Abstract
Duchenne muscular dystrophy (DMD) and limb girdle muscular dystrophy (LGMD) 2C-F result from the loss of dystrophin and the sarcoglycans, respectively. Dystrophin, a cytoskeletal protein, is closely associated with the membrane-bound sarcoglycan complex. Despite this tight biochemical association, the function of dystrophin and the sarcoglycan subunits may differ. The loss of dystrophin in skeletal muscle results in muscle that is highly susceptible to contraction-induced damage, but the skeletal muscle of mice lacking γ- or δ-sarcoglycan are less susceptible. Using mouse models of DMD, LGMD-2C, and LGMD-2F, we demonstrate that isolated cardiac myocytes from mice lacking either γ- or δ-sarcoglycan have normal compliance. In contrast, dystrophin-deficient myocytes display poor passive compliance and are susceptible to terminal contracture following mild passive extensions. Mice deficient in dystrophin and, less so, δ-sarcoglycan have reduced survival during in vivo dobutamine stress testing compared to controls. Catheter-based hemodynamic studies show deficits in both baseline and dobutamine-stimulated cardiac function in all of the dystrophic mice compared to control mice, with dystrophin-deficient mice having the poorest function. In contrast, histopathology showed increased fibrosis in the sarcoglycan-deficient hearts, but not in hearts lacking dystrophin. In summary, this study provides important insights into the unique mechanisms of disease underlying these different models of inherited dystrophic cardiomyopathy and supports a model where dystrophin, but not the sarcoglycans, protects the cardiac myocyte against mechanical damage.—Townsend, D., Yasuda, S., McNally, E., Metzger, J. M. Distinct pathophysiological mechanisms of cardiomyopathy in hearts lacking dystrophin or the sarcoglycan complex.
Keywords: muscular dystrophy, hemodynamics, cellular compliance, cardiac fibrosis
Muscular dystrophies are broadly defined as a group of diseases that primarily affect striated muscles. Muscular dystrophy has been a recognized clinical entity for more than a century (1–3). A variety of different forms of muscular dystrophy have been described, initially by differences in the clinical presentation, and more recently by linkages to mutations in specific genes. Currently, there is no cure for any of the muscular dystrophies, although improved understanding of the genetics of muscular dystrophies has led to important insights into the basic pathophysiological mechanisms. Here, we investigate these mechanisms using animal models of Duchenne muscular dystrophy (DMD) and limb-girdle muscular dystrophies (LGMD 2C and 2F), which result from the loss of dystrophin and the associated sarcoglycan (SG) proteins, respectively (4–9).
Dystrophin is a very large protein that binds to cytoskeletal actin at its N terminus and a collection of glycoproteins near its C terminus (10, 11). The loss of dystrophin is profoundly observed in striated muscle, where sarcolemmal integrity is severely compromised (12, 13). Among the glycoproteins that serve to anchor dystrophin to the membrane is the SG complex. There are 4 members of the SG complex, all of which have a single transmembrane domain and are glycoslyated. Mutations within the genes encoding α-,β-, γ-, and δ-SG result in LGMD2D, 2E, 2C, and 2F, respectively (5–9). These mutations are not localized to any particular domain and result in a recessive disease condition (14). The role of the SGs in muscle physiology is complex and incompletely understood, and may have a multitude of effects within striated muscles, as well as in the surrounding tissues.
Clinically, DMD and LGMD 2C and 2F are progressive diseases of striated muscle, with both cardiac and skeletal muscle involvement (15–17). Significant cardiac disease occurs in >90% of DMD patients. Patients with mutations in the genes encoding β-, γ-, or δ-SG also have a high incidence of cardiomyopathy. Mutations in α-SG are less likely to lead to cardiomyopathy, and this may relate to the nature of the missense mutations or the compensation by ε-SG (18). Mouse models of these dystrophies have been generated, and they also have skeletal and cardiac muscle disease (19–23). Similar to the mouse model of DMD (mdx), mouse models of LGMD demonstrate a reduction in skeletal muscle membrane integrity (19, 21–23); this, in combination with the close molecular association between dystrophin and the SG complex, suggests that a common molecular mechanism may be at work in these different diseases. However, the response to contraction-induced injury in skeletal muscle differs for these mutations. In the absence of dystrophin, lengthening contractions destroy myofibers, causing a rapid decline in force production (12, 24). In contrast, lengthening contractions do not elicit increased myofiber disruption or a decline of force in γ-SG-null mice (25). Skeletal muscle lacking δ-SG has an intermediate phenotype where lengthening contractions induce some injury but not as severe as that seen in mdx muscle lacking dystrophin (22). These findings support a model where dystrophin directly participates in a mechanically strong linkage connecting the cytoplasm to the extracellular matrix, whereas the SG complex may protect myocytes through the coordination of signaling pathways or other nonmechanical aspects of muscle biology. This model is further supported by the observation that specific signaling pathways are disrupted in skeletal muscle lacking γ-SG (26, 27).
An intriguing pathophysiological mechanism that appears to be at work in mice lacking γ- or δ-SG is the dysregulation of blood flow that results in regional ischemia (19, 28, 29). This vasoconstriction occurs secondary to disruption of a paracrine communication between the vessel and neighboring myocardium. The presence of the SG complex in the striated muscle cell/fiber prevents this inappropriate vasoconstriction (29, 30). It is not clear whether a similar vascular mechanism is also at work in dystrophin-deficient myocardium, although alterations of vascular control have been implicated in the pathophysiology of DMD (31, 32).
To gain new insight into disease mechanisms, we evaluated the mechanical role of γ-SG and δ-SG in cardiac myocytes in vitro and in vivo. Utilizing a novel carbon fiber-based method (13), we assessed the passive extension-tension properties of isolated membrane-intact myocytes and found that loss of the SG subunits does not confer the deficits seen from loss of dystrophin. In vivo studies showed that dobutamine infusion caused a decline in cardiac function in the absence of dystrophin but not γ-SG. The relative preservation of the mechanical aspects of membrane integrity in the cardiomyopathic γ-SG and δ-SG hearts highlights the differential roles of SG and dystrophin in cardiac function. We report that despite the tight molecular association of dystrophin and the SG complex, their respective absence mediates disease through distinct molecular pathogenic processes.
