Abstract
Objective
To examine late adolescent substance use outcomes in relation to childhood conduct disorder (CD) and psychostimulant treatment in urban youth found to have attention-deficit/hyperactivity disorder (ADHD) in childhood.
Methods
Ninety-seven adolescents, evaluated during childhood, were seen for follow-up on average 9.30 (SD = 1.65) years later along with a well-matched never-ADHD control group. Stimulant treatment history was coded: Never (n = 28), up to 1 year (n = 19), 1 to 5 years (n = 28), and greater than 5 years (n = 22). Substance use at outcome was coded dimensionally for severity (frequency × intensity) and categorically for substance use disorders (SUDs).
Results
Individuals with ADHD+CD in childhood had significantly higher rates of SUD and substance use severity than those with childhood ADHD and controls. The ADHD and control groups did not differ significantly. Among those with childhood ADHD, there were no significant differences in SUD status or substance use severity as a function of medication history.
Conclusions
Within an ethnically diverse urban sample, the increased rate of substance use associated with ADHD was fully accounted for by the presence of CD. These results extend previous findings indicating little impact of psychostimulant treatment on later substance use to an ethnically diverse urban sample and to individuals who received treatment for up to 12 years.
Introduction
Longitudinal studies indicate that individuals found to have attention-deficit/hyperactivity disorder (ADHD) in childhood have poorer outcomes in educational (Mannuzza et al. 1997), cognitive (Fischer et al. 1990), and social (Lambert et al. 1987; Taylor et al. 1996) functioning than the age-matched controls. A diagnosis of ADHD in childhood is additionally associated with elevated rates of substance use and substance use disorders (SUDs) during adolescence and young adulthood (Milberger et al. 1997a; Mannuzza et al. 1998; Schubiner et al. 2000; Elkins et al. 2007). The subsequent development of substance use in individuals with ADHD has been found to be largely impacted by the presence of co-morbid conduct disorder (CD) (Barkley et al. 1990; Molina et al. 2002). However, several studies have shown an association between ADHD and increased substance use over and above the risk posed by CD (Milberger et al. 1997b; Burke et al. 2001; Molina and Pelham 2003; Elkins et al. 2007), whereas others have suggested that CD mediates the relationship between ADHD and later substance misuse (Brook et al. 2010). Thus, it may be that ADHD and CD together generate a greater risk for later substance misuse than either disorder alone (Flory and Lynam 2003).
Another issue of considerable importance is whether treatment of ADHD with stimulant medication impacts the development of substance use and SUDs, as stimulants are controlled substances with high potential for abuse. Preclinical studies examining the vulnerability to addiction as a function of prior exposure to methylphenidate (MPH) have generated mixed findings. Some studies have shown that early exposure to MPH enhances later drug taking (Brandon et al. 2001; Wooters et al. 2006; Valvassori et al. 2007), whereas others have found that early exposure to MPH results in a decrease in the rewarding effects of drug (Andersen et al. 2002; Carlezon, Jr. et al. 2003; Mague et al. 2005). In addition, these studies include important differences in dose amounts, age at first exposure, and timing of stimulant exposure; variables that may moderate the relationship between early exposure to MPH and later outcome (Wooters et al. 2006). In youth with ADHD, most studies report no effect of early stimulant treatment on substance use outcomes, but there are conflicting findings.
To date, only one longitudinal study in humans has reported a positive relationship between stimulant treatment in childhood and later substance use. Lambert and Hartsough (1998) divided an ethnically diverse sample of children with ADHD from largely lower socioeconomic strata into three medication groups; those never prescribed stimulant medication, those receiving medication for less than 1 year, and those with more than 1 year of medication treatment. Children treated with stimulants in childhood had significantly higher rates of daily smoking and cocaine dependence in adulthood. In contrast, two studies have suggested a protective effect of psychostimulant treatment, such that medicated individuals exhibited reduced SUD rates compared with those with ADHD not receiving such treatment (Biederman et al. 1999; Loney et al. 2002). The largest number of studies have found that childhood psychostimulant treatment does not significantly influence the likelihood of preadolescent (Chilcoat and Breslau 1999; Molina et al. 2007) or adolescent/young adult (Barkley et al. 2003; Biederman et al. 2008; Mannuzza et al. 2008) SUD in either direction.
As shown in Table 1, the majority of findings indicate that psychostimulant treatment of ADHD does not increase risk for later SUDs. However, the variability in findings suggests that some key issues remain unresolved, perhaps due in part to the fact that naturalistic longitudinal studies, by their very nature, are uncontrolled. As such, a number of factors exist both within and across studies that can potentially account for the diverse findings. For example, the only study that reported a negative influence of psychostimulant treatment was also one of only two studies to include an ethnically diverse sample from lower socioeconomic strata, and socioeconomic and ethnic differences have been shown to clearly impact substance use trajectories (Anglin et al. 1988; Gilman et al. 2008). However, this study also failed to adequately address issues pertaining to diagnostic severity and co-morbidity. Other studies either excluded children with CD from their initial childhood sample (Mannuzza et al. 1997) or had large differences in the rate of CD between the treated and untreated groups (Biederman et al. 1999; Barkley et al. 2003). That latter circumstance can introduce important differences in baseline risk unrelated to medication status that are not easily controlled for statistically (Miller and Chapman 2001).
Table 1.
