphragm muscle fiber atrophy results, at least in part, from increased proteolysis due to elevated UPS activity (31, 42,) and enhanced autophagy (46) in the human diaphragm. In the current study, we add to this information the new observation that MV induces apoptosis in human diaphragm. We show that this occurs via selective activation of the intrinsic apoptotic pathway, resulting ultimately in caspase 3 activation and nuclear DNA fragmentation. We further suggest through in vitro experiments that oxidative stress is a potential proximal activator of all of the pathways that have been implicated in VIDD, with mitochondrial dysfunction representing a central component of this cascade. Finally, we show that Bim is probably an important mediator of the oxidative stress-induced activation of the intrinsic apoptotic pathway, and we identify Fos, FoxO1, and Stat3 as transcription factors that regulate Bim expression in vitro. We were struck throughout this series of experiments by how closely the in vivo (human diaphragm muscle) findings mimic those seen in our in vitro (muscle cell culture) oxidative stress model.
Our findings of elevated caspase 9 but not caspase 8, elevated Bim (and its transcriptional variants) but not Fas or FasL, elevated COX levels, and elevated SOD2 but not SOD1 all suggest that the intrinsic (mitochondrial) rather than the extrinsic apoptotic pathway is the primary pathway operant in MV diaphragm. In vitro oxidative stress in cultured C2C12 muscle cells also selectively induces the intrinsic apoptotic pathway: here, too, there is increased activation of caspase 9 but not caspase 8 and increased expression of Bim but not Fas. Caspase 9 is the initiator caspase in the intrinsic, or mitochondrial, apoptotic pathway. Caspase 9 with Apaf-1 and cytochrome c form the apoptosome, which cleaves and activates the apoptosis effector, caspase 3. The latter is indispensable for DNA fragmentation and ultimate cell death (56). Our RNA interference experiments, which show markedly reduced levels of H2O2-induced cleavage/activation of caspases 9 and 3 when the Bim gene is silenced, suggest that Bim is required for the oxidative stress-induced activation of intrinsic apoptosis in muscle.
We have also identified FoxO1, Fos, and Stat3 as factors that regulate the increased expression of Bim associated with oxidative stress in vitro. We find that these transcription factors also participate in the in vitro regulation of the other two major pathophysiological processes underlying VIDA: atrophy and autophagy. FoxO1 regulates LC3 expression, and Fos regulates atrogin expression in response to H2O2-induced oxidative stress. These findings are consistent with the report that in a rat model, the benefit of the antioxidant Trolox on MV-induced diaphragm atrophy does not occur entirely via FoxO-dependent mechanisms (57). Although it has been reported that the FoxO family regulates autophagy (58), atrophy (59), and apoptosis (60), specific silencing of FoxO1 with siRNA did not block oxidative stress-induced atrogin expression in our in vitro model. This may be due to compensation from other FoxO family members, which might be regulated at the post-translational level. It has been reported that MV alters the phosphorylation and thereby the nuclear location of each of FoxO1, FoxO3, and FoxO4 in rats (57), and H2O2-induced oxidative stress also alters FoxO3 and FoxO4 phosphorylation and nuclear translocation (61, 62). Because the role of FoxO is not reported consistently in all experimental studies, it would be valuable in future in vivo studies to examine the effect of MV on diaphragm after blocking FoxO pathways, both individually and jointly.
Activation of the intrinsic apoptotic pathway in MV diaphragm, as demonstrated here, may contribute to the evolution of VIDA in several ways. First, the activated caspase 3 causes the activation of nuclease, which results in damage to double-stranded DNA and the loss of myonuclei. McClung et al. (37) have demonstrated that the myonuclear domain in MV rat diaphragm is preserved, suggesting that loss of myonuclei results in loss of cytoplasm within the affected muscle fibers (which is equivalent to fiber atrophy). Second, caspase 3 activation may create myofiber atrophy by cleaving myofibrils (47, 63), yielding substrate for the UPS-mediated degradation of ubiquinated proteins (48, 49). Indeed, inhibition of caspase 3 during experimental MV in rats with the specific compound Ac-DEVD-CHO (37) reduces the excessive breakdown of myofibrillar proteins. Finally, intrinsic apoptosis is associated with mitochondrial dysfunction. Because mitochondria provide >90% of cellular energy, the resulting inefficiency in energy production would probably impair muscle contractile function.
