Abstract
Adenosine (ADO) is an extracellular signaling molecule that is an important regulator of innate lung defense. On binding ADO, the A2B receptor (A2BR) stimulates cAMP production to activate the CFTR Cl− channel, increase ciliary beating, and initiate cytokine secretion. We tested the hypothesis that CFTR served as a positive regulator of the A2BRs. We found that A2BR and CFTR coimmunoprecipitated. They also underwent ADO-dependent Förster resonance energy transfer (FRET), which increased from 5% in the absence of agonist to 18% with 100 μM ADO (EC50 1.7 μM), suggesting that they dynamically associate in the plasma membrane. In contrast, despite colocalization, no FRET was observed between CFTR and GAP43. The interaction between A2BR and CFTR had some specificity: A2BR-stimulated but not forskolin-stimulated cAMP production was ∼50% greater in the presence of CFTR, due to a CFTR-dependent increase in plasma membrane A2BR levels. These CFTR-dependent increases in A2BR levels and cAMP production resulted in significantly enhanced ciliary beating and increased cytokine secretion in normal compared to cystic fibrosis airway epithelia. Thus, we hypothesize that CFTR regulates A2BR levels in the plasma membrane to modulate cell signaling and to enhance selective components of the innate lung defense system.—Watson, M. J., Worthington, E. N., Clunes, L. A., Rasmussen, J. E., Jones, L., Tarran, R. Defective adenosine-stimulated cAMP production in cystic fibrosis airway epithelia: a novel role for CFTR in cell signaling.
Keywords: chloride, ion channel, G protein-coupled receptor, 2nd messenger
Mammalian airways are protected from infection by a thin film of airway surface liquid (ASL) that acts as a lubricant for mucus clearance. Airway epithelia secrete soluble “reporter molecules” whose dilution and concentration transmit information on ASL status to the epithelia (1). Adenosine (ADO) has been proposed as one such reporter and is present in the ASL at sufficient levels to regulate ASL volume homeostasis (1–3). ADO is an extracellular ligand that can signal through 4 different receptor subtypes (A1, A2A, A2B, and A3; ref. 4). In addition to regulating ASL volume, ADO receptors are known to regulate several processes, including nociception, inflammation, Cl− secretion, and myocyte contractility (5). cAMP is an evolutionarily conserved second messenger that is used for cell signaling in both prokaryotes and eukaryotes. Activation of G-protein-coupled receptors such as the A2B receptor (A2BR) can activate stimulatory Gs proteins to activate adenylate cyclase, which forms cAMP from intracellular ATP to activate protein kinase A (PKA) (4, 6, 7). Because cAMP is soluble, one would not expect this system to afford any discreteness. However, several mechanisms have evolved to compartmentalize cAMP signaling, including anchoring of PKA, phosphodiesterases, and cAMP pumps close to their receptors to act as diffusion-limiting barriers (8–10).
CFTR is a cAMP-regulated Cl− channel, which is expressed in many epithelial tissues, as well as in the heart and brain (11). Coordinated regulation of CFTR by A2BR is vital for the defense of mucosal surfaces (3, 12). Cystic fibrosis (CF) is a Mendelian genetic disease that is caused by a spectrum of mutations in CFTR (13). The most common mutation, a deletion of phenylalanine at position 508, accounts for ∼70% of all cases of CF. The majority of ΔF508 CFTR fails to exit the endoplasmic reticulum and is degraded (14). If ΔF508 CFTR does make it to the plasma membrane, it conducts Cl− poorly, compared to WT CFTR, and its dwell time in the membrane is also reduced (15, 16). Recent data suggest that mutations in the CFTR gene lead to dehydration of airway surfaces that ultimately produces mucus stasis, mucus adhesion, inflammation, and infection (1, 3, 17). However, this hypothesis is currently controversial, and competing theories exist to explain CF pathogenesis, including altered extracellular pH, affecting mucin rheology and altered inflammation (18–20).
CFTR interacts with a number of proteins, including the β2-adrenergic receptor (21), the epithelial Na+ channel (ENaC; refs. 22, 23), the KATP channel (24), a cAMP transporter (MRP4; ref. 10), and a phophodiesterase (PDE3A; ref. 25). The benefits of such clustering are not fully understood but may include more efficient signal transduction due to the formation of microdomains. Interestingly, activated A2BR recruits additional A2BRs to the plasma membrane (26). Thus, while many purinergic receptors exhibit rapid desensitization with time, stimulation of A2BR is prolonged, with subsequent desensitization occurring only over several hours (27). Because of the importance of ADO in regulating CFTR function (1, 3), we looked for evidence of CFTR-A2BR interactions using both human bronchial epithelial cells (HBECs) and baby hamster kidney (BHK) cells stably expressing CFTR. We found that CFTR expression enhanced A2BR surface levels, leading to increased ADO-stimulated cAMP production. Furthermore, ADO-induced ciliary beating and IL-8 secretion were potentiated by the presence of CFTR, suggesting that CFTR can exert cellular effects beyond regulating ion transport.
MATERIALS AND METHODS
Cell isolation and culture
Human excess donor lungs and excised recipient lungs were obtained at the time of lung transplantation from portions of main stem or lumbar bronchi, and cells were harvested by enzymatic digestion, as described previously, under a protocol approved by the University of North Carolina Institutional Review Board (2). Baby hamster kidney (BHK) cells and human embryonic kidney 293 (HEK293) cells were cultured in DMEM/F12 medium containing 5% FBS at 37°C in 5% CO2 on glass slides for imaging or 6-well plastic plates for biochemical experiments. If required, cultures that were ∼75% confluent were transfected for 4–6 h using Effectene (Qiagen, Valencia, CA, USA) or Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), as per the manufacturer's instructions. The transfection reagents were then removed, and the cultures were used for experimentation 24–30 h later.