MATERIALS AND METHODS
Animals
Control (C57BL/6) and mdx (C57BL/10 ScSn-Dmdmdx) mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). The δ-SG- and γ-SG-deficient mice were generated at the University of Chicago (22, 23) and backcrossed on to the C57BL/6 genetic background, as described previously (33). Mice of both sexes, fed a standard rodent diet (5001; Purina, St. Louis, MO, USA), were used with ages between 6 and 8 mo at the time of study. All of the procedures utilized in this study were approved by the University of Minnesota Animal Care and Use Committee.
Studies of isolated adult cardiac myocytes
Enzymatic isolation of adult cardiac myocytes was performed as previously outlined (13). Briefly, mice were anesthetized with sodium pentobarbital, and their hearts were rapidly excised. Following cannulation of the ascending aorta, a collagenase-containing solution was infused into the coronary vasculature. The digested hearts were then mechanically disassociated, and isolated adult myocytes were separated by low-speed centrifugation. Isolated, membrane-intact myocytes were then placed on to the stage of an inverted microscope and analyzed in a loaded or unloaded configuration. For the studies of loaded myocytes, individual myocytes were attached to microcarbon fibers (13). Once attached, myocytes were subjected to a series of passive extensions, as described elsewhere (13). The contraction of the unloaded myocytes was assessed by monitoring changes in sarcomere length (Ionoptix, Milton, MA, USA).
In vivo hemodynamics
Murine pressure-volume analysis of cardiac function was performed as described previously (13). Briefly, anesthesia was maintained by ventilating animals with 2% isoflurane in oxygen. An open chest approach was utilized and a 1.4-Fr pressure-volume catheter (Millar Instruments, Houston, TX, USA) was inserted into the left ventricle via an apical stab incision. The cardiac stress protocol consisted of collection of baseline hemodynamic data, followed by i.v. infusion of dobutamine (13, 34). Dobutamine-stimulated hemodynamic measurements were obtained 2 min following the beginning of the dobutamine infusion, a time point that represented the maximal response to the infusion. Dobutamine infusion was continued until average peak systolic pressure was <60 mmHg or the infusion time reached 30 min. The volume was calibrated in each mouse by the calculation of parallel conductance and blood-filled cuvette. Parallel conductance is a measure of the contribution of the ventricular wall to the volume signal and is determined by the brief injection of hypertonic saline. The parallel conductance is subtracted from the volume signal to give an absolute volume measurement. Occasionally, this correction resulted in a volume slightly <0. In these mice, the parallel conductance was decreased to give a minimum volume of 0, as done previously (34).
Histopathology and fibrosis quantification
Formalin-fixed sections were stained with Sirius Red staining using standard methodologies. Stained slides were then digitized using an inverted Axio Observer Z-1 with a motorized stage (Zeiss, Thornwood, NY, USA). Montage images were collected and assembled by AxioVision software (Zeiss). Composite images were quantified using Adobe Photoshop CS4 Extended (Adobe Systems, San Jose, CA, USA).
Statistics
All statistical analysis were performed using Prism 5.0c (GraphPad, La Jolla, CA, USA). Where appropriate, data were analyzed using a paired t test or 1-way-ANOVA with Dunnett's multiple-comparison posttest. Survival curves were analyzed using the Mantel-Cox log-rank test.
RESULTS
Cardiac myocyte passive properties
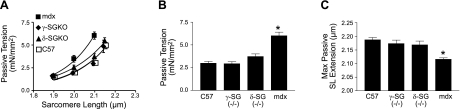
In dystrophin-deficient myocytes, the primary physiological defect is the poor passive compliance of the isolated living myocyte (13). To determine whether myocytes lacking SGs have similar deficits in passive properties, a carbon fiber-based assay was utilized to assess the passive tension-extension properties of myocytes isolated from γ- or δ-SG-knockout (KO) mice (13, 35). In previous studies, we have demonstrated that mdx mice have significantly reduced cellular compliance (Fig. 1 and ref. 13). Interestingly, intact cardiac myocytes isolated from δ-SG- or γ-SG-deficient mice had normal passive extension-tension curves (Fig. 1A, B). Furthermore, the maximal sarcomere length extension tolerated by γ- or δ-SG-null myocytes was not significantly different compared to normal myocytes (Fig. 1C). This is in marked contrast to age-matched mdx mice, whose cardiac myocytes revealed significant reductions in cellular compliance and intolerance to passive extension (Fig. 1).
Figure 1.
Passive properties of isolated membrane-intact dystrophic cardiac myocytes. A) Passive tension-extension relationships for membrane-intact myocytes isolated from C57BL/6, mdx, δ-SG-KO, and γ-SG-KO mice. B) Passive tension resulting from an extension to a sarcomere length of 2.1 μm. C) Maximum sarcomere length (SL) of passive extension tolerated before a terminal contracture. *P < 0.05 vs. control; n = 7–12 myocytes.
Myocyte contractile function
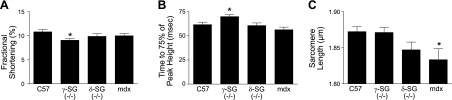
Contraction in isolated cardiac myocytes was initiated by electrical stimulation. Analysis of unloaded contractions revealed a significant decline in the contractile function of myocytes lacking γ-SG (Fig. 2A, B). Myocytes lacking either δ-SG or dystrophin had contractile function that was not significantly different from control myocytes. Myocytes isolated from dystrophin-deficient mdx mice demonstrated significant reductions in resting sarcomere length (Fig. 2C). Significant differences in resting sarcomere length were not observed in either γ-SG- or δ-SG-deficient myocytes (Fig. 2C).
Figure 2.