Summary of Longitudinal Studies Examining the Effect of Stimulant Medication on Later Substance Use in Individuals with Attention-Deficit/Hyperactivity Disorder
| Author | ADHD sample size at follow-up | Developmental stage at follow-up | Primary ethnicity | Outcome |
|---|---|---|---|---|
| Lambert and Hartsough (1998) | 174 | Young adulthood | 77% Caucasian | ↑ |
| Biederman et al. (1999) | 75 | Late adolescent | Caucasian | ↓ |
| Loney et al. (2002) | 219 | Young adulthood | 98% Caucasian | ↓ |
| Chilcoat and Breslau (1999) | 146 | Early adolescence | 46% African American | ↔ |
| Molina et al. (2007) | 486 | Early adolescence | 61% Caucasian | ↔ |
| Barkley et al. (2003) | 147 | Young adulthood | 94% Caucasian | ↔ |
| Biederman et al. (2008) | 112 | Young adulthood | Caucasian | ↔ |
| Mannuzza et al. (2008) | 176 | Young adulthood | Caucasian | ↔ |
Early adolescence = 11–13; late adolescent = 15–18; young adulthood = 21–25.
↑ = Psychostimulant medication increased risk for later substance use.
↓ = Psychostimulant medication decreased risk for later substance use.
↔ = Psychostimulant medication did not significantly impact risk for later substance use.
ADHD = attention-deficit/hyperactivity disorder.
This prospective 10-year follow-up study examining substance use outcomes in a sample of primarily non-white children from lower socioeconomic backgrounds found to have ADHD in childhood had two primary aims: (1) to compare individuals with ADHD, with and without childhood CD, to a well-matched control group on late adolescent substance use outcomes and (2) to examine, among individuals with ADHD in childhood, the degree to which stimulant medication differentially affects later substance use severity and risk for SUD. Notably, this sample was highly variable with regard to the duration of treatment (none–12 years), allowing for a detailed investigation of duration-related effects in a sample where treatment duration was unrelated to ADHD severity or the presence of CD in childhood.
Materials and Methods
Participants
Ninety-seven adolescents/young adults who were evaluated in a research protocol during childhood (mean age at baseline = 9.05 years, SD = 1.28) were seen for follow-up on average 9.30 (SD = 1.65) years later. They were drawn from a group of 169 youth who were recruited between 1990 and 1997 for a study of ADHD and aggression. Of these 169 participants, 18 refused to participate in the follow-up, 2 were known to be deceased, 7 were incarcerated, and 46 were lost to follow-up. We attempted to locate missing participants by contacting known family members and via information publicly available on the internet. However, this sample was drawn from a highly mobile inner-city population and many individuals could not be found. Nevertheless, those who were and were not assessed at follow-up did not differ significantly with regard to age at initial evaluation, rates of childhood co-morbid diagnoses, Full Scale IQ, socioeconomic status (SES), or ADHD and other disruptive behavior disorder ratings at initial assessment (all p > 0.10). Thus, although a substantial number of the original sample was lost to follow-up, the subsample that participated in the follow-up study appears to be representative of the larger group.
At baseline (ages 7–11 years), participants were evaluated using parent report on the Diagnostic Interview Schedule for Children (DISC). Parent and teacher reports using the Child Behavior Checklist and Inattention/Overactivity with Aggression (IOWA) Conners Rating Scale, respectively, were also obtained. To insure cross-situationality of ADHD symptoms, children were required to have teacher ratings on the inattention/overactivity scale of the IOWA greater than 1.5 SD above the mean for age and gender. Based upon this evaluation, 32 (33%) children met criteria for CD in addition to ADHD. As shown in Table 2, individuals with ADHD+CD in childhood had significantly higher rates of psychiatric co-morbidity, and higher parent ratings of externalizing, but not internalizing or attention problems, than those with ADHD but not CD in childhood. Teachers rated those with CD higher on both subscales of the IOWA Conners.
Table 2.
Childhood Characteristics as a Function of Presence/Absence of Conduct Disorder
| |
ADHD |
ADHD+CD |
|
|
||
|---|---|---|---|---|---|---|
| |
n = 66 |
n = 31 |
|
|
||
| Measure | Mean | SD | Mean | SD | t | p |
| Age | 9.04 | 1.36 | 9.18 | 1.17 | 0.47 | 0.64 |
| SES | 38.21 | 19.03 | 31.81 | 14.10 | 1.75 | 0.09 |
| WISC-R/III-FSIQ | 95.47 | 14.19 | 90.73 | 14.29 | 1.51 | 0.22 |
| CBCL | ||||||
| Attention problems | 71.25 | 9.95 | 74.30 | 10.14 | 1.33 | 0.19 |
| Externalizing | 66.06 | 10.66 | 78.22 | 7.32 | 3.22 | 0.03 |
| Internalizing | 63.97 | 12.30 | 67.81 | 11.07 | 2.16 | 0.10 |
| IOWA Conners | ||||||
| I/O | 10.67 | 3.27 | 12.40 | 2.75 | 2.51 | 0.01 |
| O/D | 7.14 | 4.81 | 10.30 | 3.80 | 3.15 | <0.01 |
| DISC | χ2 | p | ||||
| % ANX | 22.72 | 50.00 | 7.41 | 0.01 | ||
| % MOOD | 6.06 | 18.75 | 3.79 | 0.05 | ||
ANX = any anxiety disorder; CBCL = Child Behavior Checklist; CD = conduct disorder; DISC = Diagnostic Interview Schedule for Children; FSIQ = Full Scale IQ; IOWA = Inattention/Overactivity with Aggression Rating Scale; I/O = inattention/overactivity; MOOD = any mood disorder; O/D = oppositional/defiant; SES = socioeconomic status; WISC-R/III = Wechsler Intelligence Scale for Children, Revised/3rd Ed.
