Abstract
Over the past decade, rehabilitation hospitals have begun to incorporate robotics technologies into the daily treatment schedule of many patients. These interventions hold greater promise than simply replicating traditional therapy, because they allow therapists an unprecedented ability to specify and monitor movement features such as speed, direction, amplitude, and joint coordination patterns and to introduce controlled perturbations into therapy. We argue that to fully realize the potential of robotic devices in neurorehabilitation, it is necessary to better understand the specific aspects of movement that should be facilitated in rehabilitation. In this article, we first discuss neurorecovery in the context of motor control and learning principles that can provide guidelines to rehabilitation professionals for enhancing recovery of motor function. We then discuss how robotic devices can be used to support such activities.
Keywords: rehabilitation, robotics, spinal cord injury, stroke
Motor Learning and Rehabilitation
Before speculating on what role robotics devices should play in neurorehabilitation, it is important to look at rehabilitation in general and consider the process of functional recovery following neurological injuries such as stroke, spinal cord injury, and traumatic brain injury. A detailed discussion of this topic is beyond the scope of this article, but we wanted to touch upon a fundamental question: Do improvements in function post central nervous system (CNS) injury result from recovery processes or through compensatory strategies? Recovery would indicate that the neural substrates normally recruited to innervate a particular group of muscles prior to injury can be recruited to innervate the same muscle groups following injury. Alternatively, compensatory strategies would occur if different neural substrates were recruited after an injury to achieve a similar functional output. While any neural recovery must occur in the absence of the neurons that are no longer functional, this does not require compensation if neighboring neurons can reestablish the damaged neural circuitry.
An increasing number of studies indicate that improvements in function following CNS injuries are largely mediated by compensatory strategies rather than true recovery, particularly after the first few weeks following injury.1 For example, a recent randomized clinical trial comparing manual-assisted treadmill training to a structured home exercise program in hemiparetic stroke patients reported that individuals in all groups improved overground walking speed by over 60% yet showed almost no improvement on the Fugl-Meyer impairment scale.2 Thus, speed was improved, but little change in impairment occurred. Similarly, if we look at the results of many of the upper limb robotics studies over the last decade, subjects typically show substantial improvements in their ability to perform functional tasks, while demonstrating little change in impairment measures (see ref. 3 for review). Thus, rather than demonstrating improvements in impairment, patients are learning to use their impaired systems more effectively. If in fact functional recovery following CNS injuries mainly results from developing alternative strategies to achieve a particular task, motor control and learning principles should provide a basis for developing more effective treatments.
Motor learning has been defined as a “set of (internal) processes associated with practice or experience leading to relatively permanent changes in the capability for responding.”4 More broadly defined, motor learning can be thought of as acquiring the necessary skills to successfully plan and execute a desired movement pattern that is appropriate for a given task (see ref. 5 for a comprehensive discussion of motor learning). At this point, it is necessary to introduce a fundamental question: What constitutes a “desired movement ”? In the past, a common guiding principle used in occupational and physical therapy has often been to make movements more “normal” or similar to those exhibited by nonimpaired individuals. This idea emerged from the observation that certain characteristics of movements made by healthy individuals are fairly similar within a given task and even across tasks. For example, when reaching for an object in space, movement trajectories across healthy individuals appear fairly straight and smooth.6 Such reliability of motor behavior is particularly interesting because of the abundance of possible solutions to most movement tasks and the variety of environments in which we move. For example, one can reach for a cup of coffee with an infinite variety of joint patterns, movement speeds, and trajectories.
Regardless of these possibilities, people tend to display movement patterns that are consistent across movements and individuals.7–9 One way to arrive at a single solution when confronted with many different options is to employ optimization algorithms, which seek a minimum of a particular cost function. Previous studies have shown that optimization of certain costs reproduces many characteristics observed in human motion. For example, Flash and Hogan8 tested the idea that the smoothness of hand trajectories might reflect an important cost in the planning of reaching movements, while others speculated that mechanical costs, such as mean squared torque change,9 peak work,10 or muscle energy,11 might determine the planning of movement patterns. Harris and Wolpert12 proposed the idea that neural noise might account for the trial-to-trial variability seen during repetitions of the same task and proposed that the CNS seeks to minimize this. This work has shown that the planning of movements involves explicit performance criteria such as task accuracy as well as implicit criteria of which we are not aware, such as making energetically efficient and reliable movements. In addition, motor control and learning should be viewed as a problem-solving process, rather than a simple memorization process.