Contractile properties of isolated dystrophic cardiac myocytes. A, B) Electrically stimulated contraction of isolated cardiac myocytes from control and dystrophic cardiac myocytes, summarized as fractional shortening (A) and time to 75% of peak height (B). C) Diastolic tone of isolated cardiac myocytes is evident in the resting sarcomere length. *P < 0.05 vs. control; n = 23–57 myocytes.
Histopathology
To further address the mechanistic differences between mice lacking γ-SG or δ-SG and mdx mice lacking dystrophin, the extent of cardiac fibrosis was assessed. The areas of fibrosis were determined utilizing Sirius Red stain and quantitative morphometrics (Fig. 3). In contrast to the passive extension data, heart sections from mice lacking γ-SG or δ-SG contained significantly increased levels of fibrosis compared to wild-type mouse hearts (Fig. 3). Age-matched mdx mice were largely devoid of these lesions and had a normal level of collagen staining.
Figure 3.
Quantification of cardiac fibrosis in dystrophic cardiomyopathy. A–D) Sirius Red staining of heart sections from wild type (A), δ-SG−/− (B), γ-SG−/− (C), and mdx (D). Scale bars = 50 μm. E) Quantification of red collagen staining in heart sections shows significant increases in fibrosis in both δ-SG−/− and γ-SG−/− mice. Quantification data from 3–8 heart sections from 3–7 mice/group. *P < 0.05 vs. wild type.
Hemodynamics
The cardiac fibrotic lesions noted above are a prominent feature of the pathology of both the δ- and γ-SG-KO animals; however, the functional significance of these lesions has not been fully investigated (19, 22, 23). To assess the cardiac function of δ- and γ-SG-KO mice, catheter-based hemodynamics were performed. To study these hearts near the peak of the disease state, studies were performed in mice shortly beyond the previously reported colony half-lives of 5 mo for the γ-SG-KO (23) and 7 mo for the δ-SG-KO (22). Age-matched mdx mice were included in the analysis to assess the relative severity of these different models of dystrophic cardiomyopathy.
Diastolic dysfunction is an early indicator of cardiac disease in patients with DMD (36). Significant evidence of diastolic dysfunction was evident in the mdx mice studied here. Both the maximal rate (dP/dtmin) and the overall rate of isovolumic pressure (tau) decline were reduced in mdx mice (Fig. 4E, F and Table 1). In addition, ventricular pressures during the filling phase of diastole were also significantly elevated in mdx mice (Fig. 4G, H and Table 1). The only evidence of diastolic dysfunction in the δ- and γ-SG-KO mice was a significant reduction in the maximal rate of isovolumic relaxation (dP/dtmin; Fig. 4E), an indicator of early diastolic function (37). Previous hemodynamic assessments of younger mdx mice by our group have demonstrated a reduced diastolic volume (13, 34). Mice in the current study are older and have more advanced disease, which is manifest, in part, as a relative ventricular dilation.
Figure 4.
Catheter-based hemodynamic assessment of dystrophic cardiac function. A–D) Representative tracings of pressure-volume loops obtained from wild-type C57BL/6 (A), δ-SG-KO (B), γ-SG-KO (C), and mdx (D) mice. E–F) Measures of diastolic function derived from pressure-volume analysis of heart function: dP/dtmin (E), tau (F), minimum left ventricular (LV) pressure (G), and end-diastolic pressure (H). Values are means ± se derived from 7–11 mice. *P < 0.05 vs. control; 1-way ANOVA.
Table 1.
Baseline hemodynamic data
| Parameter | C57BL/6, n = 9 | δ-SG KO, n = 7 | γ-SG KO, n = 7 | mdx, n = 9 |
|---|---|---|---|---|
| Heart rate (bpm) | 615 ± 9 | 625 ± 20 | 592 ± 20 | 588 ± 15 |
| End-diastolic volume (μl) | 32.1 ± 1.9 | 30.1 ± 4.0 | 29.4 ± 4.4 | 31.2 ± 3.3 |
| End-stystolic volume (μl) | 13.3 ± 1.1 | 12.7 ± 2.7 | 11.9 ± 3.9 | 14.5 ± 3.0 |
| Stroke volume (μl) | 21.4 ± 1.1 | 19.5 ± 2.3 | 19.9 ± 3.4 | 20.7 ± 1.3 |
| Cardiac output (ml/min) | 13.1 ± 0.7 | 12.2 ± 1.4 | 11.8 ± 2.1 | 12.1 ± 0.7 |
| Ejection fraction (%) | 63.9 ± 2.7 | 64.5 ± 4.5 | 65.5 ± 8.7 | 65.5 ± 5.0 |
| End-diastolic pressure (mmHg) | 9.8 ± 0.7 | 7.6 ± 0.6 | 8.8 ± 0.9 | 13.5 ± 1.6* |
| Minimum pressure (mmHg) | 6.6 ± 0.6 | 5.1 ± 0.6 | 5.8 ± 0.6 | 10.3 ± 1.0* |
| Tau (ms) | 5.4 ± 0.1 | 5.4 ± 0.3 | 6.4 ± 0.4 | 7.5 ± 0.5* |
| End-systolic pressure (mmHg) | 105.1 ± 2.0 | 88.2 ± 3.4* | 85.8 ± 4.7* | 80.1 ± 3.5* |
| dP/dtmax (mmHg/s) | 10,378 ± 400 | 8563 ± 792 | 8688 ± 1280 | 7427 ± 668 |
| dP/dtmin (mmHg/s) | −11,267 ± 313 | −9173 ± 793* | −7190 ± 765* | −5922 ± 476* |
| Maximum filling rate (μl/s) | 1,087 ± 76 | 976 ± 131 | 977 ± 158 | 1093 ± 106 |
| Maximum emptying rate (μl/s) | −1,045 ± 56 | −1010 ± 110 | −1108 ± 194 | −1263 ± 72 |
| Age (mo) | 7.4 ± 0.3 | 8.3 ± 0.3 | 6.3 ± 0.2 | 7.8 ± 0.2 |
| Heart:body weight ratio (mg:g) | 4.58 ± 0.16 | 5.25 ± 0.14* | 4.41 ± 0.31 | 4.63 ± 0.10 |
Data are expressed as means ± se.