Eighty five never-ADHD controls were recruited during late adolescence/early adulthood from the same urban communities as the ADHD group. Most were identified via targeted advertisements in neighborhoods that matched the ADHD sample by zip code. Controls resembled probands on most important demographic variables, including age, gender, ethnicity, SES, and general intellectual functioning (all p > 0.05), but did not have a history of ADHD in childhood or adolescence as ascertained using the DISC ADHD module during a screening interview. Prospective controls were excluded if they had any chronic medical or neurological condition, schizophrenia, a pervasive developmental disorder, or a Full Scale IQ score below 70, as was the case for the original ADHD sample.
At follow-up, both ADHD groups exhibited higher levels of parent-rated internalizing and externalizing than controls, and the ADHD+CD group displayed higher parent ratings of externalizing and internalizing problems relative to the ADHD only group. The two ADHD groups did not differ in parent-rated attention problems although, as expected, they were both greater than controls (see Table 3). Although the ADHD and ADHD+CD groups did not differ significantly in IQ at baseline, follow-up IQ scores for the ADHD+CD group were significantly lower compared with both the ADHD group and controls.
Table 3.
Late Adolescent Characteristics as a Function of Childhood Conduct Disorder
| |
Control |
ADHD |
ADHD+CD |
|
|
|||
|---|---|---|---|---|---|---|---|---|
| |
n = 85 |
n = 66 |
n = 31 |
|
|
|||
| Measure | Mean | SD | Mean | SD | Mean | SD | F | p |
| Age | 18.51 | 1.68 | 18.23 | 1.64 | 18.83 | 2.01 | 1.33 | 0.27 |
| SES | 40.66 | 16.75 | 45.97 | 19.62 | 37.53 | 12.26 | 2.94 | 0.06 |
| WAIS-FSIQa | 96.79 | 15.33 | 96.13 | 15.25 | 87.03 | 12.29 | 4.97 | 0.01 |
| CBCL | ||||||||
| Attentionb | 51.52 | 2.89 | 60.54 | 9.77 | 63.39 | 11.52 | 33.62 | <0.01 |
| Internalizingc | 47.97 | 9.89 | 54.90 | 13.42 | 60.79 | 12.53 | 14.49 | <0.01 |
| Externalizingc | 48.35 | 10.47 | 57.97 | 13.03 | 66.75 | 10.85 | 30.28 | <0.01 |
| t | p | |||||||
| Treatment duration in years | 2.95 | 3.60 | 3.21 | 3.37 | 0.34 | 0.73 | ||
WAIS = Wechsler Adult Intelligence Scale 3rd Ed.; CBCL = Child Behavior Checklist; SES = Socioeconomic status; FSIQ = Full Scale IQ.
The ADHD+CD group was significantly different from control and ADHD groups.
Both ADHD groups were significantly different from controls.
All three groups differed significantly.
This late adolescent sample was predominately male (87.8%) and racially and ethnically diverse (26.0% African-American, 23.8% Caucasian, 35.4% Hispanic, and 14.4% mixed or other ancestry). Ages generally ranged from 16 to 22 years; however, two individuals found to have ADHD had follow-up ages of 25 and 26, and one was 15. Mean SES, estimated from parental occupation and education using the socioeconomic prestige scale (Nakao and Treas 1994), was 42.57 (SD = 17.34). The sample comprised individuals with the full range of scores on this measure (20–96), but the modal score was 20 (n = 32, 17.7%), representing, on average, a low to lower-middle status group, with a substantial portion at the poverty level.
Participants and their parents were proficient in English, and were compensated for their time and travel. All procedures were approved by the Institutional Review Boards of the participating institutions. Written informed consent was obtained from all adolescents above the age of 18 years and the parents of those under the age of 18 years. Assent was obtained from youth under 18 years old.
Medication status
At follow-up, treatment history was obtained through administration of a “Services Received Interview” where parents detailed participant exposure to psychosocial interventions and/or pharmacotherapy. Participants were specifically queried regarding duration, type, and age at which treatment occurred. Supplemental information was provided through the initial interview portion of the Kiddie-SADS Present and Lifetime Version (Kaufman et al. 1997) and a review of records from the initial assessment, which included information regarding childhood medication status and history. Using all available data, 69 (71%) individuals in the ADHD group had received some treatment with psychostimulants (mean duration = 4.26 years; SD = 3.48; range = 1 or 2 doses to 12.00 years); 28 never received medication treatment. Additionally, 23 (24%) individuals found to have ADHD received a pharmacologic intervention other than psychostimulants. Due to the high variability in treatment duration, it seemed problematic to only combine those with treatment into a single group. Therefore, we separated individuals into four independent groups of approximately equal size: Individuals with no history of stimulant medication (n = 28), those who were treated for up to 1 year (n = 19, mean = 0.49 years, SD = 0.24; range = 0.25–1.00), 1 to 5 years (n = 28, mean = 3.36 years, SD = 1.05; range = 1.5–5.0), and those who were treated for longer than 5 years (n = 22, mean = 8.67 years, SD = 1.86; range = 5.25–12.0).