Data presented here as well as previously (26, 31, 33, 36, 42) show that oxidative stress is induced in both animal and human diaphragm by MV. Because MV alters many biochemical events (activation of calpains, the E3 ubiquitin ligase-UPS, autophagy, and, as demonstrated here, apoptosis), we sought to determine whether oxidative stress may serve as a key that coordinates all of these several downstream molecular and biochemical events. We confirmed that H2O2-induced oxidative stress is able to activate the UPS, autophagy, and apoptosis. Thus, oxidative stress may be the most upstream underlying mechanism of MV-associated diaphragm atrophy. Still, the regulatory mechanism by which MV induces the “primary bolus” of oxidative stress that initiates the several downstream atrophic pathways remains incompletely understood. The initial oxidative stress may result from the sudden change in the functional status of muscle from continuous, intermittent contraction to static rest, which occurs with MV. Because noncontracting muscle fibers do not require major mitochondrial energy production, this altered functional status may place a “brake” on normal mitochondrial function. This may in turn cause mitochondrial ROS accumulation and eventual leak. It is known that limb muscle, which converts from an active to a static state by denervation, accumulates ROS (64). This effect may be even more pronounced when the active state is essentially continuous, as in the diaphragm.
Our implication here of Bim as a gene able to induce mitochondrial dysfunction is consistent with work demonstrating that Bim results in loss of mitochondrial membrane potential and enhances mitochondrial membrane permeability (65). This mitochondrial dysfunction will result in the accumulation of ROS, amplifying the degree of oxidative stress that occurs in conjunction with MV. It appears, therefore, that Bim gene expression is both induced by oxidative stress and results in mitochondrial malfunction and additional oxidative stress. This would create a positive feedback loop, potentiating rapid amplification of the atrophic effect of MV on the diaphragm. It is tempting to propose this positive feedback loop as a partial explanation for the far greater speed with which disuse atrophy occurs in the diaphragm than in locomotor muscles.
It is appropriate to address the question of the suitability of our brain-dead organ donor model as a model of clinical MV. It is, of course, impossible to eliminate all possible confounding variables in a clinical (human) model of any pathophysiological process. Thus, in the model of VIDA used here and in prior studies using diaphragm biopsies from brain-dead organ donors, there will always be clinical differences between the MV (donor) subjects and the control subjects (beyond the simple duration of time receiving MV) that might affect the characteristics of the diaphragm muscle biopsy specimens. To investigate the concern that the elevated apoptotic markers we identified in MV subjects over control subjects might result from a systemic process rather than the longer duration of MV to which the MV group was subjected, we measured Bim expression and caspase 3 and 9 activities in quadriceps muscle from MV and control patients. The findings that Bim is far more impressively elevated in MV diaphragm (∼4-fold over control) than in MV quadriceps (only 20% greater than control) and that there is selective induction of caspase 3 and 9 in diaphragm and not in quadriceps muscle, strongly suggest that the diaphragmatic apoptosis we describe does not result from a systemic process.
The interpretation that diaphragmatic changes in organ donors result from prolonged MV and not from other systemic factors is also consistent with the data presented in previous publications using the organ donor model regarding nonrespiratory (pectoralis) muscle fiber cross-sectional area (42) and locomotor muscle UPS and autophagy-associated gene expression (46). In each case, the changes in the diaphragm associated with MV were dramatic, whereas those in the nonrespiratory muscles were either absent or very modest. Further support is given by the recent finding that VIDA is a time-dependent phenomenon (i.e., that it follows a “dose”-response; ref. 43). The duplication of many of these findings in more easily controlled rodent models of MV add, of course, still further support to the concept that VIDA in each of these models results from ventilator-related mechanisms (e.g., diaphragm inactivity) rather than from systemic mechanisms.
A few agents, the nonspecific antioxidant Trolox (66), the caspase 3 inhibitor DEVD (37), the protease inhibitor leupeptin (44), the proteasome inhibitor lactacystin (31), the calpain and lysosomal protease inhibitor E-64d (31), and apocynin (67), have demonstrated some effectiveness in preventing VIDA in MV rats. Our results suggest that an antioxidant would be the ideal agent with which to prevent VIDA clinically. However, this antioxidant would ideally block pathological oxidative stress in a targeted manner. Because we found the mitochondria to be the source of the pathological reactive oxygen species in MV human diaphragm, mitochondria-targeted antioxidants would appear to be the most promising agents to explore in combating VIDD.
