Abstract
The Wnt pathway is a pivotal signaling cascade in colorectal carcinogenesis. The purpose of this work is to determine whether depletion of folate and other metabolically related B vitamins induces in vivo activation of intestinal Wnt signaling and whether this occurs in parallel with increased tumorigenesis. A hybrid mouse was created by crossing a Wnt-reporter animal (BAT-LacZ) with a model of colorectal cancer (Apc1638N). A mild depletion of folate and vitamins B2, B6, and B12 was induced over 16 wk, and the control animals in each instance were pair fed a diet containing the basal requirement of these nutrients. The multiplicity of macroscopic tumors and aberrant crypt foci both increased by ∼50% in the hybrid mice fed the depletion diet (P<0.05). A 4-fold elevation in Wnt signaling was produced by the depletion diet (P<0.05) and was accompanied by significant changes in the expression of a number of Wnt-related genes in a pattern consistent with its activation. Proliferation and apoptosis of the colonic mucosa both changed in a protransformational direction (P<0.05). In summary, mild depletion of multiple B vitamins produces in vivo activation of colonic Wnt signaling, implicating it as a key pathway by which B-vitamin inadequacies enhance intestinal tumorigenesis.—Liu, Z., Ciappio, E. D., Crott, J. W., Brooks, R. S., Nesvet, J., Smith, D. E., Choi, S.-W., Mason, J. B. Combined inadequacies of multiple B vitamins amplify colonic Wnt signaling and promote intestinal tumorigenesis in BAT-LacZ×Apc1638N mice.
Keywords: colorectal cancer, folate, methylation
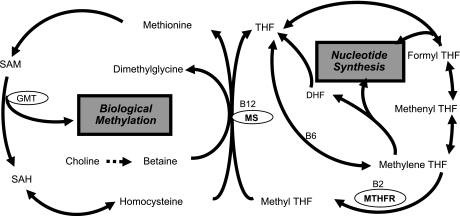
The consensus of preclinical and epidemiological studies indicates that diminished folate status increases the risk of colorectal carcinogenesis (1, 2). A flagrant deficiency of folate is not necessary to produce this increased risk of cancer: the effect is incremental, such that individuals whose folate status merely resides at the lower end of the normal range are subject to a substantially greater risk of colorectal cancer compared with others whose intake or status is greater (3). Moreover, increasing evidence indicates that other metabolically related B vitamins (B2, B6, and B12) may affect the ability of folate to modulate colorectal cancer risk (4–6). This is true because the metabolic functions of these B vitamins are highly interdependent (7), such that depletion of one often leads to biochemical phenotypes characteristic of deficiencies of the others (Fig. 1).
Figure 1.
Pathways of 1-carbon metabolism in the cytoplasm. The 4 B vitamins, folate, B2, B6, and B12, each serve important roles as cofactors. MTHFR, methylenetetrahydrofolate reductase; MS, methionine synthase; GMT, general methyltransferase family; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; THF, tetrahydrofolate; DHF, dihydrofolate.
Studying mild inadequacies of multiple B vitamins is entirely relevant to industrialized nations, where flagrant deficiencies of these vitamins are rare, but marginal deficiencies of multiple B vitamins still remain highly prevalent. The folate status of the U.S. population has improved substantially since the institution of mandatory folic acid fortification in 1996. Nevertheless, based on recent nationally representative data from the National Health and Nutrition Examination Survey (8), inadequacies in total folate intake are still felt to exist in certain segments of the population. For example, ∼22% of reproductive-age women, 19% of 14- to 18-yr-olds, 17% of 19- to 30-yr-old women, and 23% of non-Hispanic black women in U.S. still did not meet even the Estimated Average Requirements, which is a value that is lower than the Recommended Dietary Allowance. Population-based studies in industrialized nations have reported that 18–25% of adults have low B6 status, and 10–20% of healthy elders display “subclinical” B12 deficiency (9, 10). Also, the most recent nationally representative nutrition survey of the United Kingdom reveals that >50% of adults have subnormal levels of the most accurate blood marker of riboflavin status (11).
It has been suggested that 2 critical cellular functions of folate, namely the maintenance of normal patterns of biological methylation and nucleotide synthesis, are thought to be instrumental in mediating its effect on cancer risk and that these functions depend not only on the adequacy of folate but also on the availability of vitamin B2, B6, and B12 (7, 12, 13). However, the precise mechanisms by which combined inadequacies of these dietary B vitamins contribute to cancer risk have not been clearly delineated. Our prior work (14) demonstrated that mild depletion of folate, riboflavin, and vitamin B6 and B12 alters several elements of the Wnt pathway in an overall pattern consistent with activation of the cascade. This is important, since aberrant signaling along the Wnt pathway is an early event in 90% of human colorectal cancers and is thought to play an important mechanistic role at an early stage of colorectal tumorigenesis (15, 16). We embarked on the present study to provide definitive proof of in vivo Wnt activation in the colonic mucosa due to multiple B-vitamin depletion by using a Wnt-reporter mouse model and to establish that this occurs in conjunction with increased intestinal tumorigenesis, thereby implicating Wnt signaling as an avenue by which the depletion state enhances carcinogenesis.
A variety of genetic and epigenetic aberrations in components of the Wnt pathway have been identified in human colorectal neoplasms (17, 18). For instance, classical mutations of Apc are frequently observed (18), but de novo methylation of the Apc promoter region may also play an important role as a “second hit” in silencing Apc expression (19). Besides the Apc gene, epigenetic silencing of extracellular Wnt inhibitors (e.g., the SFRP family, Wnt inhibitory factor-1, and the DKK family) are frequently present in colorectal neoplasms (15, 20, 21). Folate plays a critical role in maintaining the integrity of both epigenetic and genetic features of the genome by donating 1-carbon moieties for biological methylation and nucleotide synthesis (22, 23). We have shown that severe folate depletion creates excess DNA strand breakage in the Apc gene and concomitantly diminishes its expression. More recently, we demonstrated that only a very mild depletion of folate is required to induce this molecular anomaly if it is present in conjunction with the depletion of other 1-carbon vitamins, and we further demonstrated that it alters several components of Wnt cascade (14). Other researchers have also observed changes in the expression of several extracellular modulators of the Wnt pathway in mammalian cell culture as a result of folate depletion (25). Indirect evidence that folate depletion alters the Wnt pathway can also be drawn from studies of a rodent model of folate-sensitive neural tube birth defects (26), as well as from a cell culture study (27). Therefore, disparate pieces of evidence indicate that folate depletion may induce alterations in various elements of the Wnt pathway, but it is not clear whether these alterations truly activate Wnt signaling, and by doing so, accelerate tumorigenesis. By using the BAT-LacZ mouse, which expresses a β-galactosidase reporter gene only in the presence of β-catenin-activated Wnt signaling (28), and a colorectal cancer mouse model (Apc1638N), the current study addressed the following important questions: whether 1-carbon vitamin depletion activates Wnt signaling in the murine colon and whether that activation occurs in conjunction with enhanced intestinal tumorigenesis.
