Abstract
Background
Rapid eye movement (REM) sleep is greatest in the developing brain, is driven by acetylcholine, and may represent a protected time for neuroplasticity. Recently published data from our lab observed that children with autism spent significantly less time in this state during a single night recording than did typically developing children and those with developmental delay without autism. The objective of this study was to determine whether or not donepezil can increase the REM % in children with diagnosed autism spectrum disorder (ASD) found to have REM % values of at least two standard deviations below expected for age.
Methods
Five subjects found to have an ASD (ages 2.5–6.9 years) and demonstrated deficits in REM sleep compared with within-lab controls were enrolled in a dose finding study of donepezil. Each subject was examined by polysomnography for REM sleep augmentation after drug administration.
Results
REM sleep as a percentage of Total Sleep Time was increased significantly and REM latency was decreased significantly after drug administration in all subjects. No other observed sleep parameter was changed significantly.
Conclusions
Donepezil can increase the amount of time that children with an ASD spend in the REM sleep state. A double-blind, placebo-controlled trial is needed to assess the association between REM sleep augmentation and learning, cognition, and behavior in such children.
Introduction
Studies in typical and autistic children show an association between disordered sleep and daytime behavioral disturbance and performance impairment (Sadeh et al. 2002; Schreck et al. 2004). Previous studies in children with a diagnosis of autism have identified various abnormalities in rapid eye movement (REM) sleep in particular, including immature organization, decreased quantity, abnormal twitches, undifferentiated sleep, and REM sleep behavior disorder characterized by the absence of the muscle atonia that is normal in REM sleep (Tanguay et al. 1976; Diomedi et al. 1999; Elia et al. 2000; Thirumalai et al. 2002). Recent results from the National Institute of Mental Health's Pediatrics and Developmental Neuroscience Branch support a growing body of literature demonstrating that sleep architecture is abnormal in autism. In a polysomnographic study of three cohorts, children with a diagnosis of autism, children with developmental delay without autism matched for nonverbal IQ, and typically developing children, no differences in sleep parameters between the typical versus the developmental delay group were found. The autism versus typical group revealed shorter total sleep time (TST) (p = 0.004), greater slow wave sleep (SWS) percentage (p = 0.001), and much smaller REM percentage, 14.5 versus 22.6 (p < 0.001). The autism versus the developmental delay group revealed shorter TST (p = 0.001), greater stage 1 percentage (p < 0.001), greater SWS percentage (p < 0.001), and a much smaller REM percentage, 14.5 versus 25 (p < 0.001) (Buckley et al. 2010).
It is our hypothesis that a relative deficiency of REM may indicate an abnormality in neural organization in young children with autism and other autism spectrum disorders (ASDs) that is not directly associated with or related to inherent intellectual disability but may serve as a window into understanding core neurotransmitter abnormalities unique to this disorder. If REM sleep is a privileged time for brain plasticity in the developing brain, as molecular and cellular work in animals continues to suggest (Lopez et al. 2008; Ravassaard et al. 2009), then therapeutic augmentation of this sleep state, when found to be deficient, may positively impact behaviors and influence learning.
Donepezil is a piperidine derivative that increases acetylcholine in the synaptic cleft by selectively and reversibly inhibiting the enzyme acetylcholinesterase and thereby enhancing neurotransmission on all Ach receptor subtypes. It has been shown to effectively enhance REM sleep in young healthy adults; in elderly, healthy adults; and in elderly, demented adults (Kanbayashi et al. 2002; Mizuno et al. 2004; Moraes et al. 2006; Schredl et al. 2001). To the best of our knowledge, it has not been used to augment REM sleep in pediatric populations.
Previous studies using donepezil in children found to have ASD have focused on changes in behavior and attention (but not REM sleep) and have used between 2.5 mg and 10 mg/day. To date, one controlled trial of donepezil for children found to have ASD has been published and the parameters were behavioral. Forty-three children with autism or pervasive developmental disorder-not otherwise specified (PDD-NOS) between the ages of 2 and 10 years were given 2.5 mg/day of donepezil for a 6-week double-blind period, followed by a 6-week open trial. The endpoints were assessments of autistic symptoms using the Childhood Autism Ratings Scale and assessments of receptive and expressive scores using Gardner's Expressive One-Word Picture Vocabulary Test and Gardner's Receptive One-Word Picture Vocabulary Test. The authors reported improvement in all areas by 6 weeks (Chez et al. 2003).
We report the results of donepezil treatment, starting at 1.25 mg daily, in 5 children between the ages of 2.5 and 6.9 years found to have an ASD and found to have an REM % demonstrated in our lab to be two standard deviations or more below expected values for age, or roughly <15%.