Förster resonance energy transfer (FRET)
FRET was performed with a TE300 microscope (Nikon Instruments, Melville, NY, USA) with a ×60 1.2-NA water objective lens, switchable filter wheels (Ludl, Hawthorne, NY, USA), and an Orca CCD camera (Hamamatsu, Bridgewater, NJ, USA) or with a SP5 confocal microscope (Leica Microsystems, Buffalo Grove, IL, USA) with a ×63 1.2-NA glycerol objective lens, as described previously (28).
Coimmunoprecipitation of CFTR and A2BR
BHK cells stably expressing HA-tagged CFTR were transfected with V5-tagged A2BR or empty vector and lysed on ice after 36 h with Nonidet P-40 buffer containing 0.1% Nonidet P-40, 1 μg/ml leupeptin, 2 μg/ml aprotinin, 50 μg/ml Pefabloc, 121 μg/ml benzamidine, and 3.5 μg/ml E64 (Sigma-Aldrich, St. Louis, MO, USA). After insoluble material was removed, A2BR was immunoprecipitated using an anti-V5 mouse monoclonal antibody (Abcam, Cambridge, MA, USA), and the precipitate was analyzed by immunoblot for the presence of A2BR and CFTR, as described previously (28).
Immunofluorescence
Cultures were fixed with 4% paraformaldehyde in PBS for 10 min, washed, and membranes were permeabilized (0.1% saponin or 0.2% Triton X-100). BSA (1%) and 5% goat serum were then applied for 2 h in PBS. Cells were incubated with a rabbit polyclonal antibody against A2BR (Biomol/Enzo, Plymouth Meeting, PA, USA) diluted in PBS, followed by Dylight-488-labeled goat-anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA).
Cell surface biotinylation
Cells were incubated for 30 min at 4°C with 1 mg/ml EZ-Link NHS-SS biotin (Pierce, Rockford, IL, USA). Biotinylation was terminated by washing 3 times with ice-cold PBS containing 1% BSA, followed by washing 3 times with PBS alone. Cultures were scraped into 50-ml conical tubes in the presence of quenching solution (Pierce). Culture plates were then rinsed with 4 ml TBS to remove any additional cells, and the combined washes were centrifuged at 500 g for 3 min. The supernatant was discarded, and pellets were washed with 1 ml TBS before recentrifugation. Cells were then lysed with 500 μl of PBS-1% Triton X-100 with protease inhibitors, before being disrupted by sonication for 5 s every 5 min for 30 min at 4°C. Cell lysate was then centrifuged at 10,000 g for 2 min at 4°C, and the clarified cell lysate was transferred to a new tube. Clarified cell lysate (400 μl) was then incubated with Immobilized NeutrAvidin Gel (Pierce) for 60 min at room temperature. The biotin-avidin complexes were then harvested by centrifugation, washed 3 times, and separated by electrophoresis.
Western blot analysis
Cells were lysed with Nonidet P-40 buffer on ice. Cell lysates were centrifuged at 4°C, and supernatants were collected. Cell lysates (25 μg) were loaded, separated on 3–8% Tris acetate minigels, and transferred to PVDF membrane (Invitrogen).
cAMP measurements
After vehicle or agonist addition, medium was aspirated, cells were lysed with 0.1 N HCl and centrifuged, and the supernatants were assayed using an enzyme immunoassay kit (Enzo). Pellets were assayed for protein content using the BCA method (Pierce). In some cases, cells were preincubated with 200 μM papaverine in serum-free DMEM:F12 (Invitrogen) for 15 min and then stimulated with either vehicle, 100 μM ADO, or 10 μM forskolin, as described previously (2).
IL-8 measurements
Age-matched normal and CF HBECs were placed in 12-well tissue culture plates with 1 ml of fresh serosal growth medium immediately prior to the mucosal addition of the ADO analog NECA (10 μM) or forskolin (10 μM). The serosal medium was sampled after 24 h, and IL-8 levels were measured using the OptEIA Human IL-8 ELISA set (BD Biosciences, Franklin Lakes, NJ, USA), as per the manufacturer's protocol.
Measurements of ciliary beat frequency (CBF)
CBF was measured on well differentiated HBECs using phase-contrast microscopy (Nikon TE2000, ×40 air objective) and computerized frequency spectrum analysis. All experiments were performed at room temperature (21°C). Digitized video was collected in 2.1-s segments for analysis using a MegaPlus ES310 turbo video camera (Kodak, Rochester, NY, USA). The temporal limit of the measurement system, given its dependence on the video field rate (60 Hz), is 30 Hz. Beat frequency analysis was performed on digitized video using Sisson-Ammons Video Analysis (SAVA) software (Ammons Engineering, Mt. Morris, MI, USA). Data were deemed acceptable when a single dominant frequency was observed.
Statistical methods
All data are presented as means ± se. Data were inspected by analysis of variance to demonstrate whether the data were derived from a single population and normally distributed. Statistical significance between groups was assessed using paired or unpaired t test as appropriate. If data were not normally distributed, then the Mann-Whitney U test or Wilcoxin matched pairs test was used as appropriate. Values of n refer to the number of subjects or the number of cultures used in each group. For HBECs, a minimum of 4 donors supplied cultures for each experiment. For experiments utilizing BHK and HEK293 cells, all experiments were performed on ≥3 separate occasions.
