Abstract
Background
We examined specific causes of mortality in human immunodeficiency virus type 1 (HIV-1)–infected patients who initiated antiretroviral therapy (ART) in Europe and North America from 1996 through 2006, and we quantified associations of prognostic factors with cause-specific mortality.
Methods
We retrospectively classified all deaths among 39,272 patients enrolled in 13 HIV-1 cohorts (154,667 person years of follow-up) into the categories specified in the Cause of Death (CoDe) project protocol.
Results
In 1597 (85%) of 1876 deaths, a definitive cause of death could be assigned. Among these, 792 deaths (49.5%) were AIDS related, followed by non-AIDS malignancies (189; 11.8%), non-AIDS infections (131; 8.2%), violence- and/or drug-related causes (124; 7.7%), liver disease (113; 7.0%), and cardiovascular disease (103; 6.5%). Rates of AIDS-related death (hazard ratio [HR] per 100 cell decrease, 1.43; 95% confidence interval [CI], 1.34–1.53) and death from renal failure (HR, 1.73; 95% CI, 1.18–2.55) were strongly inversely related to CD4 count at initiation of ART, whereas rates of death attributable to AIDS (HR for viral load >5 vs ≤5 log copies/mL, 1.31; 95% CI, 1.12–1.53), infection (HR, 1.85; 95% CI, 1.25–2.73), cardiovascular (HR, 1.54; 95% CI, 1.05–2.27), and respiratory causes (HR, 3.62; 95% CI, 1.30–10.09) were higher in patients with baseline viral load >5 log copies/mL than in other patients. Rates of each cause of death were higher in patients with presumed transmission via injection drug use than in other patients, with marked increases in rates of liver-related (HR for injection drug use vs non–injection drug use, 6.06; 95% CI, 4.03–9.09) and respiratory tract–related (HR, 4.94; 95% CI, 1.96–12.45) mortality. The proportion of deaths classified as AIDS related decreased with increasing duration of ART.
Conclusions
Important contributors to non-AIDS mortality in treated HIV-1–infected individuals must be addressed if decreases in mortality rates are to continue.
Combination antiretroviral therapy (ART) has dramatically improved life expectancy for human immunodeficiency virus type 1 (HIV-1)–infected patients [1], primarily because of reductions in deaths attributable to AIDS-related conditions [2]. However, an increasing number of deaths are attributable to causes not conventionally considered to be HIV-1 related [3]. These may be related to immunodeficiency and inflammation attributable to uncontrolled viremia and, thus, may still be linked to HIV-1 infection [4, 5]. Therefore, large clinical end point trials now incorporate non-AIDS events as end points [6]. With the introduction of more effective and tolerable ART regimens, it is important to monitor causes of death and assess associated risk factors.
Coding systems, such as the World Health Organization’s International Classification of Diseases, 9th Edition (ICD-9) [7] and ICD-10 [8], may not reflect recent rapid changes in our understanding of HIV-1 pathogenesis. Many AIDS-defining illnesses are poorly identified in the ICD, and some diseases (eg, central nervous system diseases) have a different etiology in HIV-1–infected patients and are, therefore, not covered or are at risk of misclassification.
We hypothesized that associations of patient characteristics with overall mortality may mask differential associations with specific causes of death. Using data on antiretroviral-naive individuals starting ART from a collaboration of HIV-1 cohorts in high-income countries, we classified reported deaths using a system designed for attribution of causes of death in HIV-1–infected persons. We described frequencies of specific causes of death, examined associations with prognostic factors at start of ART, and investigated whether causes of death differ between early and more recent ART eras and according to duration of time receiving treatment.