As shown in Table 4, comparison of treatment groups at baseline revealed that groups were generally similar with regard to parent and teacher ratings of behavior and patterns of co-morbidity. However, individuals receiving medication for 1–5 years had significantly higher teacher-rated inattention/overactivity than those receiving up to 1 year of medication, and individuals receiving treatment for up to 1 year had higher parent ratings of internalizing problems than those receiving medication for longer than 5 years. As shown in Table 5, analyses examining group differences among the medication group on follow-up measures of age, SES, and intellectual and psychological functioning revealed that individuals receiving treatment for up to 1 year had significantly higher externalizing scores than individuals never receiving medication treatment. Otherwise, groups did not differ on any other follow-up measure.
Table 4.
Childhood Characteristics of Medication Subgroups
| |
None |
One or less |
1–5 |
Greater than 5 |
|
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
n = 28 |
n = 19 |
n = 28 |
n = 22 |
|
|
||||
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p |
| Agea | 9.44 | 1.56 | 9.51 | 1.41 | 8.81 | 1.16 | 8.53 | 1.31 | 3.38 | 0.02 |
| SES | 32.19 | 16.50 | 37.78 | 17.59 | 34.52 | 14.97 | 43.73 | 21.50 | 1.78 | 0.16 |
| WISC-R/III - FSIQ | 89.07 | 14.02 | 93.55 | 11.49 | 96.74 | 13.82 | 97.10 | 16.65 | 1.76 | 0.16 |
| IOWA Conners | ||||||||||
| I/Ob | 10.39 | 3.18 | 9.61 | 3.98 | 12.73 | 2.22 | 11.23 | 2.88 | 4.69 | 0.004 |
| O/D | 7.19 | 4.59 | 9.61 | 4.73 | 9.19 | 4.61 | 7.05 | 4.84 | 1.78 | 0.16 |
| CBCL | ||||||||||
| Attention problems | 71.00 | 10.84 | 72.00 | 10.68 | 75.16 | 9.43 | 70.25 | 9.22 | 1.10 | 0.36 |
| Externalizing | 67.07 | 11.38 | 71.67 | 10.32 | 73.00 | 9.64 | 67.05 | 13.14 | 1.79 | 0.16 |
| Internalizingc | 64.00 | 13.24 | 70.00 | 10.45 | 67.28 | 10.25 | 59.30 | 12.11 | 3.07 | 0.03 |
| DISC | χ2 | p | ||||||||
| % CD | 32.14 | 21.05 | 35.71 | 36.36 | 1.42 | 0.70 | ||||
| % ANX | 35.71 | 47.37 | 21.43 | 27.27 | 3.91 | 0.27 | ||||
| % MOOD | 10.71 | 10.53 | 10.71 | 9.09 | 0.05 | 0.99 | ||||
| % Receiving other pharmacologic treatment | 10.71 | 26.32 | 25.00 | 36.36 | 6.08 | 0.11 | ||||
Post hoc tests did not reveal any significant individual group differences.
Individuals receiving medication for 1–5 years were significantly different than those receiving up to 1 year of medication.
Individuals receiving treatment for up to 1 year were significantly different than those receiving medication greater than 5 years.
Table 5.
Follow-up Characteristics of Medication Subgroups
| |
None |
One or less |
1–5 |
Greater than 5 |
|
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
n = 28 |
n = 19 |
n = 28 |
n = 22 |
|
|
||||
| Measure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p |
| Age | 18.45 | 2.05 | 18.75 | 2.30 | 18.51 | 1.34 | 17.99 | 1.44 | 0.65 | 0.59 |
| SES | 39.59 | 18.36 | 41.44 | 14.68 | 44.52 | 17.65 | 47.86 | 20.36 | 0.94 | 0.43 |
| WAIS-FSIQ | 89.86 | 10.09 | 90.00 | 13.74 | 95.52 | 15.10 | 97.32 | 19.56 | 1.51 | 0.22 |
| CBCL | ||||||||||
| Externalizinga | 55.36 | 12.75 | 66.53 | 12.04 | 63.74 | 11.83 | 59.05 | 13.69 | 3.22 | 0.03 |
| Internalizing | 53.00 | 13.42 | 63.67 | 12.73 | 57.67 | 13.92 | 55.57 | 11.99 | 2.16 | 0.10 |
Tukey's post hoc test revealed a significant difference between those that never received medication treatment and those receiving treatment for up to 1 year.
Substance use status
Determinations of substance use behaviors and SUD status were obtained using the Kiddie-SADS–PL, which was administered at follow-up to each adolescent, and separately to each participant's parent. SUDs were separated into disorders resulting from alcohol (ETOH) and illicit drug (DRUG) misuse. Evaluators were Ph.D.-level psychologists or trained psychology graduate students blind to group membership. Responses were combined across interviewee by item; if either informant indicated that the item caused significant distress or impairment, the symptom was judged to be present. Diagnoses of alcohol and drug abuse and dependence, past and present, were collapsed into binary (yes/no) categories that combined diagnoses of abuse and dependence.
