Abstract
Brain parenchymal arterioles (PAs), but not pial arteries, undergo hypotrophic outward remodeling during pregnancy that involves peroxisome proliferator-activated receptor-γ (PPARγ) activation. Relaxin, a peptide hormone produced during pregnancy, is involved in systemic and renal artery remodeling and activates PPARγ in vitro. Thus, we hypothesized that relaxin is involved in the selective outward remodeling of PAs through a PPARγ-dependent mechanism. Nonpregnant rats were treated with relaxin (4 μg/h, osmotic minipump), relaxin plus PPARγ inhibitor GW9662 (10 mg/kg/d), or vehicle for 10 d. Vascular function and structure were compared in isolated and pressurized middle cerebral arteries (MCAs) and PAs taken from the same animals. Relaxin treatment increased serum relaxin to the level of pregnancy (54 ng/ml) and increased passive wall thickness (hypertrophy; 70±5 vs. 54±4 μm in vehicle; P<0.05) and inner diameter (outward remodeling; 10.6±0.5 vs. 8.0±0.6 μm in vehicle; P<0.05) in PAs, but not in MCAs. This hypertrophic outward remodeling was prevented by GW9662 that had diameters (57±3 μm) and wall thickness (8.6±1.0 μm) similar to vehicle. GW9662 also prevented relaxin-induced changes in PPARγ target gene expression. These results suggest that relaxin produced during pregnancy may be partly responsible for selective remodeling of PAs during pregnancy through a mechanism involving PPARγ.—Chan, S.-L., Cipolla, M. J. Relaxin causes selective outward remodeling of brain parenchymal arterioles via activation of peroxisome proliferator-activated receptor-γ.
Keywords: hypertrophy, middle cerebral arteries, myogenic reactivity
Relaxin is a small peptide hormone primarily produced by the ovaries and placenta during pregnancy (1). Three relaxin genes have been identified in humans, designated as H1, H2, and H3 relaxin. H2 relaxin is the major source of circulating relaxin that is substantially increased during pregnancy (2). H2 relaxin binds to relaxin family peptide receptor 1 (RXFP1, previously known as LGR7) with high affinity (3). A growing amount of literature suggests that relaxin has extensive cardiovascular effects, even outside of pregnancy, such as promoting vasodilation and angiogenesis, and protecting against fibrosis and inflammation in systemic and renal circulations (4, 5). Recently, the therapeutic potential of relaxin has been suggested for treatment of heart failure and preeclampsia (6, 7).
The role of circulating relaxin in the cardiovascular system has been studied extensively in the systemic and renal vascular systems. Relaxin treatment of nonpregnant (NP) rats to the level that occurs in midterm pregnancy decreased peripheral vascular resistance and increased cardiac output (8, 9). In the renal circulation, relaxin enhanced glomerular filtration rate and effective renal plasma flow in NP rats, likely by reducing renal vascular resistance (10). Conversely, treating pregnant rats with relaxin-neutralizing antibody inhibited these peripheral and renal hemodynamic changes in pregnancy (11, 12). These studies demonstrate that relaxin has a significant role in hemodynamic changes in the systemic and renal circulations during pregnancy (13).
Despite substantial hemodynamic changes in the systemic and renal circulations during pregnancy, cerebral hemodynamics, including vascular resistance and blood flow, appear to remain relatively stable throughout the course of gestation (14, 15). Despite little change in overall hemodynamics, we previously found that pregnancy induces selective outward remodeling of brain parenchymal arterioles (PAs) but not larger pial arteries (16). This outward remodeling during pregnancy is characterized by increased inner diameter (ID) and decreased wall thickness (WT). The cerebral circulation is unique in that the large arteries contribute significantly to vascular resistance in the brain (17). Thus, the unchanged size of the large arteries may serve to maintain normal vascular resistance and blood flow in the brain during pregnancy in the face of significant changes in cardiac output, plasma volume, and outward remodeling of PAs (18). However, the underlying mechanism by which PAs undergo selective outward remodeling during pregnancy is not known. On the basis of the effect of relaxin in renal arteries, it is possible that relaxin has a role in selective remodeling of the cerebral vasculature during pregnancy. Thus, the first aim of this study was to test the hypothesis that relaxin promotes selective outward remodeling in PAs similar to pregnancy. To test this hypothesis, we compared structural and functional changes in PAs and middle cerebral arteries (MCAs) from vehicle- and relaxin-treated NP rats to the level of pregnancy.
Peroxisome proliferator-activated receptor-γ (PPARγ) is a nuclear receptor transcription factor shown to be involved in structural remodeling of the cerebral circulation (19). PPARγ is highly activated during pregnancy and involved in placental development and changes in maternal metabolism (20, 21). Our previous study showed that activation of PPARγ in NP rats caused outward remodeling of PAs similar to that seen in pregnancy (22). In addition, pharmacological inhibition of PPARγ in late-pregnant rats inhibited this outward remodeling in PAs (23), suggesting that PPARγ activation has a role in selective outward remodeling in the cerebral vasculature during pregnancy. Furthermore, it has also been shown in a recent study that relaxin activates PPARγ in transfected HEK-293T cells through a nonreceptor-mediated mechanism (24), leading us to hypothesize that high circulating relaxin during pregnancy may activate PPARγ in cerebral vasculature to cause structural remodeling. Thus, the second aim of this study was to determine whether selective remodeling of PAs induced by relaxin was through activation of PPARγ. This was accomplished by treating rats with a PPARγ inhibitor, GW9662, in addition to relaxin to determine whether this would prevent relaxin-induced remodeling. We also assessed the effect of relaxin and relaxin plus GW9662 on PPARγ target genes to determine whether relaxin activates PPARγ.
