Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) has neuroprotective and neurotrophic properties and is a potent α-secretase activator. As PACAP peptides and their specific receptor PAC1 are localized in central nervous system areas affected by Alzheimer's disease (AD), this study aims to examine the role of the natural peptide PACAP as a valuable approach in AD therapy. We investigated the effect of PACAP in the brain of an AD transgenic mouse model. The long-term intranasal daily PACAP application stimulated the nonamyloidogenic processing of amyloid precursor protein (APP) and increased expression of the brain-derived neurotrophic factor and of the antiapoptotic Bcl-2 protein. In addition, it caused a strong reduction of the amyloid β-peptide (Aβ) transporter receptor for advanced glycation end products (RAGE) mRNA level. PACAP, by activation of the somatostatin-neprilysin cascade, also enhanced expression of the Aβ-degrading enzyme neprilysin in the mouse brain. Furthermore, daily PAC1-receptor activation via PACAP resulted in an increased mRNA level of both the PAC1 receptor and its ligand PACAP. Our behavioral studies showed that long-term PACAP treatment of APP[V717I]-transgenic mice improved cognitive function in animals. Thus, nasal application of PACAP was effective, and our results indicate that PACAP could be of therapeutic value in treating AD.—Rat, D., Schmitt, U., Tippmann, F., Dewachter, I., Theunis, C., Wieczerzak, E, Postina, R., van Leuven, F., Fahrenholz, F., Kojro, E. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer's disease-like pathology in amyloid precursor protein-transgenic mice.
Keywords: PAC1 receptor, G-protein-coupled receptor, neuroprotection, nasal delivery
The accumulation of the amyloid β peptide (Aβ) in the brain is a central process leading to the development of Alzheimer′s disease (AD) (1). The amyloid precursor protein (APP), the source of Aβ, can be proteolytically processed by two competing pathways: the amyloidogenic (β-secretase-mediated) amyloid-β peptides generating and the nonamyloidogenic (α-secretase-mediated) pathway. Proteolytic cleavage of APP by α-secretase within the amyloid-β peptide sequence precludes formation of neurotoxic Aβ peptides and leads to the release of soluble N-terminal APP fragments (sAPPα) with neurotrophic and neuroprotective properties (2, 3). A decreased amount of sAPPα was observed in the cerebrospinal fluid (CSF) of patients with familial and sporadic AD (4, 5), and in addition, lowered levels of CSF sAPPα have been correlated with poor memory performance in patients with AD (6, 7). Indeed, endogenous sAPPα enhances hippocampal N-methyl-d-aspartate receptor function, long-term potentiation (LTP), and spatial memory in adult rats (8), and exogenous sAPPα also facilitates LTP in hippocampal slices of adult rats (9). In addition, a neuronal overexpression of the α-secretase ADAM10 in AD transgenic mice increased the secretion of sAPPα, reduced the amounts of Aβ peptides, and improved cognition (10). ADAM10 has been demonstrated to be a physiologically relevant α-secretase in neurons (11). Recently, several groups (12–14) have shown that retinoid signaling increases the nonamyloidogenic APP processing pathway via up-regulation of α-secretase ADAM10 expression.
These observations suggest that pharmacological α-secretase stimulation may be a useful strategy against AD. Several G-protein-coupled receptors (GPCRs) are known to be involved in the activation of the α-secretase-mediated pathway of APP processing (15, 16). We found that pituitary adenylate cyclase-activating polypeptide (PACAP) is a potent α-secretase activator (17). Stimulation of α-secretase is mediated by the G-protein-coupled PAC1 receptor, which is expressed in the cortex and hippocampus. It is well documented that PACAPs have neurotrophic as well as antiapoptotic properties and are involved in learning and memory processes (18, 19). PACAP actions are mediated by 3 receptor subtypes: the PACAP-selective receptor PAC1, and VPAC1 and VPAC2, which are equally sensitive to both PACAP and vasoactive intestinal peptide (VIP). All 3 receptors belong to the GPCR family and are positively coupled to the adenylyl cyclase. The PAC1 receptor, which is predominantly expressed in the central nervous system (CNS) (20), also stimulates phospholipase C and extracellular regulated kinase (ERK) pathways (19).
A down-regulation of PACAP in several AD transgenic mouse models and in the human AD temporal cortex was demonstrated by comparative analysis of cortical gene expression (21). As PACAP exerts neuroprotective and neurotrophic effects and modulates neuronal gene expression (19), the application of the natural neuropeptide PACAP may restore normal PACAP/PAC1-receptor function in the brain and therefore might be of therapeutic value for AD treatment. The therapeutic application of PACAP is mainly limited by its enzymatic degradation in blood; therefore, we chose the intranasal application in our study. Intranasal administration is a potential route for drug delivery to the brain that bypasses the blood-brain barrier (BBB) (22) and has been demonstrated to effectively deliver drugs to the human brain without inducing systemic side effects (23). The intranasal administration of insulin improves memory in patients having early AD by raising the insulin level in the CNS without affecting the plasma insulin level (24).
To test the hypothesis that activation of PACAP/PAC1 signaling provides a physiological defense mechanism against Aβ neurotoxicity in vivo, we investigated the effect of PACAP in the brain of the APP[V717I] AD transgenic mouse model. PACAP treatment resulted in enhancement of the nonamyloidogenic pathway of APP processing and in an improvement of the cognitive function. We also found increased expression of several genes and proteins responsible for neuroprotection in AD and down-regulation of neurotoxic factors.
MATERIALS AND METHODS
Materials
We used the following primary antibodies: mouse IgG 6E10 detecting sAPPα; polyclonal rabbit antibody SIG-39138 detecting sAPPβ (Covance, Princeton, NJ, USA); 6687 (kindly provided by C. Haass, Ludwig Maximilians University Munich, Munich, Germany) as an antibody against the C terminus of human APP; anti-neprilysin (56C6) and anti-Bcl-2 (C-2; Santa Cruz Biotechnology, Heidelberg, Germany); and anti-pro-brain-derived neurotrophic factor (BDNF; Sigma-Aldrich, Taufkirchen, Germany). Secondary anti-mouse and anti-rabbit antibodies 35S-labeled were from GE Healthcare Life Sciences (Solingen, Germany), and peroxidase-coupled antibodies and ECL detection reagent were from Thermo Fisher Scientific (Walldorf, Germany). Chitosan glutamate (Protasan UP G213) was obtained from NovaMatrix FMC BioPolymer (Sandvika, Norway). [125I] PACAP27 was from Perkin-Elmer Life Sciences (Wellesley, MA, USA). PACAP38 peptide was synthesized by a solid-phase method using fluorenylmethoxycarbonyl chemistry, purified by HPLC to homogeneity, and identified by amino acid analysis and mass spectrometry. BDNF EmaxImmunoAssay was from Promega (Mannheim, Germany).
Intranasal PACAP administration procedure
The generation and characterization of transgenic APP[V717I] mice, strain background FVB/N, has been described elsewhere (25). In the first study, the intranasal administration was performed according to the procedure described by Alcalay et al. (26). In short, the peptide was dissolved in a water solution, administration solution 1; each milliliter contained the following: 7.5 mg of NaCl, 1.7 mg of citric acid monohydrate, 3 mg of disodium phosphate dehydrate, and 0.2 mg of benzalkonium chloride solution (50%). The solution of PACAP38 (1 μg/μl) was administered intranasally to male APP[V717I] mice (1 mo old; n=8) 5 d/wk for 3 mo. The mice were treated with 10 μg/d (2.19 nmol/d) PACAP38, 10 μl for each mouse (5 μl/nostril); 5 μl/nostril of the inert carrier was given to the control group (n=8).
In the second study, 3-mo-old male APP[V717I] mice (15 mice/group) were also treated for 3 mo. The PACAP38 solution, administration solution 2 (1 μg/μl in 0.5% chitosan glutamate and 0.5% NaCl in water; pH 4), was prepared as described previously (27). The mice were treated with 10 μg PACAP38 daily, and the control group was treated with administration solution 2 not containing peptide, as described above.