It is important to note here that some recent observations suggest that habitual intake of supraphysiologic quantities of folate, administered to individuals who already harbor neoplastic foci, may produce a paradoxical promotion of cancer (29). The present study, however, focused on inadequacies of these nutrients, not supraphysiologic supplementation. Moreover, this study was conducted within an early time frame of tumorigenesis in the Apc1638N model, and its focus was concentrated on the Wnt pathway, which is an early event in human colorectal cancers (16). Thus, any potential paradoxical promoting effects of these nutrients were not an issue in this study.
MATERIALS AND METHODS
Animals and diets
A Wnt-reporter mouse, the BAT-LacZ animal, was used in these experiments to demonstrate in vivo activation of canonical Wnt signaling. In this mouse, the expression of the inserted β-galactosidase gene is proportional to enhanced transcription due to binding by intranuclear β-catenin to an enhancer element that normally mediates Wnt signaling (28). This mouse model has been used by several studies for definitive proof of the canonical Wnt signaling activation (30). The tumorigenic model, Apc1638N, was intentionally selected to investigate the modulation of intestinal tumorigenesis. This model possesses a mild tumorigenic phenotype (31), which is particularly useful for this type of experiment, since the very aggressive tumorigenic phenotypes present in many of the more commonly used models, such as the Apcmin mouse, readily overwhelm the modest effects produced by nutritional interventions.
Both strains, which are on the C57BL/6 background, were bred and cross-bred in our center. The protocol was approved by the Institutional Animal Care and Use Committee at the Jean Mayer U.S. Department of Agriculture (USDA) Human Nutrition Research Center on Aging at Tufts University. Eventually, 18 BAT-LacZ mice and 21 hybrid mice of the cross between the BAT-LacZ and Apc1638N mouse were generated and used in this experiment. These animals were pair fed one of two experimental diets that contained different levels of folate and vitamin B2, B6, and B12 (Table 1) for 16 wk beginning at 8 wk of age. The amino acid-defined diet was originally designed to precisely govern folate status (32) and has been used extensively in our previous studies (33). It is noteworthy that diets intended to induce very mild degrees of dietary vitamin depletion were used in this study; this issue is discussed in greater depth in the Discussion.
Table 1.
Concentrations of 1-carbon metabolism-related B vitamins in experimental diets
| Treatment | Riboflavin (mg/kg) | Pyridoxine HCl (mg/kg) | B12 (μg/kg) | Folic acid (mg/kg) |
|---|---|---|---|---|
| Folate sufficiency (control) | 6.0 | 7.0 | 5.0 | 2.0 |
| Multiple vitamin depletion | 0.5 | 0.5 | 0.0 | 0.0 |
Preparation of tissue samples and isolated colonocytes
After 16 wk of consuming the experimental diets, all mice were anesthetized and then exsanguinated by cardiac puncture. Colonocytes were isolated with 30 mM EDTA at 4°C as described previously (34) and utilized for subsequent expression arrays of Wnt elements and determination of the β-galactosidase (Wnt-reporter) gene expression. The mucosa of the cecum was gently removed by scraping after being washed with ice-cold saline. These mucosal scrapings were later used for the analyses of tissue folate and protein expression. A segment from the central portion of the colon was excised and fixed in 10% buffered formalin overnight followed by paraffin embedding for immunohistochemical assays.
Vitamin concentrations in blood and colonic mucosa
Plasma and colonic mucosa folate concentrations were determined by a conventional microbiological microtiter plate assay using a Lactobacillus casei assay (35). Vitamin B2 was measured by the erythrocyte glutathione reductase activity coefficient assay (36). Vitamin B12 was measured by a competitive protein binding assay (Bio-Rad Laboratories, Hercules, CA, USA).
Wnt pathway-specific microarray analysis
To identify genes within the Wnt pathway network whose expression is sensitive to 1-carbon vitamin depletion, we utilized a Wnt pathway-specific array (PAMM-043A; 6 arrays/group; SABiosciences, Frederick, MD, USA). This array profiles the expression of 84 genes related to Wnt-mediated signal transduction, including signaling molecules, cell-surface receptors, signal regulators, and target genes. The complete gene list is available at the manufacturer's website (http://www.sabiosciences.com).
Briefly, colonocytes were isolated from the proximal half of the colon using 30 mM EDTA at 4°C. Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) and purified using the RNeasy Mini Cleanup kit (Qiagen, Valencia, CA, USA). cDNA was synthesized using the RT2 First Strand Kit (SABiosciences). The PCR array was performed on an ABI7300 real-time PCR system (Applied Biosystems, Carlsbad, CA, USA).
Analysis of Wnt-responsive β-galactosidase expression
Wnt signaling activity was characterized by the transcriptional expression of the β-galactosidase transgene in the mouse colon. β-Galactosidase transcript was determined by real-time PCR. The relative expression of the β-galactosidase was quantified using SYBR Green dye and an ABI7300 real-time PCR machine. The forward and reverse sequences for β-galactosidase are 5′-GATCTTCCTGAGGCCGATACTG-3′ and 5′-GGCGGATTGACCGTAATGG-3′, respectively (37). Statistical analyses were performed using ΔCt, and the relative expression values were reported.