MATERIALS AND METHODS
Animals and treatment groups
All procedures were approved by the University of Vermont Animal Care and Use Committee and conducted in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. Female virgin NP Sprague-Dawley rats (250–300 g, 14–16 wk; Charles River, Senneville, QC, Canada) were used for all experiments and housed in the University of Vermont Animal Care Facility. Animals were randomly selected and grouped as untreated (n=4), treated with recombinant human relaxin-2 (relaxin, 4 μg/hr; n=14), relaxin plus a specific PPARγ inhibitor, GW9662 (10 mg/kg daily in food; n=8), or vehicle (20 mM sodium acetate; n=14) for 10 d through osmotic minipumps (Alzet 2ML2; Durect Corp., Cupertino, CA, USA). GW9662 treatment was started 1 d before and for the duration of relaxin treatment to ensure that PPARγ was inhibited. Animals were anesthetized with 3% isoflurane, and minipumps were implanted subcutaneously in the back of the neck. All animals received postsurgical analgesic (buprenorphine, 50 μg/kg s.c.). Relaxin infusion was continuous for 10 d until animals were euthanized for vessel experiments and tissue collection. The minipumps used were capable of delivering relaxin for 14 d; thus, tissue collection was within this duration.
Determination of relaxin levels in serum
Vehicle-, relaxin-, and relaxin+GW9662-treated animals were anesthetized with isoflurane (3% in oxygen) and decapitated, and trunk blood was collected for measurement of circulating relaxin. Serum was then collected and stored at −80°C for determination of relaxin levels by ELISA (Human Relaxin-2 Quantikine ELISA Kit, R&D Systems, Minneapolis, MN, USA), following manufacturer's instructions. Serum samples were diluted by 1:500 in the assay.
Vessel preparation and pressurized arteriograph system
After collecting trunk blood, the brain was quickly removed and placed in cold physiological salt solution (PSS). In the first set of experiments, PAs between the M1 and M2 region of the MCA territory and the MCAs from the same animals were carefully isolated from vehicle- or relaxin-treated animals and mounted on glass cannulas in a dual-chambered arteriograph, such that one PA and one MCA from each animal were studied simultaneously. PAs were identified as branches off the MCAs that penetrated into the brain tissue, as described previously (25). In the second set of experiments, PAs from the same brain region as above were dissected from relaxin+GW9662-treated animals and compared to PAs from vehicle- and relaxin-treated animals. MCAs were not studied in this second set of animals because there was no effect of relaxin on that segment of the vasculature. The proximal cannulas were connected to an inline pressure transducer and a servo-null pressure control system (Living Systems Instrumentation, Burlington, VT, USA) that allowed intravascular pressure to be maintained at a set pressure or changed at a variable rate. The distal cannulas were closed during the experiment to avoid flow-mediated responses. Temperature and pH were continuously measured and maintained at 37.0 ± 0.5°C and 7.40 ± 0.05, respectively. Measurements of ID and WT were made via video microscopy (Living Systems Instrumentation).
Vascular reactivity and structural characteristics in PAs and MCAs
For the first set of experiments, PAs and MCAs were equilibrated for 1 h at 25 mmHg, after which myogenic activity was determined by stepwise increases in pressure from 40 to 100 mmHg for PAs or 50 to 175 mmHg for MCAs. ID and WT were measured at each pressure once stable. Changes in ID and WT were measured in PAs only in response to small (SKCa)- and intermediate (IKCa)-conductance calcium-activated potassium channel blockers, apamin (3×10−7 M) and TRAM-34 (10−6 M), respectively. PAs are unique in that the contribution of endothelium-derived hyperpolarizing factors (EDHFs) to tone is greater than most vessels (26). Thus, inhibition of SKCa/IKCa channels, a critical component in the EDHF pathway, causes constriction in PAs (26). Therefore, we assessed the effect of relaxin on constriction of PAs in response to SKCa/IKCa channel inhibition in order to assess this unique pathway. To obtain structural measurements, ID and WT were recorded at pressures between 5 and 200 mmHg for PAs or between 5 and 175 mmHg for MCAs after papaverine (10−4 M) and diltiazem (10−5 M) were added to the bath. For the second set of experiments in which animals were treated with relaxin+GW9662, passive structural measurements were made as described above.
Determination of RXFP1 and PPARγ target gene expression
MCAs and PAs from 4 untreated rats were collected to determine expression level of RXFP1, the primary relaxin receptor, using real-time quantitative PCR (qPCR) methods. We also collected adipose tissue, pial arteries, and PAs from vehicle, relaxin and relaxin + GW9662 groups (n=6/group) for determination of PPARγ target gene expression. All collected samples were stored in RNase inhibitor (1 U/μl, RiboLock; Fermentas, Glen Burnie, MD, USA) at −80°C for comparing expression of fatty acid translocase (FAT)/CD36 and liver X receptors-α (LXRα) for adipose tissue and plasminogen activator inhibitor-1 (PAI-1) for vasculature. Standard techniques for real-time qPCR were performed by the Vermont Cancer Center DNA analysis facility at the University of Vermont, as described previously (26). Briefly, RNA was extracted from tissues, and concentration and integrity were determined by a NanoDrop ND-1000 spectrophotometer (Nanodrop, Wilmington, DE, USA) and an Agilent 2100 bioanalyzer (Agilent, Santa Clara, CA, USA), respectively. Samples with low RNA quality were excluded. cDNA was then made by a SuperScript III Kit (Invitrogen, Carlsbad, CA, USA). Real-time qPCR was set up as follows: 10 μl Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 1 μl Assay on Demand (Applied Biosystems), 8 μl water, and 1 μl cDNA. β2-Microglobulin (B2M, housekeeping control) and target genes were assessed using Assays on Demand (Applied Biosystems). All primers were validated by the manufacturer for efficiency and did not detect homologs. Primers were designed across an exon-exon junction to avoid detecting genomic DNA. Thus, no DNase treatment of samples was necessary. All samples were run in duplicates using a 7900HT Sequence Detection System (Applied Biosystems). The PCR was cycled for 2 min at 50°C, 10 min at 95°C, 40 cycles at 95°C for 15 s, and then 60°C for 1 min.