Supplementary Material
Acknowledgments
The authors thank Kevin Hoang (summer student at University of California, San Diego, CA, USA) and Donna Minagawa (Stanford University) for technical assistance and Xiaohua Zhang (Stanford University) for assistance in bioinformatics analysis.
This research was supported by a Veterans Affairs merit review grant awarded to J.B.S. and also in part by U.S. National Institutes of Health grant HL078834. The authors declare that they have no financial relationship with a commercial entity.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Gayan-Ramirez G., Decramer M. (2002) Effects of mechanical ventilation on diaphragm function and biology. Eur. Respir. J. 20, 1579–1586 [DOI] [PubMed] [Google Scholar]
- 2. Laghi F., Cattapan S., Jubran A., Parthasarathy S., Warshawsky P., Choi Y., Tobin M. (2003) Is weaning failure caused by low-frequency fatigue of the diaphragm? Am. J. Respir. Crit. Care Med. 167, 120–127 [DOI] [PubMed] [Google Scholar]
- 3. Swartz M., Marino P. (1985) Diaphragmatic strength during weaning from mechanical ventilation. Chest 88, 736–739 [DOI] [PubMed] [Google Scholar]
- 4. Alía I., Esteban A. (2000) Weaning from mechanical ventilation. Crit. Care 4, 72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Esteban A., Frutos F., Tobin M., Alía I., Solsona J., Valverdú I., Fernández R., de la Cal M., Benito S., Tomás R. (1995) A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N. Engl. J. Med. 332, 345–350 [DOI] [PubMed] [Google Scholar]
- 6. Dasgupta A., Rice R., Mascha E., Litaker D., Stoller J. (1999) Four-year experience with a unit for long-term ventilation (respiratory special care unit) at the Cleveland Clinic Foundation. Chest 116, 447–455 [DOI] [PubMed] [Google Scholar]
- 7. Martin A., Davenport P., Franceschi A., Harman E. (2002) Use of inspiratory muscle strength training to facilitate ventilator weaning: a series of 10 consecutive patients. Chest 122, 192–196 [DOI] [PubMed] [Google Scholar]
- 8. Scheinhorn D., Chao D., Stearn-Hassenpflug M., LaBree L., Heltsley D. (1997) Post-ICU mechanical ventilation: treatment of 1,123 patients at a regional weaning center. Chest 111, 1654–1659 [DOI] [PubMed] [Google Scholar]
- 9. Vassilakopoulos T., Zakynthinos S., Roussos C. (2006) Bench-to-bedside review: weaning failure—should we rest the respiratory muscles with controlled mechanical ventilation? Crit. Care 10, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sprague S., Hopkins P. (2003) Use of inspiratory strength training to wean six patients who were ventilator-dependent. Phys. Ther. 83, 171–181 [PubMed] [Google Scholar]
- 11. Scheinhorn D., Chao D., Hassenpflug M., Gracey D. (2001) Post-ICU weaning from mechanical ventilation: the role of long-term facilities. Chest 120, 482S–484S [DOI] [PubMed] [Google Scholar]
- 12. Scheinhorn D., Chao D., Stearn-Hassenpflug M. (2002) Liberation from prolonged mechanical ventilation. Crit. Care Clin. 18, 569–595 [DOI] [PubMed] [Google Scholar]
- 13. Purro A., Appendini L., De Gaetano A., Gudjonsdottir M., Donner C., Rossi A. (2000) Physiologic determinants of ventilator dependence in long-term mechanically ventilated patients. Am. J. Respir. Crit. Care Med. 161, 1115–1123 [DOI] [PubMed] [Google Scholar]
- 14. Pilcher D., Bailey M., Treacher D., Hamid S., Williams A., Davidson A. (2005) Outcomes, cost and long term survival of patients referred to a regional weaning centre. Thorax 60, 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Afessa B., Hogans L., Murphy R. (1999) Predicting 3-day and 7-day outcomes of weaning from mechanical ventilation. Chest 116, 456–461 [DOI] [PubMed] [Google Scholar]
- 16. Scheinhorn D., Chao D., Stearn-Hassenpflug M. (2000) Approach to patients with long-term weaning failure. Respir. Care Clin. N. Am. 6, 437–461, vi [DOI] [PubMed] [Google Scholar]