Evaluation of PACAP incorporation into the brain after intranasal administration
For detecting PACAP in the brain, 10 μg PACAP38 and ∼7 × 105 cpm of [125I]PACAP27 in administration solution 1 or 2 were applied intranasally to 3-mo-old mice. At designated time points (5, 10, 15, 30, and 60 min), mice were killed, and the amount of labeled PACAP in the brain tissue was determined. For each time point, 3 mice treated with PACAP in administration solution 1 or 2 were examined. The determination of the intact PACAP in the brain was performed as described previously (28). In short, mouse brains were homogenized and centrifuged, and supernatants were analyzed by using HPLC fractionation. Samples were monitored for radioactivity in a γ counter.
Mouse brain sample preparation
Mice were killed, and their brains were cut in half sagittally. Half of the brain was frozen on dry ice and then stored at −20°C for biochemical analysis; the other half was preserved in RNAlater (Qiagen, Hilden, Germany) and stored at −80°C for gene expression analysis. For biochemical analysis, brain hemispheres of 4- or 6-mo-old mice were homogenized in ice-cold buffer (20 mM Tris-HCl, pH 8.5) supplemented with a complete mixture of proteinase inhibitors (Roche Diagnostic Corp., Mannheim, Germany) and centrifuged at 100,000 g for 1 h at 4°C. The supernatant fraction was used for quantification of soluble sAPPα, sAPPβ, Aβ peptides, and BDNF.
Full-length APP, neprilysin, Bcl-2, and pro-BDNF were quantified within the membrane pellet fraction.
Quantitative real-time RT-PCR
Total RNA of mouse brain and of SK-N-MC PAC1 cells was isolated using the RNeasy Kit (Qiagen). RNA was subjected to quantitative analysis by real-time RT-PCR using the One-step QuantiTectSYBRGreen Kit (Qiagen) in a total reaction volume of 20 μl and the 7500 Fast Real-Time PCR System (Applied Biosystems, Darmstadt, Germany) according to the manufacturer's instructions. The following primers were obtained from Qiagen: QT00106351 (mouse ADAM10), QT00170338 (mouse ADAM17), QT00100317 (mouse PACAP), QT00120561 (mouse PAC1R), QT00097118 (mouse BDNF), QT00162589 (mouse neprilysin), QT01046528 (mouse somatostatin), QT00102102 [mouse receptor for advanced glycation end products (RAGE)], QT02278031 (mouse Bcl-2), QT00309099 (mouse GAPDH), QT00235368 (human BDNF), and QT01192646 (human GAPDH).
The relative mRNA quantities were calculated by the standard-curve approach, determining Ct values. Data were normalized to relative expression of GAPDH. All reactions were performed in duplicate or triplicate. Values obtained from control samples were set to 100%, and alterations in relative gene expression are presented as means ± sd.
Immunoblot analysis
Immunoblot analysis of sAPPα and sAPPβ was performed as described previously (10, 29). In brief, for detection of sAPPα and sAPPβ, membranes were probed using antibody 6E10 or an anti-sAPPβ antibody, respectively, followed by a 35S-labeled or peroxidase-coupled anti-mouse or anti-rabbit antibody. Aliquots (as indicated in figures) of membrane proteins were separated by SDS/PAGE on 10% gels or Nu-PAGE gels (Invitrogen, Karlsruhe, Germany), and proteins were blotted onto PVDF membranes. Membranes were probed applying specific antibodies against APP, neprilysin, Bcl-2, and pro-BDNF followed by a 35S-labeled or peroxidase-coupled anti-rabbit antibody (as indicated in the figures). Loading controls were performed by GAPDH detection for soluble proteins and actin detection for membrane fractions. The specific protein bands were quantified by chemiluminescence or phosphoimaging using the VersaDoc system (Bio-Rad Laboratories, Munich, Germany) or the Bio-Imaging Analyzer BAS-1800 (FujiFilm Medical Systems, Düsseldorf, Germany).
Quantification of amyloid peptides
Quantification of Aβ peptides was performed by using ELISA assays as described previously (30).
Quantification of soluble BDNF
The amount of BDNF was measured in supernatants of mouse brain homogenates. The BDNF contents were determined in triplicate by the EmaxImmunoAssay system (Promega) using 400 μg of total protein of the supernatant fraction for each measurement, according to the manufacturer's protocol.
Studies in vitro
Approximately 3 × 105 SK-N-MC cells stably expressing the PAC1 receptor were seeded and grown in DMEM supplemented with 10% FCS to ∼70% confluency on 6-well plates coated with poly-l-lysine. After being washed twice with serum-free DMEM, cells were incubated for 4 h with 300 nM PACAP27 in serum-free DMEM containing fatty acid-free BSA (10 μg/ml) and N2 supplements (Invitrogen). Neurotoxicity was induced by addition of 10 μM Aβ42 oligomeres for an additional 24 h, and then cells were used for RNA isolation or CREB determination. Analysis of phospho-CREB and CREB proteins in cell lysates was performed by using the PhosphoPlus CREB (Ser-133 antibody kit; Cell Signaling Technology, Danvers, MA, USA) according to the manufacturer's protocol. Oligomeric Aβ42 was generated according to Dahlgren et al. (31).
Statistical analysis
The results are expressed as percentage relative to control (animals treated with the inert carrier) and are the averages ± sd. Statistical significance was determined by using the unpaired Student's t test or the 1-way ANOVA/Bonferroni post hoc test analysis. Values of P < 0.05 were considered significant; degrees of significance are indicated in the figures.
Behavioral analysis
Mice were housed 1/cage at 22°C and 60% relative humidity. Food and water were provided ad libitum, and a 12-h light-dark cycle was maintained (light on from 6:00 AM until 6:00 PM). Thirty-six male mice were tested from 6 mo of age onward. Two groups of APP[V717I]-transgenic mice (treatment with administration solution 2 only, n=12; and treatment with administration solution 2+PACAP, n=12) were investigated together with age-matched wild-type FVB/N mice (controls, treated with administration solution 2; n=12). All procedures were carried out in accordance with the European Communities Council Directive regarding care and use of animals for experimental procedures and were approved by local authorities of the state of Rhineland-Palatinate.
Animals completing the treatment underwent the following tests: open field activity, enriched open field, and novel object recognition. Tests and monitoring of behavior were performed as described previously (32).
Nonparametric statistics were used for analysis. Kruskal-Wallis-Test was conducted for overall differences among the 3 groups as ANOVA. Post hoc comparisons were based on the Mann-Whitney-U test. Differences were considered significant at values of P ≤ 0.05.
RESULTS
Effect of long-term intranasal PACAP treatment on APP processing in the brain of APP[V717I]-transgenic mice
To investigate whether PACAP peptides act as activators of α-secretase in vivo, we performed experiments using APP-transgenic mice. We chose the APP[V717I]-transgenic line, which expresses the London mutation of human APP under control of the Thy-1 promoter. In this line, cognitive deficits are evident before plaque formation. An increased level of soluble Aβ peptides is detectable ∼3 mo after birth, followed by defects in long-term potentiation and learning (25).
