Abstract
Nature has evolved effective cell adhesion mechanisms to deliver inflammatory cells to inflamed tissue; however, many culture-expanded therapeutic cells are incapable of targeting diseased tissues following systemic infusion, which represents a great challenge in cell therapy. Our aim was to develop simple approaches to program cell-cell interactions that would otherwise not exist toward cell targeting and understanding the complex biology of cell-cell interactions. We employed a chemistry approach to engineer P- or L-selectin binding nucleic acid aptamers onto mesenchymal stem cells (MSCs) to enable them to engage inflamed endothelial cells and leukocytes, respectively. We show for the first time that engineered cells with a single artificial adhesion ligand can recapitulate 3 critical cell interactions in the inflammatory cell adhesion cascade under dynamic flow conditions. Aptamer-engineered MSCs adhered on respective selectin surfaces under static conditions >10 times more efficiently than controls including scrambled-DNA modified MSCs. Significantly, engineered MSCs can be directly captured from the flow stream by selectin surfaces or selectin-expressing cells under flow conditions (≤2dyn/cm2). The simple chemistry approach and the versatility of aptamers permit the concept of engineered cell-cell interactions to be generically applicable for targeting cells to diseased tissues and elucidating the biology of cell-cell interactions.—Zhao, W., Loh, W., Droujinine, I. A., Teo, W., Kumar, N., Schafer, S., Cui, C. H., Zhang, L., Sarkar, D., Karnik, R., Karp, J. M. Mimicking the inflammatory cell adhesion cascade by nucleic acid aptamer programmed cell-cell interactions.
Keywords: mesenchymal stem cell, homing, trafficking, cell therapy
Dynamic cell-cell interactions under flow conditions mediate the initial adhesion of immune cells (leukocytes and platelets) to the endothelium at targeted tissue sites, which is a critical first step for immune defense against pathogens (1–3). This event includes 3 types of cell-cell interactions: between circulating inflammatory cells and endothelial cells (ECs; refs. 1, 3), between circulating inflammatory cells and inflammatory cells arrested on ECs (4, 5), and between 2 or more circulating inflammatory cells in blood circulation (6, 7), which form cellular complexes and subsequently adhere on ECs. These intercellular interactions, which collectively deliver and accumulate immune cells to sites of inflammation, are mediated by cell surface adhesion molecules and, in particular, selectins and their ligands (8). For instance, P-selectin and its ligands [e.g., P-selectin glycoprotein ligand-1 (PSGL-1)] are involved in leukocyte-endothelium, platelet-endothelium, and leukocyte-platelet interactions (9–11). L-selectin and its ligands (e.g., PSGL-1) are exclusively responsible for leukocyte-leukocyte interactions (4, 5).
Mimicking these cell-cell interactions under dynamic flow conditions has significant implications for targeting of exogenous therapeutic cells, which do not possess natural adhesion ligands, to injured or inflamed tissues (12, 13). Enhanced engraftment of mesenchymal stem cells (MSCs), for example, at the site of injury (e.g., ischemic myocardium) has been shown to inhibit fibrous tissue formation and to promote tissue regeneration (14, 15). Here we show for the first time that cells engineered chemically with artificial ligands can engage all 3 types of cell-cell interactions under flow conditions that represent critical components of the inflammatory cell adhesion cascade. We focused on MSCs (16) or multipotent mesenchymal stromal cells (17) that are capable of repairing injured tissues (e.g., bone, cartilage, fat, and muscle) due to their multilineage differentiation potential (18) and are immune privileged and immunosuppressive (e.g., suppress T-cell activation and proliferation; ref. 19). They offer significant therapeutic potential and are being tested in >100 clinical trials for treatment of tissue injury and immune diseases, including osteogenesis imperfecta, graft-vs.-host disease, myocardial infarction, and multiple sclerosis (20). However, a significant barrier in MSC therapy, and cell therapy in general, is the inability to target systemically administrated cells to tissues of interest with high efficiency (12, 20). This is, in part, because MSCs do not express (13, 21), or lose during culture expansion (22), some key adhesion ligands (e.g., PSGL-1) that are required in the immune cell adhesion cascade.
Here we demonstrate that nucleic acid aptamers, when chemically attached onto the MSC surface, serve as artificial “adhesion ligands” and bind to protein receptors on adjacent cells. Aptamers are single-stranded nucleic acid sequences that can be isolated for virtually any protein receptor of interest through an in vitro method called systematic evolution of ligands by exponential enrichment (SELEX; refs. 23, 24). Aptamers offer great engineering versatility (25): they are synthesized by a scalable and reproducible chemical process by which functionalities such as biotin, polyethylene glycol (PEG) spacers, and fluorescent dyes can easily be incorporated. Their binding affinity to target receptors can be tailored, for example, by including mutations in their sequence during synthesis. In addition, aptamers are chemically stable and can be tuned to resist degradation in response to restriction enzymes under physiological conditions by incorporation of modifiers, including PEG, phosphorothioates, and locked nucleic acids (26–28). Indeed, aptamers have emerged as powerful tools for construction of biosensors, bioanalysis, and assembly of nanostructures and for capture and isolation of stem cells or cancer cells when immobilized on a substrate (29–36).
Herein we report the successful engineering of aptamers onto the surface of MSCs, enabling them to bind under dynamic flow conditions to ECs or leukocytes. Interactions were facilitated between MSCs in suspension and adherent ECs, MSCs in suspension and neutrophils arrested on P-selectin-coated substrates (such substrates mimic adherent EC), and neutrophils and MSCs in suspension that complex and subsequently tether to P-selectin substrates (Scheme 1). Specifically, we demonstrate that P-selectin binding aptamer engineered MSCs can tether and firmly adhere to both P-selectin-coated substrates and ECs in a P-selectin-dependent manner. We also show that L-selectin binding aptamer engineered MSCs can tether to L-selectin-coated substrates or bind to freely flowing and adherent neutrophils in an L-selectin-dependent manner. The concept of engineering specific cell-cell interactions using cell-surface-attached artificial ligands has several implications for systemic targeting of cells, construction of cellular aggregates in tissue engineering, and building simple models to study the biology of cellular interactions.
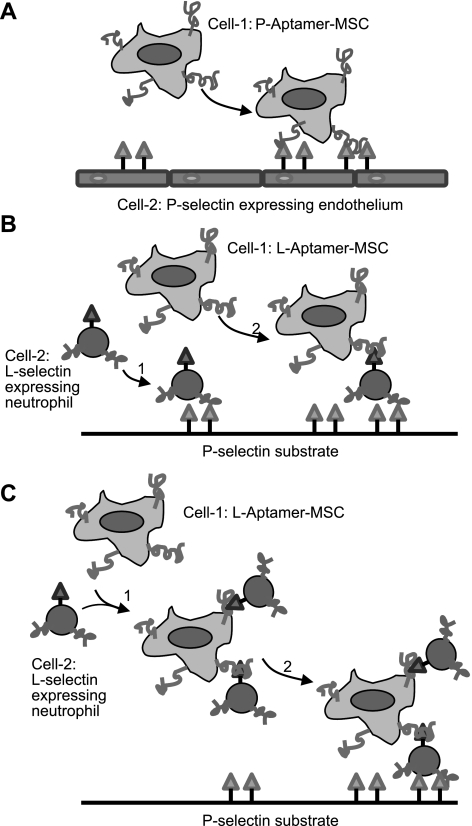
Scheme 1.