Immunohistochemical analysis for proliferation and apoptosis
Immunohistochemical quantifications of Ki-67 and cleaved caspase-3 antigens were used as measures of proliferation and apoptosis, respectively. Antibody targeting Ki-67 was from Abcam (Cambridge, MA, USA), and antibody for caspase-3 was from Cell Signaling (Danvers, MA, USA). Proliferation was determined by counting the number of positive epithelial cells per crypt divided by the total number of crypt cells (i.e., the proliferation index). Longitudinal sections of crypts were selected for scoring only if the base of the crypt touched the muscularis mucosa and had an open lumen at the top. Approximately 20 crypts were counted for each mouse in a masked procedure. Apoptosis was estimated by scoring the slides into 4 grades: 0, no staining; 1, mild staining; 2, moderate staining; and 3, heavy staining.
Histopathology for aberrant crypt foci (ACFs) and quantification of tumor incidence
Immediately after death, the entire gastrointestinal tract was examined for tumors by 2 examiners (E.D.C. and J.W.C.), both of whom were masked to the randomization scheme. The distal potion of colon from the BAT-LacZ×Apc1638N mice was used for colonic ACF quantification after formalin fixation and 0.2% methylene blue staining. The same standard criteria (38) were used for the determination and quantification of ACFs in the colon of the Apc1638N mice.
Statistical analysis
Analysis of the Wnt pathway microarray data was performed using the Web Portal provided by SABiosciences (http://www.SABiosciences.com/pcrarraydataanalysis.php). Data for other endpoint analyses were conducted by using analysis of variance (ANOVA) or χ test with SAS v9.2 software (SAS Institute, Cary, NC, USA). Dietary effects on proliferation and apoptosis were assessed using the data combined from the two genetic mouse models with the analysis of covariance (ACOVA) method to adjust the variations between the models. Values in the text are presented as means ± se.
RESULTS
Mild dietary 1-carbon vitamin depletion successfully induced vitamin depletion in blood and tissue, but without anemia or outward signs of disease
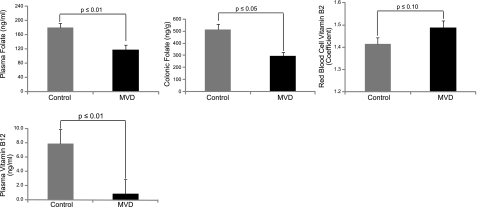
The multiple B-vitamin depletion diet formulated in this study successfully induced mild vitamin depletion, which is consistent with our previous observations (14). Vitamin status is presented in Fig. 2. There were no significant differences with respect to vitamin status between the models (BAT-LacZ and BAT-LacZ×Apc1638N). Therefore, the biochemical data reported in Fig. 2 represent pooled data from these models. Consumption of the vitamin-deficient diet resulted in a 37% reduction of plasma folate concentration (P≤0.01) compared with the vitamin-replete group. Similarly, plasma vitamin B12 was significantly diminished (P≤0.01) in the deficient group. There was also a trend toward reduction (P=0.10) in erythrocyte vitamin B2, measured in a reciprocal fashion by the activity coefficient of glutathione peroxidase. Vitamin B6 was not measured due to the limited amount of plasma that was available. However our prior published work, which used the same depletion diet and a nearly identical protocol, showed a significant 50% reduction in plasma pyridoxal 5′-phosphate at the end of the depletion period (14). The mild degree of these depletion states was further underscored by colonic folate: colonic folate concentration was 22% lower (P≤0.05) in the depletion group than in the control group.
Figure 2.
B-vitamin status in blood and colonic tissue. Means represent pooled data from the BAT-lacZ and BAT-lacZ×Apc1638N models, since the values for the models do not differ significantly. Plasma and colonic folate concentrations were determined by a conventional microbiological microtiter plate assay using L. casei. Vitamin B2 was measured by the erythrocyte glutathione reductase activity coefficient assay; B12 was determined by a competitive protein binding assay. B6 was not measured due to limited sample, although such data appears for the same diet in a prior publication (14). MVD, multiple vitamin depletion. Control, n = 19 mice; MVD, n = 21 mice.
Mild dietary 1-carbon vitamin depletion induced transcriptional alterations of multiple elements within Wnt signaling pathway
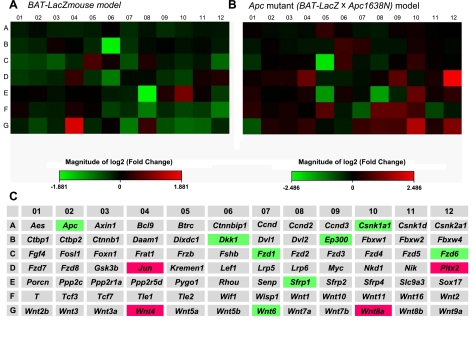
We employed a Wnt pathway-focused microarray (SAB Biosciences) to compare the gene expression profiles. As would be expected from an animal possessing a defective Apc allele, there were a large number of Wnt elements whose expressions changed in the BAT-LacZ×Apc1638N animals compared with the BAT-LacZ animals; 33 of 84 genes in the microarray underwent significant change (Supplemental Fig. S1). The observed changes in gene expression are nearly all consistent with Wnt activation in the Apc1638N colon; for instance, Apc gene expression was decreased in the Apc1638N mouse model, and downstream genes whose expression increases with Wnt signaling, such as Pitx2, Jun, and Cyclin D1, were each significantly up-regulated.
Next, we examined the effect of diet on Wnt pathway genes. In the BAT-LacZ mice, the expression of several extracellular Wnt inhibitors, including SFRP1 and DKK1, was decreased 3- to 4-fold (P=0.016 and 0.082, respectively) by multiple vitamin depletion. In the BAT-LacZ×Apc1638N mice, the spectrum of gene expression changes due to vitamin depletion were somewhat different, as might be expected. Most notably, Apc gene expression was decreased ∼27% (P=0.032) by B-vitamin depletion. Downstream target genes, such as Jun and Pitx2, were significantly increased by multiple vitamin depletion in the Apc1638N colon (Fig. 3). Details of the genes that underwent significant changes are shown in Table 2.
Figure 3.