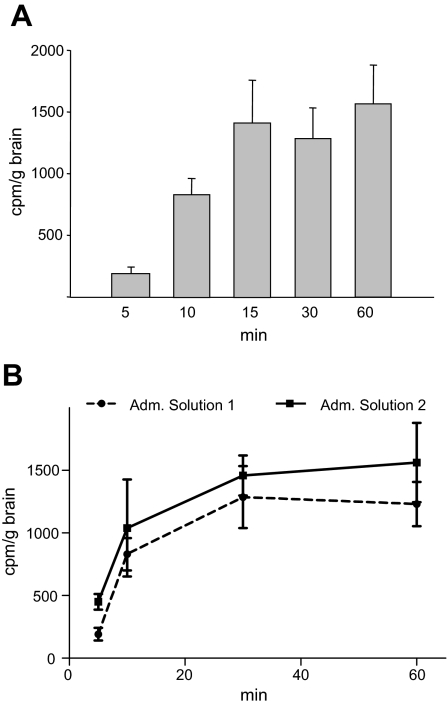
Intranasal administration was used for applying PACAP peptide to the brain of APP-transgenic mice. To determine whether PACAP peptide accesses the mouse brain, [125I]PACAP27 was applied intranasally. Results clearly showed that [125I]PACAP27 reaches the mouse brain very rapidly (after 5 min). The maximal amount of peptide, ∼0.1% of initial radioactivity, was detected 15 min after administration (Fig. 1A). The presence of ∼20% of intact PACAP peptide in the brain 30 min after application was identified by HPLC fractionation. Our results are in accordance with previous findings that showed similar brain uptake efficiency and similar stability in the brain for peptides such as VIP and NAP (28, 33). It has been shown in several animal models that chitosan enhances the nasal absorption of drugs and peptides (34, 35). To increase the delivery of PACAP into the brain, we used water-soluble chitosan glutamate for peptide administration in a second trial. Including chitosan glutamate in the administration buffer (administration solution 2) resulted in enhanced access of PACAP into the brain; ∼2-fold more radioactive PACAP was detected 5 min after application and a slightly increased amount (∼20%) in saturation conditions as compared with PACAP application in administration solution 1 (Fig. 1B). Based on these results, buffer with chitosan glutamate was used for intranasal PACAP administration in further studies.
Figure 1.
Detection of [125I]PACAP27 in the brain following intranasal administration. A) Time course of [125I]PACAP27 transport into the mouse brain. Each mouse received 10 μg PACAP38 and ∼7 × 105 cpm of [125I]PACAP27 in administration solution 1. Animals were killed at indicated times after PACAP administration, and brains were dissected, weighed, and assayed for radioactivity in a γ counter. B) Comparison of PACAP amounts in the brain after treatment using different administration solutions. Experiment was performed as described above, but PACAP peptides were applied either in administration solution 1 or in chitosan glutamate containing administration solution 2. Adm., administration.
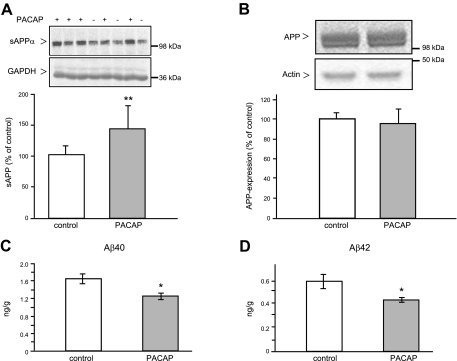
Next, we examined whether the long-term intranasal application of PACAP stimulates the nonamyloidogenic processing of APP[V717I] in the mouse brain. Mice (1 mo old) were treated with 10 μg PACAP daily for 3 mo, and the control group was treated with administration solution 1 without peptide. Following this treatment, APP processing was analyzed in brain homogenates. We observed an increase of ∼40% in sAPPα secretion (Fig. 2A) and at the same time a significant ∼25% reduction of soluble Aβ40 and Aβ42 peptides (Fig. 2C, D). The PACAP treatment had no influence on the expression level of full-length APP in the brain (Fig. 2B).
Figure 2.
Influence of intranasal PACAP38 administration on human APP processing in the brain of 4- mo-old APP[V717I]-transgenic mice. Animals (male mice, 1 mo old) were treated with administration solution 1 alone (control group) or PACAP in administration solution 1 (PACAP group) for 3 mo (5 d/wk), as described in Materials and Methods, and then APP processing was analyzed in brain homogenates. A) Representative Western blots and quantification of secreted sAPPα; 200 μg of mouse brain supernatant proteins was analyzed. B) Representative Western blots and quantitative analysis of the full-length APP expression; 50 μg of mouse brain membrane proteins was analyzed. APP proteins were detected using specific antibodies 6E10 and 6687, respectively, followed by 35S-labeled secondary antibodies. Specific bands corresponding to sAPPα or APP were quantified applying the Bio-Imaging analyzer model BAS-1800. Values are expressed as mean ± sd percentage of values from control mice (control, n=6; PACAP, n=8). GAPDH and actin detection were used as loading controls. C, D) Quantification of soluble Aβ40 (C) and Aβ42 (D) peptides detected in mouse brain supernatants by sandwich ELISA. Values are the mean ± se of the amount of each Aβ peptide per gram of mouse (control, n=6; PACAP, n=8). *P < 0.05; **P < 0.01.
As 3-mo-old APP[V717I]-transgenic mice show cognitive deficits, in the next trial we investigated whether PACAP treatment would counteract this effect in 6-mo-old animals. Mice (3 mo old) were treated daily with PACAP38 in chitosan glutamate solution for an additional 3 mo as described above, and then APP processing was analyzed in brain homogenates of 6-mo-old animals. In this study, PACAP treatment increased sAPPα production by ∼25% and at the same time reduced sAPPβ secretion by ∼20% (Fig. 3A). The expression of full-length APP was not affected (Fig. 3B). Increased nonamyloidogenic processing of APP could be caused by enhanced gene expression of α-secretases. Therefore, we examined the influence of PACAP treatment on the transcription level of ADAM10 and ADAM17 genes. Analysis of gene expression by quantitative real-time RT-PCR revealed that the enhanced level of sAPPα was not caused by increased ADAM10 and ADAM17 gene expression (Fig. 3C, D and Table 1).
Figure 3.
Influence of intranasal PACAP38 administration on human APP processing in the brain of 6-mo-old APP[V717I]-transgenic mice. Male mice (3 mo old) were treated for an additional 3 mo (5 d/wk) with administration solution 2 alone (control group) or PACAP38 in administration solution 2 (PACAP group), and then APP processing was analyzed in brain homogenates. A) Representative Western blots and quantitative analysis of secreted sAPPα and sAPPβ. B) Representative Western blots and quantitative analysis of the full-length APP expression. Western blot analysis was performed as described in Materials and Methods, but peroxidase-coupled anti-mouse or anti-rabbit antibodies were used as secondary antibodies (n=12 animals/group). C, D) Quantification of ADAM10 (C) and ADAM17 (D) mRNA levels by quantitative real-time RT-PCR. Quantification of mRNA levels was performed as described in Materials and Methods. GAPDH mRNA was used for normalization. Values are expressed as mean ± sd percentage of values from control mice (n=7 animals/group). ns, nonsignificant (P>0.05). **P < 0.01; unpaired Student's t test.
Table 1.
Quantification of brain mRNA levels
| Gene | Results (%) |
|---|---|
| ADAM10 | 114.8 ± 7.8 |
| ADAM17 | 120.0 ± 9.1 |
| Somatostatin | 137.5 ± 9.7*** |
| Neprilysin | 146.0 ± 7.1*** |
| PACAP | 154.2 ± 16.1** |
| PAC1-receptor | 167.9 ± 13.2*** |
| BDNF | 153.6 ± 15.7** |
| Bcl-2 | 124.7 ± 4.7* |
| RAGE | 50.7 ± 7.8** |
RNAs from brains of 6-mo-old APP[V717I] transgenic mice treated for 3 mo with PACAP or with administration solution were subjected to quantitative analysis by real-time RT-PCR (n=7 animals/group). Quantification of mRNA levels was performed as described in Materials and Methods. GAPDH mRNA was used for normalization. mRNA level of administration solution-treated mice was set to 100%. Results with no note were statistically nonsignificant.
P < 0.05,
P < 0.01,
P < 0.001; Student's t test.