RESULTS
CFTR and A2BR undergo FRET
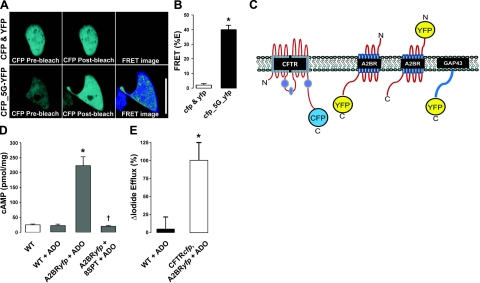
To study CFTR-A2BR interactions, we utilized a FRET-based approach. As a positive control, we linked cyan fluorescent protein (CFP) to yellow fluorescent protein (YFP) by 5 glycines. Under these conditions, we observed ∼40% FRET, which is likely to be close to the maximum amount of FRET that can be observed in our system with these fluorophores (Fig. 1A, B). We then made a series of FRET constructs to probe CFTR-A2BR interactions (Fig. 1C). To ensure that these constructs were functional, they were expressed in BHK cells for ∼24 h, and CFTRcfp/A2BRyfp function was assayed using I− efflux and ELISA, respectively (Fig. 1D, E). In both cases, transfected cells, but not nontransfected controls, responded to agonists with an increase in cAMP formation and I− efflux, respectively.
Figure 1.
FRET constructs used in this study and verification of their function. A) Images of unlinked cytoplasmic CFP and YFP (top panels) and CFP linked to YFP by 5 glycines (CFP_5G_YFP; bottom panels) before and after acceptor (YFP) photobleaching. Scale bar = 25 μm. B) Mean percentage FRET taken from A. C) Cartoon of CFP/YFP FRET constructs. The following constructs were intracellularly labeled on their C terminus: CFTRcfp, A2BRyfp, and GAP43yfp. An additional control construct was labeled extracellularly on its N terminus (yfpA2BR). D) Mean changes in the intracellular cAMP concentration measured by ELISA in BHK cells ± A2BRyfp under basal conditions (open bar) and following stimulation with 10 μM ADO ± 10 μM 8-SPT (shaded bars). All n = 6. E) Percentage change in I− efflux from BHK cells following stimulation with 10 μM ADO. Nontransfected WT control cells (solid bars, n=3) vs. CFTRcfp and A2BRyfp-transfected cells (open bars, n=5). *P < 0.05 vs. control. †P < 0.05 vs. ADO.
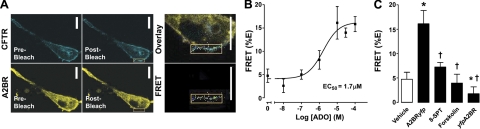
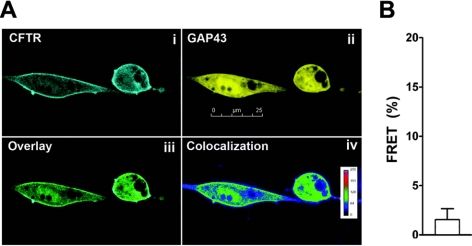
Coexpression of CFTRcfp and A2BRyfp for 24–30 h resulted in ∼5% FRET, a value significantly different from 0 (Fig. 2A, B). Surprisingly, the amount of FRET between these constructs increased in a dose-dependent manner to ∼18% as ADO was added, with an EC50 of 1.7 μM (Fig. 2B). This additional increase in FRET occurred over a relatively short time-frame (i.e., 5 min). These results were not cell-type specific, and a similar ADO-dependent FRET dose-response was observed when these constructs were expressed in HEK293 cells (EC50 0.7 μM; n=6). Surprisingly, forskolin exposure did not increase CFTR-A2BR FRET (Fig. 2C). However, the ADO-induced increase in FRET was inhibited by the ADO receptor antagonist 8-SPT (Fig. 2C). As a negative control, we N-terminally labeled A2BR with YFP (yfpA2BR; see Fig. 1C) and did not detect FRET between this construct and CFTRcfp (Fig. 2C). As an additional control, we measured FRET between GAP43yfp (29), a protein which binds to intracellular membranes, and CFTRcfp. Despite colocalization between CFTRcfp and GAP43yfp, we did not detect any significant FRET between these constructs (Fig. 3).
Figure 2.
FRET analysis reveals that the A2BR-CFTR interaction is dynamic and ADO dependent. BHK cells were transfected with the relevant FRET pairs and exposed to vehicle vs. agonist (ADO). A) Typical images of CFTRcfp and A2BRyfp before and after donor (YFP) photobleaching. Note the increase in fluorescence of CFTRcfp in the region where A2BRyfp was photobleached. B) Mean data showing the dose-dependent increase in FRET between CFTRcfp and A2BRyfp; n = 9–12. C) Mean FRET data after vehicle, with 10 μM ADO when YFP was attached to the C terminus of A2BR (A2Byfp), in the presence of ADO and 10 μM 8-SPT, with 10 μM forskolin and with ADO when YFP was attached to the N terminus of A2BR (yfpA2B). Open bar denotes vehicle. Solid bars denotes FRET in the presence of agonist (ADO unless noted) of agonist and 8-SPT. All n ≥ 9. Scale bars = 10 μm. *P < 0.05 vs. vehicle. †P < 0.05 vs. ADO.
Figure 3.
CFTR and GAP43 colocalize but do not undergo FRET. A) Images of CFTRcfp (i) and GAP43yfp (ii) coexpressed in BHK cells, along with an overlay of these two images (iii) and a graphical representation of the degree of colocalization (iv), obtained using Image J. B) Mean acceptor photobleaching percentage FRET between CFTRcfp and GAP43yfp expressed in BHK cells. Data did not differ significantly from 0; n = 6. Scale bar = 25 μm.