A, B) Changes in steady-state transcript levels in Wnt pathway genes induced by multiple B-vitamin depletion in BAT-LacZ mice (A) and Apc1638N×BAT-LacZ hybrid mice (B). C) Names of each of the 84 genes in the heat maps, highlighting genes that underwent significant changes due to depletion in either of the models: red indicates up-regulation due to depletion; green indicates down-regulation. In total, 24 Wnt-specific arrays were performed; 6 animals in each of the 2 dietary groups were compared. More details regarding these genes are displayed in Table 2.
Table 2.
Genes that underwent significant changes induced by B-vitamin depletion in the BAT-LacZ and BAT-LacZ×Apc1638N models
| Gene | Cell | Description | Fold | P |
|---|---|---|---|---|
| BAT-LacZ | ||||
| Csnk1a1 | A10 | Casein kinase 1, α 1 | −1.16 | 0.002 |
| Sfrp1 | E08 | Secreted frizzled-related protein 1 | −3.68 | 0.016 |
| Wnt6 | G07 | Wingless-related MMTV integration site 6 | −1.86 | 0.017 |
| Fzd1 | C07 | Frizzled homolog 1 (Drosophila) | −1.50 | 0.028 |
| Wnt4 | G04 | Wingless-related MMTV integration site 4 | 3.05 | 0.040 |
| Fzd6 | C12 | Frizzled homolog 6 (Drosophila) | −1.70 | 0.049 |
| Dkk1 | B06 | Dickkopf homolog 1 (Xenopus laevis) | −3.47 | 0.082 |
| BAT-LacZ×Apc1638N | ||||
| Wnt8a | G10 | Wingless-related MMTV integration site 8A | 3.34 | 0.005 |
| Jun | D04 | Jun oncogene | 2.01 | 0.027 |
| Apc | Adenomatosis polyposis coli | −1.27 | 0.032 | |
| Ep300 | B09 | E1A binding protein p300 | −1.13 | 0.033 |
| Pitx2 | D12 | Paired-like homeodomain transcription factor 2 | 5.60 | 0.039 |
Positive fold change indicates up-regulation due to vitamin depletion; negative change indicates down-regulation. Changes in expression for each model were determined by comparing 6 animals in each of the 2 dietary groups (24 arrays in total).
In vivo Wnt signaling was increased by the deplete diet
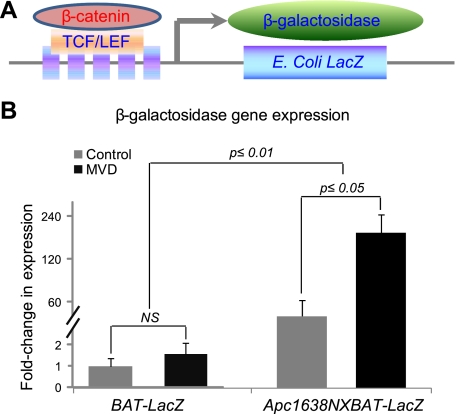
In the BAT-LacZ mouse model, the metric of functional activation of Wnt signaling is the inserted β-galactosidase transcriptional expression (28). As demonstrated in Fig. 4, the presence of a mutation in 1 Apc allele in the BAT-LacZ×Apc1638N mouse model significantly increased β-galactosidase expression when compared with the model with intact Apc alleles in the BAT-LacZ model (P<0.01). When comparing the 2 control groups, the mutant Apc allele in the BAT-LacZ×Apc1638N mouse model increased β-galactosidase expression by ∼50-fold, establishing the marked degree of Wnt activation that occurs in the colonic epithelium as a result of the defective Apc gene in this mouse strain. Mild B-vitamin depletion induced a 58% increase in the β-galactosidase transcript in the BAT-LacZ model (P>0.05), whereas a marked ∼4-fold increase was observed (P≤0.05) in the BAT-LacZ×Apc1638N animal. Thus, the presence of one damaged allele in Apc sensitizes the colonic epithelium to the effects of B-vitamin depletion on Wnt signaling.
Figure 4.
In vivo Wnt signaling in the colon, determined by β-galactosidase expression. A) BAT-LacZ construct. B) β-Galactosidase expression by real-time PCR. Multiple vitamin depletion (MVD) increased expression by 58% in the BAT-lacZ mice (not significant) and by ∼4-fold in the BAT-LacZ×Apc1638N model (P<0.05). Data analysis was performed based on ΔCt. Data for the BAT-LacZ model is presented in a linear scale; data for the BAT-LacZ×Apc1638N model is presented in a logarithmic scale. BAT-LacZ model: n = 9 mice/group. BAT-LacZ×Apc1638N model: control, n = 10 mice; MVD, n = 11. Note that the presence of a mutant Apc allele in the hybrid, independent of diet, significantly increases β-galactosidase expression when compared with the BAT-LacZ model (P<0.01).
Multiple 1-carbon vitamin depletion resulted in protransformational alterations in cell cycle kinetics in the colonic epithelium
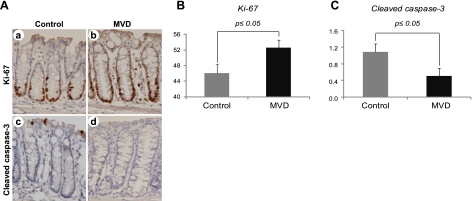
Proliferation was assessed in a blinded fashion by evaluating the expression of Ki-67, a nuclear protein that is required for cellular proliferation (39). Increases in proliferation due to the depletion diet were observed for both the BAT-LacZ and Apc1638N×BAT-LacZ models. Pooling the data from the models revealed an overall 7% increase in the proliferation index (P≤0.05) due to the imposition of the depletion diet (Fig. 5).
Figure 5.
Effects of B-vitamin depletion on proliferation and apoptosis. A) Representative photomicrographs of proliferation (Ki-67; a, b) and apoptosis (cleaved caspase-3; c, d) in control (a, c) and multiple vitamin depletion (MVD; b, d) groups. Brown color indicates positive immunohistochemical staining. B, C) MVD induced a significant increase in the proliferation index (Ki-67) in the colonic epithelial cells (B) and a decrease in apoptosis (cleaved caspase-3; C). Control, n = 19 mice; MVD, n = 21 mice.