Drugs and solutions
All isolated vessel experiments were performed in physiological salt solution (PSS) containing 119.0 mM NaCl, 24.0 mM NaHCO3, 4.7 mM KCl, 1.17 mM MgSO4, 0.026 mM EDTA, 5.0 mM CaCl2, 1.1 mM 8 KH2PO4, and 5.5 mM glucose and aerated with 5% CO2, 10% O2 and balanced N2 to maintain pH at 7.40 ± 0.05. Relaxin was a generous gift from Corthera Inc (San Carlos, CA, USA) and Novartis Pharmaceuticals (Basel, Switzerland). GW9662 was from Cayman Chemical (Ann Arbor, MI, USA). Papaverine and diltiazem were purchased from Sigma (St. Louis, MO, USA) and were made as stock solutions and stored at 4°C each week. Apamin and TRAM-34 were purchased from Tocris (Ellisville, MO, USA), mixed with vehicle, portioned into aliquots, and stored at −20°C until use.
Data calculations and statistical analysis
Percentage tone was calculated as percentage decrease in ID from the passive diameter at each intravascular pressure: [1 − (IDactive/IDpassive)] × 100%. Outer diameter (OD) was calculated from measured ID and WT: ID + 2WT. Cross-sectional area (CSA) of the wall was calculated as [(OD/2)2 − (ID/2)2] × π. Data from qPCR assays were analyzed using the −2ΔΔCT method, as described previously (27). Data were removed when the CT values of technical replicates differed by >0.5.
All data are presented as means ± se. Differences between vehicle- and relaxin-treated groups were determined with 1-way analysis of variance and a post hoc Newman-Keuls test for multiple comparisons using Graph Pad Prism 5 (Graph Pad Software, La Jolla, CA, USA). Differences were considered significant at values of P < 0.05.
RESULTS
RXFP1 gene expressions
Real-time qPCR revealed that RXFP1 expression was low in MCAs (threshold cycle: 37.4 vs. 22.2 in B2M) and undetectable in PAs (threshold cycle: >40 vs. 25.5 in B2M). Thus, RXFP1 was expressed in MCAs, albeit at lower levels than the housekeeping gene, whereas RXFP1 was not highly expressed, if at all, in PAs.
Relaxin levels in serum
Serum concentrations of relaxin in vehicle-, relaxin-, and relaxin+GW9662-treated animals were measured to confirm the extent of delivery of relaxin. Relaxin treatment increased serum relaxin to the levels found between middle and late pregnancy (54±8 in relaxin vs. 78±28 in relaxin+GW9662 vs. 0.01±0.008 ng/ml in control).
Effect of relaxin on vascular reactivity in PAs and MCAs
Previous studies have shown that relaxin decreases myogenic reactivity in renal and mesenteric arteries (28, 29). Therefore, we assessed whether relaxin had a similar effect in cerebral arteries and arterioles. Figure 1 shows active diameters of PAs and MCAs from vehicle- and relaxin-treated rats. One experiment in the vehicle group and one PA experiment in relaxin group were excluded because of technical issues. PAs from both groups of animals displayed myogenic reactivity, as ID did not significantly change with pressure (Fig. 1A). Relaxin treatment caused an increase in diameter of PAs at all pressures studied. In contrast, MCAs had less myogenic activity than PAs, and relaxin treatment had no effect on active diameter of MCAs at any pressure (Fig. 1B).
Figure 1.
Active ID vs. intraluminal pressure of PAs (A) and MCAs (B) from vehicle- or relaxin-treated NP rats. Relaxin increased active ID in PAs, but had no effect in MCAs. *P < 0.05 vs. vehicle.
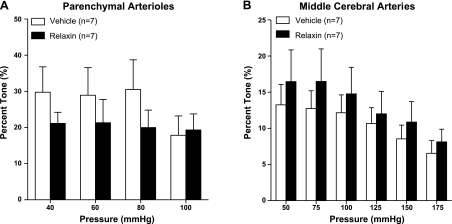
The percentage tone of PAs and MCAs from relaxin- and vehicle-treated rats is shown in Fig. 2. In both groups, PAs developed greater tone compared with MCAs, as has been shown in a previous study (26). However, relaxin did not significantly affect tone in either PAs or MCAs (Fig. 2).
Figure 2.
Percentage tone vs. intraluminal pressure of PAs (A) and MCAs (B) from vehicle- or relaxin-treated nonpregnant rats. Note that PAs had considerably more tone than MCAs. Relaxin tended to decrease tone in PAs at pressures from 40 to 80 mmHg, but not significantly. There was no effect of relaxin on tone in MCAs.