Effect of long-term PACAP treatment on somatostatin and neprilysin expression in the mouse brain
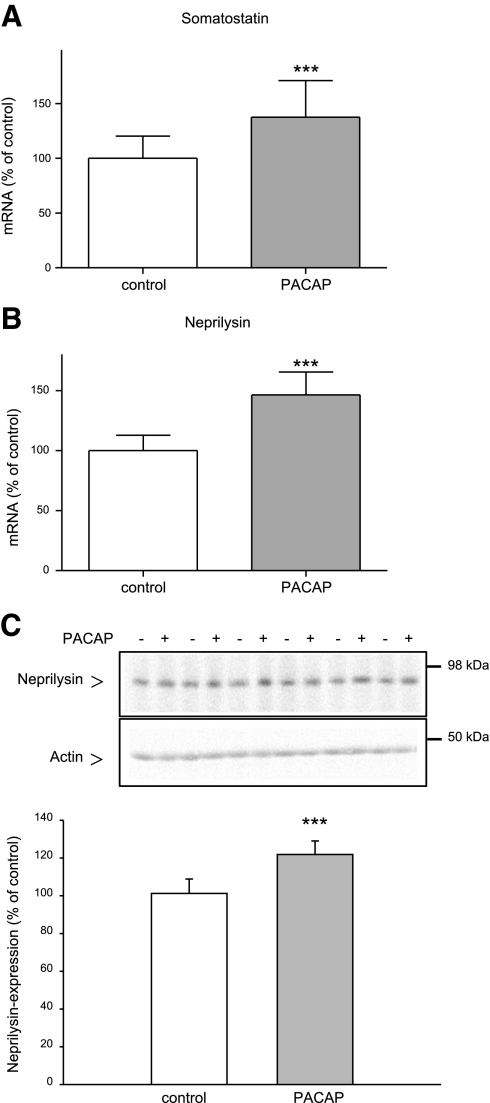
It is known that administration of PACAP enhances somatostatin gene expression in the brain (36). Furthermore, somatostatin increases the catabolism of Aβ peptides in the brain by up-regulating neprilysin activity (37). We therefore examined the influence of chronic PACAP treatment on somatostatin and neprilysin gene expression in the mouse brain. Somatostatin and neprilysin mRNA levels were increased by 37.5% (Fig. 4A and Table 1) and 46.4% (Fig. 4B and Table 1), respectively, in PACAP-treated mice as compared with administration-solution-treated mice. The protein level of neprilysin was enhanced ∼20% (Fig. 4C) after PACAP treatment. These results confirmed the close relationship among PACAP, somatostatin, and neprilysin.
Figure 4.
Effect of long-term PACAP38 treatment on the expression of somatostatin and neprilysin in the brain of 6-mo-old APP[V717I]-transgenic mice. Animals were treated as described in Fig. 3. A, B) Quantification of somatostatin (A) and neprilysin (B) mRNA levels by quantitative real-time RT-PCR (n=7 animals/group). Quantification of mRNA levels was performed as described in Fig. 3. C) Representative Western blot and quantitative analysis of neprilysin expression; 80 μg of mouse brain membrane proteins was analyzed (n=12 animals/group). ***P < 0.001.
Influence of long-term PACAP treatment on PACAP and PAC1-receptor expression in the mouse brain
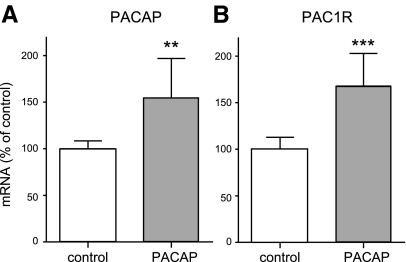
It has been reported that PACAP can increase its own expression in an autocrine manner (38). Therefore, we examined whether PACAP treatment also influences PACAP expression. Our results confirmed previous findings: ∼50% more PACAP mRNA following PACAP treatment was observed (Fig. 5A). Further, we analyzed whether durable PAC1-receptor activation by PACAP affected receptor expression. The PAC1-receptor mRNA level was enhanced by ∼70% after PACAP treatment (Fig. 5B). Thus, this 3-mo intranasal PACAP administration resulted in the elevation of mRNA levels of both the PAC1 receptor and its ligand in the mouse brain.
Figure 5.
Effect of long-term PACAP38 treatment on PACAP and PAC1-receptor expression in the brain of 6-mo-old APP[V717I]-transgenic mice. Animals were treated as described in Fig. 3. Quantification of PACAP (A) and PAC1-receptor (B) mRNA levels by quantitative real-time RT-PCR (n=7 animals/group). Quantification of mRNA levels was performed as described in Fig. 3.
Effect of long-term PACAP treatment on BDNF expression in the brain of APP[V717I]-transgenic mice and human SK-N-MC PAC1 cells
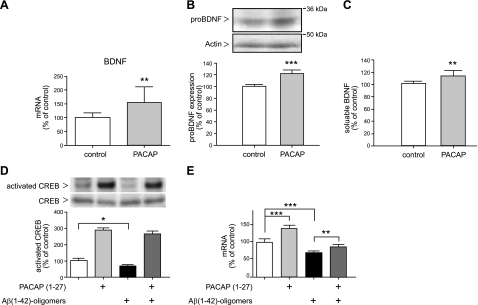
A common down-regulation of BDNF and PACAP was found in several AD transgenic mouse models and in the human AD temporal cortex (21). It is also known that PACAP, via its specific PAC1 receptor, influences BDNF expression (39, 40). Therefore, we analyzed BDNF mRNA levels following PACAP treatment in transgenic mouse brains. Quantitative real-time RT-PCR analysis revealed that PACAP treatment resulted in an increase of BDNF mRNA level exceeding 50% compared with administration-solution-treated mice (Fig. 6A and Table 1). Elevated protein expression of pro-BDNF (∼20%) and soluble BDNF (∼15%) was detected by using Western blot and ELISA, respectively (Fig. 6B, C).
Figure 6.
Effect of PACAP treatment on BDNF expression in the mouse brain and in human SK-N-MC PAC1 cells. Animals were treated as described in Fig. 3. A) Quantification of the BDNF mRNA level by quantitative real-time RT-PCR (n=7 animals/group). Quantification of mRNA levels was performed as described in Fig. 3. B) Representative Western blot and quantitative analysis of pro-BDNF expression; 100 μg of mouse brain membrane proteins was analyzed (n=12 animals/group). C) Quantification of soluble BDNF detected in mouse brain supernatants by sandwich ELISA (Promega). Values are expressed as mean ± sd percentage of values from control mice (n=7 animals/group). D) PACAP-induced CREB phosphorylation in SK-N-MC PAC1 cells. Cells were incubated for 4 h with 300 nM PACAP27 in DMEM (lanes 2, 4) or only in DMEM (lanes 1, 3), and then cells were incubated for 24 h in the absence (lanes 1, 2) or presence of 10 μM Aβ42-oligomeres (lanes 3, 4). Cells were collected, and analysis of phospho-CREB and CREB proteins in cell lysates was performed as described in Materials and Methods. E) Influence of PACAP treatment on BDNF mRNA expression in SK-N-MC PAC1 cells. Cells were treated as described above, then RNA isolation end quantitative real-time RT-PCR was performed as described in Materials and Methods. *P < 0.05, **P < 0.01, ***P < 0.001; unpaired Student's t test or 1-way ANOVA/Bonferroni post hoc test.