Severity of substance use was assessed using the Rutger's Alcohol and Drug Use Questionnaire (Labouvie et al. 1997). Adolescents were asked to report use of cigarettes, alcohol, marijuana, and other drugs (cocaine, stimulants, psychedelics, heroin, analgesics, sedatives, club drugs, and nonprescription drugs) over the past 3 years. These latter categories were combined into one group because of generally low rates of use for any individual substance. The Rutger's substance use screening measure asks adolescents about the frequency (how often) and intensity (amount) of substance use. For example, at the beginning of the cigarette use module participants were asked if they had smoked a whole cigarette at least one time during the preceding 3 years. If they indicated that they had smoked during that time they were asked to indicate the frequency (1–2 times, 3–9 times, … , 1,000 or more times) and intensity of their use when they smoked (less than 1 a day, 1–4 cigarettes, … , more than 2 packs). Similar to Labouvie et al. (1997), severity of substance use was defined as the product of frequency × intensity of use, resulting in a unitary dimensional measure of substance use severity for each drug class. The four severity variables (3-year cigarette, alcohol. marijuana, and other use) were square-root transformed to normalize their distributions.
Statistical analysis
Substance use outcomes as a function of childhood CD
A one-way multiple analysis of variance (MANOVA) was used to assess differences between the ADHD+CD, ADHD, and control groups on square-root transformed measures of 3-year substance use severity outcomes for tobacco, alcohol, marijuana, and other drug use. Following a significant Wilks' Lambda, one-way ANOVAs were conducted to determine which measures significantly distinguished the groups followed by post hoc Tukey HSD to determine specific group differences. To evaluate categorical SUD outcomes, binary logistic regression analyses were conducted using the control group as the indicator to which the ADHD+CD and ADHD groups were compared.
Relation of substance use severity and SUD to stimulant medication treatment
Among those individuals found to have ADHD in childhood, a MANOVA and chi-square tests were used to examine the relationship between medication treatment group and late adolescent substance use severity and SUD status, respectively. Previous longitudinal studies have used childhood CD and other indicators of clinical severity in an attempt to control for pre-existing differences between treated and untreated groups; however, as seen in Table 3, our groups did not differ on these measures at baseline.
Results
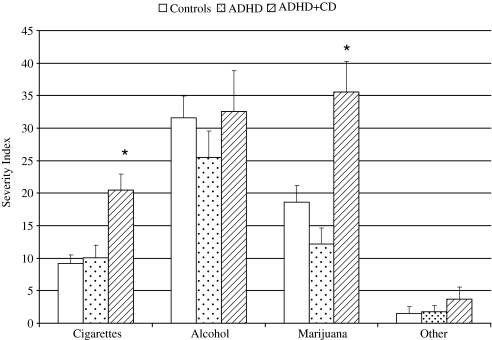
As shown in Figure 1, the one-way MANOVA examining the differences between the ADHD+CD, ADHD, and control groups on measures of 3-year substance use severity revealed a significant effect among groups, allowing for the further examination of individual ANOVAs (λ = 0.84, p ≤ 0.001, ηp2 = 0.082). Results of the one-way ANOVAs examining late adolescent substance use severity revealed significant differences among measures of 3-year cigarette, F(2, 177) = 7.92, p = 0.001, and marijuana, F(2, 177) = 10.58, p < 0.001 use. Post hoc tests revealed that the ADHD+CD group had significantly higher rates of 3-year cigarette and marijuana use severity than both the control and ADHD groups (all p < 0.01). The ADHD and control groups did not differ on either measure.
FIG. 1.
Childhood CD predicts 3-year severity of cigarette and marijuana use. *ADHD+CD group had significantly higher rates of 3-year cigarette and marijuana use severity than the control and ADHD groups (all p < 0.01). CD = conduct disorder.
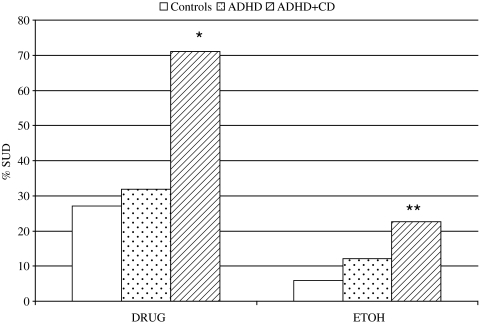
Logistic regression analyses of SUD outcomes are presented in Figure 2. A diagnosis of CD in childhood resulted in a significantly greater likelihood to have a drug use disorder by late adolescence relative to controls (OR = 6.59, p < 0.001, CI.95=2.65–16.39) and individuals found to have ADHD but not CD (OR = 5.24, p = 0.001, CI.95=2.06–13.31). A childhood diagnosis of CD resulted in a greater likelihood to have a late adolescent alcohol use disorder relative to controls (OR = 4.67, p = 0.01, CI.95=1.36–16.05), but not when compared with the ADHD group. The ADHD and control groups did not differ on either measure of SUD.
FIG. 2.
Childhood CD predicts drug and alcohol SUD. *Greater than controls and ADHD; p < 0.01. **Greater than controls; p < 0.01. DRUG = any substance use disorder other than alcohol; ETOH = any alcohol use disorder; SUD = substance use disorder.