The research described above has led to the understanding that motor control and learning involve not only the acquisition of new motor patterns, but also the development of more accurate motor predictions. To better understand the nature of motor learning, Conditt et al13 examined subjects adapt their arm movements while holding onto the handle of a robot that could impose forces during their movements. They were asked to make straight-line movements between 2 targets while the robot produced a strong viscous field that had the tendency to skew their movements (ie, similar to trying to move across a flowing river). After a short period of time, individuals were able to compensate for the externally imposed viscous field and straighten out their movements. After learning the force field by making point-to-point movements, subjects were also able to move through circular patterns, which would not have been possible if they compensated for the field through rote learning. Similar findings of adaptation to novel task dynamics have been demonstrated for inertial,14 curl field,15 and coriolis forces.16 Although generalization across the workspace is often limited, subjects always generalize outside of the space that they had practiced. These findings emphasize that we do not learn rote patterns of coordination, but rather learn to make predictions about how motor commands influence movements in a particular set of environmental and task contexts. Such predictions are based on internal models of our body in the context of the local environment. These models are then used to predict the motor commands needed to produce a variety of movements that extend beyond those experienced during practice. These types of predictions underlie motor generalization and are critical to solve optimal control problems, such as those described previously.
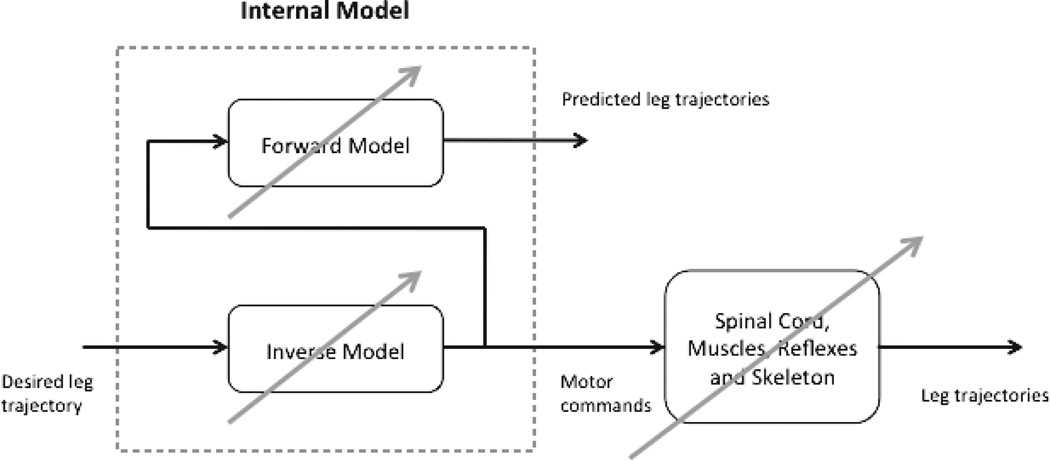
Internal models of our limbs are thought to be developed at a young age and are continuously adjusted as we progress from infancy to adulthood.17 Yet what happens when someone has a stroke, spinal cord injury, or other injury to the CNS? As illustrated in Figure 1, for the intact nervous system, we have an internal model of our limbs and the environment that allows us to predict the motor commands necessary to achieve a desired movement trajectory.
Figure 1.
The motor control system after stroke or spinal cord injury is altered so that the internal model is no longer appropriate to the postinjury sensory-motor system. As a result, predicted motor commands are likely to be inaccurate in achieving a desired movement trajectory.
A forward model is used to compare the predicted movement patterns with the actual movements; if there are errors between the two, the internal model can be updated and refined for future movements. After CNS injury, the internal model utilized throughout adulthood is no longer appropriate because damage to CNS neural substrates often leads to impairments such as weakness,18–20 spasticity,21 and other disturbances.22 As a result, the predicted motor commands no longer produce the desired movement trajectories, often resulting in disturbances in movement patterns. Even if the ability to accurately model the damaged system is left intact, optimal movement solutions would not be expected to mimic the patterns of healthy individuals due to changes in the central and peripheral system.