To investigate whether PACAP treatment also regulates human BDNF expression, we used human neuroblastoma SK-N-MC cells overexpressing the PAC1 receptor (SK-N-MC PAC1 cells). Transcriptional activation of the BDNF gene is commonly regulated by a key transcription factor, the cAMP response element-binding protein (CREB) (41, 42), and it has been reported that PACAP induces CREB phosphorylation (43). Treatment with oligomeric Aβ42 peptides reduced basal levels of BDNF mRNA in human neuroblastoma cells (44). Therefore, we investigated whether PACAP treatment can counteract this reduction. PACAP treatment resulted in a >3-fold induced CREB phosphorylation (Fig. 6D, lanes 1 vs. 2) and in a 40% elevation of BDNF mRNA expression (Fig. 6E, lanes 1 vs. 2). Treatment of cells with Aβ42 oligomers for 24 h caused reduction (∼30%) of both CREB phosphorylation (Fig. 6D, lanes 1 vs. 3) and BDNF mRNA expression (Fig. 6E, lanes 1 vs. 3). However, cells preincubated with PACAP for 4 h, and then simultaneously treated with PACAP and Aβ42 oligomers for 24 h, displayed enhanced CREB phosphorylation (Fig. 6D, lanes 3 vs. 4) and increased BDNF mRNA expression (20%) as compared with Aβ42-oligomer-treated cells (Fig. 6E, lanes 3 vs. 4). Apparently, PACAP treatment can maintain BDNF mRNA expression in the presence of Aβ42 oligomers.
Alleviation of Aβ toxicity by PACAP
There is compelling evidence to implicate caspases in the pathogenesis of AD, as Aβ peptides can induce apoptosis through caspase activation (45). PACAP exerts antiapoptotic activities in physiological and pathological conditions (19). By increasing Bcl-2 expression, PACAP prevents the release of cytochrome c into the cytosol, and this way may prevent caspase activation (46). Therefore, we examined the influence of long-term PACAP treatment on antiapoptotic Bcl-2 expression in the brain of APP[V717I]-transgenic mice. We observed an increased expression of Bcl-2 of both the gene (∼25%; Fig. 7A and Table 1) and protein (∼30%; Fig. 7B) level in the brain of APP-transgenic mice after PACAP treatment.
Figure 7.
Influence of intranasal PACAP38 administration on expression of Bcl-2 and RAGE in the brain of 6-mo-old APP[V717I]-transgenic mice. Animals were treated as described in Fig. 3. A) Quantification of Bcl-2 mRNA level by quantitative real-time RT-PCR (n=7 animals/group). B) Representative Western blots and quantitative analysis of Bcl-2 expression; 160 μg of mouse brain membrane proteins was analyzed (n=12 animals/group). C) Quantification of RAGE mRNA level by quantitative real-time RT-PCR (n=7 animals/group). Quantification of mRNA levels was performed as described in Fig. 3D. *P < 0.05, **P < 0.01, ***P < 0.001.
Rapid Aβ toxic effects have been associated with a prooxidant effect of the peptide (47) and may, in part, be mediated by RAGE (48). RAGE binds soluble Aβ and is thought to be the primary transporter of Aβ from the systemic circulation across the BBB into the brain. RAGE ligand interaction induces activation of the proinflammatory transcription factor NF-κB (49). Several studies (50, 51) have shown inhibition of NF-κB by VIP and PACAP via different signal transduction pathways in activated microglial and monocytic cells. To study the possible crosstalk between RAGE and PACAP in the brain of APP-transgenic mice, we examined the influence of PACAP treatment on RAGE gene expression. Quantitative real-time RT-PCR analysis revealed that PACAP treatment caused a strong reduction (∼50%) of the RAGE mRNA level compared with administration solution-treated mice (Fig. 7C).
Behavioral analysis of APP[V717I]-transgenic mice following long-term PACAP treatment
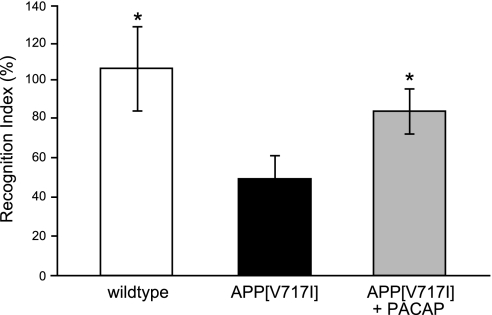
Three groups of mice were tested in the novel object recognition test following 3 mo of daily treatment: wild-type mice treated with administration solution, APP[V717I]-transgenic mice treated with administration solution, and APP[V717I]-transgenic mice treated with PACAP. Wild-type mice spent 50% more time with the novel object compared with APP[V717I]-transgenic mice (Fig. 8). AD transgenic mice, due to their memory deficits, did not discriminate as well between novel and familiar objects. PACAP treatment significantly rescued this impaired recognition; treated mice spent ∼30% more time with the novel object compared with control APP[V717I]-transgenic mice [Fig. 8; index H(2;33)=7.754; P≤0.05].
Figure 8.
Cognitive assessment of APP[V717I]-transgenic mice following PACAP treatment, applying the novel object recognition test. Three groups of mice (6 mo old; n=12 animals/group) were tested in the novel object recognition test following 3 mo of daily treatment: wild-type mice treated with administration solution (left bar), APP[V717I]-transgenic mice treated with administration solution (center bar), and APP[V717I]-transgenic mice treated with PACAP (right bar). Object recognition test results are expressed by the recognition index. Recognition index gives the proportion of investigating the novel displaced object relative to the known objects of the previous trial. *P <0.05 vs. APP[V717I] treated with administration solution.
The results of other behavioral tests (open field activity and enriched open field) revealed no differences in activity and exploration-related parameters between the tested groups (data not shown).
DISCUSSION
As PACAP peptides and PAC1 receptors are localized in brain areas affected by AD, the aim of this study was to explore the application of the natural peptide PACAP as a valuable approach regarding AD therapy. We found that long-term treatment of APP[V717I]-transgenic mice with the PACAP peptide by intranasal administration increased secretion of the neuroprotective sAPPα and positively influenced expression levels of AD-relevant genes and proteins, such as BDNF, Bcl-2, and RAGE. Finally, PACAP treatment resulted in improved cognition in transgenic animals.
Our study has shown that PACAP treatment influences APP processing in vivo by increasing the nonamyloidogenic pathway of APP. The PACAP peptide acts as an α-secretase activator, which resulted in enhanced secretion of neuroprotective sAPPα and decreased secretion of sAPPβ. PACAP treatment had no influence on the expression level of α-secretases ADAM10 and ADAM17 and on the substrate APP. This is in accordance with our previous in vitro findings, demonstrating that PACAP-induced α-secretase stimulation was primarily mediated by activation of MAP-kinase and phosphatidylinositol 3-kinase pathways and was not an effect of increased α-secretase expression (17). Since α-secretase activity is diminished in the brain of AD patients (4, 7, 52), the α-secretase stimulation by PACAP may have beneficial effects. Parallel to increased secretion of sAPPα, a reduction of soluble Aβ40 and Aβ42 peptides was observed. The activation of the somatostatin-neprilysin cascade by PACAP enhanced neprilysin expression, which may increase Aβ peptide degradation. The reduction of soluble Aβ peptides could therefore be caused by two effects: an increase of the nonamyloidogenic pathway of APP and an enhancement of Aβ peptide catabolism.
Multiple lines of evidence suggest that PACAP exerts a large array of pharmacological effects and biological functions. As PACAP acts neuroprotective as well as neurotrophic and modulates neuronal gene expression (19), we analyzed whether nasal PACAP treatment also caused these beneficial effects in the brain of APP-transgenic mice.
We found that 3 mo of daily PAC1-receptor activation with PACAP increased mRNA levels of both the PAC1 receptor and its ligand PACAP. Our results confirmed previous findings that PACAP can increase its own expression (38, 53). We observed a clear increase (70%) of PAC1-receptor mRNA expression, which suggests that PACAP could act as an autocrine factor for the transcription of its own receptor. Thus, the PACAP/PAC1-receptor system undergoes self-regulation and via this way, short-term PACAP exposure might be turned into a long-term action of the endogenous PACAP/PAC1-receptor system.