Apoptosis was evaluated by measuring the expression of cleaved caspase-3, an integral component of the caspase cascade that occurs in apoptosis (40). The depletion diet produced a numerical decline in apoptosis in both animal models. An analysis of the combined data from the models demonstrates a significant decrease in apoptosis (P≤0.05) resulting from multiple 1-carbon vitamin depletion (Fig. 5).
Tumor multiplicity and ACFs increased in the mouse fed a multiple 1-carbon vitamin depletion diet
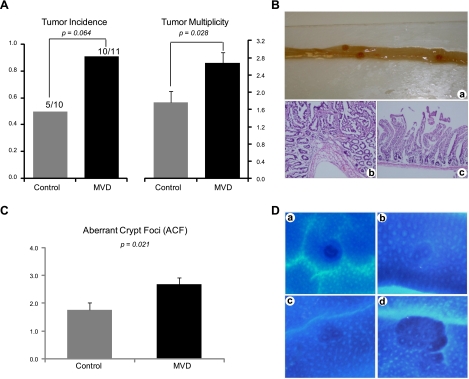
The numbers of tumors in the small intestine and colon, which were assessed in a masked fashion, were significantly different between the control group and the vitamin depletion group (Fig. 6A). In the control group, tumors were observed in 5 of 10 mice, whereas 10 of 11 mice possessed tumors in the depletion group (P=0.064). Tumor multiplicity, which indicates the number of tumors per mouse, was significantly greater (P=0.028) in the depletion group than in the control group. Histopathological pictures of representative tumors are shown in Fig. 6B.
Figure 6.
Effects of B-vitamin depletion on tumorigenesis in the BAT-LacZ×Apc1638N hybrid. A) Effect of multiple vitamin depletion (MVD) on tumor incidence (percentage of mice bearing tumors) and tumor multiplicity (average number of tumors per mouse in each group). B) Representative mouse intestine bearing 3 tumors (a), histopathology of a typical adenoma (b), and adjacent normal intestine (c). C) Effect of MVD on ACFs. D) Representative ACFs identified in BAT-LacZ×Apc1638N mice, containing different numbers of aberrant crypts: 1 aberrant crypt (a), 2 aberrant crypts (b), 3 aberrant crypts (c), and multiple aberrant crypts (d). Control, n = 10 hybrid mice; MVD, n = 11 mice.
ACFs were quantitated in the distal half of each colon of the hybrid animals. The number of ACFs is significantly greater (P=0.021) in the depletion group (Fig. 6C, D) when compared with the control. We observed far fewer ACFs in the Apc1638N mice than was reported by Pretlow et al. (31). This is probably because ACFs were counted only in the distal half of the colon in our experiment, and we utilized a more stringent definition for ACFs than Pretlow et al. (31).
DISCUSSION
The present study provides definitive evidence of in vivo activation of Wnt signaling in the colon resulting from mild depletion of multiple B vitamins that are metabolically interrelated in 1-carbon metabolism. Moreover, by crossing the Wnt-reporter mouse with a model of intestinal tumorigenesis, we demonstrate that the activation of the Wnt pathway occurs in parallel with increased micro- and macroscopic tumorigenesis due to the depletion diet, thereby implicating this signaling pathway as one that is mechanistically involved in mediating the protumorigenic effect. This contrasts with our previous study (14), in which activation of the colonic Wnt pathway could only be inferred indirectly from the pattern of changes that occurred in various elements of the Wnt pathway and in which no links with tumorigenesis were established.
Altered expression of several elements in the Wnt cascade occurred, as would be expected from a perturbation that increases throughput through this pathway. It is difficult to fully integrate all the observed changes in the Wnt pathway-specific microarray into a single coherent mechanistic scheme, since there does not yet exist a comprehensive understanding of the feedback mechanisms, post-transcriptional processing, and regulation that exists in this pathway. However, nearly all of the observed changes in gene expression due to the Apc1638N allele, or the combined depletion of multiple B vitamins, are consistent with Wnt activation. Notably, a significant decrease in Apc gene expression was shown in the BAT-LacZ×Apc1638N mice fed a depletion diet, an observation that recapitulates our prior observations in the rodent colon (14, 24).
It is potentially of considerable relevance that, in contrast to the hybrid mice, the decrease in Apc expression in the BAT-LacZ mice due to the diet failed to reach statistical significance. This indicates that the modest effect produced by this dietary perturbation is more likely to have a molecular effect in the colon under circumstances where the expression of Apc is already handicapped by the presence of one damaged allele. Moreover, this differential between the models was also quite evident in overall Wnt activation. The “2-hit” hypothesis of tumor suppressor gene inactivation postulates that the process of carcinogenesis only proceeds when both alleles of a critical tumor suppressor are inactivated (41). Our observations suggest that B-vitamin depletion is much more effective at suppressing Apc expression when one Apc allele is already damaged, as it is in the BAT-LacZ×Apc1638N hybrid. Similarly, on a functional level, our data demonstrate that the ability of B-vitamin depletion to activate Wnt signaling is considerably greater when one Apc allele is already inactivated. Although the precise means by which B-vitamin depletion effects these changes remains unclear, our prior work has shown that the depletion state induces DNA strand breaks in the coding region of the Apc gene and that this occurs in parallel with diminished Apc expression (14, 24). Strand breaks have been reported to interfere with gene expression (42), and therefore, this may be the mechanism in question.
Altered expression of components of the Wnt signaling cascade due to the diet was also observed in upstream regulators including Wnt ligand molecules (Wnt4, Wnt6, and Wnt8a), soluble Wnt ligand antagonists (SFRP1 and DKK1), as well as the Wnt receptors (Fzd1 and Fzd6). Interestingly, epigenetic alterations in colorectal carcinogenesis have been frequently observed in 2 Wnt antagonists: SFRP1 (15) and DKK1 (20). Thus, dietary depletion of B vitamins might contribute to the decreased expression of these genes and increased Wnt signaling through an epigenetic mechanism. As for the downstream genes, increased expression of Jun and Pitx2 was induced by the multiple vitamin depletion diet. Both Jun and Pitx2 are involved in numerous cellular processes, including proliferation, transformation, and apoptosis, and are well characterized as genes whose expression is upregulated by the Wnt signaling (43, 44). Nevertheless, there are ∼50 genes whose expression is thought to be partially regulated by canonical Wnt signaling (http://www.stanford.edu/group/nusselab/cgi-bin/wnt/), and not all of these genes were observed to be upregulated. However, most of these genes are also regulated by a number of other pathways, so it is not surprising that not all downstream genes would follow an identical pattern.