P < 0.05 vs. control C57BL/6; 1-way ANOVA.
The cardiac output and ejection fraction were largely preserved in all of the dystrophic hearts examined, indicating that the pumping function of the heart is maintained under baseline conditions (Table 1). However, the significant reduction in systolic pressures observed in all dystrophic mice compared to control mice suggests that the contractile function of the myocardium is compromised (Table 1). It is important to note that both the heart and the systemic vasculature determine systolic blood pressure and that the hypotension observed may be secondary to alterations in vascular function. Cardiac stress testing was implemented to determine the contractile capacity of the dystrophic myocardium.
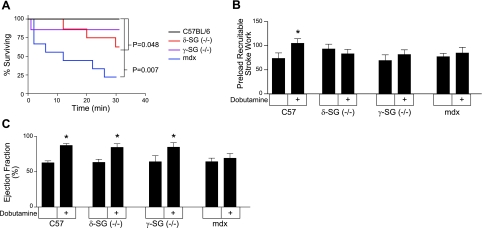
Cardiac stress testing is a useful and clinically relevant method to determine the presence of subclinical cardiac disease (38, 39). This study utilized a chemical stress test in the form of i.v. infusion of dobutamine (42 μg/kg/min). This dose of dobutamine maximally activates the heart and reveals any reduction in cardiac reserve. During the course of the 30-min dobutamine stress test, both mdx and, to a lesser extent, the δ-SG-KO mice displayed significantly increased mortality compared to wild-type mice, all of whom survived (Fig. 5A). This increased mortality was preceded by evidence of limited cardiac reserve present in all lines of dystrophic mice studied. This reduction in cardiac reserve was evident in several indices of cardiac function assessed shortly after the initiation of the dobutamine infusion (Table 2). Cardiac pump function is determined by both the contraction of the myocardium and the tone of the vasculature to which it couples. Ventricular function can be assessed using measures of function intrinsic to the heart, such as preload recruitable stroke work (PRSW) or by utilizing measures that are also dependent on the vasculature (e.g., cardiac output and maximum pressure derivative, dP/dtmax). In control mice, PRSW, dP/dtmax, and cardiac output were significantly increased in response to dobutamine infusion. The dobutamine-induced increases of these functional indices were significantly blunted in dystrophic hearts (Fig. 5B, C and Table 2). Ejection fraction is frequently used clinically as a measure of global cardiac function. In this study, control mice and mice lacking either γ-SG or δ-SG exhibited significant increases in ejection fraction during dobutamine infusion (Fig. 5C). Interestingly, the mdx mice had no significant changes in ejection fraction or cardiac output in response to dobutamine infusion (Fig. 5C). These reduced responses to dobutamine in the mdx heart are secondary to persistent elevations of the left ventricular end-systolic volume (Table 1).
Figure 5.
Dobutamine cardiac stress testing of dystrophic hearts. Intravenous infusion of dobutamine (42 μg/kg/min) was used to assess cardiac reserve in several models of dystrophic cardiomyopathy. A) Dobutamine infusion begins at time 0 and proceeds for 30 min or until a minimal pressure cutoff was reached. Kaplan-Meier survival curve analysis provided the P values compared to control mice. B) PRSW provides evidence of limited cardiac reserve in dystrophic hearts. C) Ejection fraction is a clinically relevant measure of global cardiac pump function, which was significantly increased by dobutamine in wild-type and γ- and δ-SG-null mice, but not in mdx mice. Results were derived from 7–9 animals. *P < 0.05 vs. baseline cardiac function; t test.
Table 2.
Dobutamine-stimulated hemodynamics
| Parameter | C57BL/6, n = 9 | δ-SG KO, n = 7 | γ-SG KO, n = 7 | mdx, n = 9 |
|---|---|---|---|---|
| Heart rate (bpm) | 631 ± 8 | 641 ± 19 | 611 ± 18 | 590 ± 8 |
| End-diastolic volume (μl) | 30.6 ± 2.0 | 27.1 ± 4.5 | 27.6 ± 4.9 | 30.0 ± 2.2 |
| End-systolic volume (μl) | 4.8 ± 0.7 | 6.7 ± 2.1 | 5.6 ± 2.0 | 12.6 ± 3.1* |
| Stroke volume (μl) | 29.8 ± 1.9 | 23.6 ± 3.4 | 24.7 ± 4.2 | 21.6 ± 1.2 |
| Cardiac output (ml/min) | 18.7 ± 1.1 | 15.1 ± 2.2 | 14.9 ± 2.4 | 12.7 ± 0.7* |
| End-diastolic pressure (mmHg) | 8.7 ± 0.4 | 7.5 ± 0.7 | 8.4 ± 0.9 | 12.3 ± 1.1* |
| Minimum pressure (mmHg) | 4.7 ± 0.6 | 4.9 ± 0.8 | 5.5 ± 0.7 | 9.9 ± 0.9* |
| Tau (ms) | 5.3 ± 0.1 | 5.1 ± 0.2 | 6.2 ± 0.3 | 7.4 ± 0.5* |
| End-systolic pressure (mmHg) | 127.5 ± 11.8 | 84.3 ± 3.8* | 80.8 ± 4.0* | 76.1 ± 5.3* |
| dP/dtmax (mmHg/s) | 15,764 ± 289 | 10,256 ± 978* | 9930 ± 1,066* | 7756 ± 915* |
| dP/dtmin (mmHg/s) | −10,168 ± 601 | −7785 ± 585* | −5637 ± 311* | −5066 ± 488* |
| Maximum filling rate (μl/s) | 1362 ± 125 | 1094 ± 171 | 1253 ± 254 | 991 ± 79 |
| Maximum emptying rate (μl/s) | −1650 ± 125 | −1403 ± 194 | −1676 ± 295 | −1444 ± 122 |
Data are expressed as means ± se.