Relaxin has been shown to affect endothelial function of renal and mesenteric arteries (28, 29). Thus, we studied the effect of relaxin on endothelial function in cerebral arterioles. We tested endothelial function through inhibition of SKCa and IKCa because these channels are involved in inhibiting tone in PAs but not MCAs (26). Figure 3 shows the effect of apamin and TRAM-34 on ID of PAs in both groups of animals. We excluded the data from one PA experiment from the relaxin group because the PAs dilated in response to apamin, likely due to depolarization of calcitonin gene-related peptide-containing perivascular nerve fibers that are few on these vessels (30). Apamin induced minimal vasoconstriction in PAs, while TRAM-34 caused greater vasoconstriction, suggesting a greater influence of IKCa vs. SKCa channels in inhibiting tone. However, there was no difference between vehicle- and relaxin-treated rats in response to SKCa/IKCa inhibition.
Figure 3.
Percentage constriction of PAs in response to cumulative addition of apamin (3×10−5 M) and TRAM-34 (10−6 M) from vehicle- or relaxin-treated nonpregnant rats. Apamin caused little change in diameter of PAs, whereas TRAM-34 had a larger effect. There was no difference in percentage constriction of PAs between vehicle- and relaxin-treated groups.
Effect of relaxin on the structure of PAs and MCAs
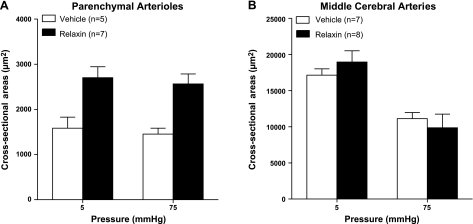
Relaxin has been shown to have significant effects on the structure of renal and mesenteric arteries (11, 31, 32). Thus, we measured ID and WT at various pressures under passive conditions and calculated OD and CSA. Figure 4 shows passive ID, WT, and OD in PAs and MCAs from vehicle- and relaxin-treated rats. Two experiments in PAs from vehicle-treated animals were excluded because of technical reasons. In PAs, relaxin treatment significantly increased ID at all pressures studied (Fig. 4A), demonstrating that similar to other vascular beds (and pregnancy), relaxin caused outward remodeling in PAs. Relaxin-induced remodeling of PAs was hypertrophic in nature, as these vessels also had increased WT and CSA (Fig. 4C and Fig. 5A). In addition, relaxin increased OD of PAs (Fig. 4E), confirming that vessels from relaxin-treated rats underwent hypertrophic outward remodeling. The effect of relaxin on structural remodeling appeared to be selective to PAs because relaxin had no effect on the structure of MCAs (Figs. 4B, D, F, and 5B).
Figure 4.
Passive ID of PAs (A), ID of MCAs (B), WT of PAs (C), WT of MCAs (D), OD of PAs (E), and OD of MCAs (F) against intraluminal pressure from vehicle- or relaxin-treated NP rats. Relaxin significantly increased ID, WT, and OD at all pressures in PAs, but had no effect on the structure of MCAs. *P < 0.05 vs. vehicle controls.
Figure 5.
CSA at 5 and 75 mmHg of PAs (A) and MCAs (B) from vehicle- or relaxin-treated NP rats. Relaxin increased CSA in PAs at both 5 and 75 mmHg, but not in MCAs. *P < 0.05 vs. vehicle.
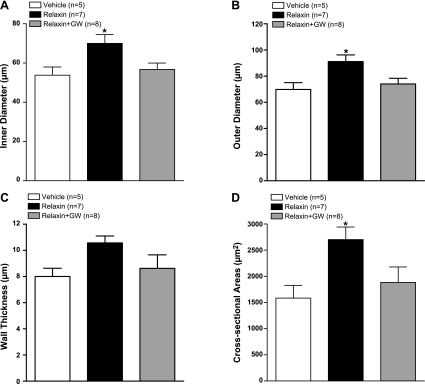
Effect of GW9662 on relaxin-induced remodeling in PAs
PPARγ has been previously shown to be involved in pregnancy-induced outward remodeling of PAs (33). Thus, we investigated whether PPARγ also has a role in relaxin-induced outward remodeling. Figure 6 compares the structural properties of PAs from vehicle- and relaxin-treated animals to those in which PPARγ was inhibited with GW9662 during relaxin infusions. We compared structural remodeling at a low pressure (5 mmHg) to avoid differences in structure that may be induced by changes in distensibility at higher pressures. We found that treatment with GW9662 prevented the structural changes induced by relaxin. For example, PAs from relaxin + GW9662-treated animals had similar ID (Fig. 6A), OD (Fig. 6B), and CSA (Fig. 6D) as vehicle and significantly decreased compared to relaxin treatment alone. WT of PAs from relaxin + GW9662 animals was also similar to that of vehicle-treated animals, although this was not statistically significant compared with the relaxin-treated group (Fig. 6C). Thus, PPARγ inhibition prevented structural remodeling of PAs in relaxin-treated animals.
Figure 6.
Passive ID (A), OD (B), WT (C), and CSA (D) in PAs at 5 mmHg from vehicle-treated (n=5), relaxin-treated (n=7), or relaxin+GW9662-treated (n=8) NP rats. GW9662 treatment inhibited relaxin-induced increase in ID, OD, and CSA. *P < 0.05 vs. vehicle or relaxin+GW9662.