The inserted β-galactosidase element in the BAT-LacZ mice is expressed only under conditions of activated Wnt signaling due to the presence of β-catenin-mediated stimulation of transcription (28), enabling us to definitively demonstrate that combined depletion of multiple B vitamins results in increased canonical Wnt signaling in the mouse colon. The depletion induced a 58% increase of β-galactosidase expression in the BAT-LacZ mouse model in which both Apc alleles are intact, whereas the magnitude increased to 4-fold in the BAT-LacZ×Apc1638N mice. As stated above, with a mutant Apc allele, Wnt activation in the BAT-LacZ×Apc1638N mouse model appears to be far more susceptible to the dietary perturbation utilized in these studies.
Accompanying the amplification in Wnt signaling produced by the diet were alterations in cell cycle kinetics of the colonic epithelium that would be expected from Wnt activation: increased proliferation and decreased apoptosis. Indeed, it is these changes in the cell cycle that are likely to be responsible, in part, for the enhancement of tumorigenesis that accompanies inappropriate Wnt activation. This was borne out by our observations of increased ACF and multiplicity of macroscopic neoplasms.
It is well recognized that all nonhuman models of colorectal cancer have limitations, and this includes the Apc1638N mice. Virtually all genetic models of colorectal carcinogenesis in rodents (including the widely used Apcmin mice) have a strong predilection for forming small intestinal, rather than colonic, neoplasms, and we have found this to be true with the Apc1638N mouse model as well. Although the original characterization of this model (45) indicated that it develops colorectal tumors as well, we have found this to be a relatively rare event. Nevertheless, genetically induced models of colorectal carcinogenesis have been used with considerable effectiveness to study the effects of diet on cancer development, since the small intestinal tumorigenesis generally responds in a fashion that mimics the effects of diet on the colon, and this has certainly been true of the Apc1638N mice (46, 47). Also, the Wnt pathway is known to modulate proliferation and apoptosis in both the small intestinal epithelium and the colon, and to this extent one can infer changes in cell cycle due to the mutation from one organ to the other.
It is noteworthy that only mild dietary vitamin depletion was used in this study. As documented in detail in our earlier publication in which the same diet was used (14), the very mild systemic vitamin depletion in this study is different from our previous studies (5, 48) in which moderate or severe depletion diets were used. By utilizing the mild diet, plasma folate decreases by only 30–50%, and colon folate decreases by ∼40%, whereas plasma and colon folate are decreased by 10 and 2 times, respectively, by moderate dietary folate depletion in rats (48). Plasma homocysteine, which can be perceived as a measure of the integrated capability for methylation by 1-carbon metabolism, increases only slightly (∼30%) in the multiple depleted group compared with the replete group, whereas it increases ∼4-fold in rodents on a diet moderately deficient in folate (48). Also, when compared with earlier rodent studies (5, 49, 50), the magnitude of the riboflavin, vitamin B6, and vitamin B12 deficiencies imposed in this study were of a considerably milder degree. Physiologically, no anemia or retardation in weight gain occurs with these mild levels of depletion, which are sometimes evident with more severe degrees of depletion.
In summary, these observations provide conclusive evidence that mild depletion of the 4 B vitamins that participate in 1-carbon metabolism, a nutritional condition that has relevance to populations in industrialized nations, amplifies Wnt signaling in the colonic epithelium, produces protransformational changes in the cell cycle, and enhances tumorigenesis. Mechanistic insight into the molecular basis by which inadequate intake of folate, or other B vitamins, enhance colorectal cancer risk is an important step in devising strategies to exploit diet to minimize the burden of this cancer in our society.
Supplementary Material
Acknowledgments
This work was supported in part by the Prevent Cancer Foundation (to Z.L.), U.S. National Institutes of Health grant R21 ES019102 (to J.B.M.), and the USDA Agricultural Research Service (agreement 1950-074-01S). Any opinions, findings, conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Giovannucci E. (2002) Epidemiologic studies of folate and colorectal neoplasia: a review. J. Nutr. 132, 2350S–2355S [DOI] [PubMed] [Google Scholar]
- 2. Kim Y. I. (2004) Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ. Mol. Mutagen. 44, 10–25 [DOI] [PubMed] [Google Scholar]
- 3. Fuchs C. S., Willett W. C., Colditz G. A., Hunter D. J., Stampfer M. J., Speizer F. E., Giovannucci E. L. (2002) The influence of folate and multivitamin use on the familial risk of colon cancer in women. Cancer Epidemiol. Biomarkers Prev. 11, 227–234 [PubMed] [Google Scholar]
- 4. Wei E. K., Giovannucci E., Selhub J., Fuchs C. S., Hankinson S. E., Ma J. (2005) Plasma vitamin B6 and the risk of colorectal cancer and adenoma in women. J. Natl. Cancer Inst. 97, 684–692 [DOI] [PubMed] [Google Scholar]
- 5. Choi S. W., Friso S., Ghandour H., Bagley P. J., Selhub J., Mason J. B. (2004) Vitamin B-12 deficiency induces anomalies of base substitution and methylation in the DNA of rat colonic epithelium. J. Nutr. 134, 750–755 [DOI] [PubMed] [Google Scholar]