Materials and Methods
The National Institutes of Health's (NIH) Combined Neurosciences Institutional Review Board approved the protocol. Five boys, between the ages of 2.5 and 6.9 years, were enrolled in the study after their parents consented to study participation. Subjects were evaluated via a two-step process.
First, behavioral evaluation at the NIH assessed eligibility for a diagnosis of an ASD. Autism symptoms were assessed using the Autism Diagnostic Observation Schedule (Lord et al. 1999) and the Autism Diagnostic Interview–Revised (Lord et al. 1994). The diagnostic assessment included behavioral testing using the Vineland Adaptive Behavior Scales, Second Edition (Sparrow et al. 2005), and cognitive testing: 4 subjects received the Mullen Scales of Early Learning (Mullen 1995) and 1 child received the Differential Ability Scales (Elliott 2007). Nonverbal ratio IQs were obtained for each child. The ratio score was calculated as the mean of the age equivalents (of the subtests measuring nonverbal abilities) divided by chronological age and multiplied by 100.
Second, children were evaluated during a two-night polysomnogram (PSG) observation in the NIH sleep lab. The first night was used as an acclimation or accommodation night, and the second was used for study entry. Children who slept >7 hours on the second night and were found to have an REM % more than two standard deviations below expected values for age were offered the treatment.
Sixteen children completed the two-night sleep study. Eight of the sixteen were eligible for the drug portion of the study. One child did not begin drug due to very frequent epileptiform discharges at baseline and parents' decision to seek treatment locally. One child withdrew from the study due to mother's concerns that she did not see any behavioral benefit at the 1.25 mg dose. One child experienced a complex seizure with fever while on the 1.25 mg dose and was withdrawn from the study. Five of the eight who were eligible for drug completed the trial, and their results are included below. None of the children was taking other medication at study entry or during the trial. Four subjects met Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994), criteria for a diagnosis of autism, and one child met criteria for a diagnosis of PDD-NOS.
The primary outcome was the amount of time each child spent in REM sleep while on drug compared with baseline. Due to the short and variable duration of participation in this trial, measurement changes in core symptoms of autism pre and post-treatment with donepezil could not be evaluated. Total sleep time, sleep efficiency, latencies to sleep and to REM sleep, wake after sleep onset, and sleep stages 1, 2, and 3 were also evaluated before and after drug. Donepezil was administered in 1.25 mg capsules, prepared by the NIH Clinical Center Pharmacy.
All subjects began at a start dose of 1.25 mg. The half-life of donepezil is 72 hours. Accordingly, subjects were brought back after a minimum of 2 weeks to ensure steady-state concentrations. All subjects were reevaluated by PSG between 2 and 4 weeks after commencement of drug. If the subject's REM sleep changed to within one standard deviation of expected values, the subject was maintained on that dose for 2 months and then brought back for a final PSG. If REM % was outside of one standard deviation of reported values for age, then dose was doubled and subject was reevaluated in 4 weeks. This algorithm could be repeated twice to a maximum dose of 5 mg/day. If parents or clinicians felt subject's sleep or other behavior to be adversely affected by donepezil, a clinical decision could be made to stop or decrease dose.
Clinical readings were provided by the sleep lab at the National Institute of Neurological Disorders and Stroke and then re-read by two sleep specialists blind to drug dose (K.S. and A.J.R.). All subjects had a baseline EKG and safety labs drawn at the time of study entry and with each dose increase.
Results
There were no statistically significant changes between baseline evaluation and postdrug administration at the 1.25 mg dose for the following measurements: TST, stage 1, stage 2, stage 3, wake after sleep onset time, sleep latency, or sleep efficiency. Subjects experienced a statistically significant (p = 0.02, two-tailed) increase in REM % at the initial 1.25 mg dose as depicted in Table 1. Mean REM % at baseline for the group of 5 subjects was 10 (standard deviation = 4.2). Mean REM % after treatment with 1.25 mg donepezil was 19 (5.6). Subjects also experienced a statistically significant decrease in latency to REM sleep from baseline (p = 0.02) with an observed mean of 230.9 minutes (37) before treatment and an observed mean of 142.6 minutes (25.6) after 1 month at the 1.25 mg dose. No other sleep parameters were found to have statistically significant changes pre- and post-treatment.
Table 1.