P < 0.05 vs. control C57BL/6; 1-way ANOVA.
DISCUSSION
In both mouse models of and human patients with DMD or LGMD2C and 2F, there is a significant loss of membrane integrity, as demonstrated by the presence of creatine kinase in the serum and extracellular proteins within muscle fibers (16, 18, 19, 21–23). This similar clinical phenotype and the close biochemical association between dystrophin and the SG complex suggested that a common mechanical mechanism of disease might underlie these forms of dystrophic cardiomyopathy. However, this study demonstrates that the loss of these related proteins results in distinct pathophysiological mechanisms of dystrophic cardiomyopathy resulting from the loss of dystrophin or the SG complex.
In the dystrophin-deficient heart, the membrane surrounding cardiac myocytes becomes susceptible to the formation of microtears during passive extension (13). A small amount of calcium enters through these holes and partially engages the myofilaments, generating tension that is observed as a reduction in passive compliance. Furthermore, the persistent elevation of Ca2+ during diastole results in significant reductions of resting sarcomere length in the mdx myocyte (Fig. 2C and refs. 13, 40). Passive extension of sufficient magnitude causes enough calcium to enter the cell that massive calcium release is triggered, resulting in a terminal contracture. This membrane instability represents a major pathophysiological mechanism of the cardiomyopathy resulting from the loss of dystrophin in the heart. We show here that this type of mechanical disruption of membrane integrity is not present in either δ-SG-KO or γ-SG-KO mice. The normal passive properties of myocytes from mice lacking γ-SG or δ-SG indicate that the SG complex is not critically important in protecting these cells from the passive extension-induced membrane disruptions that are so damaging to dystrophin-deficient cells. These data support the hypothesis that membrane damage observed in the absence of the SG complex results from deficits in nonmechanical cellular processes. This is consistent with studies from skeletal muscle suggesting that dystrophin is an important mechanical stabilizer of the muscle membrane and that the myopathy resulting from the loss of the SGs results from some other nonmechanical mechanism (23, 25).
Despite this lack of membrane instability in response to passive extension, rodent models lacking γ- or δ-SG do have significant cardiac lesions that are evident by histopathology, as shown here (Fig. 3) and elsewhere (19, 22, 23). It has been observed that both γ- and δ-SG-deficient mice have significant amounts of vascular spasm (19, 28, 29). The presence of these spasms may explain the increased fibrosis evident in the myocardium of these animals. Furthermore, the failure to detect significant fibrosis in dystrophin-deficient mdx mice, where membrane instability is present, suggests that adaptive responses are present to limit cell death and/or fibrosis.
The severe cardiac fibrosis and premature death of rodents null for the SG complex (21–23, 41) suggested that the cardiac function of the SG-null mice would be markedly worse than that of the dystrophin-deficient mice. In contrast to this view, the deficits in diastolic function observed in mdx mice are greater than those observed in γ- and δ-SG-null mice (Fig. 4). These changes in diastolic function are consistent with the altered passive extension-tension properties observed in isolated dystrophin-deficient myocytes. The poor passive compliance of the individual cardiac myocyte in the absence of dystrophin might be expected to increase the rate of cardiac myocyte necrosis, and thus an increase in myocardial fibrosis. However, there is relatively little evidence of fibrosis in the hearts of the young adult mdx mice studied here (Fig. 3D). In contrast, hearts from age matched γ- and δ-SG-null mice have significantly more fibrotic lesions throughout the myocardium (Fig. 3). The apparent uncoupling of ventricular function and fibrosis is an interesting phenomenon, suggesting that dystrophin and the SG complex have distinct effects on cardiac function and that these effects are independent of the presence of fibrotic tissue. Another potential mechanism for this uncoupling is that loss of the SG complex alters the binding of the myocyte to the extracellular matrix in such a way that myocardial function is improved but increases the stress on a subset of cardiac myocytes that are damaged and subsequently replaced with fibrosis.
The mortality observed during the dobutamine stress testing suggests that increased β-adrenergic stimulation may be causing damage to the dystrophic hearts. The severity of this damage is greatest in the mdx mice, followed by the δ-SG-null mice, with the γ-SG-null mice being very mildly affected. Interestingly, isolated γ-SG-deficient myocytes have a significantly reduced systolic contraction. This mild decrease in the contractile properties of the isolated cell may provide protection against any injury resulting from dobutamine stimulation. These results are also very reminiscent of those seen with damage induced by lengthening contractions in skeletal muscle (12, 23–25).
Mice lacking γ- or δ-SG displayed significant increases in ejection fraction (EF) shortly after the beginning of the dobutamine infusion (Fig. 5C). However, intrinsic myocardial contraction was not increased by dobutamine in any of the dystrophic mice (Fig. 5B). This limited cardiac reserve may result from increased basal sympathetic tone or perhaps be an indication of ongoing myocardial damage. The observed increase in EF is driven primarily by a decrease in end-systolic volume (Table 2). Reduction in end-systolic volume can occur secondary to increases in myocardial contractility and/or reduction in the afterload on the heart. The failure of dobutamine to increase myocardial contractility suggests that the changes in end-systolic volume are secondary to reductions in afterload, presumably via peripheral vasodilation. It follows that this dobutamine-induced vascular relaxation may not occur in the dystrophin-deficient mdx mice, resulting in a constant afterload and maintenance of the baseline systolic volume.