CFTR enhances A2BR plasma membrane levels leading to increased cAMP production
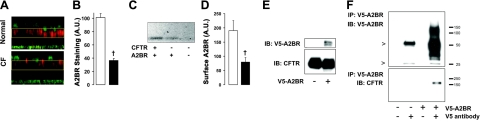
Because we could detect significant FRET between CFTR and the A2BR, we then turned our attention to the A2BR itself and found reduced numbers of this receptor in the apical membrane of both CF HBECs (Fig. 4A, B) and BHK cells not expressing CFTR (Fig. 4C, D). Because CFTR undergoes FRET with A2BR and influences the amount of A2BR in the plasma membrane, we then tested whether these two proteins existed as members of a macromolecular complex. We immunoprecipitated V5-tagged A2BR and probed for CFTR binding using an antibody directed to nuclear binding fold 1 (Fig. 4E and ref. 30). CFTR was found in the immunoprecipitate of A2BR, indicating that these two molecules can associate (Fig. 4F).
Figure 4.
CFTR interacts with the A2BR and increases A2BR surface levels. A) Confocal micrographs showing increased A2BR levels (red) in normal HBECs. Wheat germ agglutinin (green) was used to stain cilia. Yellow boxes approximately denote the region of interest used to quantify A2BR staining. B) Mean fluorescence taken from A. Open bars, normal (n=6); solid bars, CF (n=6). C) Representative Western blot of biotinylated V5-A2BR expressed in BHK and BHKCFTR cells. D) Mean cell surface V5-A2BR levels taken from C. Open bars denote normal (n=6); solid bars denote CF (n=6). E) Immunoblot (IB) analysis of A2BR expression (top panel) and CFTR expression (bottom panel) in BHK cells. Protein loaded represents 10% of input into the immunoprecipitation (IP). F) Top panel: immunoblot showing immunoprecipitation of A2BR-V5 using an antibody for the V5 epitope. Arrowheads denote heavy and light chain of IgG. Bottom panel: CFTR coimmunoprecipitated with A2BR. †P < 0.05 vs. ±CFTR.
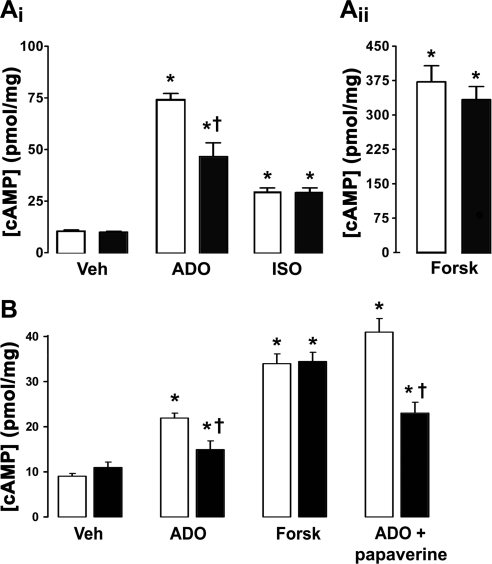
Because CFTR interacted with A2BR and influenced the amount of A2BR in the plasma membrane, we compared cAMP levels in normal and CF HBECs to determine whether there were functional consequences of this interaction. In response to ADO exposure, normal HBECs produced significantly more cAMP than CF HBECs homogenously expressing ΔF508 CFTR (Fig. 5Ai). However, no significant difference in cAMP production was observed between genotypes following stimulation of the β2 adrenergic receptor with isoproterenol (ISO; Fig. 5Ai) or following direct stimulation of adenylate cyclase with forskolin (Fig. 5Aii). To confirm that this phenomenon was due to changes in CFTR expression, we measured ADO-induced cAMP production in BHK cells ± CFTR (31). These cells failed to respond to ADO unless transfected with the A2BR (Fig. 5B). However, as with HBECs, ADO elicited a significantly larger cAMP response when CFTR was expressed (Fig. 5B). Again, the forskolin-induced cAMP response was CFTR independent (Fig. 5B). To determine whether the increase in cAMP levels was due to an increase in cAMP production vs. a decrease in cAMP degradation, we preincubated BHK cells with the phosphodiesterase inhibitor papaverine. Under these conditions, ADO-stimulated cAMP production was still greater in the presence of CFTR, suggesting that cAMP production was predominantly affected (Fig. 5B).
Figure 5.
Adenosine-stimulated cAMP production is CFTR dependent. A) Intracellular cAMP levels in normal and CF HBECs following addition of 100 μM ADO and 10 μM ISO (i) or 10 μM forskolin (ii). Open bars denote normal; solid bars denote CF. All n = 8. B) cAMP levels in BHK cells stably transfected with CFTR (BHKCFTR; open bars) and wild-type BHK cells lacking CFTR (solid bars), both transfected with A2BR and exposed to vehicle (Veh), 100 μM ADO ± 100 μM papaverine, or 10 μM forskolin. All n ≥ 7. *P < 0.05 vs. vehicle. †P < 0.05 vs. ±CFTR.