- 6. Eussen S. J., Vollset S. E., Hustad S., Midttun O., Meyer K., Fredriksen A., Ueland P. M., Jenab M., Slimani N., Boffetta P., Overvad K., Thorlacius-Ussing O., Tjonneland A., Olsen A., Clavel-Chapelon F., Boutron-Ruault M. C., Morois S., Weikert C., Pischon T., Linseisen J., Kaaks R., Trichopoulou A., Zilis D., Katsoulis M., Palli D., Pala V., Vineis P., Tumino R., Panico S., Peeters P. H., Bueno-de-Mesquita H. B., van Duijnhoven F. J., Skeie G., Munoz X., Martinez C., Dorronsoro M., Ardanaz E., Navarro C., Rodriguez L., VanGuelpen B., Palmqvist R., Manjer J., Ericson U., Bingham S., Khaw K. T., Norat T., Riboli E. Plasma vitamins B2, B6, and B12, and related genetic variants as predictors of colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 19, 2549–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ulrey C. L., Liu L., Andrews L. G., Tollefsbol T. O. (2005) The impact of metabolism on DNA methylation. Hum. Mol. Genet. 14(Spec. 1), R139–R147 [DOI] [PubMed] [Google Scholar]
- 8. Bailey R. L., Dodd K. W., Gahche J. J., Dwyer J. T., McDowell M. A., Yetley E. A., Sempos C. A., Burt V. L., Radimer K. L., Picciano M. F. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am. J. Clin. Nutr. 91, 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Planells E., Sanchez C., Montellano M. A., Mataix J., Llopis J. (2003) Vitamins B6 and B12 and folate status in an adult Mediterranean population. Eur. J. Clin. Nutr. 57, 777–785 [DOI] [PubMed] [Google Scholar]
- 10. Lindenbaum J., Rosenberg I. H., Wilson P. W., Stabler S. P., Allen R. H. (1994) Prevalence of cobalamin deficiency in the Framingham elderly population. Am. J. Clin. Nutr. 60, 2–11 [DOI] [PubMed] [Google Scholar]
- 11. Henderson L., Irving K., Gregory J. (2004) National Diet and Nutrition Survey: Adults Aged 19–64, Office for National Statistics and Medical Research Council Human Nutrition Research, London, UK [Google Scholar]
- 12. Van den Veyver I. B. (2002) Genetic effects of methylation diets. Annu. Rev. Nutr. 22, 255–282 [DOI] [PubMed] [Google Scholar]
- 13. Davis C. D., Uthus E. O. (2004) DNA methylation, cancer susceptibility, and nutrient interactions. Exp. Biol. Med. (Maywood) 229, 988–995 [DOI] [PubMed] [Google Scholar]
- 14. Liu Z., Choi S. W., Crott J. W., Keyes M. K., Jang H., Smith D. E., Kim M., Laird P. W., Bronson R., Mason J. B. (2007) Mild depletion of dietary folate combined with other B vitamins alters multiple components of the Wnt pathway in mouse colon. J. Nutr. 137, 2701–2708 [DOI] [PubMed] [Google Scholar]
- 15. Suzuki H., Watkins D. N., Jair K. W., Schuebel K. E., Markowitz S. D., Chen W. D., Pretlow T. P., Yang B., Akiyama Y., Van Engeland M., Toyota M., Tokino T., Hinoda Y., Imai K., Herman J. G., Baylin S. B. (2004) Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat. Genet. 36, 417–422 [DOI] [PubMed] [Google Scholar]
- 16. Fodde R., Smits R., Clevers H. (2001) APC, signal transduction and genetic instability in colorectal cancer. Nat. Rev. Cancer 1, 55–67 [DOI] [PubMed] [Google Scholar]
- 17. Baylin S. B., Ohm J. E. (2006) Epigenetic gene silencing in cancer–a mechanism for early oncogenic pathway addiction? Nat. Rev. Cancer 6, 107–116 [DOI] [PubMed] [Google Scholar]
- 18. Su L. K., Kinzler K. W., Vogelstein B., Preisinger A. C., Moser A. R., Luongo C., Gould K. A., Dove W. F. (1992) Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256, 668–670 [DOI] [PubMed] [Google Scholar]
- 19. Arnold C. N., Goel A., Niedzwiecki D., Dowell J. M., Wasserman L., Compton C., Mayer R. J., Bertagnolli M. M., Boland C. R. (2004) APC promoter hypermethylation contributes to the loss of APC expression in colorectal cancers with allelic loss on 5q. Cancer Biol. Ther. 3, 960–964 [DOI] [PubMed] [Google Scholar]
- 20. Aguilera O., Fraga M. F., Ballestar E., Paz M. F., Herranz M., Espada J., Garcia J. M., Munoz A., Esteller M., Gonzalez-Sancho J. M. (2006) Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene 25, 4116–4121 [DOI] [PubMed] [Google Scholar]
- 21. Taniguchi H., Yamamoto H., Hirata T., Miyamoto N., Oki M., Nosho K., Adachi Y., Endo T., Imai K., Shinomura Y. (2005) Frequent epigenetic inactivation of Wnt inhibitory factor-1 in human gastrointestinal cancers. Oncogene 24, 7946–7952 [DOI] [PubMed] [Google Scholar]
- 22. Pufulete M., Al-Ghnaniem R., Leather A. J., Appleby P., Gout S., Terry C., Emery P. W., Sanders T. A. (2003) Folate status, genomic DNA hypomethylation, and risk of colorectal adenoma and cancer: a case control study. Gastroenterology 124, 1240–1248 [DOI] [PubMed] [Google Scholar]
- 23. Blount B. C., Mack M. M., Wehr C. M., MacGregor J. T., Hiatt R. A., Wang G., Wickramasinghe S. N., Everson R. B., Ames B. N. (1997) Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc. Natl. Acad. Sci. U. S. A. 94, 3290–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim Y. I., Shirwadkar S., Choi S. W., Puchyr M., Wang Y., Mason J. B. (2000) Effects of dietary folate on DNA strand breaks within mutation-prone exons of the p53 gene in rat colon. Gastroenterology 119, 151–161 [DOI] [PubMed] [Google Scholar]
- 25. Katula K. S., Heinloth A. N., Paules R. S. (2007) Folate deficiency in normal human fibroblasts leads to altered expression of genes primarily linked to cell signaling, the cytoskeleton and extracellular matrix. J. Nutr. Biochem. 18, 541–552 [DOI] [PubMed] [Google Scholar]