METHODS
Cohorts and patients
The ART Cohort Collaboration was established in 2000 to estimate prognosis of HIV-1–infected patients initiating ART in Europe and North America. The collaboration is described in detail elsewhere [9–11] (http://www.art-cohort-collaboration.org). Patients naive to ART, aged >16 years, and starting ART with a combination of ≥3 drugs were followed. The dataset was assembled during 2007 and includes patients starting ART during 1996–2006 from 13 cohorts (Appendix, which appears only on the online version of the journal). All cohorts had systematic (though different) approaches to capturing information regarding deaths, predominantly using linkages to death registries or hospital records. At all sites, institutional review boards approved data collection. All cohorts provided anonymized data on a predefined set of demographic, laboratory, and clinical variables, which were pooled and analyzed centrally.
Coding cause of death
We initially classified deaths into the 30 categories specified in the Cause of Death (CoDe) project protocol (http://www.cphiv.dk/). AIDS-related deaths were further classified, where possible, as AIDS infection (CoDe 01.1) or AIDS malignancy (CoDe 01.2). We produced summary tables with information on each death that included ICD-9, ICD-10, or free text coding of deaths; patient characteristics at ART initiation (age, sex, pre-existing AIDS-defining conditions, risk transmission group); details and timing of new AIDS events during receipt of treatment; time from treatment initiation to death; CD4 count obtained closest to death; and whether patients were receiving ART at time of death. Cause of death for each patient was classified by a clinician and also was automatically based on ICD codes alone, with use of computer algorithms developed by the Mortalité 2000–2005 Study Group [12, 13]. Disagreements between clinicians and/or computer-assigned codes, as well as infrequent causes of death, were independently examined by pairs of reviewers and, if necessary, were further discussed by the review panel.
Death was coded as AIDS (CoDe 01) if the patient experienced a serious AIDS-defining condition(s) close to death and/ or had a low CD4 count (<50 cells/mm3) prior to death and a diagnosis compatible with AIDS as cause of death. Death due to Hodgkin lymphoma was classified as AIDS related. Deaths described as due to sepsis/septicemia were coded as bacterial infection with sepsis (CoDe 2.1.1). Deaths attributed to unspecified liver failure were classified as hepatitis (CoDe 03) if the patient was positive for hepatitis C antibody at baseline or if hepatitis C status was unknown but HIV-1 transmission was through injection drug use. If the patient was not known to be an injection drug user or hepatitis C positive, then such deaths were coded as liver failure (CoDe 14). If a patient was positive for hepatitis C or B antibodies at baseline and died of “liver cancer,” we assumed the cancer was secondary to hepatitis [14]. Deaths described as accidental/violent with a history of self-harm in ICD-9 were coded as suicide (CoDe 17). If it was unclear whether death was attributable to suicide, the less specific code of accidental/violent death (CoDe 16) was assigned. Deaths described as due to cardiac arrest or respiratory failure with no other information were coded as unknown (CoDe 92). Deaths due to myocarditis and endocarditis were coded as non-AIDS infection (CoDe 02). Deaths with insufficient information to achieve a consensus were coded as cause unknown (CoDe 92).
Statistical analyses
We grouped causes associated with <20 deaths as “other.” For the main analyses, we grouped causes of death as AIDS (CoDe 01, 01.1, and 01.2), non-AIDS infection (CoDe 02), liver-related deaths (hepatitis and liver failure [CoDe 03 and 14]), non-AIDS malignancy (CoDe 04), cardiovascular disease (myocardial infarction/ischemic heart disease, stroke, heart failure/unspecified, and other heart disease [CoDe 08, 09, and 24]), respiratory disease (CoDe 13 and 25), renal failure (CoDe 15), violent deaths (including accident, suicide, and overdose [CoDe 16, 17, and 19]), and other. In secondary analyses we separated deaths due to AIDS into unspecified, infection, and malignancy; liver disease into hepatitis-related and other liver disease; cardiovascular disease into myocardial infarction/ischemic heart disease, stroke, and other heart-related deaths; and violent deaths into suicide, substance abuse, and other (including homicide, accident and nonattributed violent death). We also classified deaths as either AIDS or non-AIDS related.