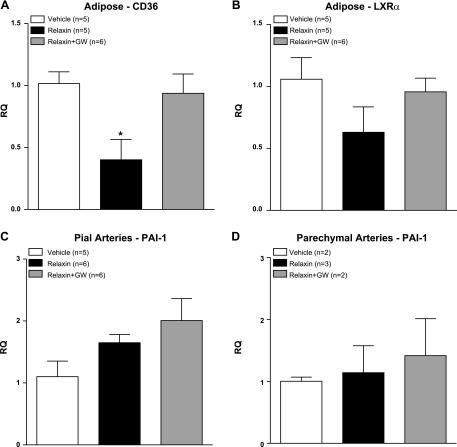
PPARγ target gene expression
To confirm activation of PPARγ by relaxin, we determined gene expression of PPARγ target genes in adipose tissue, pial arteries, and PAs. In adipose tissue in which PPARγ is highly expressed, relaxin decreased gene expression of CD36, which was normalized by GW9662 treatment (Fig. 7A). A similar trend was observed with LXR-α in adipose tissue, although the changes were not statistically significant (Fig. 7B). In vascular segments, relaxin did not significantly change PAI-1 in pial arteries (Fig. 7C) or PAs (Fig. 7D). In PAs, the low sample numbers were due to both bad RNA quality and high CT variability between replicates; therefore, these samples were removed.
Figure 7.
Relative quantification (RQ) of mRNA expression of CD36 in adipose tissue (A), liver X receptor-α in adipose tissue (B), PAI-1 in pial arteries (C), and PAI-1 in parenchymal arterioles (D). Relaxin decreased CD36 expression in adipose tissue, which was normalized by GW9662 treatment. There was no effect of relaxin or GW9662 on PAI-1 expression in either vascular segment. *P < 0.05 vs. vehicle or relaxin+GW9662.
DISCUSSION
The major finding of this study was that treating NP rats with relaxin to serum levels of mid- to late-pregnancy caused hypertrophic outward remodeling in PAs, but not in MCAs, demonstrating that similar to pregnancy, relaxin causes selective remodeling of the cerebral vasculature. This selective outward remodeling by relaxin was inhibited by treatment with GW9662, suggesting that relaxin activates PPARγ to cause remodeling of PAs. In addition, relaxin caused changes in PPARγ target gene expression that was prevented by GW9662 in adipose tissue, confirming that relaxin activates PPARγ in this tissue. Although relaxin caused structural remodeling in PAs, relaxin did not significantly affect myogenic reactivity, tone, or endothelial function in these vessels. Thus, this is the first study demonstrating an effect of relaxin on structural remodeling in the cerebral circulation and that this effect is dependent on PPARγ activation.
PPARγ activation has been shown to cause outward remodeling in PAs from NP rats similar to that seen in pregnancy (33). In this study, we found that PPARγ activation was also involved in relaxin-induced outward remodeling in PAs, as suggested by the inhibitory effect of GW9662 on relaxin-induced outward remodeling. Activation of PPARγ was further confirmed by GW9662 treatment that prevented relaxin-induced changes in PPARγ target gene expression in adipose tissue, in which PPARγ is highly expressed. To our knowledge, this report is the first to show that relaxin activates PPARγ after in vivo relaxin treatment and is consistent with a previous study that reported that PPARγ was activated by relaxin in transfected HEK-293T cells in vitro (24). There are a number of circulating hormones and factors produced in high levels during pregnancy. In addition, circulating endogenous ligands of PPARγ are increased during pregnancy, leading to increased PPARγ activation (20). Results from the present study suggest that relaxin may be another endogenous activator of PPARγ that is increased during pregnancy. This finding may have physiological significance because PPARγ is involved in many functions during pregnancy, such as changes in maternal metabolism and early placental and fetal development (21, 34–36). In addition, PPARγ has a significant role in vascular function and structure, as demonstrated in our and other studies (19, 22, 23, 37). Further, PPARγ activation is decreased in preeclampsia (20), and mutation of the PPARγ gene P465L causes symptoms of preeclampsia, including hypertension and vascular dysfunction (19, 38). Relaxin is thought to have therapeutic potential for the treatment of preeclampsia, mostly based on its positive effect on endothelium-dependent vasodilation in renal and systemic circulations (7, 12, 28). Together, these findings suggest that the protective effects of relaxin may also be related to its ability to activate PPARγ.
How relaxin activates PPARγ is largely unknown. In this study, we found that expression of CD36 was decreased by relaxin in adipose tissue. This effect was prevented by a selective PPARγ inhibitor, GW9662. A similar trend was also observed in another PPARγ target gene, LXR-α, although the results were not statistically significant. These results are contrary to a previous study that showed that relaxin increased expression of CD36 and LXR-α in transfected HEK-293T cells and that it was unaffected by GW9662 (24). These results suggest the effect of relaxin may be tissue-specific and may work through different mechanisms to affect PPARγ activity. Because GW9662 inhibits binding of PPARγ ligands, the mechanism of PPARγ activation in adipose tissue may be different from that of HEK-293T cells, which was through a non-ligand-binding mechanism. Moreover, the PPARγ target gene PAI-1 was not significantly changed in vascular segments regardless of relaxin or GW9662 treatment. This result is not surprising, as RXFP1 expression was very low in MCAs and undetectable in PA. Thus, if PPARγ acts through the relaxin receptor, as previously suggested (24), we would not see much effect in vascular tissue that has very low or undetectable RXFP1 expression. These results also suggest that PPARγ-dependent selective outward remodeling of PAs may not be a direct effect of relaxin that involves activation of RXFP1 in the vasculature. One possibility is that PPARγ is activated in astrocytes (39), exerting a paracrine effect to the closely associated PAs. As astrocytes are not present around pial arteries, this paracrine effect may explain the PPARγ-dependent selective remodeling of PAs.