In other words, damage to the CNS and associated secondary changes in the musculoskeletal system can induce changes in the set of possible solutions as well as the costs associated with any given task. Therefore, patients may arrive at solutions that may not look “normal,” but may be “optimal” given their specific pathologies. Therefore, it is important to understand the biomechanical costs associated with different tasks, rather than simply attempting to make movements look more “normal.” Many clinical assessments of function include either the ability to perform certain activity of daily living (ADL) tasks23 (FIM*) or the ability to perform simulated ADL tasks in particular time constraints24 (Jebson-Taylor Hand Function Test [JHFT]). We now propose that analysis of energetic efficiency should provide an important supplement to such clinical tests. Such analysis is important; when ADLs are completed independently, yet with too high energetic costs, one should expect minimal carryover into the patient’s spontaneous behavior.
Based on the arguments presented above, we propose that the focus of rehabilitation should be to retrain an individual’s abilities to adapt to a task in variety of contexts, exploiting the “modeling” abilities of the CNS, rather than to practice a particular movement pattern repetitiously. After patients have lost some aspect of their motor function, it is necessary for them to develop new models of their body that account for their residual capacities and allow them to develop optimal coordination in the context of their disabilities. Indeed, it is likely that some deficits will make executing certain tasks difficult if not impossible. The goal for such patients should be to try and develop the optimal control strategies for functional tasks within the constraints of their abilities.
Applications to Rehabilitation
What do we know about motor learning that may be applicable to rehabilitation? First, repetition (or practice) is extremely important.4 There is little doubt that the ability to perform a task improves with practice, yet in order for these improvements to generalize, there needs to be some degree of variability. For example, having subjects repeatedly move an object between 2 points may improve their ability to perform the task for that particular object, however it may not allow them to perform similar movements with other objects. Along similar lines, it is important for individuals to practice a diverse set of activities, otherwise generalization will be limited. For example, spinalized cats trained to stand can stand well, whereas spinalized cats trained to step can step well. However spinalized cats trained to stand often step worse than cats that received no training at all.25 It could be theorized that this lack of task variability is one reason manual-assisted treadmill training in humans has not proven to be more effective than conventional gait training in improving overground walking ability after stroke2 or spinal cord injury.26 With treadmill training, individuals only practice stepping on a moving belt and do not get to practice balance tasks, postural control, or walking over uneven terrain or obstacles.
The research that we have summarized indicates that motor learning involves the development of optimal coordination patterns that satisfy costs associated with task performance and energetic costs. This planning process is dependent on predicting the effects of movement plans. However, the nervous system also appears to set control policies that modulate reflexes27 to account for perturbations from unexpected changes in environmental or internal conditions. The importance of recognizing both of these features of control in rehabilitation is fundamentally important, because brain damage due to stroke can have differential effects on these 2 aspects of coordination. For example, although it is well-established that spasticity interferes with accurate reflex control, Beer et al28 showed that hemiparesis also disrupts optimal intersegmental coordination, resulting in inefficient coordination that fails to account for the dynamic interactions between the segments. Thus, both these aspects of coordination must be addressed to optimize functional movement control in patients with CNS damage.
Traditional therapeutic strategies, as well as more recent robot-aided rehabilitation strategies, tend to target the optimal control process by practicing fairly consistent patterns of coordination and reinforcing task success. We agree that this type of repetition is critical for improving coordination through repetition. However, focusing on repetitive movements under consistent environmental conditions should only represent the first step in the rehabilitation process. With repetition of consistent patterns alone, patients may become adept at the training protocols but show limited transfer to ADLs. We suggest that as patients improve their movement patterns under predictable repetitive practice, training protocols should progressively incorporate unpredictable conditions. Such conditions should introduce unpredictable perturbations. Robotic devices that can be precisely programmed to provide perturbing forces of various magnitude and times are perfectly suited to such training.
It should also be stressed that visual feedback can be manipulated and enhanced using computer-based robotics technologies. Scheidt et al29 tested the importance of visual feedback compared to proprioceptive feedback by having subjects make point-to-point arm movements while connected to a robotic manipulandum. The robot created a viscous field the subjects had to move within (similar to the description above of the flowing river) such that when the field was first turned on, the subject’s accuracy was diminished. When subjects received full visual and proprioceptive feedback or only proprioceptive feedback, they corrected their movements after a short time and their accuracy increased to the level prior to the disturbance field turning on. However, when subjects were given false visual feedback of their performance (ie, the visual display showed them hitting the target even when they were not accurate), despite having proprioceptive feedback, the subjects never corrected their movements and their accuracy remained low. Visual feedback of performance is clearly a critical factor in learning to adapt to new movement conditions.