Hazard ratios (HRs) for death attributed to all and specific causes were estimated using multivariable Cox models. In models for specific causes, patients who died of other causes were censored at date of death. We estimated cause-specific HR for the following characteristics measured at start of ART (baseline): female sex (vs male sex), age (per 10 years), injection drug user (vs non–injection drug user), prior AIDS diagnosis (vs no AIDS), CD4 count (per 100 cells/mm3 decrease), and viral load (HIV-1 RNA level ≥5 vs <5 log copies/mL). All HRs were adjusted for these characteristics as well as year of ART initiation and were stratified by cohort. Linearity of relationships of cause-specific log hazards with age and CD4 count were assessed using categories of these variables and fractional polynomials [15]. There was evidence of nonlinearity in the effect of CD4 count because death rates did not vary greatly among individuals with baseline CD4 counts >500 cells/mm3. We therefore replaced baseline CD4 counts that were >500 cells/mm3 with 500 cells/mm3, and there was then little evidence of nonlinearity. We checked for between-cohort heterogeneity in the association of each risk factor with AIDS- and non–AIDS-related deaths. There was little evidence of such heterogeneity (all P values >.05), except for the effect of viral load on non–AIDS-related deaths, for which there was weak evidence of heterogeneity (P = .04). All subsequent analyses assumed that the effect of risk factors was the same across cohorts. Using random effects meta-analysis, we estimated the between-cohort variance in rates of AIDS- and non–AIDS-related deaths to be 0.19 and 0.02, respectively.
We used Poisson regression models to estimate overall and cause-specific mortality adjusted incidence rate ratios by calendar period of starting ART (1996–1997, 1998–1999, 2000–2002, 2003–2006) and duration of time receiving ART (<1, 1–1.99, ≥2 years) with 1996–1997 and duration of ART <1 year as reference categories. We estimated cumulative incidence functions for AIDS-related, non–AIDS-related, and unknown deaths with use of nonparametric modelling that accounts for censoring because of competing causes of death [16]. These were stacked to illustrate the contribution of each to total mortality.
RESULTS
Data on 39,272 patients with a median follow-up of 3.6 years (interquartile range [IQR], 1.6–6.1) were available. Table 1 shows patient characteristics at baseline with corresponding years of follow-up and numbers of deaths. Median age was 37 years (IQR, 31–44) and median calendar month of initiation of ART was December 2000 (October 1998–April 2003). The initial antiretroviral regimen contained ≥4 drugs (excluding low-dose ritonavir) in 2264 (6%) and 3 drugs in 37,008 (94%) patients. A protease inhibitor was used in 24,267 (62%) and a nonnucleoside reverse-transcriptase inhibitor in 12,688 (32%) initial regimens.
Table 1.