- 17. Douglas S., Daly B., Gordon N., Brennan P. (2002) Survival and quality of life: short-term versus long-term ventilator patients. Crit. Care Med. 30, 2655–2662 [DOI] [PubMed] [Google Scholar]
- 18. Modawal A., Candadai N., Mandell K., Moore E., Hornung R., Ho M., Tsevat J. (2002) Weaning success among ventilator-dependent patients in a rehabilitation facility. Arch. Phys. Med. Rehabil. 83, 154–157 [DOI] [PubMed] [Google Scholar]
- 19. Safdar N., Dezfulian C., Collard H. R., Saint S. (2005) Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit. Care Med. 33, 2184–2193 [DOI] [PubMed] [Google Scholar]
- 20. Buczko W. (2010) Ventilator-associated pneumonia among elderly Medicare beneficiaries in long-term care hospitals. Health Care Financ. Rev. 31, 1–10 [PMC free article] [PubMed] [Google Scholar]
- 21. Restrepo M. I., Anzueto A., Arroliga A. C., Afessa B., Atkinson M. J., Ho N. J., Schinner R., Bracken R. L., Kollef M. H. (2010) Economic burden of ventilator-associated pneumonia based on total resource utilization. Infect. Control Hosp. Epidemiol. 31, 509–515 [DOI] [PubMed] [Google Scholar]
- 22. Muscedere J. G., Martin C. M., Heyland D. K. (2008) The impact of ventilator-associated pneumonia on the Canadian health care system. J. Crit. Care 23, 5–10 [DOI] [PubMed] [Google Scholar]
- 23. Testelmans D., Maes K., Wouters P., Gosselin N., Deruisseau K., Powers S., Sciot R., Decramer M., Gayan-Ramirez G. (2006) Rocuronium exacerbates mechanical ventilation-induced diaphragm dysfunction in rats. Crit. Care Med. 34, 3018–3023 [DOI] [PubMed] [Google Scholar]
- 24. Sassoon C., Caiozzo V., Manka A., Sieck G. (2002) Altered diaphragm contractile properties with controlled mechanical ventilation. J. Appl. Physiol. 92, 2585–2595 [DOI] [PubMed] [Google Scholar]
- 25. Sassoon C., Zhu E., Caiozzo V. (2004) Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am. J. Respir. Crit. Care Med. 170, 626–632 [DOI] [PubMed] [Google Scholar]
- 26. Betters J., Criswell D., Shanely R., Van Gammeren D., Falk D., Deruisseau K., Deering M., Yimlamai T., Powers S. (2004) Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am. J. Respir. Crit. Care Med. 170, 1179–1184 [DOI] [PubMed] [Google Scholar]
- 27. Shanely R., Van Gammeren D., Deruisseau K., Zergeroglu A., McKenzie M., Yarasheski K., Powers S. (2004) Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am. J. Respir. Crit. Care Med. 170, 994–999 [DOI] [PubMed] [Google Scholar]
- 28. Criswell D., Shanely R., Betters J., McKenzie M., Sellman J., Van Gammeren D., Powers S. (2003) Cumulative effects of aging and mechanical ventilation on in vitro diaphragm function. Chest 124, 2302–2308 [DOI] [PubMed] [Google Scholar]
- 29. DeRuisseau K., Shanely R., Akunuri N., Hamilton M., Van Gammeren D., Zergeroglu A., McKenzie M., Powers S. (2005) Diaphragm unloading via controlled mechanical ventilation alters the gene expression profile. Am. J. Respir. Crit. Care Med. 172, 1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DeRuisseau K. C., Kavazis A. N., Deering M. A., Falk D. J., Van Gammeren D., Yimlamai T., Ordway G. A., Powers S. K. (2005) Mechanical ventilation induces alterations of the ubiquitin-proteasome pathway in the diaphragm. J. Appl. Physiol. 98, 1314–1321 [DOI] [PubMed] [Google Scholar]
- 31. Shanely R., Zergeroglu M., Lennon S., Sugiura T., Yimlamai T., Enns D., Belcastro A., Powers S. (2002) Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am. J. Respir. Crit. Care Med. 166, 1369–1374 [DOI] [PubMed] [Google Scholar]
- 32. Powers S., Shanely R., Coombes J., Koesterer T., McKenzie M., Van Gammeren D., Cicale M., Dodd S. (2002) Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J. Appl. Physiol. 92, 1851–1858 [DOI] [PubMed] [Google Scholar]
- 33. Zergeroglu M., McKenzie M., Shanely R., Van Gammeren D., DeRuisseau K., Powers S. (2003) Mechanical ventilation-induced oxidative stress in the diaphragm. J. Appl. Physiol. 95, 1116–1124 [DOI] [PubMed] [Google Scholar]