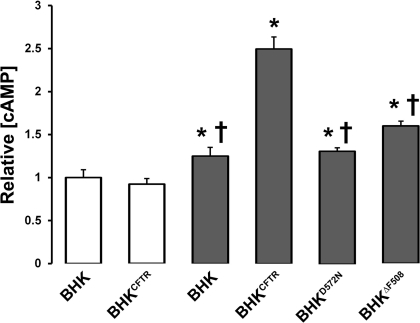
To better understand how CFTR influences A2BR-stimulated cAMP production, we compared the effects of functional wild-type CFTR on cAMP production vs. no CFTR, CFTR mutants that never leave the endoplasmic reticulum (D572N; ref. 32), or CFTR mutants that are misprocessed and dysfunctional (ΔF508; ref. 33). ADO-stimulated cAMP production was reduced with D572N CFTR to similar level as to CFTR-null cells (Fig. 6). However, there was a small but significant elevation in cAMP production in ΔF508 CFTR-expressing cells above baseline (Fig. 6).
Figure 6.
Wild-type CFTR regulates ADO-stimulated cAMP production. cAMP production was measured by ELISA in BHK cells and those stably transfected with either CFTR (BHKCFTR) or mutant CFTRs (D572N and ΔF508). Open bars denote vehicle; shaded bars denote 100 μM ADO. All n ≥ 12. *P < 0.05 vs. vehicle. †P < 0.05 vs. nonmutant CFTR with ADO.
ADO-stimulated ciliary beating and IL-8 secretion are reduced in CF bronchial epithelia
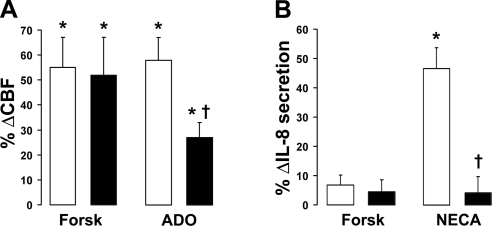
Because of a lack of CFTR, ADO-stimulated Cl− ASL secretion is absent in CF airway epithelia (1), but other components of innate immunity regulated by ADO signaling are thought to be preserved. However, despite exhibiting identical baseline CBF, the ability of ΔF508 CF HBECs to increase CBF in response to ADO was significantly diminished compared to normal controls (Fig. 7A). In contrast, CBF increased normally in response to forskolin exposure, indicating that functions downstream of A2BR are also impaired in CF airway epithelia. Because ADO and its analog NECA also stimulate the secretion of several cytokines (1), we measured IL-8 secretion as an additional marker of altered A2BR function. Baseline IL-8 secretion was moderately elevated in CF airways. However, A2BR- but not forskolin-stimulated IL-8 secretion was significantly diminished in CF HBECs compared to normal controls (Fig. 7B).
Figure 7.
ADO- but not forskolin-stimulated ciliary beating and IL-8 production are reduced in the absence of CFTR. Open bars denote normal; solid bars denote CF. A) CBF, measured by high-speed video microscopy in HBECs following either 100 μM ADO or 10 μM forskolin applied mucosally. Normal, n = 10; CF, n = 9. Baseline CBF was 5.2 ± 0.3 and 5.4 ± 0.3 Hz for normal and CF HBECs, respectively. B) The ADO analog NECA (10 μM) vs. forskolin (10 μM) was added mucosally to normal (n=11) and CF (n=13) HBECs for 24 h, and IL-8 secretion into the serosal media was measured by ELISA. Baseline IL-8 production was 3329 ± 227 and 5359 ± 716 for normal (n=11) and CF (n=12) HBECs, respectively (P<0.05). *P < 0.05 vs. vehicle. †P < 0.05 vs. ±CFTR.
DISCUSSION
CFTR and A2BR dynamically associate
To probe CFTR-A2BR interactions in live cells, we utilized a FRET pair consisting of cfp-tagged CFTR and yfp-tagged A2BR (Fig. 1). We observed significant FRET between these constructs under basal conditions (∼5%; Fig. 2) and could also coimmunoprecipitate these proteins (Fig. 4E, F). CFTR has previously been shown both to coimmunoprecipitate and to undergo a similar amount of FRET (∼7%) with ENaC, which may contribute to CFTR's regulation of ENaC (23). Together, our data suggest that we have observed a significant baseline level of interaction between CFTR and A2BR that is comparable to the interaction seen with ENaC. In contrast, CFTR does not undergo FRET with either the Ca2+-activated Cl− channel (Ano1; ref. 28) or GAP43 (Fig. 3), indicating that CFTR is somewhat selective in its interactions. As a control, we extracellularly labeled A2BR (Figs. 1 and 2) and failed to detect FRET between this construct and CFTR, which verifies our FRET assay. Surprisingly, the amount of FRET between CFTR and A2BR increased on exposure to ADO with an EC50 (1.7 μM; Fig. 2B) that was almost identical to the EC50 for native ADO-stimulated Cl− secretion in HBECs (1.6 μM) (2).
Further investigation revealed that the expression of wild-type CFTR caused an increase in A2BR plasma membrane densities in both HBECs and BHK cells (Fig. 4A–D). We speculate that this effect was caused by a physical interaction between the two molecules, although whether this is direct or indirect remains to be determined (Fig. 4E, F). Certainly, FRET places the fluorophores on these two molecules in proximity. However, the coimmunoprecipitation experiments do not reveal the mode of association, and they may interact via common scaffold proteins, or in a protein matrix. Forskolin-stimulated cAMP production has previously been shown to directly increase both A2BR and CFTR trafficking to the plasma membrane (34, 35). However, despite raising cAMP, forskolin exposure failed to increase CFTR/A2BR FRET (Fig. 2C), suggesting that the ADO-dependent increase in FRET was not due to an increase in the number of either CFTR or A2BR at the plasma membrane. A2BR has also been shown to activate phospholipase A2 to produce arachidonic acid (36). Since an increase in cAMP levels does not appear to be the trigger for the increase in FRET, it is possible that noncanonical signaling via arachidonic acid may serve as the signal to reorder CFTR and A2BR in the plasma membrane. Conformational changes in the α2A adrenergic receptor have previously been detected on ligand binding by measuring FRET between a YFP inserted into the third cytoplasmic loop and CFP fused to the C terminus (37). Thus, we cannot exclude the possibility that the agonist-dependent increase in A2BR-CFTR FRET may have reflected a conformational change in the A2BR on ADO binding, which moved its C termini closer to CFTR.