The interaction between dystrophin and the SG complex is well documented. In skeletal muscle, the loss of dystrophin dramatically reduces the levels of the SG proteins (42, 43). In the heart, the dystrophin dependence of SG expression is less clear (34, 44–46). The results presented here suggest that the levels of the SG complex are not likely to have significant effects on the mechanical properties of the dystrophin-deficient cardiac myocyte membrane. The nature of the nonmechanical function of the SG complex remains unclear. The SG complex has been implicated in several signaling pathways (26, 27, 47). However, many of these same pathways are also activated in the dystrophin-deficient muscle (47, 48), suggesting that they are not specific to the loss of the SG complex. The SG complex has been shown to associate with filamin C and a vacuolar H+-ATPase subunit (49, 50), but the physiological significance of these interactions is currently unknown. Furthermore, the importance of dystrophin for these nonmechanical functions of the SG complex is unclear, and their disruption may also contribute to the disease process in muscle lacking dystrophin. Recent data from skeletal muscle indicated that the absence of both dystrophin and the SG complex result in a synergistic pathology (51). This observation indicates that dystrophin and the SG complex also have distinct physiological functions in skeletal muscle.
It is important to note that correction of myocyte-passive properties only partially restores mdx cardiac function relative to dystrophin-replete animals (13). This suggests that nonmechanical pathological pathways are also present in the dystrophin-deficient heart. Dystrophin is also associated with a wide variety of signaling proteins, whose function in the heart remains largely unknown and may be important in the progression of dystrophic cardiomyopathy (52–55). In addition to providing insights into new therapeutic approaches for these genetic diseases, elucidation of the physiological roles of dystrophin and SGs will aid in the understanding of several forms of acquired heart disease, in which dystrophin and associated proteins are affected, including viral myocarditis and heart failure (56, 57).
Acknowledgments
This work was supported by grants from the Muscular Dystrophy Association (68419 to D.T.) and the U.S. National Institutes of Health (HL61322 to E.M., HL102066 to D.T., and HL086790 to J.M.).
REFERENCES
- 1. Duchenne G. B. (1867) The pathology of paralysis with muscular degeneration (paralysie myosclerotique), or paralysis with apparent hypertrophy. Br. Med. J. 2, 541–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ross J. (1883) On a case of pseudohypertrophic paralysis. Br. Med. J. 1, 200–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walton J. N., Nattrass F. J. (1954) On the classification, natural history and treatment of the myopathies. Brain 77, 169–231 [DOI] [PubMed] [Google Scholar]
- 4. Hoffman E. P., Brown R. H., Jr., Kunkel L. M. (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 [DOI] [PubMed] [Google Scholar]
- 5. Roberds S. L., Leturcq F., Allamand V., Piccolo F., Jeanpierre M., Anderson R. D., Lim L. E., Lee J. C., Tome F. M., Romero N. B., Fardeau M., Beckmann J. S., Kaplan J.-C., Campbell K. P. (1994) Missense mutations in the adhalin gene linked to autosomal recessive muscular dystrophy. Cell 78, 625–633 [DOI] [PubMed] [Google Scholar]
- 6. Bonnemann C. G., Modi R., Noguchi S., Mizuno Y., Yoshida M., Gussoni E., McNally E. M., Duggan D. J., Angelini C., Hoffman E. P. (1995) Beta-sarcoglycan (A3b) mutations cause autosomal recessive muscular dystrophy with loss of the sarcoglycan complex. Nat. Genet. 11, 266–273 [DOI] [PubMed] [Google Scholar]
- 7. Lim L. E., Duclos F., Broux O., Bourg N., Sunada Y., Allamand V., Meyer J., Richard I., Moomaw C., Slaughter C., Tomé F. M. S., Fardeau M., Jackson C. E., Beckmann J. S., Campbell K. P. (1995) Beta-sarcoglycan: characterization and role in limb-girdle muscular dystrophy linked to 4q12. Nat. Genet. 11, 257–265 [DOI] [PubMed] [Google Scholar]
- 8. Noguchi S., McNally E. M., Ben Othmane K., Hagiwara Y., Mizuno Y., Yoshida M., Yamamoto H., Bonnemann C. G., Gussoni E., Denton P. H., Kyriakides T., Middleton L., Hentati F., Ben Hamida M., Nonaka I., Vance J. M., Kunkel L. M., Ozawa E. (1995) Mutations in the dystrophin-associated protein gamma-sarcoglycan in chromosome 13 muscular dystrophy. Science 270, 819–822 [DOI] [PubMed] [Google Scholar]
- 9. Nigro V., de Sa Moreira E., Piluso G., Vainzof M., Belsito A., Politano L., Puca A. A., Passos-Bueno M. R., Zatz M. (1996) Autosomal recessive limb-girdle muscular dystrophy, LGMD2F, is caused by a mutation in the delta-sarcoglycan gene. Nat. Genet. 14, 195–198 [DOI] [PubMed] [Google Scholar]
- 10. Ervasti J. M., Campbell K. P. (1991) Membrane organization of the dystrophin-glycoprotein complex. Cell 66, 1121–1131 [DOI] [PubMed] [Google Scholar]
- 11. Koenig M., Monaco A. P., Kunkel L. M. (1988) The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53, 219–228 [DOI] [PubMed] [Google Scholar]
- 12. Petrof B. J., Shrager J. B., Stedman H. H., Kelly A. M., Sweeney H. L. (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. U. S. A. 90, 3710–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yasuda S., Townsend D., Michele D. E., Favre E. G., Day S. M., Metzger J. M. (2005) Dystrophic heart failure blocked by membrane sealant poloxamer. Nature 436, 1025–1029 [DOI] [PubMed] [Google Scholar]