Secondary analyses were conducted to assess the contribution of key group differences at baseline and follow-up. As such, among individuals found to have ADHD with and without CD, further analyses were generated controlling for childhood differences in teacher rated ADHD symptoms (IOWA Conners I/O), childhood internalizing disorders (Mood/Anxiety), and SES. Secondary analyses were also conducted examining adolescent substance use severity and SUD as a function of group status while controlling for adolescent SES and FSIQ. Of the childhood variables controlled for, only childhood SES was found to be a significant predictor of adolescent cigarette (p = 0.03) and marijuana use (p = 0.02). SES in adolescence was associated with adolescent marijuana use (p = 0.001) and SUD (p = 0.002). However, primary outcomes were maintained across all secondary analyses.
As indicated in Table 6, duration of stimulant medication treatment, as assessed by the overall Wilks' Lambda, did not result in significant differences among the dimensional measures of substance use severity (λ = 0.92, p = 0.79), and there were no group differences in categorical drug (χ2 = 1.84, p = 0.62) or alcohol use disorders (χ2 = 2.55, p = 0.47).
Table 6.
Percentage of Substance Use Disorder Diagnoses and 3-Year Substance Use Severity as a Function of Medication Subgroups
| |
None |
1 year or less |
1–5 years |
Greater than 5 years |
|
|
|---|---|---|---|---|---|---|
| % Diagnosis | n = 28 | n = 19 | n = 28 | n = 22 | χ2 | p |
| ETOH | 14.29 | 5.26 | 17.86 | 22.72 | 2.55 | 0.47 |
| DRUG | 35.71 | 52.63 | 50.00 | 40.91 | 1.84 | 0.62 |
| Severity index | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | ηp2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cigarettes | 11.29 | 17.70 | 11.59 | 13.06 | 15.81 | 14.75 | 14.57 | 16.01 | 0.51 | 0.68 | 0.02 |
| Alcohol | 24.86 | 28.05 | 23.37 | 24.27 | 31.85 | 31.90 | 30.38 | 45.92 | 0.36 | 0.78 | 0.01 |
| Marijuana | 15.36 | 20.91 | 22.11 | 26.68 | 27.11 | 28.90 | 13.14 | 18.11 | 1.73 | 0.17 | 0.05 |
| Other | 3.68 | 13.95 | 3.05 | 12.13 | 4.26 | 12.78 | 4.62 | 16.54 | 0.05 | 0.99 | 0.002 |
DRUG = any substance use disorder other than alcohol; ETOH = any alcohol use disorder; SUD = substance use disorder.
Discussion
The primary aims of this study were to further clarify the relationships among childhood CD, history of stimulant medication treatment, and late adolescent substance use behaviors in a sample of ethnically diverse urban adolescents from largely lower socioeconomic backgrounds. Consistent with findings generated from previous longitudinal studies (e.g., Barkley et al. 1990; Molina et al. 2002; Molina and Pelham, Jr. 2003), our data indicate that childhood CD is a robust predictor of later substance abusing behaviors. Prior studies have suggested that ADHD symptomatology, in addition to CD, further increases risk for later substance using outcomes (Milberger et al. 1997b; Burke et al. 2001; Molina and Pelham 2003; Elkins et al. 2007), whereas others suggest that CD mediates the relationship between ADHD and later substance misuse (Brook et al. 2010). Our data indicate that risk for later substance abuse is primarily carried by the childhood CD diagnosis and not ADHD per se. Those with childhood ADHD but not CD did not differ from the never-ADHD comparison group on any measure of later substance use.
Our two groups with childhood ADHD (i.e., with and with CD) did not differ on measures of parent-rated attention problems. While individuals found to have CD in childhood had higher rates of teacher-rated ADHD symptoms, controlling for this variable did not alter the impact of CD on either dimensional or categorical measures of late adolescent substance misuse. Additionally, results revealed that the ADHD only group and control group were similar on all measures of substance use severity and SUD status, further suggesting that in this sample of urban, largely minority youth, ADHD symptomatology alone did not portend late adolescent substance use outcomes.
Consistent with findings derived from most studies of middle class Caucasian samples (Barkley et al. 1990; Biederman et al. 2008), our data indicate that psychostimulant treatment neither increases risk nor serves as a protective factor in relation to later substance use. The variability in stimulant medication history in our sample allowed for the identification of four independent medication history groups of approximately equal size. Dividing the sample in this manner facilitates comparisons that improve upon the group distinctions seen in previous studies, and better approximates the variability in medication history seen clinically. The lack of impact on later substance use and abuse remained consistently nonsignificant whether categorical diagnostic or dimensional severity outcome measures were employed. Thus, it seems unlikely that the singular finding of Lambert and Hartsough (1998), indicating increased risk related to treatment, is due to the unique demographic characteristics of their sample.
This study has several key findings that further inform the literature on the impact of stimulant medication on later substance use, and provide insights into the misuse of substances among individuals with ADHD. That stimulant medication treatment was not found to impact later substance use replicates previous findings in the literature and extends these findings to an ethnically diverse urban sample of individuals with well characterized childhood co-morbidity. Additionally, there was not a protective effect of treatment. This was the case even in the subgroup who received treatment for more than 5 years (on average nearly 9 years), in whom one would be most likely to see such an effect if it were present. Perhaps stimulant medication is only protective in adolescents who are actively being treated, as was largely the case in one previous study (Biederman et al. 1999).