Mimicking 3 types of cell-cell interactions under dynamic flow conditions through engineering the cell surface with aptamers. A) Flowing cell 1 (P-selectin aptamer-MSC) tethers to adherent cell 2 (P-selectin-expressing endothelial cell). B) Flowing cell 2 (L-selectin-expressing leukocyte) tethers to a P-selectin-coated substrate using the native leukocyte-P-selectin interaction and then captures flowing cell 1 (L-selectin aptamer-MSC). C) Cell 1 (L-selectin aptamer-MSC) and cell 2 (L-selectin-expressing leukocyte) first complex in the flowing stream and then tether to a P-selectin-coated substrate.
MATERIALS AND METHODS
Materials
Recombinant P-selectin-Fc, L-selectin-Fc, and all antibodies used for flow cytometry were purchased from R&D Systems (Minneapolis, MN, USA). DNA and RNA molecules were purchased from Integrated DNA Technologies (IDT; Coralville, IA, USA) and Keck Biotechnology Resource Laboratory (Yale University, New Haven, CT, USA), respectively. Primary human MSCs were obtained from the Center for Gene Therapy (Texas A&M, College Station, TX, USA). α-MEM, l-glutamine, and Penn-Strep were purchased from Invitrogen. Human umbilical vein endothelial cells (HUVECs) and their medium were obtained from Lonza (Walkersville, MD, USA). FBS was purchased from Atlanta Biologicals (Lawrencevilla, GA, USA). KG1a and HL-60 cell lines and their medium were purchased from the American Type Culture Collection (ATCC; Manasses, VA, USA) and cultured according to the protocols provided from ATCC. Protein A-coated polystyrene beads were obtained from Bangs Laboratories (Fishers, IN, USA). Fresh whole blood isolated from healthy donors was purchased from Research Blood Components (Brighton, MA, USA) in a sodium heparin tube, shipped immediately, and received within 1 h. Neutrophils were isolated from whole blood using a previously reported protocol (37) and used within 4 h. All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) and were used without further modification unless specified.
MSC culture and characterization
Primary human MSCs were isolated from human marrow of healthy consenting donors and thoroughly characterized by the Institute for Regenerative Medicine (Texas A&M Health Science Center College of Medicine, College Station, TX, USA), as described previously (21, 38). Before use, we confirmed the surface expression of CD90, CD29, CD106 and the absence of CD34 and CD45 on MSCs using flow cytometry. MSCs were adherent on tissue culture surfaces and were maintained in MSC medium containing 15% FBS, 1% (v/v) l-glutamine, 1% (v/v) Penn-Strep, and α-MEM. To detach adherent cells for passaging or for experiments, cells were incubated in 1× trypsin/EDTA solution at 37°C for 3 min. MSCs at passage number 4–6 with a confluency of <80% were used for all experiments.
HUVEC culture and activation
HUVECs were cultured on gelatin-coated surfaces (Corning, Corning, NY, USA) and maintained in a medium comprising endothelial cell basal medium 2 (EBM-2), EGM-2 SingleQuots kit, and 10% FBS at 37°C and 5% CO2, according to a protocol provided by Lonza. Medium was changed every 2 d. Cells were subcultured by incubating them in 1× trypsin/EDTA solution at 37°C for 2 min, washed by PBS using centrifuge, and subsequently plated at an appropriate density. Cells of passage 3–5 were used for all experiments. Activation of HUVEC monolayer on the dish by histamine (10−4 M) was performed in EBM-2 at 37°C for 10 min immediately before flow chamber assays.
Engineering aptamers onto the MSC surface
Typically, MSCs were first detached from tissue culture flask by trypsin/EDTA, washed once in PBS, and centrifuged (1500 rpm, 3 min) to pellet the cells. MSCs (∼106) were then incubated with 1 ml of 1 mM sulfonated biotinyl-N-hydroxy-succinimide (NHS-biotin) in PBS for 10 min at room temperature (RT) and then washed with PBS by centrifugation. Biotinylated cells were incubated with 1 ml of 50 μg/ml streptavidin solution in PBS for 5 min at RT and washed. Finally, streptavidin-modified cells were treated with 200 μl of 3.3 μM biotin-modified aptamer solution for 5 min at RT and washed. The reaction was monitored using a dye-modified aptamer (FAM-L-aptamer-biotin; Table 1) by flow cytometry (BD FACS Calibur flow cytometer; BD Biosciences, San Jose, CA, USA) and analyzed using Cell Quest software (BD Biosciences).
Table 1.
Aptamers and DNA sequences utilized in this study
| Name | Sequence |
|---|---|
| FAM-L-aptamer-biotin | 5′-FAM-tagccaaggtaaccagtacaaggtgctaaacgtaatggcttcggcttac-biotin-3′ |
| L-aptamer-biotina | 5′-biotin-tagccaaggtaaccagtacaaggtgctaaacgtaatggcttcggcttac-invert T-3′ |
| Scrambled sequence | 5′-gatgtagggacagtcaaatggagtggttcaaccgcccatcttcaacaat-biotin-3′ |
| FAM-scrambled sequence | 5′-gatgtagggacagtcaaatggagtggttcaaccgcccatcttcaacaat-FAM-3′ |
| P-aptamerb | 5′-biotin-cucaacgagccaggaacaucgacgucagcaaacgcgag-3′ |
| FAM-antisense | 5′-tacgtttagcaccttgtactggttacc-FAM-3′ |
This molecule is modified with an invert T base at the 3′ end to improve stability towards restriction enzyme digestion.
C and U bases in this RNA molecule are modified with fluoro groups at 2′ to increase the RNA stability towards restriction enzyme digestion.
Stability and accessibility of cell-surface-tethered aptamers
Aptamer modified MSCs were plated in 12-well plates in MSC medium and cultured at 37°C. At multiple time points after modification, cells were washed 3 times in PBS. A complementary DNA attached to a dye (FAM; FAM-antisense; Table 1; 2 μM in PBS with Ca/Mg) was then incubated with cells for 30 min at RT. Cells were then washed 3 times in PBS and analyzed by fluorescent microscopy (TE2000-U Inverted Nikon Microscope with a DS-Qi1 Monochrome Cooled Digital Camera; Nikon, Tokyo, Japan).
Viability, adhesion, proliferation, and multilineage differentiation characteristics
The characterization of MSC phenotype was performed according to our previous study (21).
Preparation of L- or P-selectin surfaces for flow chamber assay
L- or P-selectin solution in PBS (1.5 ml, 3.3 μg/ml) was added to 35- × 10-mm BD Falcon tissue culture dishes (BD Biosciences) and incubated at 4°C overnight. All L- or P-selectin surfaces were prepared immediately before use in flow chamber assays.