The BDNF, which plays a critical role in learning and memory is essential for the synaptic function, plasticity, and neuronal survival. Both the protein and the mRNA level of BDNF are significantly decreased in AD and in mildly cognitive impaired subjects (54, 55). Several studies (39, 40) have demonstrated that PACAP via its specific PAC1 receptor plays a pivotal role in the transcriptional regulation of the neurotrophic factor BDNF. BDNF expression was significantly reduced in PAC1-receptor-deficient mice (40). In accordance with these findings, our results clearly demonstrated that intranasal PACAP treatment enhances BDNF expression on mRNA and protein levels in the brain of APP-transgenic mice. We also provided evidence in SK-N-MC PAC1 cells that a decreased level of human BDNF mRNA caused by treatment with Aβ42 oligomers could be partly recovered by treatment with PACAP. The reduction of the BDNF gene expression could be caused by down-regulation of CREB activation via Aβ peptides (56). In our experiments, PACAP, by inducing CREB phosphorylation, counteracted this effect, resulting in an enhancement of BDNF mRNA levels.
The Aβ42 peptide induces cell death and caspase 3 activation in human neuroblastoma cells via increase of the Bax/Bcl-2 ratio (57). We demonstrate that intranasal PACAP treatment increased expression of the antiapoptotic Bcl-2 protein in the mouse brain. Our results confirm previous findings demonstrating that PACAP influences Bcl-2 expression positively. It has been shown that PACAP(+/−) and PACAP(−/−) mice exhibit a lower Bcl-2 expression and higher cytoplasmic cytochrom c level than wild-type animals. The neurological deficits in PACAP(+/−) and PACAP(−/−) mice could be prevented by PACAP38 injection (46). The neuroprotective effects of the PACAP/PAC1-receptor system are mediated through inhibition of caspase 3 activity (58, 59). PACAP probably acts on the mitochondrial apoptotic pathway, by increasing Bcl-2 expression. Bcl-2 impedes the release of cytochrome c into the cytosol, and therefore prevents activation of caspase 9 and subsequently caspase 3. In this way, PACAP may impede the Aβ-peptide-induced toxicity.
RAGE has been shown to play a crucial role in chronic inflammatory diseases, late diabetic complications, atherosclerosis, and AD (60). Since RAGE-ligand interaction mediates the activation of the proinflammatory transcription factor NF-κB and PACAP inhibits this pathway, we studied the influence of PACAP treatment on RAGE expression in the brain of AD transgenic mice. We observed significant down-regulation of RAGE gene expression after PACAP treatment. This diminished RAGE expression may cause an inhibition of the Aβ transport into the brain, and, finally, it may slow down inflammatory signaling in the mouse brain.
Behavioral study results clearly mirrored the influence of these beneficial effects, described above, on cognitive function. In APP[V717I]-transgenic mice used in this study, cognitive deficits were already detectable at ∼3 mo after birth. Treatment of 3-mo-old animals with PACAP for an additional 3 mo resulted in significant cognitive improvements; the memory deficits in APP-transgenic mice were nearly completely abolished after peptide application. The improvements in cognitive function might be a result of the combined effects, such as increased amounts of the neuroprotective sAPPα and BDNF, reduction of the Aβ level, and inhibition of Aβ toxicity.
As the PAC1-receptor gene is predominantly expressed in neurons and analyzed proteins are also expressed in neurons, PACAP probably exerts its beneficial effect on APP[V717I] mice by acting directly on neurons. It was demonstrated that IL-6, which is colocalized with PAC1 receptor in neurons, is involved in neuroprotective mechanisms mediated by PACAP after ischemia (46). However, the PACAP receptor is also expressed in glia cells, including activated astrocytes (61, 62). Various studies have demonstrated the relationship between Aβ-activated glia cells and progression of AD. On activation with Aβ, microglial cells produce proinflammatory cytokines, such as IL-1β and TNF-α; the chemokine IL-8; macrophage inflammatory protein-1 (MIP-1); and oxidative stress-related enzymes (63). PACAP in astrocytes increases the production of various neurotrophic factors, which can promote neuronal proliferation and differentiation (64). PACAP treatment also increases IL-6 level in cultured astrocytes (65) and protects astroglial cells against oxidative stress-induced apoptosis (66). Thus, it is possible that PACAP also exerts its beneficial effects on APP[V717I] mice in glia cells. However, the PACAP effect on APP[V717I] processing should be restricted to neurons, since transgenic APP is solely expressed in neurons.
PACAP delivery to the CNS either orally or intravenously is problematic, since the half-life of PACAP38 in human blood ranges from 5 to 10 min (67); therefore, intranasal administration may be a good alternative for PACAP application. It has been shown that inhaled PACAP38 is well tolerated without systemic side-effects in healthy human male subjects (68). In summary, our results suggest that restoring or increasing PACAP/PAC1-receptor function in the brain may provide therapeutic benefits and that nasal application of natural neuropeptide PACAP may be valuable for AD treatment.
Acknowledgments
The authors thank Dr. C. Haass (Ludwig Maximilians University Munich, Munich, Germany) for providing antibodies and H. Pearson for critically reading the manuscript. The authors also appreciate the technical help of A. Kanarek.
This work was supported by a grant from the Alzheimer Forschung Initiative e.V. (Düsseldorf, Germany) to E.K and F.F (07807).
REFERENCES
- 1. Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 2. Mattson M. P., Cheng B., Culwell A. R., Esch F. S., Lieberburg I., Rydel R. E. (1993) Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron 10, 243–254 [DOI] [PubMed] [Google Scholar]
- 3. Caille I., Allinquant B., Dupont E., Bouillot C., Langer A., Muller U., Prochiantz A. (2004) Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 131, 2173–2181 [DOI] [PubMed] [Google Scholar]
- 4. Lannfelt L., Basun H., Wahlund L. O., Rowe B. A., Wagner S. L. (1995) Decreased alpha-secretase-cleaved amyloid precursor protein as a diagnostic marker for Alzheimer's disease. Nat. Med. 1, 829–832 [DOI] [PubMed] [Google Scholar]
- 5. Sennvik K., Fastbom J., Blomberg M., Wahlund L. O., Winblad B., Benedikz E. (2000) Levels of alpha- and beta-secretase cleaved amyloid precursor protein in the cerebrospinal fluid of Alzheimer's disease patients. Neurosci. Lett. 278, 169–172 [DOI] [PubMed] [Google Scholar]
- 6. Almkvist O., Basun H., Wagner S. L., Rowe B. A., Wahlund L. O., Lannfelt L. (1997) Cerebrospinal fluid levels of alpha-secretase-cleaved soluble amyloid precursor protein mirror cognition in a Swedish family with Alzheimer disease and a gene mutation Arch. Neurol. 54, 641–644 [DOI] [PubMed] [Google Scholar]
- 7. Fellgiebel A., Kojro E., Muller M. J., Scheurich A., Schmidt L. G., Fahrenholz F. (2009) CSF APPs alpha and phosphorylated tau protein levels in mild cognitive impairment and dementia of Alzheimer's type. J. Geriatr. Psychiatry Neurol. 22, 3–9 [DOI] [PubMed] [Google Scholar]