The results of this study must be viewed within the context of several study limitations. First, and most importantly, we were unable to follow a substantial portion of the 169 youth who originally participated in the childhood study, although available data suggest that the subsample that was reevaluated was representative of the original group. Of note, this type of highly mobile urban sample of relatively low SES is very difficult to find once lost. In several cases, we were able to locate the parents, but often even they were unsure of their child's location. While we did manage to locate some of the cohort through social networking sites such as Facebook and My Space, in general, our greatest success on the internet was for locating those who were in prison and unavailable for reassessment. A second limitation is that the proportion of females in this study did not permit analysis of the possible gender differences in outcome measures. However, among those found to have ADHD, there was no difference in the proportion of females with and without CD. Additionally, post hoc analyses conducted without female participants yielded results similar to the found in the full group. Third, we do not have childhood data for the controls, since they were not recruited until the adolescent follow-up commenced and our determination that they never had ADHD is based solely upon retrospective assessment. Another possible limitation to this study is that SES may be confounded with childhood status; those with CD in childhood tended to have lower SES than individuals with ADHD alone. It may be the case that the differences seen between groups on measures of adolescent substance use is not being driven by CD so much, but rather a function of higher SES serving as a protective factor. Lastly, the design of this study did not allow for evaluation/monitoring of adherence to treatment. This is potentially problematic given our findings that stimulant treatment had no effect on later substance misuse—since the negative finding could be in part attributable to poor adherence to treatment. Treatment adherence has been shown to be problematic in individuals with ADHD being treated with stimulants (Pappadopulos et al. 2009), and this is a concern for any study that attempts to elucidate the impact of stimulant treatment on later outcome.
Conclusion
The results of this study extend the findings that stimulant medication treatment does not significantly impact later substance using behaviors by examining this question in a sample of ethnically diverse urban adolescents/young adults and in a subgroup that received treatment for an extended period of time. In addition, results examining differences in SUD and substance use severity suggest that among individuals found to have ADHD, a co-morbid diagnosis of CD increases the risk for experiencing drug-related poor outcomes and impairment; those with ADHD without co-morbid CD may not be at elevated risk for later substance misuse relative to their never ADHD peers.
Clinical Significance
This study extends the findings that stimulant medication treatment does not significantly impact later substance using behaviors in individuals with ADHD by examining this question in a sample of ethnically diverse urban adolescents/young adults and in a subgroup that received treatment for an extended period of time. Such findings further support the argument that prescribed stimulant medication for individuals found to have ADHD does not increase the likelihood of later substance use involvement. Among individuals found to have ADHD, a co-morbid diagnosis of CD was found to fully account for adverse drug-related outcomes, suggesting that those with ADHD without co-morbid CD may not be at elevated risk for later substance misuse relative to their never ADHD peers.
Disclosures
Dr. Newcorn is a recipient of grants for research support from Eli Lilly, McNeil, Novartis, and Shire; an advisor/consultant for Astra-Zeneca, Biobehavioral Diagnostics, Eli Lilly, Ortho-McNeil-Janssen, Schering-Plough, and Shire; and a speaker for McNeil. The other authors have no financial relationships to disclose.
Acknowledgments
This research was supported by grants RO1 MH046448 and RO1 MH060698 from the National Institute of Mental Health.
References
- Andersen SL. Arvanitogiannis A. Pliakas AM. LeBlanc C. Carlezon WA., Jr. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Anglin MD. Booth MW. Ryan TM. Hser YI. Ethnic differences in narcotics addiction. II. Chicano and Anglo addiction career patterns. Int J Addict. 1988;23:1011–1027. doi: 10.3109/10826088809056182. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Fischer M. Edelbrock CS. Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Fischer M. Smallish L. Fletcher K. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111:97–109. doi: 10.1542/peds.111.1.97. [DOI] [PubMed] [Google Scholar]
- Biederman J. Monuteaux MC. Spencer T. Wilens TE. Macpherson HA. Faraone SV. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: A naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165:597–603. doi: 10.1176/appi.ajp.2007.07091486. [DOI] [PubMed] [Google Scholar]
- Biederman J. Wilens T. Mick E. Spencer T. Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Brandon CL. Marinelli M. Baker LK. White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brook DW. Brook JS. Zhang C. Koppel J. Association between attention-deficit/hyperactivity disorder in adolescence and substance use disorders in adulthood. Arch Pediatr Adolesc Med. 2010;164:930–934. doi: 10.1001/archpediatrics.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JD. Loeber R. Lahey BB. Which aspects of ADHD are associated with tobacco use in early adolescence? J Child Psychol Psychiatry. 2001;42:493–502. [PubMed] [Google Scholar]