Static cell adhesion assay
L- or P-selectin solution in PBS (500 μl, 3.3 μg/ml) was added to each well of 24-well plates, incubated at 4°C overnight, and then rinsed by PBS 3 times. Then, ∼25,000 modified or unmodified MSCs were added and incubated at 37°C for 10 min. Nonadherent cells were removed by washing with PBS (with Ca and Mg), and adherent cells were then counted by hemocytometer.
Flow chamber assay for assessing MSC interactions on a selectin-coated Petri dish
Modified or unmodified MSCs (∼5×105 in 1 ml MSC medium) were perfused through a circular parallel plate flow chamber (Glycotech, Gaithersburg, MD, USA; gasket thickness 127 μm, width 2.5 mm), which was directly mounted onto a selectin-coated Petri dish. The shear stress was controlled by a syringe pump (PHD3000; Harvard Apparatus, Holliston, MA, USA). Cells were permitted to interact with the surface for 1 and 5 min in the cases of L-aptamer-MSCs and P-aptamer-MSCs, respectively. To monitor cell adhesion, phase-contrast microscopy was utilized; images were acquired at 1-s intervals at ×10.
Flow chamber assay for assessing cell-cell interactions under dynamic conditions
For experiments involving P-aptamer-MSCs and HUVECs, HUVECs were cultured directly on 35- × 10-mm BD tissue culture dishes on which the flow chamber was mounted. HUVECs were treated with histamine immediately before use in the flow chamber assay. Modified and unmodified MSCs (∼5×105 in 1 ml PBS with Ca/Mg) were perfused through the chamber and allowed to interact with HUVECs for 5 min before shear forces were applied.
For experiments involving L-aptamer-MSCs and neutrophils, neutrophils (∼2×106) and L-aptamer-MSCs (∼5×105) were suspended in 1 ml PBS with Ca/Mg and perfused over a P-selectin-coated flow chamber surface. Shear forces at different rates were applied immediately after cells reached the field of view (i.e., zero delay before shear was applied).
RESULTS
Engineering aptamer ligands on the surface of MSCs
Conjugation of aptamers to the cell surface
The RNA and DNA aptamers we used in this study bind specifically to human P- and L-selectin with a Kd of ∼30 pM and 2 nM, respectively (39, 40) (all nucleic acid sequences are listed in Table 1). Given that RNA is chemically less stable than DNA and therefore more difficult to handle, we chose to use L-selectin binding DNA aptamer to systematically study and optimize the MSC surface engineering steps. We first confirmed, by flow cytometry, the specific binding between the aptamer sequence adopted from the literature and L-selectin using dye-modified aptamer and L-selectin-conjugated polystyrene beads or cells (i.e., KG1a) that express L-selectin (data not shown).
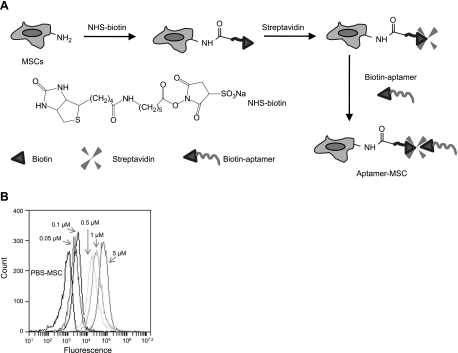
We then conjugated aptamers to the MSC surface using a simple chemical approach that we recently established (21). Specifically, the 3-step modification process includes treatment of cells (in a suspension after trypsinization) with NHS-biotin to introduce biotin groups on the cell surface, complexing with streptavidin, and coupling with biotinylated aptamers (Fig. 1A). We have previously optimized the conjugation conditions (i.e., reagent concentrations and reaction times) to achieve a desirable site density of attached aptamer molecules with minimal quantities of reagents and coupling durations (data not shown). It was found that typically ∼21,000 molecules/MSC were attached using this procedure (data not shown). The successful conjugation of aptamers (conjugated with a fluorescent dye, FAM, a fluorescein derivative; FAM-L-aptamer-biotin; Table 1) on MSC was confirmed using flow cytometry (Fig. 1B). Notably, the site density of aptamers on the cell surface could be readily tuned by adjusting the aptamer concentration used in the conjugation (Fig. 1B).
Figure 1.
A) Schematic illustration depicting the chemical immobilization of aptamers onto the MSC surface using a 3-step procedure. B) Successful conjugation of aptamers on the MSC surface was confirmed by flow cytometry. A positive fluorescence signal was observed for MSCs modified with aptamer-dye, and the intensity of the signal is directly related to the concentration of aptamer used during the conjugation process. Control experiments using MSCs with aptamer-FAM that lacked biotin modification (FAM-L-aptamer; Table 1) exhibited minimal fluorescence (data not shown).
Aptamer stability and accessibility on the MSC surface
It is crucial to investigate the stability and accessibility of aptamers on the cell membrane under physiological conditions, given the potential for cell internalization and restriction enzyme degradation. We addressed this question by staining the L-selectin binding aptamer-modified MSCs (L-aptamer-MSCs) at multiple time points after modification, with a complementary DNA conjugated to a dye (FAM; FAM-antisense; Table 1) followed by fluorescent analysis. We confirmed that aptamers on the MSC surface were accessible to FAM-antisense by flow cytometry immediately after modification (data not shown). Modified cells in a 24-well plate were used to study the accessibility of cell bound aptamers through addition of the FAM at multiple time points and examination with fluorescence microscopy. Aptamers remained stable and accessible on the cell membrane for ≥24 h in MSC cell culture medium at 37°C (mimicking physiological conditions), as evidenced by strong positive fluorescent staining compared with the unmodified PBS-MSC controls (Supplemental Fig. S1).
Effect of aptamer modification on MSC phenotype
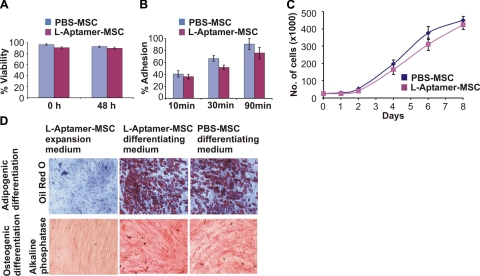
To examine the potential effect of aptamer conjugation on cell phenotype, we examined the viability, adhesion, proliferation, and multilineage differentiation potential of L-aptamer-MSC. The modification of MSCs with aptamers had minimal effect on MSC phenotype (Fig. 2), which is consistent with our previous study where we conjugated sialyl Lewis X to MSC surfaces using a similar technique (21).
Figure 2.
A) Viability of L-aptamer-MSCs and unmodified PBS-MSCs immediately after modification (0 h) and after 48 h. B) Adherence of L-aptamer-MSCs and PBS-MSCs measured at 10, 30, and 90 min. C) Proliferation of L-aptamer-MSCs and PBS-MSCs over 8 d. D) Alkaline phosphatase (ALP) and oil red O (ORO) staining, 23 d after addition of osteogenic and adipogenic differentiation medium, respectively. Negative controls (L-aptamer-MSCs cultured in expansion medium) showed no ORO or ALP staining. Positive controls (PBS-MSCs in differentiating medium) showed positive ORO and ALP staining. Experimental group (L-aptamer-MSCs in respective differentiating medium) showed positive staining for both ORO and ALP.