In the context of rehabilitation robotics, visual feedback is critical; subjects are often strapped into these devices and may not be fully aware of their performance based on proprioception alone. For example, our randomized controlled trial (RCT) comparing robotic-assisted gait training to conventional gait training in subacute stroke patients found that the conventional group improved their walking speed by twice as much as the robot-trained group and improved their walking distance (ie, endurance) 66% greater than the robot group.30 In subsequent analysis of the motor control strategies being utilized by the robot-trained subjects while walking in the robot, we found that they were often inappropriate for the task, yet the individuals were unaware that they were executing the wrong strategy because they were strapped into the robot which enforced a normal gait pattern.22 The visual feedback of watching themselves in a mirror and achieving a beautiful gait pattern was stronger than the proprioceptive feedback they received, which likely diminished their ability to learn the appropriate control strategies needed to walk.
Robotics and Rehabilitation
To reiterate some principles of motor learning we believe are important in the context of rehabilitation, individuals with CNS injury should be able to practice a diverse set of tasks repeatedly and should receive accurate visual feedback of their performance. In addition, these devices should allow natural variability in environmental conditions and/or the ability to program variations in environmental forces in order to develop error correction mechanisms. Do robotic devices support such activities? In some ways, robotic devices are perfectly suited for rehabilitation; in other ways, they are not. To illustrate our point, we compare and contrast 2 commercially available devices being used for gait training. The first device, called the Lokomat, is shown in Figure 2A (Hocoma AG, Volketswil, Switzerland). The device has 2 robotic arms that attach to the patients’ legs and can assist hip and knee movements as they walk on a treadmill. In parallel, a portion of the subjects’ weight is supported by an overhead unloading system, making it possible for patients with extreme weakness to begin training in the early stages of their injuries. The Lokomat offers some key advantages: patients can practice large volumes of steps, the device offers patients a high degree of safety, and training sessions can be quite long for higher functioning patients. In addition, the Lokomat may provide more predictable assistance than that provide by a therapist, which may help to reestablish an appropriate internal model. Some of these advantages are negated by motor learning principles not supported by the Lokomat. For example, when subjects walk in the Lokomat, they are provided a basic visual display, yet it does not truly capture their resulting leg movements. It is often difficult for the patients being trained to determine whether the motor control strategy they implemented resulted in a desired leg movement or whether the robot simply forced their leg through the correct pattern. The Lokomat also only allows patients to be trained in one therapeutic mode – walking on a treadmill. It does not allow patients to practice important activities such as balance and postural control tasks, stepping over objects, or walking on surfaces other than one that is smooth and flat. These activities provide important environmental and task variations that facilitate the development of error correction processes and the generalization of motor learning. As mentioned previously, the limitations in providing stereotypic practice may outweigh the potential advantages of such devices and may help explain the results of our RCT that compared Lokomat gait training to conventional gait training.
Figure 2.
(A) Lokomat robotic exoskeleton (Hocoma AG, Volketswil, Switzerland) assists patients as they walk on a treadmill. (B) ZeroG overground gait and balance training system (Aretech, LLC, Ashburn, Virginia).
An alternative robotic device being used for gait training is ZeroG (Figure 2B; Aretech, LLC, Ashburn, Virginia). The device is an overground body weight–support system that rides along an overhead track. The system can alleviate the patients of a percentage of their body weight as they practice walking overground, balance and postural tasks, and stepping up and down a couple of steps. The key advantage of ZeroG over other robotic exoskeletons is that patients can practice a wide range of activities in a safe, controlled manner. In addition, variability in movement performance that arises from within the patients’ motor system and from the environment remains intact. Because the system is ceiling-mounted, patients can practice walking on uneven terrain, walking up and down a step or two, standing and sitting, and other ADLs. They also receive appropriate visual feedback of their performance; if they take a step and their resulting leg trajectory is incorrect, they will immediately know. The disadvantage of ZeroG is that it requires patients to have some residual function in their lower extremities; otherwise the therapist is needed to assist their legs when attempting a particular movement pattern. Each of these devices supports various aspects of motor learning, and they both have strengths and weaknesses.