Comparing Sleep Parameters of Subjects at Baseline and 1.25 mg Donepezil
| Baseline mean (standard deviation) | Drug mean (standard deviation) | Difference (standard error), p | |
|---|---|---|---|
| Total sleep time (min) | 517.3 (85.2) | 512.3 (61.5) | −5.1 (45.9), 0.92 |
| Stage 1 (percent) | 6.2 (3.3) | 4.1 (3.1) | −2.1 (1.4), 0.22 |
| Stage 2 (percent) | 50.6 (12.5) | 44.4 (14.4) | −6.2 (4.0), 0.20 |
| SWS (percent) | 33.0 (10.4) | 32.0 (15.0) | −1.0 (4.5), 0.84 |
| REM (percent) | 10.2 (4.2) | 19.4 (5.6) | 9.2 (2.6), 0.02 |
| WASO (percent) | 43.8 (28.6) | 52.7 (52.9) | 8.9 (28.6), 0.77 |
| Sleep latency (min) | 20.6 (14.2) | 31.7 (24.6) | 11.1 (9.9), 0.32 |
| REM latency (min) | 230.9 (37.0) | 142.6 (25.6) | −88.3 (24.7), 0.02 |
| Sleep efficiency (percent) | 89.1 (6.2) | 86.6 (7.2) | −2.5 (4.7), 0.62 |
Standard error and p-value in difference column are based on paired t-tests. All p-values are two-tailed. Number of observations = 5.
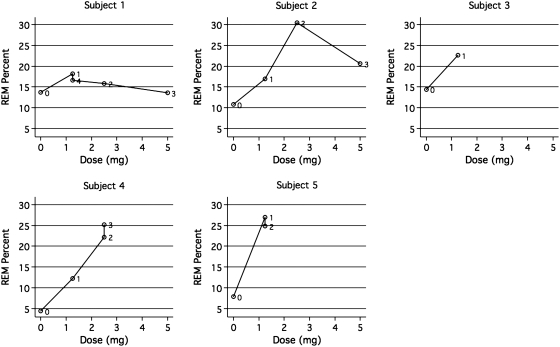
Figure 1 presents plots of REM % versus dose for each subject (plots are labeled with numbers indicating the temporal sequence of doses). Subject 1 was a 4.9-year-old boy with a nonverbal cognitive ratio IQ (NVDQ) of 55.18. He experienced a modest increase in REM % from baseline at the 1.25 mg dose and was increased to the 2.5 mg dose and restudied 1 month later. His 2.5 mg study revealed a decrease in REM %. He was further increased to 5 mg with a resultant further decline. At this dose, parents reported that he was “not himself” and his polysomnogram examination revealed a significant increase in wake after sleep onset time at this dose versus baseline (131 minutes vs. 48). The clinical decision was made to return his dose to 1.25 mg and he was reevaluated by PSG after a month on that dose (depicted as point “4” in Fig. 1, subject 1). When re-evaluated at 1.25 mg, REM sleep returned to above the baseline value and exceeded the values obtained at the 2.5 and 5 mg doses. Mother reported child “back to himself” at this dose.
FIG. 1.
Response of REM % to dose, by subject. Note: Points are labeled in temporal order (0 = baseline).
Subject 2 was a 2.5-year-old boy with an NVDQ of 72.35. He experienced an increase in REM % at the initial dose. He was increased to the 2.5 mg daily dose and restudied 4 weeks later as per protocol. At this dose, his REM % reached >30% of his total sleep time. As this result was greater than two standard deviations outside the target range for age, a clinical decision was made to increase his dose to 5 mg/day as per protocol and restudy him in 4 weeks. His PSG at 5 mg revealed an REM % of 20. The subject was maintained at 5 mg/day for 8 more weeks. He returned for his final study, but spiked a temperature while in hospital. His final PSG evaluation is not used in this data set as fever can alter sleep organization (Kryger et al. 2005).
Subject 3 was a 5.3-year-old boy with an NVDQ of 45.74. He had an initial drug study at the 1.25 mg dose that could not be used in this dataset as he slept for <6 hours during that examination. Given that his mother reported that he was “better,” described as “more attentive” and “less frustrated” since beginning the trial, a clinical decision was made to maintain him at this entry dose for a further 2 months and restudy him at the end of that time. On his final examination, his REM % had normalized at 22.6%.
Subject 4 was a 6.9-year-old boy with an NVDQ of 93.37. He tripled his REM % from baseline after 1 month at 1.25 mg donepezil. As he was still well below standard norms for age at 12.2%, his dose was increased to 2.5 mg daily. His 2.5 mg follow-up study revealed normalization at 22.2% REM. He was held at this dose for 2 more months and restudied at this dose. His final REM % was 25.2%.