Characteristics of 39,272 Patients Included in Analyses at the Start of Anti-retroviral Therapy (ART), with Follow-up Time and Number of Deaths
| Characteristics | Overall, no (%) of patients |
Duration of follow-up, person-years |
Deaths, no (%) of patients |
|---|---|---|---|
| Total | 39,272 (100) | 154,667 | 1876 (4.8) |
| Sex | |||
| Male | 28,487 (73) | 115,719 | 1530 (5.4) |
| Female | 10,785 (27) | 38,947 | 346 (3.2) |
| Presumed mode of transmission | |||
| MSM | 14,058 (36) | 60,639 | 539 (3.8) |
| Heterosexual | 16168 (41) | 59,115 | 598 (3.7) |
| IDU | 4987 (13) | 20,121 | 452 (9.1) |
| Other | 4059 (10) | 14,909 | 287 (7.1) |
| Age, years | |||
| 16–29 | 6970 (18) | 26,806 | 163 (2.3) |
| 30–39 | 16,958 (43) | 69,978 | 716 (4.2) |
| 40–49 | 10,051 (26) | 38,358 | 542 (5.4) |
| ≥50 | 5293 (13) | 19,525 | 455 (8.6) |
| CDC disease stage | |||
| A/B | 30,030 (76) | 119,019 | 989 (3.3) |
| C | 9242 (24) | 35,648 | 887 (9.6) |
| CD4 cell count, cells/mm3 | |||
| 0–99 | 10,561 (27) | 40,825 | 884 (8.4) |
| 100–199 | 7883 (20) | 28,991 | 385 (4.9) |
| 200–349 | 11,544 (29) | 42,354 | 356 (3.1) |
| ≥350 | 9284 (24) | 42,497 | 251 (2.7) |
| HIV-1 RNA, log copies/mL | |||
| <4 | 5205 (13) | 20,230 | 174 (3.4) |
| 4–5 | 15,708 (40) | 63,114 | 551 (3.5) |
| ≥5 | 18,359 (47) | 71,322 | 1151 (6.3) |
| Year of starting ART | |||
| 1996–1997 | 6015 (15) | 41,060 | 526 (8.7) |
| 1998–1999 | 9696 (25) | 53,360 | 633 (6.5) |
| 2000–2002 | 12,445 (32) | 44,974 | 509 (4.1) |
| 2003–2006 | 11,116 (28) | 15,272 | 208 (1.9) |
NOTE. CDC, Centers for Disease Control and Prevention; HIV-1, human immunodeficiency virus type 1; IDU, injection drug use; MSM, men who have sex with men.
During 154,667 years of follow-up, 1876 (4.8%) patients died. Of these deaths, 1597 (85%) were assigned a cause according to the CoDe categories. Table 2 shows the number of deaths in each of these categories. The crude all-cause death rate was 12.1 deaths per 1000 person-years (95% confidence interval [CI], 11.6–12.7 deaths per 1000 person-years). Those who died had lower median CD4 counts at baseline (110 cells/mm3; IQR, 33–247 cells/mm3) than those who survived (217 cells/mm3; IQR, 94–343 cells/mm3). In those who died, the median time to death from baseline was 1.8 years (IQR, 0.6–3.8). Compared with patients with an assigned cause of death, the 279 patients with unknown cause of death were more likely to be male, to be an injection drug user, and to have less advanced HIV-1 disease at baseline. At the last clinic visit before death, they were more likely to be receiving treatment and had higher median CD4 counts (215 vs 110 cells/mm3).
Table 2.
Frequencies of Specific Causes of Death According to the Cause of Death (CoDe) Classification in the 1876 Patients Who Died.
| Cause of death (N = 1597) | N (%) | Incidence rate (95% CI) per 1000 years |
|---|---|---|
| AIDS | 792 (49.6) | 5.12 (4.78–5.49) |
| Non-specified AIDS | 190 (11.9) | 1.23 (1.07–1.42) |
| AIDS infection | 366 (22.9) | 2.37 (2.14–2.62) |
| AIDS malignancy | 236 (14.8) | 1.52 (1.34–1.73) |
| Non AIDS malignancy | 189 (11.8) | 1.22 (1.06–1.41) |
| Non-AIDS infection | 131 (8.2) | 0.85 (0.71–1.01) |
| CVD1 | 126 (7.9) | 0.81 (0.68–0.97) |
| MI/IHD2 | 51 (3.2) | 0.33 (0.25–0.43) |
| Stroke | 23 (1.4) | 0.15 (0.10–0.22) |
| Other heart disease | 52 (3.3) | 0.34 (0.26–0.44) |
| Violence3 | 124 (7.8) | 0.80 (0.67–0.96) |
| Suicide | 48 (3.0) | 0.31 (0.23–0.41) |
| Substance abuse | 42 (2.6) | 0.41 (0.32–0.52) |
| Other violent death | 34 (2.1) | 0.22 (0.16–0.31) |
| Liver-related | 113 (7.1) | 0.73 (0.61–0.88) |
| Hepatitis-related | 63 (3.9) | 0.41 (0.32–0.52) |
| Other liver-related | 50 (3.1) | 0.32 (0.25–0.43) |
| Respiratory disease | 25 (1.6) | 0.16 (0.11–0.24) |
| Renal failure | 24 (1.5) | 0.16 (0.10–0.23) |
| Other causes with N<20 | 73 (4.6) | 0.47 (0.38–0.59) |
CVD cardiovascular disease (includes MI/IHD, stroke, heart failure/unspecified and other heart disease)
MI/IHD myocardial infarction/ischemic heart disease
Violent includes homicide, accident, suicide and substance abuse as well as ill-defined violent deaths.