L-aptamer-MSCs bind to L-selectin-coated substrates under dynamic flow conditions
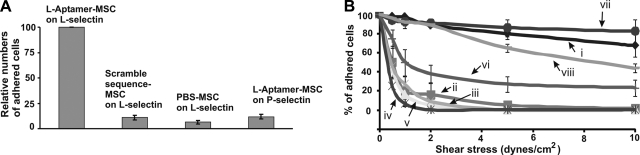
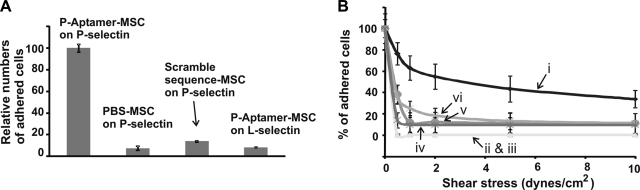
After we confirmed the successful conjugation and the availability of aptamers on the MSC surface, we investigated the interactions between L-aptamer-MSCs and L-selectin-coated surfaces under both static and flow conditions. For the static adhesion assay, aptamer-modified and unmodified MSCs were incubated with L-selectin-coated surfaces for 10 min, and unbound cells were then removed through rinsing. As shown in Fig. 3A, the number of L-aptamer-MSCs that adhered to L-selectin surfaces (normalized to 100) was significantly higher than in control groups (8.95±2.23, 6.4±1.78, and 9.6±2.3 for scrambled sequence aptamer-modified MSCs on L-selectin, PBS MSCs on L-selectin, and L-aptamer-MSCs on P-selectin, respectively).
Figure 3.
A) Static adhesion of L-aptamer-MSCs on an L-selectin-coated substrate with controls including scrambled sequence DNA modified MSCs on L-selectin, PBS MSC on L-selectin, and L-aptamer-MSCs on P-selectin. Adherent cell numbers in controls were normalized using the number of L-aptamer-MSCs on L-selectin as 100. B) Adhesion of modified and unmodified MSCs on L-selectin or P-selectin-coated substrates under flow conditions. i) L-aptamer-MSCs on L-selectin-coated surfaces (5 μM aptamer was used in the conjugation). ii) PBS-MSCs on an L-selectin surface. iii) L-aptamer-MSCs on a P-selectin surface. iv) L-aptamer-MSCs on an L-selectin surface in the presence of 5 mM EDTA. v) Scrambled sequence DNA-modified MSCs on L-selectin-coated surfaces. vi) L-aptamer-MSCs on L-selectin surface preblocked with L-selectin aptamers. vii) Native HL60 cells on L-selectin-coated surfaces. viii) L-aptamer-MSCs (0.5 μM aptamer was used in conjugation) on L-selectin-coated surfaces. Note that only cells that were initially in the fields of view before shear stress was applied were considered (i.e., newly incoming cells were not considered). Flow was continuously applied from lower to higher shear stresses, with 1 min of flow at each shear stress.
We then investigated the adhesion of L-aptamer-MSCs on L-selectin-coated surfaces under dynamic flow conditions using a parallel flow chamber. Specifically, cells were perfused into a flow chamber and then permitted to settle and interact with the substrate for 1 min before resuming flow conditions. The number of cells remaining on the surface was plotted as a percentage of the number of cells present before flow conditions were applied (y axis) as a function of shear stress (x axis) (Fig. 3B). L-aptamer-MSCs showed significantly stronger binding to the L-selectin-coated surface than the controls. Controls included PBS-MSCs on L-selectin-coated surfaces; L-aptamer-MSCs on P-selectin-coated surfaces; L-aptamer-MSCs on L-selectin-coated surfaces in the presence of 5 mM EDTA (EDTA removes divalent cations, e.g., Ca2+, that are essential for aptamer binding to L-selectin; ref. 40); scrambled sequence DNA-modified MSCs on L-selectin-coated surfaces; and L-aptamer-MSCs on L-selectin-coated surfaces blocked with L-selectin aptamers. Significantly, the ability of L-aptamer-MSCs to adhere to L-selectin-coated surfaces was comparable to that of native HL-60 cells on L-selectin (Fig. 3B, trace vii). HL-60 cells can adhere strongly to L-selectin, up to shear stresses of 10 dyn/cm2 (41). Notably, we can modulate the binding strength between L-aptamer-MSCs and L-selectin-coated surfaces by simply titrating the aptamer site density on the MSC surface (i.e., by modulating the avidity). For instance, L-aptamer-MSCs with a lower site density of aptamer (prepared with 0.5 μM aptamer, Fig. 1B) showed significant but decreased binding to L-selectin-coated surfaces (Fig. 3B, trace viii) compared with L-aptamer-MSCs prepared using 5 μM aptamer (Fig. 3B, trace i).
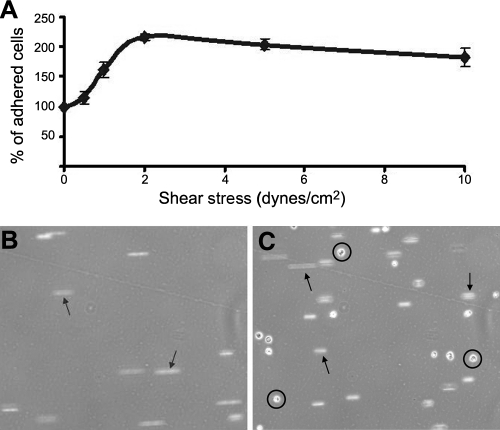
Figure 3B shows the adhesion behavior of the cells that initially settled in the field of view before shear stress was applied (newly incoming cells that entered the field of view on the application of shear flow were ignored). When newly incoming cells were considered, we observed a significant accumulation of L-aptamer-MSCs on L-selectin-coated surface. As we increased shear stress, the total number of adhered cells in the field of view initially increased (up to 2 dyn/cm2) and then started decreasing at higher shear stresses (i.e., 2–10 dyn/cm2; Fig. 4A). Strikingly, L-aptamer-MSCs could be directly captured from the flowing cell suspension by L-selectin-coated substrates under physiologically relevant flow conditions (i.e., up to 1.5 dyn/cm2; Fig. 4B, C and Supplemental Video S1). Cell-cell interactions leading to cell accumulation under dynamic flow conditions are critical in both normal physiology and in some cell-based therapies. For example, tethering of leukocytes or systemically infused therapeutic cells requires contact and interaction with the endothelium under shear flow (12).
Figure 4.
Engineered tethering of aptamer-engineered MSCs under dynamic flow conditions. A) Accumulation of L-aptamer-MSCs on an L-selectin-coated substrate. Cells were permitted to interact with the surface for 1 min under no-flow conditions. Next, gradually increasing shear stresses were applied for 1 min at each shear stress. Relative cell numbers in the same field of view were enumerated before each transition to the next shear stress. B, C) Representative snapshots of tethering for L-aptamer-MSCs on L-selectin-coated substrate under continuous flow condition (i.e., cells were not permitted to interact with surface) at 0.75 dyn/cm2. Images were acquired under flow conditions at time 0 (B) and 5 min (C). Arrows and circles indicate floating cells in the free stream and tethered cells, respectively. See Supplemental Video S1 for a full-time video.