Subject 5 was a 5.8-year-old boy with an NDVQ of 47.22. He increased his REM % from 7.9% to 27% after 1 month at 1.25 mg/day. He was held at this dose and restudied after 2 months. His final REM % at 1.25 mg maintained normalization of REM at 24.8%. Mother reported that child was “much better” at the end of the study. She reported that subject began to wake up spontaneously after having slept for 9 or 10 hours, whereas previously he would be “dead tired” in the mornings. She also reported that he became continent overnight during the course of the study where previously he had “wet the bed almost every night.”
In addition, as depicted in Table 1, each subject had an initial decrease in REM latency at the 1.25 mg dose. The parents of all 5 subjects who completed the study opted to continue taking donepezil at their final study dose, and a further 3-month supply was furnished by the NIH. There were no adverse side effects other than the unspecified behavioral change noted above in subject 1 at the 5 mg dose.
The 5 subjects reported here had a total of 18 separate sleep studies at 3 possible doses. Despite the small sample size, it is possible to use this additional data to make a rough estimate of the (possibly nonlinear) response in REM % and latency to REM to doses over the entire observed range. Accordingly,
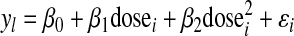 |
we fit independent quadratic regression models for the two outcome measures (yi) of the form:
The two models were estimated using least squares with robust (“sandwich”) standard errors clustered by subject (to account for nonindependence of observations). All coefficients in each model were found to be statistically significant (p < 0.05, two-tailed) and the models appear to fit the data reasonably well overall (R2 = 0.52 and 0.54, respectively, and the omnibus F test was rejected at p < 0.05 for both models). Based on the results of these models, the ideal dose for maximizing REM % and minimizing the REM latency appears to be approximately between 2.5 and 3.5 mg. Complete results are available on request.
Discussion
Rapid-eye-movement sleep may serve a particular role in normal development. The increased ponto-thalamo-cortical activity evident during REM sleep may provide the endogenous stimulation needed to form and stabilize durable synaptic connections in the developing brain (Roffwarg et al. 1966; Marks 1995; Hobson 2009). This sleep state is driven by acetylcholine, and a relative REM deficiency found on PSG in children with autism disorders may reflect a relative deficiency of this neurotransmitter. Donepezil is a cholinesterase inhibitor with very minimal side effects that has been used in special populations to augment memory and attention (Yoo 2007). This pilot trial demonstrates that at 12.5% of the standard adult dose, donepezil can normalize REM sleep percentages and decrease REM latency in children with ASD found to spend an abnormally short sleep period time in REM.
This study has several limitations. This is an open-label study without controls. The magnitude of the change in REM sleep parameters with no other significant change in other sleep measurements would strongly suggest that the cholinergic enhancement achieved by the administration of donepezil is responsible for the change. However, with only 5 subjects and the inherent variability of these measures across individuals and time, these results must be considered preliminary. Change measurements in symptom severity, including parent reports of cognition and other behavior, could not be evaluated due to variable length and dose, and the lack of a placebo group. A randomized, controlled trial with a larger sample size might be able to address the larger questions. That investigation should assess changes in language and socialization associated with improved REM sleep, as a positive impact on development is the ultimate goal of this line of research.
Clinical Significance
It is our hypothesis that diminished cholinergic tone is a common mediating mechanism that contributes to both a reduction in REM sleep and the production of core symptoms of ASD. Therapeutic augmentation of this state, in conjunction with objective, reliable, and quantifiable measures of symptom improvement, could establish cholinergic activation as a safe, rational, and effective treatment for young children with ASD.
Disclosures
This was not an industry-supported study. The authors have indicated no financial conflicts of interest.