PPARγ activation caused both hypertrophy and remodeling in PAs that may be affected differently by relaxin or PPARγ. Previous studies in cerebral arterioles revealed that pulse pressure, the renin-angiotensin system, sympathetic nerves, oxidative stress, and nitric oxide are major determinants of hypertrophy (40–44). How PPARγ affects or regulates these factors remains to be studied; however, it is unlikely that PPARγ activation works through angiotensin type 1 receptors (AT1Rs) or superoxide to cause hypertrophy because PPARγ down-regulates AT1Rs and has antioxidant properties (45, 46). In addition, the mechanism of remodeling may be independent of hypertrophy. Previous studies have shown that PPARγ activation normalized angiotensin II-induced vascular remodeling and decreased expression of AT1Rs (46, 47). The renin-angiotensin system has been considered an important determinant of inward remodeling in cerebral arterioles (43, 48). Therefore, it is possible that PPARγ activation causes outward remodeling via down-regulation of AT1Rs. Furthermore, in addition to a PPARγ-dependent mechanism, relaxin may induce outward remodeling through up-regulation of gelatinases (MMP2 and MMP9), as has been previously shown (31, 49), which would encourage turnover of extracellular components and allow smooth muscle cell migration. Lastly, a previous study showed that relaxin increased arterial compliance in systemic vasculature (9). However, in the present study, relaxin did not change passive distensibility (data not shown), a mean of measuring stiffness, suggesting arterial compliance did not change. The difference between our findings and others may be due to different vessel type. In cerebral arterioles, hypertrophic outward remodeling may not necessarily be accompanied by changes in distensibility (44).
The physiological significance of selective outward remodeling induced by relaxin in PAs is not clear, but it may be similar to that of pregnancy. Because relaxin did not affect the structure of MCAs, overall cerebral vascular resistance is likely to be unchanged. This is consistent with relatively unchanged cerebral blood flow in normotensive conditions during pregnancy (33). However, as PAs contribute to local vascular resistance, and, thus, perfusion to downstream microcirculation, increased ID may decrease local vascular resistance and, hence, increase blood flow locally that may be important for changes in local brain metabolism during pregnancy. Previous findings suggest that neurotransmitter turnover and neuronal activity increase during pregnancy (50, 51). However, it should be noted that relaxin- and pregnancy-induced remodeling in PAs are somewhat different. Previously, we found that pregnancy causes selective outward remodeling of PAs with a thinner vascular wall, i.e., hypotrophic (23), not thicker, or hypertrophic, as shown in this study. This difference between relaxin- and pregnancy-induced remodeling in PAs is unclear, however, because pregnancy is a state of altered hormones and receptor profiles, it is unlikely that relaxin is the only factor involved in cerebral vascular adaptation to pregnancy. For example, we showed previously that pregnancy decreased AT1R expression in cerebral vasculature (52), an effect that may inhibit hypertrophic remodeling in the presence of relaxin.
Relaxin has been shown to decrease myogenic reactivity in small renal arteries and mesenteric arteries in NP rats (10, 28), which may affect renal and systemic vascular resistance and blood flow (10, 11). However, we found that relaxin had no effect on myogenic reactivity and tone in either vascular segment. Moreover, endothelial function of PAs, as evaluated by constriction to SKCa/IKCa channel inhibition, was also unaffected by relaxin. Thus, the increased active diameters of PAs by relaxin were likely due to outward remodeling. These results suggest that, unlike the renal and systemic circulations, relaxin did not appear to affect endothelial and smooth muscle functions in cerebral vasculature, but it had a significant effect on PA structure.
In summary, we provide the first evidence that relaxin activates PPARγ in vivo, suggesting that relaxin may be another endogenous activator of PPARγ in pregnancy. PPARγ activation appears to be involved in the underlying mechanism by which relaxin caused outward remodeling of PAs, but not larger upstream arteries. These results also suggest that relaxin may have a role in outward remodeling in PAs during pregnancy; however, other circulating hormonal and/or metabolic factors in pregnancy are also likely involved.
Acknowledgments
The authors thank Dr. Dennis Stewart (Corthera, Inc., San Carlos, CA, USA) and Novartis Pharmaceuticals (Basel, Switzerland) for providing recombinant human relaxin-2. The authors thank Mr. Timothy Hunter, Ms. Mary Lou Shane, and the Vermont Cancer Center DNA analysis facility at the University of Vermont for their technical expertise and help with PCR. The authors also thank Ms. Julie Sweet for technical expertise with the PA experiments.
The authors gratefully acknowledge the continued support from the U.S. National Institutes of Health (NS045940), the American Recovery and Reinvestment Act supplement (NS045940-05S1), the American Heart Association Established Investigator Award (0540081N), and the Totman Medical Research Trust. Recombinant human relaxin-2 was a gift from Corthera Inc. and Novartis Pharmaceuticals.