P-selectin aptamer-MSCs bind to P-selectin-coated surfaces
After establishing utility for the L-selectin binding DNA aptamer-MSC system, we then used the same procedure to conjugate P-selectin binding RNA aptamers (39) onto MSCs (P-aptamer-MSCs) and subsequently investigated their interactions with P-selectin-coated surfaces under both static and flow conditions. As expected, cell-surface-tethered P-selectin aptamers facilitated the binding of MSC to P-selectin-coated surfaces (Fig. 5), which was otherwise absent under investigated conditions. Interestingly, the binding of P-aptamer-MSCs to P-selectin-coated surfaces under flow conditions was not as effective as the L-aptamer-MSC system: fewer cells remained adhered under high shear stress (Fig. 5B), and the tethering of cells on the substrate under continuous flow conditions was observed only up to 0.75 dyn/cm2 (data not shown). This was a surprise, particularly considering that the P-selectin aptamer had a lower Kd (∼30 pM) than the L-selectin DNA aptamer (∼2 nM) (39, 40), and is not fully understood at the current stage.
Figure 5.
A) Static adhesion of P-aptamer-MSCs on P-selectin-coated substrates compared with controls, including scrambled sequence DNA-modified MSCs on P-selectin, PBS-treated MSCs on P-selectin, and P-aptamer-MSCs on L-selectin. Adherent cell numbers in controls were normalized using P-aptamer-MSCs on P-selectin as 100. B) Adhesion of modified and unmodified MSCs on P-selectin or L-selectin-coated substrates under flow conditions. i) P-aptamer-MSCs on P-selectin surface. ii) PBS-MSCs on P-selectin surface. iii) Scrambled sequence-modified MSCs on P-selectin surface. iv) P-aptamer-MSCs on L-selectin surface. v) P-aptamer-MSCs on P-selectin surface in the presence of 5 mM EDTA. vi) P-aptamer-MSCs on P-selectin surface preblocked with P-selectin aptamers. Note that only cells that were initially in the field of view were considered.
Aptamer-promoted cell-cell interactions
P-selectin aptamer-promoted MSC-EC interactions
After demonstrating that aptamer-engineered MSCs can bind specifically to selectin-coated substrates, we investigated aptamer-promoted cell-cell interactions. We started with the first mechanism (Scheme 1A) to determine whether the aptamer could promote a direct interaction between flowing MSCs and adherent ECs activated by inflammatory cytokines. HUVECs were used as a model system, as they are well known to express P-selectin when treated by inflammatory molecules such as histamine (9–11). In this study, we treated HUVECs with histamine for 10 min at 37°C and confirmed the up-regulation of P-selectin on HUVECs on treatment using immunostaining (Supplemental Fig. S4) and flow cytometry (data not shown).
We then studied P-aptamer-MSC (with controls) and HUVEC interactions using a parallel flow chamber assay. Specifically, a confluent monolayer of HUVECs was first cultured. After histamine treatment, P-aptamer-MSCs were perfused on the endothelium under controlled shear stress in the flow chamber. Significantly, P-aptamer-MSCs bound to HUVECs under static conditions and accumulated on the HUVEC plate when shear stresses were applied, up to 0.75 dyn/cm2 (Fig. 6A and Supplemental Video S2). Approximately 60% of MSCs that were initially present before flow conditions remained attached to HUVECs even at stresses up to 5 dyn/cm2, which is significantly higher than controls, including MSCs without aptamer modification, scrambled sequence DNA-modified MSCs, and P-aptamer-MSCs on HUVECs preblocked with P-selectin aptamers (Fig. 6B). This strongly suggests that P-selectin binding aptamer conjugation to the MSC surface promoted strong and specific interactions between MSCs and HUVECs.
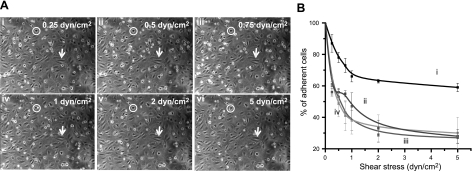
Figure 6.
P-aptamer-MSCs bind to histamine treated HUVECs. A) Representative images demonstrate the accumulation of P-aptamer-MSCs on HUVECs when low to high shear stresses were applied; total number of adhered cells in the same field of view first increases to 0.75 dyn/cm2 and then starts decreasing at higher shear stresses (i.e., 1–5 dyn/cm2). Cell numbers at 0.25 (i), 0.5 (ii), and 0.75 (iii), 1 (iv), 2 (v), and 5 dyn/cm2 (vi) are 54, 63, 76, 55, 50, and 42, respectively. See Supplemental Video S2 for a representative video. Note that perfused circular MSCs (circles) and adherent spindle-shaped HUVECs (arrows) can be easily distinguished by their differing shapes, which was confirmed by immunostaining (Supplemental Fig. 3A, B). B) Percentage of adherent MSCs as a function of shear stress. Only MSCs initially present in the field of view were considered. i) P-aptamer-MSCs. ii) MSCs treated with PBS instead of P-selectin aptamer in the third step of modification. iii) Scrambled sequence-modified MSCs. iv) P-aptamer-MSCs on HUVECs preblocked with P-selectin aptamers.
L-selectin aptamer-promoted MSC-neutrophil interactions
We next studied the L-selectin aptamer promoted cell-cell interactions between MSCs and leukocytes (i.e., neutrophils). Neutrophils exhibit robust rolling and adhesion on activated endothelium and P-selectin-coated surfaces (which resemble activated endothelium; refs. 9–11). In addition, neutrophils that adhere on activated endothelium further capture free-flowing neutrophils via interactions between L-selectin and its ligands (i.e., PSGL-1), which are both expressed on neutrophils (4, 5, 42). We first validated these native properties of neutrophils using the parallel flow chamber assay and observed robust neutrophil rolling, adhesion, and secondary tethering events on P-selectin-coated surfaces (data not shown), confirming that P-selectin ligands and L-selectin expressed on neutrophils are viable and functional and confirming the reliability of using such an assay to study MSC-neutrophil interactions, as described below.
We then investigated the interactions between L-aptamer-MSCs and L-selectin-expressing neutrophils. In the flow chamber assay, we first mixed neutrophils (∼2×106) and L-aptamer-MSCs (∼5×105) and then perfused them immediately over a P-selectin-coated surface. Strikingly, we observed that arrested neutrophils on the P-selectin-coated surface captured free-flowing L-aptamer-MSCs (Fig. 7A and Scheme 1B), and neutrophils first complexed with the L-aptamer-MSCs in the free-flowing stream, which facilitated tethering of the MSCs onto the P-selectin-coated surface (Fig. 7B and Scheme 1C). Several combinations of MSC-neutrophil complexes were formed in the flow stream: In addition to MSC-neutrophil pairs, MSCs were commonly conjugated to 2 or more neutrophils, and in some cases, large multicellular MSC-neutrophil aggregates formed (Fig. 8 and Supplemental Video S3). Note that these large aggregates could tether to P-selectin-coated surfaces under flow conditions through neutrophil-P-selectin interactions (Supplemental Video S3). In contrast, for control experiments, minimal MSC-neutrophil interactions or neutrophil-mediated capture of MSCs on P-selectin surface were observed where neutrophils and PBS-MSCs, neutrophils and scrambled sequence aptamer-modified MSCs, or neutrophils blocked with L-selectin aptamers and L-aptamer-MSCs were investigated (Supplemental Fig. S2). Interestingly, unlike interactions between L-aptamer-MSCs on L-selectin-coated surfaces that were sustained well above 1.5 dyn/cm2, L-aptamer-MSCs and neutrophil interactions were only effective under flow conditions at shear stresses of 0.5 dyn/cm2 or lower. It is unclear whether this is due to cell-cell interactions being ineffective at higher shear stresses and/or the shedding of L-selectins from neutrophil surfaces at higher shear stresses (4, 5, 42, 43).