- Carlezon WA., Jr. Mague SD. Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Chilcoat HD. Breslau N. Pathways from ADHD to early drug use. J Am Acad Child Adolesc Psychiatry. 1999;38:1347–1354. doi: 10.1097/00004583-199911000-00008. [DOI] [PubMed] [Google Scholar]
- Elkins IJ. McGue M. Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Fischer M. Barkley RA. Edelbrock CS. Smallish L. The Adolescent Outcome of Hyperactive-Children Diagnosed by Research Criteria .2. Academic, Attentional, and Neuropsychological Status. J Consult Clin Psychol. 1990;58:580–588. doi: 10.1037//0022-006x.58.5.580. [DOI] [PubMed] [Google Scholar]
- Flory K. Lynam DR. The relation between attention deficit hyperactivity disorder and substance abuse: What role does conduct disorder play? Clin Child Fam Psychol Rev. 2003;6:1–16. doi: 10.1023/a:1022260221570. [DOI] [PubMed] [Google Scholar]
- Gilman SE. Breslau J. Conron KJ. Koenen KC. Subramanian SV. Zaslavsky AM. Education and race-ethnicity differences in the lifetime risk of alcohol dependence. J Epidemiol Community Health. 2008;62:224–230. doi: 10.1136/jech.2006.059022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Labouvie E. Bates ME. Pandina RJ. Age of first use: Its reliability and predictive utility. J Stud Alcohol. 1997;58:638–643. doi: 10.15288/jsa.1997.58.638. [DOI] [PubMed] [Google Scholar]
- Lambert NM. Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Lambert NM. Hartsough CS. Sassone D. Sandoval J. Persistence of hyperactivity symptoms from childhood to adolescence and associated outcomes. Am J Orthopsychiatry. 1987;57:22–32. doi: 10.1111/j.1939-0025.1987.tb03505.x. [DOI] [PubMed] [Google Scholar]
- Loney J. Kramer JR. Salisbury H. Medicated versus unmedicated ADHD children: Adult involvement with legal, illegal drugs. In: Jensen PS, editor; Cooper JR, editor. Attention Deficit Hyperactivity Disorder. Kingston, NJ: Civic Research Institute; 2002. pp. 17-1–17-16. [Google Scholar]
- Mague SD. Andersen SL. Carlezon WA., Jr. Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiatry. 2005;57:120–125. doi: 10.1016/j.biopsych.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Mannuzza S. Klein RG. Bessler A. Malloy P. Hynes ME. Educational and occupational outcome of hyperactive boys grown up. J Am Acad Child Adolesc Psychiatry. 1997;36:1222–1227. doi: 10.1097/00004583-199709000-00014. [DOI] [PubMed] [Google Scholar]
- Mannuzza S. Klein RG. Bessler A. Malloy P. LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155:493–498. doi: 10.1176/ajp.155.4.493. [DOI] [PubMed] [Google Scholar]
- Mannuzza S. Klein RG. Truong NL. Moulton JL., III Roizen ER. Howell KH. Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: Prospective follow-up into adulthood. Am J Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberger S. Biederman J. Faraone SV. Chen L. Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1997a;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Milberger S. Biederman J. Faraone SV. Wilens T. Chu MP. Associations between ADHD and psychoactive substance use disorders. Findings from a longitudinal study of high-risk siblings of ADHD children. Am J Addict. 1997b;6:318–329. [PubMed] [Google Scholar]
- Miller GA. Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Molina BS. Bukstein OG. Lynch KG. Attention-deficit/hyperactivity disorder and conduct disorder symptomatology in adolescents with alcohol use disorder. Psychol Addict Behav. 2002;16:161–164. doi: 10.1037//0893-164x.16.2.161. [DOI] [PubMed] [Google Scholar]
- Molina BS. Pelham WE., Jr. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Molina BSG. Flory K. Hinshaw SP. Greiner AR. Arnold LE. Swanson JM. Hectman L. Jensen PS. Vitiello B. Hoza B. Pelham WE. Elliott GR. Wells KC. Abikoff HB. Gibbons RD. Marcus S. Conners K. Epstein JN. Greenhill LL. March JS. Newcorn JH. Severe JB. Wigal T. Delinquent behavior and emerging substance use in the MTA at 36 months: Prevalence, course, and treatment effects. J Am Acad Child Adolesc Psychiatry. 2007;46:1027–1039. doi: 10.1097/chi.0b013e3180686d96. [DOI] [PubMed] [Google Scholar]
- Nakao K. Treas J. Updating occupational prestige and socioeconomic scores: How the new measures measure up. Sociol Methodol. 1994;24:1–72. [Google Scholar]
- Pappadopulos E. Jensen PS. Chait AR. Arnold LE. Swanson JM. Greenhill LL. Hechtman L. Chuang S. Wells KC. Pelham W. Cooper T. Elliott G. Newcorn JH. Medication adherence in the MTA: Saliva methylphenidate samples versus parent report and mediating effect of concomitant behavioral treatment. J Am Acad Child Adolesc Psychiatry. 2009;48:501–510. doi: 10.1097/CHI.0b013e31819c23ed. [DOI] [PubMed] [Google Scholar]
- Schubiner H. Tzelepis A. Milberger S. Lockhart N. Kruger M. Kelley BJ. Schoener EP. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000;61:244–251. doi: 10.4088/jcp.v61n0402. [DOI] [PubMed] [Google Scholar]
- Taylor E. Chadwick O. Heptinstall E. Danckaerts M. Hyperactivity and conduct problems as risk factors for adolescent development. J Am Acad Child Adolesc Psychiatry. 1996;35:1213–1226. doi: 10.1097/00004583-199609000-00019. [DOI] [PubMed] [Google Scholar]
- Valvassori SS. Frey BN. Martins MR. Reus GZ. Schimidtz F. Inacio CG. Kapczinski F. Quevedo J. Sensitization and cross-sensitization after chronic treatment with methylphenidate in adolescent Wistar rats. Behav Pharmacol. 2007;18:205–212. doi: 10.1097/FBP.0b013e328153daf5. [DOI] [PubMed] [Google Scholar]
- Wooters TE. Dwoskin LP. Bardo MT. Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacology. 2006;188:18–27. doi: 10.1007/s00213-006-0445-9. [DOI] [PubMed] [Google Scholar]