Table 3 shows frequencies of the 8 grouped causes of death. Seven hundred ninety-two (49.6%) of the deaths for which a cause was assigned were due to AIDS, of which 366 (46.2%) could be further classified as infection and 236 (29.8%) as malignancy. The most frequent non-AIDS causes of death were non-AIDS malignancy (11.8%), non-AIDS infection (8.2%), cardiovascular disease (7.9%, of which 40% were myocardial infarction/ischemic heart disease and 18% stroke), violence (7.8%, approximately one-third each of suicide, substance abuse, and homicide/accident/unspecified), and liver disease (7.1%, of which 55.8% were hepatitis related). The most frequent sites for non-AIDS malignancies were respiratory tract or intrathoracic organs (36.7%); digestive organs and peritoneum (28.7%); lip, oral cavity, and pharynx (6.0%); and skin (4.7%). During the first year of ART, 63% of deaths were attributable to AIDS; this subsequently decreased to 43%. Table 3 shows crude incidence rates per 1000 years of different causes of death; note that these are underestimates because 15% of deaths could not be classified. Table 4 shows frequencies of cause-specific death by year of death. The proportion of AIDS-related deaths decreased from 58.5% in 1996–1999 to 43.7% in 2003–2006. The proportion of deaths due to AIDS-defining cancers decreased from 20.5% to 12.5%, and the proportion of deaths due to non–AIDS-defining cancers increased from 7.3% to 15.4% over the same time periods.
Table 3.
Frequencies of Specific Causes of Death in the 1597 Patients Who Died, with Crude Incidence Rates per 1000 Person-Years of Follow-up
| Cause of death | No (%) of patientsa (n = 1597) |
Incidence rate (95% CI) per 1000 years |
|---|---|---|
| AIDS | ||
| All | 792 (49.6) | 5.12 (4.78–5.49) |
| Nonspecified AIDS | 190 (11.9) | 1.23 (1.07–1.42) |
| AIDS infection | 366 (22.9) | 2.37 (2.14–2.62) |
| AIDS malignancy | 236 (14.8) | 1.52 (1.34–1.73) |
| Non-AIDS malignancy | 189 (11.8) | 1.22 (1.06–1.41) |
| Non-AIDS infection | 131 (8.2) | 0.85 (0.71–1.01) |
| CVDb | ||
| All | 126 (7.9) | 0.81 (0.68–0.97) |
| MI/IHD | 51 (3.2) | 0.33 (0.25–0.43) |
| Stroke | 23 (1.4) | 0.15 (0.10–0.22) |
| Other heart disease | 52 (3.3) | 0.34 (0.26–0.44) |
| Violencec | ||
| All | 124 (7.8) | 0.80 (0.67–0.96) |
| Suicide | 48 (3.0) | 0.31 (0.23–0.41) |
| Substance abuse | 42 (2.6) | 0.41 (0.32–0.52) |
| Other violent death | 34 (2.1) | 0.22 (0.16–0.31) |
| Liver related | ||
| All | 113 (7.1) | 0.73 (0.61–0.88) |
| Hepatitis related | 63 (3.9) | 0.41 (0.32–0.52) |
| Other liver related | 50 (3.1) | 0.32 (0.25–0.43) |
| Respiratory disease | 25 (1.6) | 0.16 (0.11–0.24) |
| Renal failure | 24 (1.5) | 0.16 (0.10–0.23) |
| Other causes with n < 20 | 73 (4.6) | 0.47 (0.38–0.59) |
NOTE. CI, confidence interval; CVD, cardiovascular disease; MI/IHD, myocardial infarction/ischemic heart disease.