Rather than providing a list of pros and cons of all available rehabilitation robotic technologies commercially available today, we will instead provide a general framework outlining some of the key principles these devices should follow. First, if in fact improvements in a patient’s ability to perform a task are the result of motor learning and perhaps training a new internal model, robotic devices should support a wide range of activities that allow the individual the ability to practice them in high volume. The ability to practice something over and over again is important to “getting it right”; however, in order to facilitate learning, the individual must generate errors and receive accurate error information. Thus an accurate representation of performance must be provided. This feedback will be highly dependent on the robot control strategies utilized during the training. For example, if the robot assists the subject’s limbs to move through a prescribed kinematic trajectory, there will have to be a visual display indicating the magnitude and direction of assistance provided. This is likely to be quite confusing to the patient and may be misinterpreted. Alternatively, if the robot is run in some type of impedance mode, the visual feedback could simply be where the limbs end up.
Another essential characteristic that robotic devices should posses is diversity. Motor learning often generalizes, particularly if an accurate internal model is developed, but that generalization can only go so far. For example, if a basketball develops the perfect motor control strategy for making free throws, there is no guarantee they will be an accurate 3-point shooters. The idea is that robotic devices should allow individuals to practice a wide variety of activities and not just walking or reaching in a horizontal plane. In addition, these variations should include natural and programmed perturbations to develop patients’ error correction capabilities.
Finally, related to the previous few points, it is important that during rehabilitation, patients are allowed to fail at the activities they are trying to perform. Failure allows them to try the activity with a particular motor control strategy, see if it works, and then adjust their strategy. It also allows the development of error correction mechanisms, such as impedance control through modulation of reflex circuits. Such error correction is fundamental to motor learning. Error experience also allows patients to test the boundaries of task success. In gait, for example, it is important for patients to understand how large of a step they can take while still maintaining their balance and stability. Robotic devices are perfectly suited for such activities; they can provide the patient a safe environment for taking risks and testing their limits and allow them to learn what their motor capacity is in their new motor control system.
It is unclear what the future of rehabili-tation robotics will be, however we believe that the next generation of robotic devices must support activities based on solid scientific principles rather than just being really cool pieces of technology. As more and more studies seem to support the notion that improvements in function following many CNS injuries are coming through compensatory strategies rather than pure recovery, we believe new devices should support motor learning principles, which may help enhance these patients’ ability to perform the functional tasks needed at home and in society.
Acknowledgments
A portion of this work was supported by grant 1R01HD059783 awarded by the National Institutes of Health, National Institute of Child Health and Human Development (PI: Dr. Robert Sainburg).
Footnotes
FIM™ is a trademark of Uniform Data System for Medical Rehabilitation, a division of UB Foundation Activities, Inc.
REFERENCES
- 1.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004;22:281–299. [PubMed] [Google Scholar]
- 2.Duncan PW, et al. Prototocl for the locomotor experience applied post-stroke (LEAPS) trial: a randomized controlled trial. BMC Neurol. 2007;7:39. doi: 10.1186/1471-2377-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hidler J, Nichols D, Pelliccio M, Brady K. Advances in the understanding and treatment of stroke impairment using robotic devices. Top Stroke Rehabil. 2005;12(2):21–33. doi: 10.1310/RYT5-62N4-CTVX-8JTE. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt RA, Lee TD. Motor Control and Learning. 3rd ed. Champaign, IL: Human Kinetics Publishing; 1999. [Google Scholar]
- 5.Schadmehr R, Wise SP. The Computational Neurobiology of Reaching and Pointing: A Foundation for Motor Learning. Cambridge, MA: The MIT Press; 2005. [Google Scholar]