- 14. Trabelsi M., Kavian N., Daoud F., Commere V., Deburgrave N., Beugnet C., Llense S., Barbot J. C., Vasson A., Kaplan J. C., Leturcq F., Chelly J. (2008) Revised spectrum of mutations in sarcoglycanopathies. Eur. J. Hum. Genet. 16, 793–803 [DOI] [PubMed] [Google Scholar]
- 15. Van der Kooi A. J., de Voogt W. G., Barth P. G., Busch H. F., Jennekens F. G., Jongen P. J., de Visser M. (1998) The heart in limb girdle muscular dystrophy. Heart 79, 73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bushby K. M. (1999) The limb-girdle muscular dystrophies-multiple genes, multiple mechanisms. Hum. Mol. Genet. 8, 1875–1882 [DOI] [PubMed] [Google Scholar]
- 17. Politano L., Nigro V., Passamano L., Petretta V., Comi L. I., Papparella S., Nigro G., Rambaldi P. F., Raia P., Pini A., Mora M., Giugliano M. A., Esposito M. G. (2001) Evaluation of cardiac and respiratory involvement in sarcoglycanopathies. Neuromuscul. Disord. 11, 178–185 [DOI] [PubMed] [Google Scholar]
- 18. Norwood F., de Visser M., Eymard B., Lochmuller H., Bushby K. (2007) EFNS guideline on diagnosis and management of limb girdle muscular dystrophies. Eur. J. Neurol. 14, 1305–1312 [DOI] [PubMed] [Google Scholar]
- 19. Coral-Vazquez R., Cohn R. D., Moore S. A., Hill J. A., Weiss R. M., Davisson R. L., Straub V., Barresi R., Bansal D., Hrstka R. F., Williamson R., Campbell K. P. (1999) Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell 98, 465–474 [DOI] [PubMed] [Google Scholar]
- 20. Duclos F., Straub V., Moore S. A., Venzke D. P., Hrstka R. F., Crosbie R. H., Durbeej M., Lebakken C. S., Ettinger A. J., van der Meulen J., Holt K. H., Lim L. E., Sanes J. R., Davidson B. L., Faulkner J. A., Williamson R., Campbell K. P. (1998) Progressive muscular dystrophy in alpha-sarcoglycan-deficient mice. J. Cell Biol. 142, 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Durbeej M., Cohn R. D., Hrstka R. F., Moore S. A., Allamand V., Davidson B. L., Williamson R. A., Campbell K. P. (2000) Disruption of the beta-sarcoglycan gene reveals pathogenetic complexity of limb-girdle muscular dystrophy type 2E. Mol. Cell 5, 141–151 [DOI] [PubMed] [Google Scholar]
- 22. Hack A. A., Lam M. Y., Cordier L., Shoturma D. I., Ly C. T., Hadhazy M. A., Hadhazy M. R., Sweeney H. L., McNally E. M. (2000) Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophin-glycoprotein complex. J. Cell Sci. 113, 2535–2544 [DOI] [PubMed] [Google Scholar]
- 23. Hack A. A., Ly C. T., Jiang F., Clendenin C. J., Sigrist K. S., Wollmann R. L., McNally E. M. (1998) Gamma-sarcoglycan deficiency leads to muscle membrane defects and apoptosis independent of dystrophin. J. Cell Biol. 142, 1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cox G. A., Cole N. M., Matsumura K., Phelps S. F., Hauschka S. D., Campbell K. P., Faulkner J. A., Chamberlain J. S. (1993) Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature 364, 725–729 [DOI] [PubMed] [Google Scholar]
- 25. Hack A. A., Cordier L., Shoturma D. I., Lam M. Y., Sweeney H. L., McNally E. M. (1999) Muscle degeneration without mechanical injury in sarcoglycan deficiency. Proc. Natl. Acad. Sci. U. S. A. 96, 10723–10728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barton E. R. (2006) Impact of sarcoglycan complex on mechanical signal transduction in murine skeletal muscle. Am. J. Physiol. Cell Physiol. 290, C411–C419 [DOI] [PubMed] [Google Scholar]
- 27. Barton E. R. (2010) Restoration of gamma-sarcoglycan localization and mechanical signal transduction are independent in murine skeletal muscle. J. Biol. Chem. 285, 17263–17270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohn R. D., Durbeej M., Moore S. A., Coral-Vazquez R., Prouty S., Campbell K. P. (2001) Prevention of cardiomyopathy in mouse models lacking the smooth muscle sarcoglycan-sarcospan complex. J. Clin. Invest. 107, R1–R7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wheeler M. T., Korcarz C. E., Collins K. A., Lapidos K. A., Hack A. A., Lyons M. R., Zarnegar S., Earley J. U., Lang R. M., McNally E. M. (2004) Secondary coronary artery vasospasm promotes cardiomyopathy progression. Am. J. Pathol. 164, 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Durbeej M., Sawatzki S. M., Barresi R., Schmainda K. M., Allamand V., Michele D. E., Campbell K. P. (2003) Gene transfer establishes primacy of striated vs. smooth muscle sarcoglycan complex in limb-girdle muscular dystrophy. Proc. Natl. Acad. Sci. U. S. A. 100, 8910–8915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sander M., Chavoshan B., Harris S. A., Iannaccone S. T., Stull J. T., Thomas G. D., Victor R. G. (2000) Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. U. S. A. 97, 13818–13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas G. D., Sander M., Lau K. S., Huang P. L., Stull J. T., Victor R. G. (1998) Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 95, 15090–15095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heydemann A., Huber J. M., Demonbreun A., Hadhazy M., McNally E. M. (2005) Genetic background influences muscular dystrophy. Neuromuscul. Disord. 15, 601–609 [DOI] [PubMed] [Google Scholar]
- 34. Townsend D., Blankinship M. J., Allen J. M., Gregorevic P., Chamberlain J. S., Metzger J. M. (2007) Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol. Ther. 15, 1086–1092 [DOI] [PubMed] [Google Scholar]
- 35. Townsend D., Yasuda S., Li S., Chamberlain J. S., Metzger J. M. (2008) Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol. Ther. 16, 832–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Markham L. W., Michelfelder E. C., Border W. L., Khoury P. R., Spicer R. L., Wong B. L., Benson D. W., Cripe L. H. (2006) Abnormalities of diastolic function precede dilated cardiomyopathy associated with Duchenne muscular dystrophy. J. Am. Soc. Echocardiogr. 19, 865–871 [DOI] [PubMed] [Google Scholar]