- 34. Le Bourdelles G., Viires N., Boczkowski J., Seta N., Pavlovic D., Aubier M. (1994) Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am. J. Respir. Crit. Care Med. 149, 1539–1544 [DOI] [PubMed] [Google Scholar]
- 35. Rácz G., Gayan-Ramirez G., Testelmans D., Cadot P., De Paepe K., Zádor E., Wuytack F., Decramer M. (2003) Early changes in rat diaphragm biology with mechanical ventilation. Am. J. Respir. Crit. Care Med. 168, 297–304 [DOI] [PubMed] [Google Scholar]
- 36. Falk D., Deruisseau K., Van Gammeren D., Deering M., Kavazis A., Powers S. (2006) Mechanical ventilation promotes redox status alterations in the diaphragm. J. Appl. Physiol. 101, 1017–1024 [DOI] [PubMed] [Google Scholar]
- 37. McClung J., Kavazis A., DeRuisseau K., Falk D., Deering M., Lee Y., Sugiura T., Powers S. (2007) Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am. J. Respir. Crit. Care Med. 175, 150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shanely R., Coombes J., Zergeroglu A., Webb A., Powers S. (2003) Short-duration mechanical ventilation enhances diaphragmatic fatigue resistance but impairs force production. Chest 123, 195–201 [DOI] [PubMed] [Google Scholar]
- 39. Zhu E., Sassoon C., Nelson R., Pham H., Zhu L., Baker M., Caiozzo V. (2005) Early effects of mechanical ventilation on isotonic contractile properties and MAF-box gene expression in the diaphragm. J. Appl. Physiol. 99, 747–756 [DOI] [PubMed] [Google Scholar]
- 40. Capdevila X., Lopez S., Bernard N., Rabischong E., Ramonatxo M., Martinazzo G., Prefaut C. (2003) Effects of controlled mechanical ventilation on respiratory muscle contractile properties in rabbits. Intensive Care Med. 29, 103–110 [DOI] [PubMed] [Google Scholar]
- 41. Anzueto A., Peters J., Tobin M., de los Santos R., Seidenfeld J., Moore G., Cox W., Coalson J. (1997) Effects of prolonged controlled mechanical ventilation on diaphragmatic function in healthy adult baboons. Crit. Care Med. 25, 1187–1190 [DOI] [PubMed] [Google Scholar]
- 42. Levine S., Nguyen T., Taylor N., Friscia M., Budak M., Rothenberg P., Zhu J., Sachdeva R., Sonnad S., Kaiser L., Rubinstein N., Powers S., Shrager J. (2008) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 358, 1327–1335 [DOI] [PubMed] [Google Scholar]
- 43. Jaber S., Petrof B. J., Jung B., Chanques G., Berthet J. P., Rabuel C., Bouyabrine H., Courouble P., Koechlin C., Sebbane M., Similowski T., Scheuermann V., Mebazaa A., Capdevila X., Mornet D., Mercier J., Lacampagne A., Philips A., Matecki S. (2011) Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am. J. Respir. Crit. Care Med. 183, 364–371 [DOI] [PubMed] [Google Scholar]
- 44. Maes K., Testelmans D., Powers S., Decramer M., Gayan-Ramirez G. (2007) Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am. J. Respir. Crit. Care Med. 175, 1134–1138 [DOI] [PubMed] [Google Scholar]
- 45. Levine S., Biswas C., Dierov J., Barsotti R., Shrager J., Nguyen T., Sonnad S., Kucharchzuk J., Kaiser L., Singhal S., Budak M. (2011) Increased proteolysis, myosin depletion and atrophic AKT-FOXO signaling in human diaphragm disuse. Am. J. Respir. Crit. Care Med. 183, 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hussain S., Mofarrahi M., Sigala I., Kim H., Vassilakopoulos T., Maltais F., Bellenis I., Chaturvedi R., Gottfried S., Metrakos P., Danialou G., Matecki S., Jaber S., Petrof B., Goldberg P. (2010) Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am. J. Respir. Crit. Care Med. 182, 1377–1386 [DOI] [PubMed] [Google Scholar]
- 47. Du J., Wang X., Miereles C., Bailey J., Debigare R., Zheng B., Price S., Mitch W. (2004) Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J. Clin. Invest. 113, 115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Du J., Hu Z., Mitch W. E. (2005) Molecular mechanisms activating muscle protein degradation in chronic kidney disease and other catabolic conditions. Eur. J. Clin. Invest. 35, 157–163 [DOI] [PubMed] [Google Scholar]