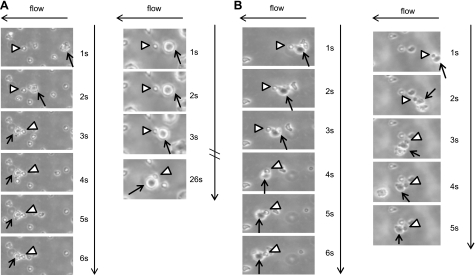
Figure 7.
Interactions between L-aptamer-MSCs and neutrophils on a P-selectin substrate under flow conditions at 0.25 dyn/cm2. Note that neutrophils and MSCs, ∼8 and ∼20–25 μm in diameter, respectively (determined by examining pure populations of neutrophils and MSCs by microscopy), can be easily distinguished from each other by size, which was confirmed by immunostaining (Supplemental Fig. 3C, D). Representative examples of an adherent neutrophil (arrowhead) capturing a flowing MSC (arrow) (A) and a neutrophil (arrowhead) complexed with MSCs (arrow) first in the flowing stream and then tethered onto the P-selectin surface (B). Note that once captured, MSCs always shift their position to the left of the immobilized neutrophils due to the flow direction (from right to left in this case), which clearly demonstrates that the capture of MSCs on P-selectin was mediated by binding of neutrophils to the P-selectin-coated substrate vs. MSCs binding to the P-selectin-coated substrate.
Figure 8.
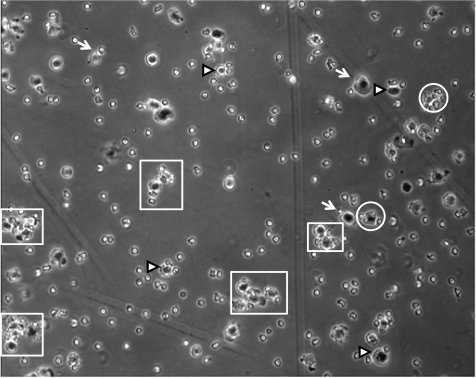
Representative image of typical L-aptamer-MSC-neutrophil interactions in the flow stream at a shear stress of 0.25 dyn/cm2. Arrows, arrowheads, and circles indicate MSC-neutrophil complexes with a MSC:neutrophil ratio of 1:1, 1:2, and 1:3 (or 3+), respectively. Larger cellular aggregates, i.e., comprising ≥2 MSCs and multiple neutrophils, were also observed (highlighted in boxes). See Supplemental Video S3 for a representative video of L-aptamer-MSC-neutrophil interactions and their binding to a P-selectin surface under flow conditions.
DISCUSSION
In nature, leukocytes have evolved effective yet complex intercellular adhesion mechanisms to target inflamed sites in the body. Thus, there is significant interest in studying these interactions for the creation of novel therapeutic strategies (13, 21, 44–49). In particular, leukocyte-endothelium interactions can be emulated to engineer systemically infused therapeutic (stem) cells to target endothelium at injured or diseased sites (13, 21, 41).
We have demonstrated for the first time that MSCs engineered with nucleic acid aptamers on their surface can specifically engage their respective protein receptors on a substrate or on adjacent cells. We chose P- and L-selectin as target receptors because they are expressed on inflamed endothelial cells and leukocytes, respectively, and serve major roles in mediating leukocyte-endothelium, leukocyte-leukocyte, and leukocyte-platelet interactions in the inflammatory cell adhesion cascade (4, 5, 9–11). We have demonstrated that MSCs engineered with aptamers, which target adhesion receptors on leukocytes, can recapitulate 3 key types of cell-cell interactions under flow conditions that represent critical components of the inflammatory cell adhesion cascade (Scheme 1). Significantly, to our knowledge, this is one of the first studies to report engineered cell tethering under flow conditions, and the approach can be used to emulate many steps in the natural leukocyte homing cascade by using a single engineered cell surface ligand. For instance, L-aptamer-MSCs can directly tether onto L-selectin substrate under physiologically relevant flow conditions (Fig. 4 and Supplemental Video S1). The same ligand can be used to enhance the interaction of flowing cells with a substrate, which mimics activated endothelium within inflamed tissue by taking advantage of natural homing properties of neutrophils to promote MSC-neutrophil interactions (Figs. 7, 8 and Supplemental Video S3). This strategy enabled MSCs to mimic the neutrophil-neutrophil interactions that promote MSC adhesion to inflamed endothelium and may offer a unique cell targeting route in which therapeutic cells are delivered to site of interest via naturally homing cells (e.g., neutrophils). In addition, engineered cell-cell interactions may provide a platform to interrogate how cell-cell contact affects cellular functions and intercellular communications, perhaps similar to how in vitro systems utilize beads with immobilized selectin ligands to roll on selectin-coated surfaces, which have been employed to help model in vivo events within the leukocyte adhesion cascade (50). In particular, we have shown that a single engineered cell surface aptamer ligand can allow the cells to be directly arrested on the target receptor surface without a cell rolling step that is required in the traditional paradigm of leukocyte homing cascade. This simple in vitro model system may therefore be useful to the study cell adhesion mechanism, which in turn provides guidance for engineering cells toward systemic targeting.
We have used a simple chemical approach to engineer aptamers on the cell surface. Conducting chemistry at the cell surface has recently been emerging as a powerful tool for cell labeling and imaging, drug delivery and discovery, and even manipulating cell fate (44, 45, 51, 52). The chemical approach is appealing, particularly when compared with genetic and enzymatic engineering, due to its simplicity and broad applicability. For instance, aptamer conjugation takes <30 min and can be conducted at RT or 37°C, and multiple types of ligands can be attached simultaneously. The site density of cell surface attached ligands can be readily tuned to adjust avidity, for example, by adjusting the concentration of reagents used in the conjugation (Figs. 1B and 3B, traces i, viii). Notably, we have shown that this modification has a minimal effect on cell phenotype, including viability, adhesion, proliferation, secretion of paracrine factors, multilineage differentiation, and homing ability, including transendothelial migration (data herein and not shown). Furthermore, cell-surface-attached ligands are stable and accessible for a reasonably long period of time. For instance, we have shown that the biotin moieties conjugated on the cell surface using the NHS-biotin treatment are stable for ≥7 d (21). In the present study, aptamers on the MSC surface remain accessible, for ≥24 h under physiological conditions in vitro (Supplemental Fig. 1). Note also that aptamer stability toward restriction enzyme degradation (which can occur in vivo) is tunable by incorporating a variety of modifiers, including polyethylene glycol, phosphorothioates, and locked nucleic acids (26–28). For in vivo applications, aptamers can be engineered to have a half-life on the cell surface that is sufficient to engage target cells but subsequently degrade to minimize nonspecific interactions. In addition, given that aptamers are synthesized chemically using a DNA synthesizer, which permits many other functionalities to be incorporated, the cells can be engineered to possess multiple functions (25). For instance, cells modified with aptamers that are conjugated to fluorescent dyes can fulfill both targeting and in vivo cell monitoring and imaging functions.