- 37. Brutsaert D. L., Sys S. U. (1989) Relaxation and diastole of the heart. Physiol. Rev. 69, 1228–1315 [DOI] [PubMed] [Google Scholar]
- 38. Bosser G., Lucron H., Lethor J. P., Burger G., Beltramo F., Marie P. Y., Marcon F. (2004) Evidence of early impairments in both right and left ventricular inotropic reserves in children with Duchenne's muscular dystrophy. Am. J. Cardiol. 93, 724–727 [DOI] [PubMed] [Google Scholar]
- 39. Pratali L., Picano E., Otasevic P., Vigna C., Palinkas A., Cortigiani L., Dodi C., Bojic D., Varga A., Csanady M., Landi P. (2001) Prognostic significance of the dobutamine echocardiography test in idiopathic dilated cardiomyopathy. Am. J. Cardiol. 88, 1374–1378 [DOI] [PubMed] [Google Scholar]
- 40. Turner P. R., Fong P. Y., Denetclaw W. F., Steinhardt R. A. (1991) Increased calcium influx in dystrophic muscle. J. Cell Biol. 115, 1701–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nigro V., Okazaki Y., Belsito A., Piluso G., Matsuda Y., Politano L., Nigro G., Ventura C., Abbondanza C., Molinari A. M., Acampora D., Nishimura M., Hayashizaki Y., Puca G. A. (1997) Identification of the Syrian hamster cardiomyopathy gene. Hum. Mol. Genet. 6, 601–607 [DOI] [PubMed] [Google Scholar]
- 42. Ervasti J. M., Ohlendieck K., Kahl S. D., Gaver M. G., Campbell K. P. (1990) Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 345, 315–319 [DOI] [PubMed] [Google Scholar]
- 43. Ohlendieck K., Campbell K. P. (1991) Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J. Cell Biol. 115, 1685–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bies R. D., Maeda M., Roberds S. L., Holder E., Bohlmeyer T., Young J. B., Campbell K. P. (1997) A 5′ dystrophin duplication mutation causes membrane deficiency of alpha-dystroglycan in a family with X-linked cardiomyopathy. J. Mol. Cell. Cardiol. 29, 3175–3188 [DOI] [PubMed] [Google Scholar]
- 45. Hainsey T. A., Senapati S., Kuhn D. E., Rafael J. A. (2003) Cardiomyopathic features associated with muscular dystrophy are independent of dystrophin absence in cardiovasculature. Neuromusc. Disord. 13, 294–302 [DOI] [PubMed] [Google Scholar]
- 46. Matsumura K., Ervasti J. M., Ohlendieck K., Kahl S. D., Campbell K. P. (1992) Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 360, 588–591 [DOI] [PubMed] [Google Scholar]
- 47. Griffin M. A., Feng H., Tewari M., Acosta P., Kawana M., Sweeney H. L., Discher D. E. (2005) gamma-sarcoglycan deficiency increases cell contractility, apoptosis and MAPK pathway activation but does not affect adhesion. J. Cell Sci. 118, 1405–1416 [DOI] [PubMed] [Google Scholar]
- 48. Sen S., Tewari M., Zajac A., Barton E., Sweeney H. L., Discher D. E. (2010) Upregulation of paxillin and focal adhesion signaling follows dystroglycan complex deletions and promotes a hypertensive state of differentiation. Eur J. Cell Biol. 90, 249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen J., Skinner M. A., Shi W., Yu Q. C., Wildeman A. G., Chan Y. M. (2007) The 16 kDa subunit of vacuolar H+-ATPase is a novel sarcoglycan-interacting protein. Biochim. Biophys. Acta 1772, 570–579 [DOI] [PubMed] [Google Scholar]
- 50. Thompson T. G., Chan Y. M., Hack A. A., Brosius M., Rajala M., Lidov H. G., McNally E. M., Watkins S., Kunkel L. M. (2000) Filamin 2 (FLN2): A muscle-specific sarcoglycan interacting protein. J. Cell Biol. 148, 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li D., Long C., Yue Y., Duan D. (2009) Sub-physiological sarcoglycan expression contributes to compensatory muscle protection in mdx mice. Hum. Mol. Genet. 18, 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gavillet B., Rougier J. S., Domenighetti A. A., Behar R., Boixel C., Ruchat P., Lehr H. A., Pedrazzini T., Abriel H. (2006) Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ. Res. 99, 407–414 [DOI] [PubMed] [Google Scholar]
- 53. Iwata Y., Shigekawa M., Wakabayashi S. (2005) Cardiac syntrophin isoforms: species-dependent expression, association with dystrophin complex and subcellular localization. Mol. Cell. Biochem. 268, 59–66 [DOI] [PubMed] [Google Scholar]
- 54. Leonoudakis D., Conti L. R., Anderson S., Radeke C. M., McGuire L. M., Adams M. E., Froehner S. C., Yates J. R., 3rd, Vandenberg C. A. (2004) Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)-associated proteins. J. Biol. Chem. 279, 22331–22346 [DOI] [PubMed] [Google Scholar]
- 55. Williams J. C., Armesilla A. L., Mohamed T. M., Hagarty C. L., McIntyre F. H., Schomburg S., Zaki A. O., Oceandy D., Cartwright E. J., Buch M. H., Emerson M., Neyses L. (2006) The sarcolemmal calcium pump, alpha-1 syntrophin, and neuronal nitric-oxide synthase are parts of a macromolecular protein complex. J. Biol. Chem. 281, 23341–23348 [DOI] [PubMed] [Google Scholar]
- 56. Badorff C., Lee G. H., Lamphear B. J., Martone M. E., Campbell K. P., Rhoads R. E., Knowlton K. U. (1999) Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat. Med. 5, 320–326 [DOI] [PubMed] [Google Scholar]
- 57. Vatta M., Stetson S. J., Jimenez S., Entman M. L., Noon G. P., Bowles N. E., Towbin J. A., Torre-Amione G. (2004) Molecular normalization of dystrophin in the failing left and right ventricle of patients treated with either pulsatile or continuous flow-type ventricular assist devices. J. Am. Coll. Cardiol. 43, 811–817 [DOI] [PubMed] [Google Scholar]