REFERENCES
- 1. Sherwood O. D. (2004) Relaxin's physiological roles and other diverse actions. Endocr. Rev. 25, 205–234 [DOI] [PubMed] [Google Scholar]
- 2. Sherwood O. D. (1994) Relaxin. In The Physiology of Reproduction, Vol. 1 (Knobil E., Neill J. D. eds) pp. 861–1009, Raven, New York [Google Scholar]
- 3. Bathgate R. A., Ivell R., Sanborn B. M., Sherwood O. D., Summers R. J. (2006) International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol. Rev. 58, 7–31 [DOI] [PubMed] [Google Scholar]
- 4. Bani D. (2008) Relaxin as a natural agent for vascular health. Vasc. Health Risk Manag. 4, 515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conrad K. P., Novak J. (2004) Emerging role of relaxin in renal and cardiovascular function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R250–R261 [DOI] [PubMed] [Google Scholar]
- 6. Teerlink J. R., Metra M., Felker G. M., Ponikowski P., Voors A. A., Weatherley B. D., Marmor A., Katz A., Grzybowski J., Unemori E., Teichman S. L., Cotter G. (2009) Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet 373, 1429–1439 [DOI] [PubMed] [Google Scholar]
- 7. Unemori E., Sibai B., Teichman S. L. (2009) Scientific rationale and design of a phase I safety study of relaxin in women with severe preeclampsia. Ann. N. Y. Acad. Sci. 1160, 381–384 [DOI] [PubMed] [Google Scholar]
- 8. Debrah D. O., Conrad K. P., Danielson L. A., Shroff S. G. (2005) Effects of relaxin on systemic arterial hemodynamics and mechanical properties in conscious rats: sex dependency and dose response. J. Appl. Physiol. 98, 1013–1020 [DOI] [PubMed] [Google Scholar]
- 9. Conrad K. P., Debrah D. O., Novak J., Danielson L. A., Shroff S. G. (2004) Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology 145, 3289–3296 [DOI] [PubMed] [Google Scholar]
- 10. Danielson L. A., Conrad K. P. (2003) Time course and dose response of relaxin-mediated renal vasodilation, hyperfiltration, and changes in plasma osmolality in conscious rats. J. Appl. Physiol. 95, 1509–1514 [DOI] [PubMed] [Google Scholar]
- 11. Debrah D. O., Novak J., Matthews J. E., Ramirez R. J., Shroff S. G., Conrad K. P. (2006) Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology 147, 5126–5131 [DOI] [PubMed] [Google Scholar]
- 12. Novak J., Danielson L. A., Kerchner L. J., Sherwood O. D., Ramirez R. J., Moalli P. A., Conrad K. P. (2001) Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J. Clin. Invest. 107, 1469–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Samuel C. S., Hewitson T. D. (2006) Relaxin in cardiovascular and renal disease. Kidney Int. 69, 1498–1502 [DOI] [PubMed] [Google Scholar]
- 14. Cipolla M. J., Bullinger L. (2008) Pregnancy decreases cerebrovascular resistance and increases blood-brain barrier permeability during acute hypertension: a role in eclampsia? Reprod. Sci. 15, 288A [Google Scholar]
- 15. Williams K. P., Wilson S. (1998) Variation in cerebral perfusion pressure with different hypertensive states in pregnancy. Am. J. Obstet. Gynecol. 179, 1200–1203 [DOI] [PubMed] [Google Scholar]
- 16. Cipolla M. J., Sweet J. G. (2008) Pregnancy decreases myogenic activity of brain parenchymal arterioles: role of estrogen. FASEB J. 22, 1151–1158 [Google Scholar]
- 17. Faraci F. M., Heistad D. D. (1990) Regulation of large cerebral arteries and cerebral microvascular pressure. Circ. Res. 66, 8–17 [DOI] [PubMed] [Google Scholar]
- 18. Pohl U., De Wit C., Gloe T. (2000) Large arterioles in the control of blood flow: role of endothelium-dependent dilation. Acta Physiol. Scand. 168, 505–510 [DOI] [PubMed] [Google Scholar]
- 19. Beyer A. M., Baumbach G. L., Halabi C. M., Modrick M. L., Lynch C. M., Gerhold T. D., Ghoneim S. M., de Lange W. J., Keen H. L., Tsai Y. S., Maeda N., Sigmund C. D., Faraci F. M. (2008) Interference with PPARγ signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension 51, 867–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waite L. L., Louie R. E., Taylor R. N. (2005) Circulating activators of peroxisome proliferator-activated receptors are reduced in preeclamptic pregnancy. J. Clin. Endocrinol. Metab. 90, 620–626 [DOI] [PubMed] [Google Scholar]
- 21. Wieser F., Waite L., Depoix C., Taylor R. N. (2008) PPAR action in human placental development and pregnancy and its complications. PPAR Res. 2008, 527048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cipolla M. J., Bullinger L., Godfrey J. (2009) Pregnancy and PPARg activation cause small vessel remodeling in the maternal brain and diminished cerebrovascular resistance: a role in eclampsia? Reprod Sci. 16, 91A [Google Scholar]
- 23. Cipolla M. J., Chan S.-L., Chapman A. C., Godfrey J. A. (2010) Inhibition of PPARγ during pregnancy causes inward remodeling of brain parenchymal arterioles. FASEB J. 24, 979.4 (abstr.) [Google Scholar]
- 24. Singh S., Bennett R. G. (2010) Relaxin signaling activates peroxisome proliferator-activated receptor gamma. Mol. Cell. Endocrinol. 315, 239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cipolla M. J., Bullinger L. V. (2008) Reactivity of brain parenchymal arterioles after ischemia and reperfusion. Microcirculation 15, 495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cipolla M. J., Smith J., Kohlmeyer M. M., Godfrey J. A. (2009) SKCa and IKCa channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke 40, 1451–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 28. Li Y., Brookes Z. L., Kaufman S. (2005) Acute and chronic effects of relaxin on vasoactivity, myogenic reactivity and compliance of the rat mesenteric arterial and venous vasculature. Regul. Pept. 132, 41–46 [DOI] [PubMed] [Google Scholar]