MSCs are known to possess immunomodulatory properties and have a profound suppressive effect on a variety of immune cells, including T and B lymphocytes (T and B cells), neutrophils, dendritic cells, and natural killer cells (12, 53) making MSCs a candidate to treat inflammation and graft rejection in bone marrow or solid organ transplantations. However, the routes by which MSCs exert these effects on immune cells are controversial: it is unclear whether the underlying mechanisms involve direct cell-cell contact and/or soluble factors (12, 53). Simple in vitro models that utilize engineered aptamer mediated intercellular interactions should prove useful in elucidating the biological functions of contact between MSCs and immune or other types of cells.
The concept of engineering cells with artificial aptamer ligands should be generally applicable where modulating cell-cell contact is beneficial. For instance, we have demonstrated that it is possible to construct cellular aggregates using aptamer-engaged cell-cell contact. As shown in Fig. 8 and Supplemental Video S3, L-aptamer-MSCs are commonly conjugated to 2 or more neutrophils that form large MSC-neutrophil aggregates. Recently, Gartner and Bertozzi (44) elegantly demonstrated that DNA-modified cells can be assembled into microtissues in a well-defined manner (i.e., structures were controlled by varying the DNA site density on the cell surface, cell concentration, and DNA sequence); however, their approach requires the modification of both cell types. We reason that aptamer-programmed cell-cell contact can be used to build tissue constructs in tissue engineering. Factors such as stoichiometry, aptamer length and rigidity, and aptamer type can be modulated to obtain desired 3-dimensional cellular constructs. By incorporating different cell types with defined cell-cell distances, this strategy could be applied to building functional tissues. In addition, such integrated tissue constructs may potentially be used as in vitro models for examining cell-cell communication and cell biology, investigating pathology and disease states, modulating cell microenvironments or niches, and testing and screening new drugs (44–47).
In summary, we have demonstrated the use of a simple chemical approach to conjugate aptamers to the cell surface, which can induce specific cell-cell interactions that would otherwise not exist. This approach is broadly applicable given that an aptamer for virtually any target protein receptor can be isolated through SELEX (23, 24). The engineered cell-cell interactions may provide a platform to interrogate how cell-cell contact affects cellular functions and intercellular communications and should be useful for assembling 3-dimensional tissue constructs, systemic targeting of cells to sites of inflammation or to specific tissues, and elucidating the biology of cellular interactions.
Supplementary Material
Acknowledgments
The authors thank Uli von Andrian for helpful discussions.
This work was supported by National Institute of Health (NIH) grants HL-097172, HL-095722, and DE-019191 (to J.M.K.) and by American Heart Association grant 0970178N (to J.M.K). W.Z. is supported by an International Human Frontier Science Program Organization postdoctoral fellowship. Primary human MSCs were obtained from the Center for Gene Therapy (Texas A&M, College Station, TX, USA), which received funding through NIH National Center for Research Resources grant P40RR017447.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 2. Laird D. J., von Andrian U. H., Wagers A. J. (2008) Stem cell trafficking in tissue development, growth, and disease. Cell 132, 612–630 [DOI] [PubMed] [Google Scholar]
- 3. Luster A. D., Alon R., von Andrian U. H. (2005) Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 6, 1182–1190 [DOI] [PubMed] [Google Scholar]
- 4. Simon S. I., Rochon Y. P., Lynam E. B., Smith C. W., Anderson D. C., Sklar L. A. (1993) Beta 2-integrin and L-selectin are obligatory receptors in neutrophil aggregation. Blood 82, 1097–1106 [PubMed] [Google Scholar]
- 5. Walcheck B., Moore K., McEver R., Kishimoto T. (1996) Neutrophil–neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. J. Clin. Invest. 98, 1081–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diacovo T., Catalina M., Siegelman M., von Andrian U. (1998) Circulating activated platelets reconstitute lymphocyte homing and immunity in L-selectin-deficient mice. J. Exp. Med. 187, 197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ludwig J., Schultz J., Boehncke W., Podda M., Tandi C., Krombach F., Baatz H., Kaufmann R., von Andrian U., Zollner T. (2004) Activated, not resting, platelets increase leukocyte rolling in murine skin utilizing a distinct set of adhesion molecules. J. Invest. Dermatol. 122, 830–836 [DOI] [PubMed] [Google Scholar]
- 8. Kansas G. (1996) Selectins and their ligands: current concepts and controversies. Blood 88, 3259–3287 [PubMed] [Google Scholar]
- 9. Jones D. A., Abbassi O., McIntire L.V., McEver R. P., Smith C. W. (1993) P-selectin mediates neutrophil rolling on histamine-stimulated endothelial cells. Biophys. J. 65, 1560–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yao L., Pan J., Setiadi H., Patel K. D., McEver R. P. (1996) Interleukin 4 or oncostatin M induces a prolonged increase in P-selectin mRNA and protein in human endothelial cells. J. Exp. Med. 184, 81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sugama Y., Tiruppathi C., Janakidevi K., Anderson T. T., Fenton J. W., 2nd, Malik A. B. (1992) Thrombin-induced expression of endothelial P-selectin and intercellular adhesion molecule-1: a mechanism for stabilizing neutrophil adhesion. J. Cell Biol. 119, 935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karp J. M., Teo G. S. L. (2009) Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4, 206–216 [DOI] [PubMed] [Google Scholar]
- 13. Sackstein R., Merzaban J. S., Cain D. W., Dagia N. M., Spencer J. A., Lin C. P., Wohlgemuth R. (2008) Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 14, 181–187 [DOI] [PubMed] [Google Scholar]
- 14. Huang J., Zhang Z., Guo J., Ni A., Deb A., Zhang L., Mirotsou M., Pratt R. E., Dzau V. J. (2010) Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ. Res. 106, 1753–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hodgkinson C. P., Gomez J. A., Mirotsou M., Dzau V. (2010) Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum. Gene Ther. 21, 1513–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caplan A. I. (1991) Mesenchymal stem cells. J. Orthop. Res. 9, 641–650 [DOI] [PubMed] [Google Scholar]
- 17. Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F. C., Krause D. S., Deans R. J., Keating A., Prockop D. J., Horwitz E. M. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 [DOI] [PubMed] [Google Scholar]