Acknowledgments
All research was funded by intramural NIH and by Award Number T32MH067763 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- Buckley AW. Rodriguez A. Jennison K. Buckley J. Thurm A. Sato S. Swedo S. REM sleep percentage in children with autism compared to children with developmental delay and typical development. Arch Pediatr Adolesc Med. 2010;164:1032–1037. doi: 10.1001/archpediatrics.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chez MG. Buchanan Becker TM. Kessler M. Aimonovitch MC. Mrazek SR. Donepezil hydrochloride: A double-blind study in autistic children. J Ped Neurol. 2003;1:83–88. [Google Scholar]
- Diomedi M. Curatolo P. Scalise A. Placidid F. Caretto F. Gigli GL. Sleep abnormalities in mentally retarded autistic subjects: Down's syndrome with mental retardation and normal subject. Brain Dev. 1999;21:548–553. doi: 10.1016/s0387-7604(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Elia M. Ferri R. Musumeci SA. Del Gracco S. Bottitta M. Scuderi C. Miano G. Panerai S. Bertrand T. Grubar JC. Sleep subjects with autistic disorder: A neuropsychological and psychological study. Brain Dev. 2000;22:88–92. doi: 10.1016/s0387-7604(99)00119-9. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Manual for the Differential Ability Scales. second. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- Hobson JA. REM sleep and dreaming: towards a theory of protoconsciousness. Nat Rev Neurosci. 2009;10:803–813. doi: 10.1038/nrn2716. [DOI] [PubMed] [Google Scholar]
- Kanbayashi T. Sugiyama T. Aizawa R. Saito Y. Ogawa Y. Kitajima T. Kaneko Y. Abe M. Shimizu T. Effects of donepezil (Aricept) on rapid eye movement sleep of normal subjects. Psychiatry Clin Neurosci. 2002;56:307–308. doi: 10.1046/j.1440-1819.2002.01008.x. [DOI] [PubMed] [Google Scholar]
- Kryger M. Roth T. Dement W. Principles and Practice of Sleep Medicine. fourth. Philadelphia: Elsevier Saunders; 2005. [Google Scholar]
- Lopez J. Roffwarg HP. Dreher A. Bissette G. Karolewicz B. Shaffery JP. Rapid eye movement sleep deprivation decreases long-term potentiation stability and affects some glutamatergic signaling proteins during hippocampal development. Neuroscience. 2008;153:44–53. doi: 10.1016/j.neuroscience.2008.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C. Rutter M. DiLavore P. Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Lord C. Rutter M. le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Marks GA. Shaffery JP. Oksenberg A. Speciale SG. Roffwarg A. A functional role for REM sleep in brain maturation. Behav Brain Res. 1995;69:1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- Mizuno S. Kameda A. Inagaki T. Horiguchi J. Effects of donepezil on Alzheimer's disease: The relationship between cognitive function and rapid eye movement sleep. Psychiatry Clin Neurosci. 2004;58:660–665. doi: 10.1111/j.1440-1819.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- Moraes WS. Poyores DR. Guilleminault C. Ramos LR. Bertolucci PH. Tutili S. The effects of donepezil on sleep in patients with Alzheimer's disease: A double-blind placebo-controlled study. Sleep. 2006;29:199–205. doi: 10.1093/sleep/29.2.199. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Ravassaard P. Pachoud B. Comte JC. Mejia-Perez C. Scote-Blachon C. Gay N. Claustrat B. Touret M. Luppi PH. Salin PA. Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep. 2009;32:227–240. doi: 10.1093/sleep/32.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffwarg HP. Muzio NN. Dement WC. Ontogenetic development of the human sleep- dream cycle. Science. 1966;152:602–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Sadeh A. Gruber R. Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002;73:405–417. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- Schreck KA. Mulick JA. Smith AF. Sleep problems as possible predictors of intensified symptoms of autism. Res Dev Disabil. 2004;25:57–66. doi: 10.1016/j.ridd.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Schredl M. Hornung O. Regen F. Albrecht N. Danker-Hopfe Heuser I. The effect of donepezil on sleep in elderly, healthy persons: A double-blind placebo-controlled study. Pharmacopsychiatry. 2006;39:205–208. doi: 10.1055/s-2006-950396. [DOI] [PubMed] [Google Scholar]
- Schredl M. Weber B. Leins ML. Heuser I. Donepezil-induced REM sleep augmentation enhances memory performance in elderly, healthy persons. Exp Gerontol. 2001;36:353–361. doi: 10.1016/s0531-5565(00)00206-0. [DOI] [PubMed] [Google Scholar]
- Sparrow SS. Cicchetti DV. Balla DA. Vineland Adaptive Behavior Scales. Second. Circle Pines, MN: AGS Publishing; 2005. [Google Scholar]
- Tanguay PE. Ornitz EM. Forsythe AB. Ritvo ER. Rapid eye movement (REM) activitiy in normal and autistic children during REM sleep. J Austim Child Schizophr. 1976;6:275–288. doi: 10.1007/BF01543468. [DOI] [PubMed] [Google Scholar]
- Thirumalai SS. Shubin RA. Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol. 2002;17:173–178. doi: 10.1177/088307380201700304. [DOI] [PubMed] [Google Scholar]
- Yoo JH. Relevance of donepezil in enhancing learning and memory in special populations: A review of the literature. J Autism Dev Dis. 2007;37:1883–1901. doi: 10.1007/s10803-006-0322-8. [DOI] [PubMed] [Google Scholar]