39,272 patients with 154,667 years of follow-up.
CVD includes MI/IHD, stroke, heart failure/unspecified, and other heart disease.
Violence includes homicide, accident, suicide, and substance abuse, as well as ill-defined violent deaths.
Table 4.
Frequencies of Specific Causes of Death in the 1597 Patients Who Died by Year of Death
| Cause of death | N = 1876 | % |
| Assigned | 1597 | 85 |
| Could not be assigned | 279 | 15 |
| CoDe cause of death | N = 1597 | % |
| 01 AIDS (not further specified) | 190 | 11.9 |
| 01.1 AIDS infection | 366 | 22.9 |
| 01.2 AIDS malignancy | 236 | 14.8 |
| 02 Non-AIDS infection | 131 | 8.2 |
| 03 Chronic viral hepatitis | 63 | 3.9 |
| 04 Non-AIDS malignancy | 189 | 11.6 |
| 05 Diabetes Mellitus | 2 | 0.1 |
| 06 Pancreetitis | 5 | 0.3 |
| 07 Lactic acidosis | 8 | 0.5 |
| 08 Myocardial infarction/ischemic heart disease | 51 | 3.2 |
| 09 Stroke | 23 | 1.4 |
| 10 Gastro Intestinal haemborrage | 12 | 0.8 |
| 11 Primary pulmonary hypertension | 2 | 0.1 |
| 12 Lung embolus | 10 | 0.6 |
| 13 Chronic obstructive pulmonary disease | 3 | 0.2 |
| 14 Liver failure | 50 | 3.1 |
| 15 Renal failure | 24 | 1.5 |
| 16 Accidentiviolent | 34 | 2.1 |
| 17 Suicide | 48 | 3.0 |
| 18 Euthanasia | 0 | 0 |
| 19 Substance abuse | 42 | 2.6 |
| 20 Haemantological disease | 9 | 0.5 |
| 21 Endocrine disease | 0 | 0 |
| 22 Psychiatric disease | 3 | 0.2 |
| 23 Central nervous system disease | 11 | 0.7 |
| 24 Heartivascular | 52 | 3.3 |
| 25 Respiratory disease | 22 | 1.4 |
| 26 Digestive system disease | 2 | 0.1 |
| 27 Skin and motor system disease | 0 | 0 |
| 28 Urogenital | 1 | 0.1 |
| 29 Obstetric complications | 0 | 0 |
| 20 Congenital disorders | 0 | 0 |
| 90 Other1 | 8 | 0.5 |
8 deaths classified as "other" were: foreign body in respiratory tract, acidosis (2), amyloidosis, instantaneous death, toxic epidermal necrolysis, terminal pulmonary hypertension with arterial occlusion of the extremities, intra-cerebral hematoma
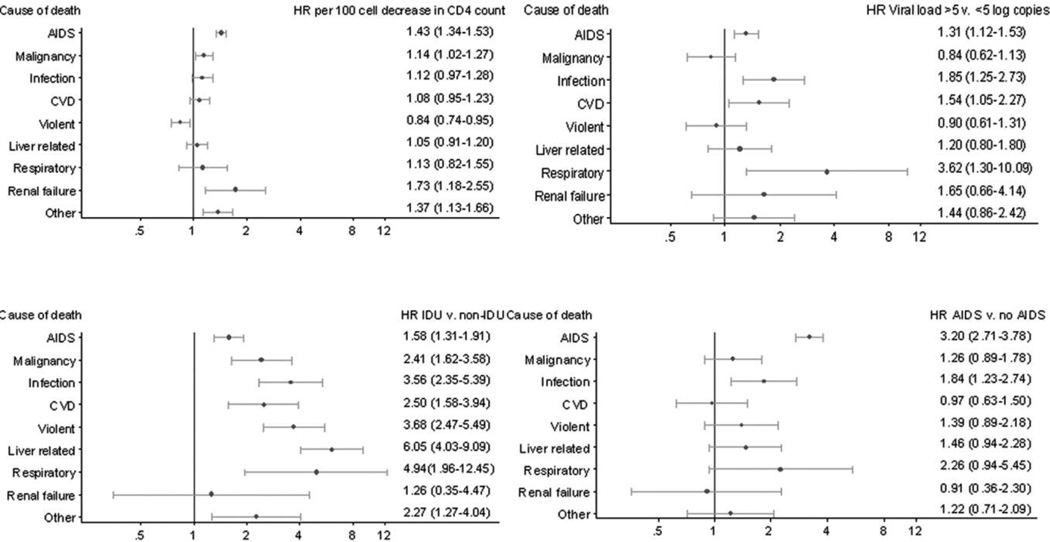