ADO-stimulated cAMP production
CF HBECs displayed a diminished capacity to respond to ADO compared to normal HBECs (Fig. 5A). We have previously shown that A2BR is the only ADO receptor expressed in our HBEC system (2), making it highly likely that these effects were due to the A2BR receptor. CFTR-dependent cAMP production could be reprised in BHK cells expressing A2BR, suggesting that the use of these cells to better understand this process was valid (Figs. 5 and 6). Since cAMP production was also decreased in the presence of the phosphordiesterase inhibitor papaverine (Fig. 5), the decrease in ADO-dependent cAMP production was unlikely to be due to increased cAMP degradation and rather, was due to increased cAMP formation. Expression of D572N CFTR, which does not escape the endoplasmic reticulum (32), did not potentiate ADO-stimulated cAMP production, suggesting that CFTR must be in the plasma membrane to regulate A2BR function (Fig. 6). Expression of ΔF508 CFTR, which traffics extremely poorly to the plasma membrane (33), had a small but significant increase in ADO-stimulated cAMP production, i.e., greater than CFTR-null cells, but much below cAMP levels in wild-type CFTR-expressing cells (Fig. 6).
Further investigation revealed that expression of wild-type CFTR caused an increase in A2BR plasma membrane densities in both HBECs and BHK cells (Fig. 4A–D). This was likely caused by an association between the two molecules (Fig. 4E, F). CFTR interacts with ENaC through NHERF1 via postsynaptic density, Disc large, and ZO-1 protein (PDZ)-binding mechanisms (38, 39). The A2BR interacts with NHERF2 through a similar PDZ mechanism, which may serve to anchor A2BR in a signaling complex in the plasma membrane (26). However, appending fluorescent proteins to the C terminus of CFTR has previously been shown to affect CFTR surface mobility and to reduce PDZ binding, since the PDZ-binding motifs are close to the C termini (40, 41). Thus, it is unlikely that the CFTR-A2BR interaction that we observed by FRET (Fig. 2) is mediated by PDZ-type binding. However, CFTR has also been shown to interact with other proteins, such as filamin via its N terminus (42), and we speculate that this type of binding may be involved in facilitating CFTR-A2BR interactions.
A2BR and cytokine secretion
ADO-stimulated, but not forskolin- or isoproterenol- stimulated, cAMP production was reduced in the absence of CFTR (Figs. 5 and 6), which likely accounted for the decrease in CBF and IL-8 secretion in CF compared to normal HBECs (Fig. 7). These data indicate that the most distal components of the ADO signaling cascade are also impaired by a lack of cAMP production in CF airway epithelia and suggest that specific components of innate lung defense may also be compromised in CF-affected epithelia, although whether or not depression of the A2BR signaling cascade has a significant effect on CF lung pathogenesis in vivo remains to be determined. We used IL-8 as a marker to test whether cytokine production was altered in a similar fashion as CBF. However, other ADO-stimulated cytokines may also be affected by CFTR-dependent ADO signaling and remain to be tested. For example, IL-6 secretion is stimulated by A2BR activation in a similar fashion to IL-8 (43), and we would also predict CFTR expression to potentiate IL-6 secretion.
CFTR-mediated ADO/A2BR enhancement may provide a mechanism to potentiate local cAMP production whenever these two proteins are coexpressed, allowing for a richly complex array of responses to physiological agonists. Cytokines such as IL-1β increase CFTR expression in airway epithelia (44), and we speculate that an IL-1β increase in CFTR levels may cause a concomitant increase in the number of receptor molecules (i.e., the A2BR) in the plasma membrane. This mechanism may permit fine tuning and an increased capacity of ADO-dependent activities, including Cl− and fluid secretion, as well as increased CBF and IL-8 when required, without the need to drastically alter global cAMP signaling.
CONCLUSIONS
How the absence of CFTR contributes to CF lung disease is controversial, and several mechanisms detailing how CFTR may contribute to chronic airway infections have been proposed, including ASL dehydration/mucus stasis due to abnormal ion transport, CFTR-dependent changes in epithelial inflammatory status, altered ASL pH, and altered bacterial binding (1, 3, 18–20). The effect of reduced ADO-dependent cAMP signaling on functions other than Cl− secretion has not previously been considered in CF-affected epithelia. Such processes, including regulation of CBF and cytokine secretion, are important components of innate immunity, and their lessened function, as predicted by our results, could, in part, account for the increased susceptibility to bacterial colonization seen in CF airways. Thus, we predict that CF airways may exhibit lowered innate defense due to defective ADO/cAMP signaling, not only from a lack of Cl− secretion, but also from an impaired ability to raise ciliary beating and secrete cytokines, which may limit their ability to respond to and resolve airway infections.
Acknowledgments
The authors thank Dr. Claire Brown (McGill University, Montreal, QC, Canada) for the GAP43 construct and Drs. Tamas Hegudus, Martina Gentzsch and Jack Riordan [University of North Carolina (UNC)-Chapel Hill] for providing the CFTRcfp construct and the BHK cell lines. The authors also thank Drs. Eduardo Lazarowski and Jack Stutts for critical reading of this manuscript. The help of the UNC Immunotechnology Core and the UNC CF Center Molecular and Tissue Culture Cores is gratefully acknowledged.