- 18. Pittenger M., Mackay A., Beck S., Jaiswal R., Douglas R., Mosca J., Moorman M., Simonetti D., Craig S., Marshak D. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 [DOI] [PubMed] [Google Scholar]
- 19. Le Blanc K., Tammik C., Rosendahl K., Zetterberg E., Ringden O. (2003) HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 31, 890–896 [DOI] [PubMed] [Google Scholar]
- 20. Ankrum J., Karp J. (2010) Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol. Med. 16, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sarkar D., Vemula P. K., Teo G. S. L., Spelke D., Karnik R., Wee L. Y., Karp J. M. (2008) Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjug. Chem. 19, 2105–2109 [DOI] [PubMed] [Google Scholar]
- 22. Rombouts W. J. C., Ploemacher R. E. (2003) Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 17, 160–170 [DOI] [PubMed] [Google Scholar]
- 23. Ellington A., Szostak J. (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 [DOI] [PubMed] [Google Scholar]
- 24. Tuerk C., Gold L. (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 [DOI] [PubMed] [Google Scholar]
- 25. Jayasena S. (1999) Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 45, 1628–1650 [PubMed] [Google Scholar]
- 26. Detzer A., Sczakiel G. (2009) Phosphorothioate-stimulated uptake of siRNA by mammalian cells: a novel route for delivery. Curr. Top. Med. Chem. 9, 1109–1116 [DOI] [PubMed] [Google Scholar]
- 27. Veedu R., Wengel J. (2009) Locked nucleic acid as a novel class of therapeutic agents. RNA Biol. 6, 321–323 [DOI] [PubMed] [Google Scholar]
- 28. Luo D., Saltzman W. (2000) Synthetic DNA delivery systems. Nat. Biotech. 18, 33–37 [DOI] [PubMed] [Google Scholar]
- 29. Zhao W., Ali M. M., Brook M. A., Li Y. (2008) Rolling circle amplification: Applications in nanotechnology and biodetection with functional nucleic acids. Angew. Chem. Int. Ed. Engl. 47, 6330–6337 [DOI] [PubMed] [Google Scholar]
- 30. Liu J., Cao Z., Lu Y. (2009) Functional nucleic acid sensors. Chem. Rev. 109, 1948–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao W., Chiuman W., Lam J. C., McManus S. A., Chen W., Cui Y., Pelton R., Brook M. A., Li Y. (2008) DNA aptamer folding on gold nanoparticle: from colloid chemistry to biosensors. J. Am. Chem. Soc. 130, 3610–3618 [DOI] [PubMed] [Google Scholar]
- 32. Zhao W., Chiuman W., Brook M. A., Li Y. (2007) Simple and rapid colorimetric biosensors based on DNA aptamer and non-crosslinking gold nanoparticle aggregation. Chem. Biochem. 8, 727. [DOI] [PubMed] [Google Scholar]
- 33. Zhao W., Brook M. A., Li Y. (2008) Design of gold nanoparticle based colorimetric biosensing assays. Chem. Biochem. 9, 2363–2371 [DOI] [PubMed] [Google Scholar]
- 34. Phillips J., Xu Y., Xia Z., Fan Z., Tan W. (2009) Enrichment of cancer cells using aptamers immobilized on a microfluidic channel. Anal. Chem. 81, 1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo K. T., Schäfer R., Paul A., Gerber A., Ziemer G., Wendel H. P. (2006) A new technique for the isolation and surface immobilization of mesenchymal stem cells from whole bone marrow using high-specific DNA aptamers. Stem Cells 24, 2220–2231 [DOI] [PubMed] [Google Scholar]
- 36. Wu Y., Sefah K., Liu H., Wang R., Tan W. (2010) DNA aptamer-micelle as an efficient detection/delivery vehicle toward cancer cells. Proc. Natl. Acad. Sci. U. S. A. 107, 5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oh H., Siano B., Diamond S. (2008) Neutrophil isolation protocol. J. Vis. Exp. 17, 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sekiya I., Larson B. L., Smith J., Pochampally R., Cui J., Prockop D. J. (2002) Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells 20, 530–541 [DOI] [PubMed] [Google Scholar]
- 39. Jenison R., Jennings S., Walker D., Bargatze R., Parma D. (1998) Oligonucleotide inhibitors of P-selectin-dependent neutrophil-platelet adhesion. Antisense Nucl. Acid Drug Dev. 8, 265–279 [DOI] [PubMed] [Google Scholar]
- 40. Hicke B., Watson S., Koenig A., Lynott C. K., Bargatze R., Chang Y., Ringquist S., Moon-McDermott L., Jennings S., Fitzwater T., Han H., Varki N., Albinana I., Willis M., Varki A., Parma D. (1996) DNA aptamers block L-selectin function in vivo. J. Clin. Invest. 98, 2688–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dimitroff C. J., Lee J. Y., Schor K. S., Sandmaier B. M., Sackstein R. (2001) Differential L-selectin binding activities of human hematopoietic cell L-selectin ligands, HCELL and PSGL-1. J. Biol. Chem. 276, 47623–47631 [DOI] [PubMed] [Google Scholar]
- 42. Bargatze R., Kurk S., Butcher E., Jutila M. (1994) Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J. Exp. Med. 180, 1785–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weyrich A. S., Zimmerman G. A. (2004) Platelets: signaling cells in the immune continuum. Trends Immunol. 25, 489–495 [DOI] [PubMed] [Google Scholar]
- 44. Gartner Z., Bertozzi C. (2009) Programmed assembly of 3-dimensional microtissues with defined cellular connectivity. Proc. Natl. Acad. Sci. 106, 4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao W., Teo G., Kumar N., Karp J. (2010) Chemistry and material science at the cell surface. Materials Today 13, 14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Bank P., Hou Q., Warner R., Wood I., Ali B., MacNeil S., Kendall D., Kellam B., Shakesheff K., Buttery L. (2007) Accelerated formation of multicellular 3-D structures by cell-to-cell cross-linking. Biotechnol. Bioengin. 97, 1617–1625 [DOI] [PubMed] [Google Scholar]
- 47. Hui E., Bhatia S. (2007) Micromechanical control of cell–cell interactions. Proc. Natl. Acad. Sci. U. S. A. 104, 5722–5726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fleming T. (2002) Cell-Cell Interactions: A Practical Approach, 2nd Ed., Oxford University Press, Oxford, UK [Google Scholar]
- 49. Wolf E., Hofmeister R., Kufer P., Schlereth B., Baeuerle P. A. (2005) BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Disc. Today 10, 1237–1244 [DOI] [PubMed] [Google Scholar]
- 50. Yago T., Leppanen A., Qiu H., Marcus W. D., Nollert M. U., Zhu C., Cummings R. D., McEver R. P. (2002) Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow. J. Cell Biol. 158, 787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hsiao S. C., Shum B. J., Onoe H., Douglas E. S., Gartner Z. J., Mathies R. A., Bertozzi C. R., Francis M. B. (2009) Direct cell surface modification with DNA for the capture of primary cells and the investigation of myotube formation on defined patterns. Langmuir 25, 6985–6991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chandra R. A., Douglas E. S., Mathies R. A., Bertozzi C. R., Francis M. B. (2006) Programmable cell adhesion encoded by DNA hybridization. Angew. Chem. Int. Ed. Engl. 45, 896–901 [DOI] [PubMed] [Google Scholar]
- 53. Nauta A. J., Fibbe W. E. (2007) Immunomodulatory properties of mesenchymal stromal cells. Blood 110, 3499–3506 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.