- 29. Novak J., Ramirez R. J., Gandley R. E., Sherwood O. D., Conrad K. P. (2002) Myogenic reactivity is reduced in small renal arteries isolated from relaxin-treated rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R349–R355 [DOI] [PubMed] [Google Scholar]
- 30. Cipolla M. J., Li R., Vitullo L. (2004) Perivascular innervation of penetrating brain parenchymal arterioles. J. Cardiovasc. Pharmacol. 44, 1–8 [DOI] [PubMed] [Google Scholar]
- 31. Jeyabalan A., Kerchner L. J., Fisher M. C., McGuane J. T., Doty K. D., Conrad K. P. (2006) Matrix metalloproteinase-2 activity, protein, mRNA, and tissue inhibitors in small arteries from pregnant and relaxin-treated nonpregnant rats. J. Appl. Physiol. 100, 1955–1963 [DOI] [PubMed] [Google Scholar]
- 32. Jeyabalan A., Novak J., Danielson L. A., Kerchner L. J., Opett S. L., Conrad K. P. (2003) Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ. Res. 93, 1249–1257 [DOI] [PubMed] [Google Scholar]
- 33. Cipolla M. J., Sweet J. G., Chan S. L. (2011) Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J. Appl. Physiol. 110, 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fournier T., Therond P., Handschuh K., Tsatsaris V., Evain-Brion D. (2008) PPARgamma and early human placental development. Curr. Med. Chem. 15, 3011–3024 [DOI] [PubMed] [Google Scholar]
- 35. Toth B., Hornung D., Scholz C., Djalali S., Friese K., Jeschke U. (2007) Peroxisome proliferator-activated receptors: new players in the field of reproduction. Am. J. Reprod. Immunol. 58, 289–310 [DOI] [PubMed] [Google Scholar]
- 36. Arck P., Toth B., Pestka A., Jeschke U. Nuclear receptors of the peroxisome proliferator-activated receptor (PPAR) family in gestational diabetes: from animal models to clinical trials. Biol. Reprod. 83, 168–176 [DOI] [PubMed] [Google Scholar]
- 37. Halabi C. M., Beyer A. M., de Lange W. J., Keen H. L., Baumbach G. L., Faraci F. M., Sigmund C. D. (2008) Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 7, 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsai Y. S., Kim H. J., Takahashi N., Kim H. S., Hagaman J. R., Kim J. K., Maeda N. (2004) Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARγ. J. Clin. Invest. 114, 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dello Russo C., Gavrilyuk V., Weinberg G., Almeida A., Bolanos J. P., Palmer J., Pelligrino D., Galea E., Feinstein D. L. (2003) Peroxisome proliferator-activated receptor gamma thiazolidinedione agonists increase glucose metabolism in astrocytes. J. Biol. Chem. 278, 5828–5836 [DOI] [PubMed] [Google Scholar]
- 40. Baumbach G. L. (1996) Effects of increased pulse pressure on cerebral arterioles. Hypertension 27, 159–167 [DOI] [PubMed] [Google Scholar]
- 41. Baumbach G. L., Chillon J. M. (2000) Effects of angiotensin-converting enzyme inhibitors on cerebral vascular structure in chronic hypertension. J. Hypertens. Suppl. 18, S7–S11 [PubMed] [Google Scholar]
- 42. Baumbach G. L., Heistad D. D., Siems J. E. (1989) Effect of sympathetic nerves on composition and distensibility of cerebral arterioles in rats. J. Physiol. 416, 123–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baumbach G. L., Sigmund C. D., Faraci F. M. (2003) Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension 41, 50–55 [DOI] [PubMed] [Google Scholar]
- 44. Baumbach G. L., Sigmund C. D., Faraci F. M. (2004) Structure of cerebral arterioles in mice deficient in expression of the gene for endothelial nitric oxide synthase. Circ. Res. 95, 822–829 [DOI] [PubMed] [Google Scholar]
- 45. Inoue I., Goto S., Matsunaga T., Nakajima T., Awata T., Hokari S., Komoda T., Katayama S. (2001) The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARα) and PPARγ increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism 50, 3–11 [DOI] [PubMed] [Google Scholar]
- 46. Sugawara A., Takeuchi K., Uruno A., Ikeda Y., Arima S., Kudo M., Sato K., Taniyama Y., Ito S. (2001) Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferator-activated receptor-gamma in vascular smooth muscle cells. Endocrinology 142, 3125–3134 [DOI] [PubMed] [Google Scholar]
- 47. Diep Q. N., El Mabrouk M., Cohn J. S., Endemann D., Amiri F., Virdis A., Neves M. F., Schiffrin E. L. (2002) Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation 105, 2296–2302 [DOI] [PubMed] [Google Scholar]
- 48. Chillon J. M., Baumbach G. L. (1999) Effects of an angiotensin-converting enzyme inhibitor and a beta-blocker on cerebral arterioles in rats. Hypertension 33, 856–861 [DOI] [PubMed] [Google Scholar]
- 49. Jeyabalan A., Novak J., Doty K. D., Matthews J., Fisher M. C., Kerchner L. J., Conrad K. P. (2007) Vascular matrix metalloproteinase-9 mediates the inhibition of myogenic reactivity in small arteries isolated from rats after short-term administration of relaxin. Endocrinology 148, 189–197 [DOI] [PubMed] [Google Scholar]
- 50. Desan P. H., Woodmansee W. W., Ryan S. M., Smock T. K., Maier S. F. (1988) Monoamine neurotransmitters and metabolites during the estrous cycle, pregnancy, and the postpartum period. Pharmacol. Biochem. Behav. 30, 563–568 [DOI] [PubMed] [Google Scholar]
- 51. Gregg C. (2009) Pregnancy, prolactin and white matter regeneration. J. Neurol. Sci. 285, 22–27 [DOI] [PubMed] [Google Scholar]
- 52. Chan S.-L., Chapman A. C., Sweet J. G., Gokina N. I., Cipolla M. J. (2010) Effect of PPARγ inhibition during pregnancy on posterior cerebral artery function and structure. Front. Vasc. Physiol. 1, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]