- 8. Taylor C. J., Ireland D. R., Ballagh I., Bourne K., Marechal N. M., Turner P. R., Bilkey D. K., Tate W. P., Abraham W. C. (2008) Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol. Dis. 31, 250–260 [DOI] [PubMed] [Google Scholar]
- 9. Ishida A., Furukawa K., Keller J. N., Mattson M. P. (1997) Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport 8, 2133–2137 [DOI] [PubMed] [Google Scholar]
- 10. Postina R., Schroeder A., Dewachter I., Bohl J., Schmitt U., Kojro E., Prinzen C., Endres K., Hiemke C., Blessing M., Flamez P., Dequenne A., Godaux E., van Leuven F., Fahrenholz F. (2004) A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J. Clin. Invest. 113, 1456–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuhn P. H., Wang H., Dislich B., Colombo A., Zeitschel U., Ellwart J. W., Kremmer E., Rossner S., Lichtenthaler S. F. (2010) ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J. 29, 3020–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tippmann F., Hundt J., Schneider A., Endres K., Fahrenholz F. (2009) Up-regulation of the alpha-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J. 23, 1643–1654 [DOI] [PubMed] [Google Scholar]
- 13. Donmez G., Wang D., Cohen D. E., Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell 142, 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Jarvis C. I., Goncalves M. B., Clarke E., Dogruel M., Kalindjian S. B., Thomas S. A., Maden M., Corcoran J. P. (2010) Retinoic acid receptor-alpha signalling antagonizes both intracellular and extracellular amyloid-beta production and prevents neuronal cell death caused by amyloid-beta. Eur. J. Neurosci. 32, 1246–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nitsch R. M., Deng M., Growdon J. H., Wurtman R. J. (1996) Serotonin 5-HT2a and 5-HT2c receptors stimulate amyloid precursor protein ectodomain secretion. J. Biol. Chem. 271, 4188–4194 [DOI] [PubMed] [Google Scholar]
- 16. Caccamo A., Oddo S., Billings L. M., Green K. N., Martinez-Coria H., Fisher A., LaFerla F. M. (2006) M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron 49, 671–682 [DOI] [PubMed] [Google Scholar]
- 17. Kojro E., Postina R., Buro C., Meiringer C., Gehrig-Burger K., Fahrenholz F. (2006) The neuropeptide PACAP promotes the alpha-secretase pathway for processing the Alzheimer amyloid precursor protein. FASEB J. 20, 512–514 [DOI] [PubMed] [Google Scholar]
- 18. Uchida D., Arimura A., Somogyvari-Vigh A., Shioda S., Banks W. A. (1996) Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res. 736, 280–286 [DOI] [PubMed] [Google Scholar]
- 19. Vaudry D., Falluel-Morel A., Bourgault S., Basille M., Burel D., Wurtz O., Fournier A., Chow B. K., Hashimoto H., Galas L., Vaudry H. (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 61, 283–357 [DOI] [PubMed] [Google Scholar]
- 20. Spengler D., Waeber C., Pantaloni C., Holsboer F., Bockaert J., Seeburg P. H., Journot L. (1993) Differential signal transduction by five splice variants of the PACAP receptor. Nature 365, 170–175 [DOI] [PubMed] [Google Scholar]
- 21. Wu Z. L., Ciallella J. R., Flood D. G., O'Kane T. M., Bozyczko-Coyne D., Savage M. J. (2006) Comparative analysis of cortical gene expression in mouse models of Alzheimer's disease. Neurobiol. Aging 27, 377–386 [DOI] [PubMed] [Google Scholar]
- 22. Hanson L. R., Frey W. H. (2007) Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS. J. Neuroimmune Pharmacol. 2, 81–86 [DOI] [PubMed] [Google Scholar]
- 23. Born J., Lange T., Kern W., McGregor G. P., Bickel U., Fehm H. L. (2002) Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci. 5, 514–516 [DOI] [PubMed] [Google Scholar]
- 24. Reger M. A., Watson G. S., Green P. S., Wilkinson C. W., Baker L. D., Cholerton B., Fishel M. A., Plymate S. R., Breitner J. C., DeGroodt W., Mehta P., Craft S. (2008) Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70, 440–448 [DOI] [PubMed] [Google Scholar]
- 25. Moechars D., Dewachter I., Lorent K., Reverse D., Baekelandt V., Naidu A., Tesseur I., Spittaels K., Van den Haute C., Checler F., Godaux E., Cordell B., Van Leuven F. (1999) Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J. Biol. Chem. 274, 6483–6492 [DOI] [PubMed] [Google Scholar]
- 26. Alcalay R. N., Giladi E., Pick C. G., Gozes I. (2004) Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci. Lett. 361, 128–131 [DOI] [PubMed] [Google Scholar]
- 27. Illum L., Watts P., Fisher A. N., Hinchcliffe M., Norbury H., Jabbal-Gill I., Nankervis R., Davis S. S. (2002) Intranasal delivery of morphine. J. Pharmacol. Exp. Ther. 301, 391–400 [DOI] [PubMed] [Google Scholar]
- 28. Gozes I., Giladi E., Pinhasov A., Bardea A., Brenneman D. E. (2000) Activity-dependent neurotrophic factor: intranasal administration of femtomolar-acting peptides improve performance in a water maze. J. Pharmacol. Exp. Ther. 293, 1091–1098 [PubMed] [Google Scholar]
- 29. Kojro E., Fuger P., Prinzen C., Kanarek A. M., Rat D., Endres K., Fahrenholz F., Postina R. (2010) Statins and the squalene synthase inhibitor zaragozic acid stimulate the non-amyloidogenic pathway of amyloid-beta protein precursor processing by suppression of cholesterol synthesis. J. Alzheimers Dis. 20, 1215–1231 [DOI] [PubMed] [Google Scholar]
- 30. Dewachter I., Reverse D., Caluwaerts N., Ris L., Kuiperi C., Van den Haute C., Spittaels K., Umans L., Serneels L., Thiry E., Moechars D., Mercken M., Godaux E., Van Leuven F. (2002) Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. J. Neurosci. 22, 3445–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dahlgren K. N., Manelli A. M., Stine W. B., Jr., Baker L. K., Krafft G. A., LaDu M. J. (2002) Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability J. Biol. Chem. 277, 32046–32053 [DOI] [PubMed] [Google Scholar]
- 32. Schmitt U., Tanimoto N., Seeliger M., Schaeffel F., Leube R. E. (2009) Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience 162, 234–243 [DOI] [PubMed] [Google Scholar]
- 33. Dufes C., Olivier J. C., Gaillard F., Gaillard A., Couet W., Muller J. M. (2003) Brain delivery of vasoactive intestinal peptide (VIP) following nasal administration to rats. Int. J. Pharm. 255, 87–97 [DOI] [PubMed] [Google Scholar]
- 34. Illum L. (2000) Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 11, 1–18 [DOI] [PubMed] [Google Scholar]
- 35. Illum L. (2006) Nasal clearance in health and disease. J. Aerosol Med. 19, 92–99 [DOI] [PubMed] [Google Scholar]
- 36. Li S., Grinevich V., Fournier A., Pelletier G. (1996) Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on gonadotropin-releasing hormone and somatostatin gene expression in the rat brain. Brain Res. Mol. Brain Res. 41, 157–162 [DOI] [PubMed] [Google Scholar]
- 37. Saito T., Iwata N., Tsubuki S., Takaki Y., Takano J., Huang S. M., Suemoto T., Higuchi M., Saido T. C. (2005) Somatostatin regulates brain amyloid beta peptide Abeta42 through modulation of proteolytic degradation. Nat. Med. 11, 434–439 [DOI] [PubMed] [Google Scholar]
- 38. Hashimoto H., Hagihara N., Koga K., Yamamoto K., Shintani N., Tomimoto S., Mori W., Koyama Y., Matsuda T., Baba A. (2000) Synergistic induction of pituitary adenylate cyclase-activating polypeptide (PACAP) gene expression by nerve growth factor and PACAP in PC12 cells. J. Neurochem. 74, 501–507 [DOI] [PubMed] [Google Scholar]
- 39. Pellegri G., Magistretti P. J., Martin J. L. (1998) VIP and PACAP potentiate the action of glutamate on BDNF expression in mouse cortical neurones. Eur. J. Neurosci. 10, 272–280 [DOI] [PubMed] [Google Scholar]