- 26. Carter M., Chen X., Slowinska B., Minnerath S., Glickstein S., Shi L., Campagne F., Weinstein H., Ross M. E. (2005) Crooked tail (Cd) model of human folate-responsive neural tube defects is mutated in Wnt coreceptor lipoprotein receptor-related protein 6. Proc. Natl. Acad. Sci. U. S. A. 102, 12843–12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han S. P., Pan Y., Peng Y. Z., Gu X. Q., Chen R. H., Guo X. R. (2009) Folbp1 promotes embryonic myocardial cell proliferation and apoptosis through the WNT signal transduction pathway. Int. J. Mol. Med. 23, 321–330 [DOI] [PubMed] [Google Scholar]
- 28. Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A. B., Volpin D., Bressan G. M., Piccolo S. (2003) Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. U. S. A. 100, 3299–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mason J. B. (2009) Folate, cancer risk, and the Greek god, Proteus: a tale of two chameleons. Nutr. Rev. 67, 206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ye F., Chen Y., Hoang T., Montgomery R. L., Zhao X. H., Bu H., Hu T., Taketo M. M., van Es J. H., Clevers H., Hsieh J., Bassel-Duby R., Olson E. N., Lu Q. R. (2009) HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat. Neurosci. 12, 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pretlow T. P., Edelmann W., Kucherlapati R., Pretlow T. G., Augenlicht L. H. (2003) Spontaneous aberrant crypt foci in Apc1638N mice with a mutant Apc allele. Am. J. Pathol. 163, 1757–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clifford A. J., Wilson D. S., Bills N. D. (1989) Repletion of folate-depleted rats with an amino acid-based diet supplemented with folic acid. J. Nutr. 119, 1956–1961 [DOI] [PubMed] [Google Scholar]
- 33. Song J., Medline A., Mason J. B., Gallinger S., Kim Y. I. (2000) Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 60, 5434–5440 [PubMed] [Google Scholar]
- 34. Bjerknes M., Cheng H. (1981) Methods for the isolation of intact epithelium from the mouse intestine. Anat. Rec. 199, 565–574 [DOI] [PubMed] [Google Scholar]
- 35. O'Broin S., Kelleher B. (1992) Microbiological assay on microtitre plates of folate in serum and red cells. J. Clin. Pathol. 45, 344–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nichoalds G. E. (1974) Assessment of status riboflavin nutriture by assay of erythrocyte glutathione reductase activity. Clin. Chem. 20, 624–628 [PubMed] [Google Scholar]
- 37. Davies P. S., Dismuke A. D., Powell A. E., Carroll K. H., Wong M. H. (2008) Wnt-reporter expression pattern in the mouse intestine during homeostasis. BMC Gastroenterol. 8, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bird R. P. (1987) Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 37, 147–151 [DOI] [PubMed] [Google Scholar]
- 39. Scholzen T., Gerdes J. (2000) The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182, 311–322 [DOI] [PubMed] [Google Scholar]
- 40. Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A., Munday N. A., Raju S. M., Smulson M. E., Yamin T.-T., Yu V. L., Miller D. K. (1995) Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376, 37–43 [DOI] [PubMed] [Google Scholar]
- 41. Lasko D., Cavenee W., Nordenskjold M. (1991) Loss of constitutional heterozygosity in human cancer. Annu. Rev. Genet. 25, 281–314 [DOI] [PubMed] [Google Scholar]
- 42. Kathe S. D., Shen G. P., Wallace S. S. (2004) Single-stranded breaks in DNA but not oxidative DNA base damages block transcriptional elongation by RNA polymerase II in HeLa cell nuclear extracts. J. Biol. Chem. 279, 18511–18520 [DOI] [PubMed] [Google Scholar]
- 43. Mann B., Gelos M., Siedow A., Hanski M. L., Gratchev A., Ilyas M., Bodmer W. F., Moyer M. P., Riecken E. O., Buhr H. J., Hanski C. (1999) Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. U. S. A. 96, 1603–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kioussi C., Briata P., Baek S. H., Rose D. W., Hamblet N. S., Herman T., Ohgi K. A., Lin C., Gleiberman A., Wang J., Brault V., Ruiz-Lozano P., Nguyen H. D., Kemler R., Glass C. K., Wynshaw-Boris A., Rosenfeld M. G. (2002) Identification of a Wnt/Dvl/beta-catenin-> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 111, 673–685 [DOI] [PubMed] [Google Scholar]
- 45. Fodde R., Edelmann W., Yang K., van Leeuwen C., Carlson C., Renault B., Breukel C., Alt E., Lipkin M., Khan P. M., Kucherlapati R. (1994) A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc. Natl. Acad. Sci. U. S. A. 91, 8969–8973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang W. C., Mathew J., Velcich A., Edelmann W., Kucherlapati R., Lipkin M., Yang K., Augenlicht L. H. (2001) Targeted inactivation of the p21(WAF1/cip1) gene enhances Apc-initiated tumor formation and the tumor-promoting activity of a Western-style high-risk diet by altering cell maturation in the intestinal mucosal. Cancer Res. 61, 565–569 [PubMed] [Google Scholar]
- 47. Yang K., Edelmann W., Fan K., Lau K., Leung D., Newmark H., Kucherlapati R., Lipkin M. (1998) Dietary modulation of carcinoma development in a mouse model for human familial adenomatous polyposis. Cancer Res. 58, 5713–5717 [PubMed] [Google Scholar]
- 48. Choi S. W., Friso S., Dolnikowski G. G., Bagley P. J., Edmondson A. N., Smith D. E., Mason J. B. (2003) Biochemical and molecular aberrations in the rat colon due to folate depletion are age-specific. J. Nutr. 133, 1206–1212 [DOI] [PubMed] [Google Scholar]
- 49. Yates C. A., Evans G. S., Powers H. J. (2001) Riboflavin deficiency: early effects on post-weaning development of the duodenum in rats. Br. J. Nutr. 86, 593–599 [DOI] [PubMed] [Google Scholar]
- 50. Mackey A. D., Lieu S. O., Carman C., Gregory J. F., 3rd (2003) Hydrolytic activity toward pyridoxine-5′-beta-D-glucoside in rat intestinal mucosa is not increased by vitamin B-6 deficiency: effect of basal diet composition and pyridoxine intake. J. Nutr. 133, 1362–1367 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.