- 6.Morasso P. Spatial control of arm movements. Exp Brain Res. 1981;42(2):223–227. doi: 10.1007/BF00236911. [DOI] [PubMed] [Google Scholar]
- 7.Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394(6695):780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- 8.Flash T, Hogan N. The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci. 1985;5(7):1688–1703. doi: 10.1523/JNEUROSCI.05-07-01688.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uno Y, Kawato M, Suzuki R. Formation and control of optimal trajectory in human multijoint arm movement. Minimum torque-change model. Biol Cybernetics. 1989;61(2):89–100. doi: 10.1007/BF00204593. [DOI] [PubMed] [Google Scholar]
- 10.Soechting JF, Buneo CA, Herrmann U, Flanders M. Moving effortlessly in three dimensions: does Donders' law apply to arm movement? J Neurosci. 1995;15(9):6271–6280. doi: 10.1523/JNEUROSCI.15-09-06271.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goble JA, Zhang Y, Shimansky Y, Sharma S, Dounskaia NV. Directional biases reveal utilization of arm's biomechanical properties for optimization of motor behavior. J Neurophysiol. 2007;98(3):1240–1252. doi: 10.1152/jn.00582.2007. [DOI] [PubMed] [Google Scholar]
- 12.Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394(6695):780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- 13.Conditt MA, Gandolfo F, Mussa-Ivaldi FA. The motor system does not learn the dynamics of the arm by rote memorization of past experience. J Neurophysiol. 1997;78:554–560. doi: 10.1152/jn.1997.78.1.554. [DOI] [PubMed] [Google Scholar]
- 14.Sainburg RL, Ghez C, Kalakanis D. Intersegmental dynamics are controlled by sequential anticipatory, error correction, and positional control mechanisms. J Neurophysiol. 1999;81:1045–1056. doi: 10.1152/jn.1999.81.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14(5 Pt 2):3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dizio P, Lackner JR. Motor adaptation to Coriolis force perturbations of reaching movements: endpoint but not trajectory adaptation transfers to the nonexposed arm. J Neurophysiol. 1995;74(4):1787–1792. doi: 10.1152/jn.1995.74.4.1787. [DOI] [PubMed] [Google Scholar]
- 17.Thelen E. Motor development: A new synthesis. Am Psychol. 1995;50(2):79–95. doi: 10.1037//0003-066x.50.2.79. [DOI] [PubMed] [Google Scholar]
- 18.Hidler J, Schmit BD. Evidence for force-feedback inhibition in chronic stroke. IEEE Trans Neural Syst Rehabil Engineer. 2004;12:166–176. doi: 10.1109/TNSRE.2004.828428. [DOI] [PubMed] [Google Scholar]
- 19.Hidler J, Carroll M, Federovich E. Strength and coordination in the paretic leg of individuals following acute stroke. IEEE Trans Neural Syst Rehabil Engineer. 2007;15(4):526–534. doi: 10.1109/TNSRE.2007.907689. [DOI] [PubMed] [Google Scholar]
- 20.Neckel N, Nichols D, Pelliccio M, Hidler J. Abnormal synergy patterns and weakness in individuals with chronic stroke. J NeuroEngineer Rehabil. 2006;3:17. doi: 10.1186/1743-0003-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black I, Nichols D, Pelliccio M, Hidler J. Quantification of reflex activity in stroke survivors during an imposed multi-joint leg extension movement. Exp Brain Res. 2007;183(2):271–281. doi: 10.1007/s00221-007-1045-6. [DOI] [PubMed] [Google Scholar]
- 22.Neckel N, Blonien N, Nichols D, Hidler J. Abnormal joint torque patterns exhibited by chronic stroke subjects while walking with a prescribed physiological gait pattern. J NeuroEngineer Rehabil. 2008;5:19. doi: 10.1186/1743-0003-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright J. The FIM(TM) The Center for Outcome Measurement in Brain Injury. 2000 doi: 10.1097/00001199-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 06. 1969;50(6):311–319. [PubMed] [Google Scholar]
- 25.de Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J Neurophysiol. 1999;82(1):359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- 26.Dobkin B, et al. Weight-supported treadmill vs overground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutha PK, Boulinguez P, Sainburg RL. Visual modulation of proprioceptive reflexes during movement. Brain Res. 2008;1246:54–69. doi: 10.1016/j.brainres.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131(3):305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- 29.Scheidt RA, Conditt MA, Secco EL, Mussa-Ivaldi FA. Interaction of visual and proprioceptive feedback during adaptation of human reaching movements. J Neurophysiol. 2005;93(6):3200–3213. doi: 10.1152/jn.00947.2004. [DOI] [PubMed] [Google Scholar]
- 30.Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, Hornby TG. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009;23(1):5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]