- 49. Lee S. W., Dai G., Hu Z., Wang X., Du J., Mitch W. E. (2004) Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J. Am. Soc. Nephrol. 15, 1537–1545 [DOI] [PubMed] [Google Scholar]
- 50. Kavazis A., Talbert E., Smuder A., Hudson M., Nelson W., Powers S. (2009) Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic. Biol. Med. 46, 842–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nguyen T., Rubinstein N., Vijayasarathy C., Rome L., Kaiser L., Shrager J., Levine S. (2005) Effect of chronic obstructive pulmonary disease on calcium pump ATPase expression in human diaphragm. J. Appl. Physiol. 98, 2004–2010 [DOI] [PubMed] [Google Scholar]
- 52. Powers S., Kavazis A., Levine S. (2009) Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit. Care Med. 37, S347–S353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Laghi F., Topeli A., Tobin M. (1998) Does resistive loading decrease diaphragmatic contractility before task failure? J. Appl. Physiol. 85, 1103–1112 [DOI] [PubMed] [Google Scholar]
- 54. Cattapan S., Laghi F., Tobin M. (2003) Can diaphragmatic contractility be assessed by airway twitch pressure in mechanically ventilated patients? Thorax 58, 58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Watson A., Hughes P., Louise Harris M., Hart N., Ware R., Wendon J., Green M., Moxham J. (2001) Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit. Care Med. 29, 1325–1331 [DOI] [PubMed] [Google Scholar]
- 56. Jänicke R., Sprengart M., Wati M., Porter A. (1998) Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 273, 9357–9360 [DOI] [PubMed] [Google Scholar]
- 57. McClung J., Kavazis A., Whidden M., DeRuisseau K., Falk D., Criswell D., Powers S. (2007) Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J. Physiol. 585, 203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhao J., Brault J., Schild A., Cao P., Sandri M., Schiaffino S., Lecker S., Goldberg A. (2007) FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 6, 472–483 [DOI] [PubMed] [Google Scholar]
- 59. Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S., Goldberg A. (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gilley J., Coffer P., Ham J. (2003) FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J. Cell Biol. 162, 613–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Essers M. A., Weijzen S., de Vries-Smits A. M., Saarloos I., de Ruiter N. D., Bos J. L., Burgering B. M. (2004) FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 23, 4802–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jang S. W., Yang S. J., Srinivasan S., Ye K. (2007) Akt phosphorylates MstI and prevents its proteolytic activation, blocking FOXO3 phosphorylation and nuclear translocation. J. Biol. Chem. 282, 30836–30844 [DOI] [PubMed] [Google Scholar]
- 63. Argilés J. M., López-Soriano F. J., Busquets S. (2008) Apoptosis signalling is essential and precedes protein degradation in wasting skeletal muscle during catabolic conditions. Int. J. Biochem. Cell Biol. 40, 1674–1678 [DOI] [PubMed] [Google Scholar]
- 64. Muller F. L., Song W., Jang Y. C., Liu Y., Sabia M., Richardson A., Van Remmen H. (2007) Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1159–R1168 [DOI] [PubMed] [Google Scholar]
- 65. Sugiyama T., Shimizu S., Matsuoka Y., Yoneda Y., Tsujimoto Y. (2002) Activation of mitochondrial voltage-dependent anion channel by apro-apoptotic BH3-only protein Bim. Oncogene 21, 4944–4956 [DOI] [PubMed] [Google Scholar]
- 66. McClung J., Whidden M., Kavazis A., Falk D., Deruisseau K., Powers S. (2008) Redox regulation of diaphragm proteolysis during mechanical ventilation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1608–R1617 [DOI] [PubMed] [Google Scholar]
- 67. McClung J. M., Van Gammeren D., Whidden M. A., Falk D. J., Kavazis A. N., Hudson M. B., Gayan-Ramirez G., Decramer M., DeRuisseau K. C., Powers S. K. (2009) Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit. Care Med. 37, 1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.