This study was supported by U.S. National Institutes of Health grants P50HL084934, R01HL74158–3, and P30DK34987, by British American Tobacco, and by the Cystic Fibrosis Foundation.
REFERENCES
- 1. Com G., Clancy J. P. (2009) Adenosine receptors, cystic fibrosis, and airway hydration. Handb. Exp. Pharmacol. 363–381 [DOI] [PubMed] [Google Scholar]
- 2. Rollins B. M., Burn M., Coakley R. D., Chambers L. A., Hirsh A. J., Clunes M. T., Lethem M. I., Donaldson S. H., Tarran R. (2008) A2B adenosine receptors regulate the mucus clearance component of the lung's innate defense system. Am. J. Respir. Cell Mol. Biol. 39, 190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tarran R., Button B., Boucher R. C. (2006) Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu. Rev. Physiol. 68, 543–561 [DOI] [PubMed] [Google Scholar]
- 4. Bucheimer R. E., Linden J. (2004) Purinergic regulation of epithelial transport. J. Physiol. 555, 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burnstock G. (2006) Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 58, 58–86 [DOI] [PubMed] [Google Scholar]
- 6. Brunton L. L. (2003) PDE4: arrested at the border. Sci. STKE. 2003, PE44. [DOI] [PubMed] [Google Scholar]
- 7. Fredholm B. B., Arslan G., Halldner L., Kull B., Schulte G., Wasserman W. (2000) Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch. Pharmacol. 362, 364–374 [DOI] [PubMed] [Google Scholar]
- 8. McConnachie G., Langeberg L. K., Scott J. D. (2006) AKAP signaling complexes: getting to the heart of the matter. Trends Mol. Med. 12, 317–323 [DOI] [PubMed] [Google Scholar]
- 9. Cooper D. M., Crossthwaite A. J. (2006) Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol. Sci. 27, 426–431 [DOI] [PubMed] [Google Scholar]
- 10. Li C., Krishnamurthy P. C., Penmatsa H., Marrs K. L., Wang X. Q., Zaccolo M., Jalink K., Li M., Nelson D. J., Schuetz J. D., Naren A. P. (2007) Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell 131, 940–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson M. P., Sheppard D. N., Berger H. A., Welsh M. J. (1992) Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. Am. J. Physiol. 263, L1–L14 [DOI] [PubMed] [Google Scholar]
- 12. Antonioli L., Fornai M., Colucci R., Ghisu N., Tuccori M., Del Tacca M., Blandizzi C. (2008) Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol. Ther. 120, 233–253 [DOI] [PubMed] [Google Scholar]
- 13. Harris A., Argent B. E. (1993) The cystic fibrosis gene and its product CFTR. Semin. Cell Biol. 4, 37–44 [DOI] [PubMed] [Google Scholar]
- 14. Skach W. R. (2000) Defects in processing and trafficking of the cystic fibrosis transmembrane conductance regulator. Kidney Int. 57, 825–831 [DOI] [PubMed] [Google Scholar]
- 15. Dalemans W., Barbry P., Champigny G., Jallat S., Dott K., Dreyer D., Crystal R. G., Pavirani A., Lecocq J. P., Lazdunski M. (1991) Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature 354, 526–528 [DOI] [PubMed] [Google Scholar]
- 16. Cholon D. M., O'Neal W. K., Randell S. H., Riordan J. R., Gentzsch M. Modulation of endocytic trafficking and apical stability of CFTR in primary human airway epithelial cultures. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L304–L314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chmiel J. F., Davis P. B. (2003) State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir. Res. 4, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Campodonico V. L., Gadjeva M., Paradis-Bleau C., Uluer A., Pier G. B. (2008) Airway epithelial control of Pseudomonas aeruginosa infection in cystic fibrosis. Trends Mol. Med. 14, 120–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nichols D., Chmiel J., Berger M. (2008) Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin. Rev. Allergy Immunol. 34, 146–162 [DOI] [PubMed] [Google Scholar]
- 20. Quinton P. M. (2008) Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372, 415–417 [DOI] [PubMed] [Google Scholar]
- 21. Naren A. P., Cobb B., Li C., Roy K., Nelson D., Heda G. D., Liao J., Kirk K. L., Sorscher E. J., Hanrahan J., Clancy J. P. (2003) A macromolecular complex of beta 2 adrenergic receptor, CFTR, and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc. Natl. Acad. Sci. U. S. A. 100, 342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kunzelmann K., Schreiber R., Nitschke R., Mall M. (2000) Control of epithelial Na+ conductance by the cystic fibrosis transmembrane conductance regulator. Pflügers Arch. 440, 193–201 [DOI] [PubMed] [Google Scholar]
- 23. Berdiev B. K., Cormet-Boyaka E., Tousson A., Qadri Y. J., Oosterveld-Hut H. M., Hong J. S., Gonzales P. A., Fuller C. M., Sorscher E. J., Lukacs G. L., Benos D. J. (2007) Molecular proximity of cystic fibrosis transmembrane conductance regulator and epithelial sodium channel assessed by fluorescence resonance energy transfer. J. Biol. Chem. 282, 36481–36488 [DOI] [PubMed] [Google Scholar]
- 24. Ho K. (1998) The ROMK-cystic fibrosis transmembrane conductance regulator connection: new insights into the relationship between ROMK and cystic fibrosis transmembrane conductance regulator channels. Curr. Opin. Nephrol. Hypertens. 7, 49–58 [DOI] [PubMed] [Google Scholar]