- 40. Zink M., Otto C., Zorner B., Zacher C., Schutz G., Henn F. A., Gass P. (2004) Reduced expression of brain-derived neurotrophic factor in mice deficient for pituitary adenylate cyclase activating polypeptide type-I-receptor Neurosci. Lett. 360, 106–108 [DOI] [PubMed] [Google Scholar]
- 41. Shieh P. B., Hu S. C., Bobb K., Timmusk T., Ghosh A. (1998) Identification of a signaling pathway involved in calcium regulation of BDNF expression Neuron 20, 727–740 [DOI] [PubMed] [Google Scholar]
- 42. Tao X., Finkbeiner S., Arnold D. B., Shaywitz A. J., Greenberg M. E. (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20, 709–726 [DOI] [PubMed] [Google Scholar]
- 43. Kopp M., Meissl H., Korf H. W. (1997) The pituitary adenylate cyclase-activating polypeptide-induced phosphorylation of the transcription factor CREB (cAMP response element binding protein) in the rat suprachiasmatic nucleus is inhibited by melatonin. Neurosci. Lett. 227, 145–148 [DOI] [PubMed] [Google Scholar]
- 44. Garzon D. J., Fahnestock M. (2007) Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. J. Neurosci. 27, 2628–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Loo D. T., Copani A., Pike C. J., Whittemore E. R., Walencewicz A. J., Cotman C. W. (1993) Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. Proc. Natl. Acad. Sci. U. S. A. 90, 7951–7955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ohtaki H., Nakamachi T., Dohi K., Aizawa Y., Takaki A., Hodoyama K., Yofu S., Hashimoto H., Shintani N., Baba A., Kopf M., Iwakura Y., Matsuda K., Arimura A., Shioda S. (2006) Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6, Proc. Natl. Acad. Sci. U. S. A. 103, 7488–7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Behl C., Davis J. B., Lesley R., Schubert D. (1994) Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 77, 817–827 [DOI] [PubMed] [Google Scholar]
- 48. Yan S. D., Chen X., Fu J., Chen M., Zhu H., Roher A., Slattery T., Zhao L., Nagashima M., Morser J., Migheli A., Nawroth P., Stern D., Schmidt A. M. (1996) RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature 382, 685–691 [DOI] [PubMed] [Google Scholar]
- 49. Bierhaus A., Schiekofer S., Schwaninger M., Andrassy M., Humpert P. M., Chen J., Hong M., Luther T., Henle T., Kloting I., Morcos M., Hofmann M., Tritschler H., Weigle B., Kasper M., Smith M., Perry G., Schmidt A. M., Stern D. M., Haring H. U., Schleicher E., Nawroth P. P. (2001). Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 50, 2792–2808 [DOI] [PubMed] [Google Scholar]
- 50. Delgado M., Ganea D. (2001) Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit nuclear factor-kappa B-dependent gene activation at multiple levels in the human monocytic cell line THP-1. J. Biol. Chem. 276, 369–380 [DOI] [PubMed] [Google Scholar]
- 51. Delgado M. (2002) Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit CBP-NF-kappaB interaction in activated microglia. Biochem. Biophys. Res. Commun. 297, 1181–1185 [DOI] [PubMed] [Google Scholar]
- 52. Tyler S. J., Dawbarn D., Wilcock G. K., Allen S. J. (2002) alpha- and beta-secretase: profound changes in Alzheimer's disease. Biochem. Biophys. Res. Commun. 299, 373–376 [DOI] [PubMed] [Google Scholar]
- 53. Braas K. M., Schutz K. C., Bond J. P., Vizzard M. A., Girard B. M., May V.(2007) Microarray analyses of pituitary adenylate cyclase activating polypeptide (PACAP)-regulated gene targets in sympathetic neurons Peptides 28, 1856–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Phillips H. S., Hains J. M., Armanini M., Laramee G. R., Johnson S. A., Winslow J. W. (1991) BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron 7, 695–702 [DOI] [PubMed] [Google Scholar]
- 55. Holsinger R. M., Schnarr J., Henry P., Castelo V. T., Fahnestock M. (2000) Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer's disease. Brain Res. Mol. Brain Res. 76, 347–354 [DOI] [PubMed] [Google Scholar]
- 56. Tong L., Balazs R., Thornton P. L., Cotman C. W. (2004) Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J. Neurosci. 24, 6799–6809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clementi M. E., Pezzotti M., Orsini F., Sampaolese B., Mezzogori D., Grassi C., Giardina B., Misiti F. (2006) Alzheimer's amyloid beta-peptide (1–42) induces cell death in human neuroblastoma via bax/bcl-2 ratio increase: an intriguing role for methionine 35. Biochem. Biophys. Res. Commun. 342, 206–213 [DOI] [PubMed] [Google Scholar]
- 58. Vaudry D., Gonzalez B. J., Basille M., Pamantung T. F., Fontaine M., Fournier A., Vaudry H. (2000) The neuroprotective effect of pituitary adenylate cyclase-activating polypeptide on cerebellar granule cells is mediated through inhibition of the CED3-related cysteine protease caspase-3/CPP32. Proc. Natl. Acad. Sci. U. S. A. 97, 13390–13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang G., Qi C., Fan G. H., Zhou H. Y., Chen S. D. (2005) PACAP protects neuronal differentiated PC12 cells against the neurotoxicity induced by a mitochondrial complex I inhibitor, rotenone. FEBS Lett. 579, 4005–4011 [DOI] [PubMed] [Google Scholar]
- 60. Bierhaus A., Humpert P. M., Morcos M., Wendt T., Chavakis T., Arnold B., Stern D. M., Nawroth P. P. (2005) Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 83, 876–886 [DOI] [PubMed] [Google Scholar]
- 61. Tatsuno I., Gottschall P. E., Koves K., Arimura A. (1990) Demonstration of specific binding sites for pituitary adenylate cyclase activating polypeptide (PACAP) in rat astrocytes. Biochem. Biophys. Res. Commun. 168, 1027–1033 [DOI] [PubMed] [Google Scholar]
- 62. Suzuki R., Arata S., Nakajo S., Ikenaka K., Kikuyama S., Shioda S. (2003) Expression of the receptor for pituitary adenylate cyclase-activating polypeptide (PAC1-R) in reactive astrocytes. Brain Res. Mol. Brain Res. 115, 10–20 [DOI] [PubMed] [Google Scholar]
- 63. Lue L. F., Rydel R., Brigham E. F., Yang L. B., Hampel H., Murphy G. M., Jr., Brachova L., Yan S. D., Walker D. G., Shen Y., Rogers J. (2001). Inflammatory repertoire of Alzheimer's disease and nondemented elderly microglia in vitro. Glia 35, 72–79 [DOI] [PubMed] [Google Scholar]
- 64. Masmoudi-Kouki O., Gandolfo P., Castel H., Leprince J., Fournier A., Dejda A., Vaudry H., Tonon M. C. (2007) Role of PACAP and VIP in astroglial functions. Peptides 28, 1753–1760 [DOI] [PubMed] [Google Scholar]
- 65. Gottschall P. E., Tatsuno I., Arimura A. (1994) Regulation of interleukin-6 (IL-6) secretion in primary cultured rat astrocytes: synergism of interleukin-1 (IL-1) and pituitary adenylate cyclase activating polypeptide (PACAP). Brain Res. 637, 197–203 [DOI] [PubMed] [Google Scholar]
- 66. Masmoudi-Kouki O., Douiri S., Hamdi Y., Kaddour H., Bahdoudi S., Vaudry D., Basille M., Leprince J., Fournier A., Vaudry H., Tonon M. C., Amri M. (2011) Pituitary adenylate cyclase-activating polypeptide protects astroglial cells against oxidative stress-induced apoptosis. J. Neurochem. 117, 403–411 [DOI] [PubMed] [Google Scholar]
- 67. Li M., Maderdrut J. L., Lertora J. J., Batuman V. (2007) Intravenous infusion of pituitary adenylate cyclase-activating polypeptide (PACAP) in a patient with multiple myeloma and myeloma kidney: a case study. Peptides 28, 1891–1895 [DOI] [PubMed] [Google Scholar]
- 68. Doberer D., Gschwandtner M., Mosgoeller W., Bieglmayer C., Heinzl H., Petkov V. (2007) Pulmonary and systemic effects of inhaled PACAP38 in healthy male subjects. Eur. J. Clin. Invest. 37, 665–672 [DOI] [PubMed] [Google Scholar]