- 25. Penmatsa H., Zhang W., Yarlagadda S., Li C., Conoley V. G., Yue J., Bahouth S. W., Buddington R. K., Zhang G., Nelson D. J., Sonecha M. D., Manganiello V., Wine J. J., Naren A. P. (2010) Compartmentalized cyclic adenosine 3′,5′-monophosphate at the plasma membrane clusters PDE3A and cystic fibrosis transmembrane conductance regulator into microdomains. Mol. Biol. Cell. 21, 1097–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sitaraman S. V., Wang L., Wong M., Bruewer M., Hobert M., Yun C. H., Merlin D., Madara J. L. (2002) The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J. Biol. Chem. 277, 33188–33195 [DOI] [PubMed] [Google Scholar]
- 27. Sitaraman S. V., Si-Tahar M., Merlin D., Strohmeier G. R., Madara J. L. (2000) Polarity of A2b adenosine receptor expression determines characteristics of receptor desensitization. Am. J. Physiol. Cell Physiol. 278, C1230–C1236 [DOI] [PubMed] [Google Scholar]
- 28. Sheridan J. T., Worthington E. N., Yu K., Gabriel S. E., Hartzell H. C., Tarran R. (2011) Characterization of the oligomeric structure of the Ca2+-activated Cl- channel Ano1/TMEM16A. J. Biol. Chem. 286, 1381–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Y., Fisher D. A., Storm D. R. (1993) Analysis of the palmitoylation and membrane targeting domain of neuromodulin (GAP-43) by site-specific mutagenesis. Biochemistry 32, 10714–10719 [DOI] [PubMed] [Google Scholar]
- 30. Cui L., Aleksandrov L., Chang X. B., Hou Y. X., He L., Hegedus T., Gentzsch M., Aleksandrov A., Balch W. E., Riordan J. R. (2007) Domain interdependence in the biosynthetic assembly of CFTR. J. Mol. Biol. 365, 981–994 [DOI] [PubMed] [Google Scholar]
- 31. Gentzsch M., Chang X. B., Cui L., Wu Y., Ozols V. V., Choudhury A., Pagano R. E., Riordan J. R. (2004) Endocytic trafficking routes of wild type and DeltaF508 cystic fibrosis transmembrane conductance regulator. Mol. Biol. Cell 15, 2684–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gentzsch M., Choudhury A., Chang X. B., Pagano R. E., Riordan J. R. (2007) Misassembled mutant DeltaF508 CFTR in the distal secretory pathway alters cellular lipid trafficking. J. Cell Sci. 120, 447–455 [DOI] [PubMed] [Google Scholar]
- 33. Turnbull E. L., Rosser M. F., Cyr D. M. (2007) The role of the UPS in cystic fibrosis. BMC Biochem. 8(Suppl. 1), S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bradbury N. A., Jilling T., Berta G., Sorscher E. J., Bridges R. J., Kirk K. L. (1992) Regulation of plasma membrane recycling by CFTR. Science 256, 530–532 [DOI] [PubMed] [Google Scholar]
- 35. Wang L., Kolachala V., Walia B., Balasubramanian S., Hall R. A., Merlin D., Sitaraman S. V. (2004) Agonist-induced polarized trafficking and surface expression of the adenosine 2b receptor in intestinal epithelial cells: role of SNARE proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1100–G1107 [DOI] [PubMed] [Google Scholar]
- 36. Cobb B. R., Ruiz F., King C. M., Fortenberry J., Greer H., Kovacs T., Sorscher E. J., Clancy J. P. (2002) A2 adenosine receptors regulate CFTR through PKA and PLA2. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L12–L25 [DOI] [PubMed] [Google Scholar]
- 37. Vilardaga J. P., Bunemann M., Krasel C., Castro M., Lohse M. J. (2003) Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat. Biotechnol. 21, 807–812 [DOI] [PubMed] [Google Scholar]
- 38. Guggino W. B. (2004) The cystic fibrosis transmembrane regulator forms macromolecular complexes with PDZ domain scaffold proteins. Proc. Am. Thorac. Soc. 1, 28–32 [DOI] [PubMed] [Google Scholar]
- 39. Li C., Naren A. P. (2005) Macromolecular complexes of cystic fibrosis transmembrane conductance regulator and its interacting partners. Pharmacol. Ther. 108, 208–223 [DOI] [PubMed] [Google Scholar]
- 40. Haggie P. M., Kim J. K., Lukacs G. L., Verkman A. S. (2006) Tracking of quantum dot-labeled CFTR shows near immobilization by C-terminal PDZ interactions. Mol. Biol. Cell 17, 4937–4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haggie P. M., Stanton B. A., Verkman A. S. (2004) Increased diffusional mobility of CFTR at the plasma membrane after deletion of its C-terminal PDZ binding motif. J. Biol. Chem. 279, 5494–5500 [DOI] [PubMed] [Google Scholar]
- 42. Thelin W. R., Chen Y., Gentzsch M., Kreda S. M., Sallee J. L., Scarlett C. O., Borchers C. H., Jacobson K., Stutts M. J., Milgram S. L. (2007) Direct interaction with filamins modulates the stability and plasma membrane expression of CFTR. J. Clin. Invest. 117, 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun Y., Wu F., Sun F., Huang P. (2008) Adenosine promotes IL-6 release in airway epithelia. J. Immunol. 180, 4173–4181 [DOI] [PubMed] [Google Scholar]
- 44. Gray T., Coakley R., Hirsh A., Thornton D., Kirkham S., Koo J. S., Burch L., Boucher R., Nettesheim P. (2004) Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1β in human bronchial epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L320–L330 [DOI] [PubMed] [Google Scholar]