Figure 1 shows adjusted HRs for the association of prognostic factors at baseline with specific causes of death. Lower CD4 counts were associated with higher rates of deaths due to AIDS, non-AIDS malignancy, renal failure, and other causes, as well as with death due to AIDS infection (HR per 100 cell decrease, 1.69; 95% CI, 1.52–1.88) but not AIDS malignancies (HR, 1.05; 95% CI, 0.95–1.17). Higher baseline CD4 counts were associated with higher rates of death due to violent causes. Rates of all specific causes of death except renal failure were higher in injection drug users, with particularly strong associations for liver-related death, respiratory, death, violent death, and death due to infection. AIDS before baseline was associated with higher subsequent rates of death due to both AIDS and non-AIDS infections. Patients with higher baseline viral load had higher rates of death due to AIDS, non-AIDS infection, cardiovascular disease, and respiratory disease.
Figure 1.
Adjusted hazard ratios (HRs) and 95% confidence intervals of risk factors at start of antiretroviral therapy for specific causes of death from Cox models with CD4 cell count (per 100 cell decrease), transmission risk group (injection drug user [IDU] vs non-IDU), viral load (human immunodeficiency virus type 1 RNA load ≥5 vs <5 log copies/mL), and prior AIDS diagnosis (vs no AIDS). Models were mutually adjusted for age, sex, IDU, CD4 count, viral load, prior AIDS diagnosis, cohort, and year of starting antiretroviral therapy. CVD, cardiovascular disease.
Table 5 gives further information on associations of prognostic factors with specific causes of death. Older age was strongly associated with increased rates of non-AIDS malignancy (HR per 10 years, 2.32; 95% CI, 2.04–2.63) and cardiovascular disease (HR per 10 years, 2.05; 95% CI, 1.76–2.39). The cause of death least strongly associated with age was AIDS (HR per 10 years, 1.19; 95% CI, 1.11–1.28). There was a marked increase in rates of renal death in patients aged >60 years. Compared with male patients, female patients had lower rates of all-cause mortality (HR, 0.84; 95% CI, 0.74–0.94) and, in particular, lower rates of death due to AIDS malignancies (HR, 0.58; 95% CI, 0.40–0.84) and non-AIDS malignancies (HR, 0.50; 95% CI, 0.31–0.79).
Table 5.
Mutually Adjusted Hazard Ratios for Specific Causes of Death (Frequency, >20%) for Risk Factors at Start of Antiretroviral Therapy (ART), from Multivariable Cox Models Stratified by Cohort and Calendar Year of Starting ART.
| Adjusted1 HR (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Cause of death | Female | age per 10 years | IDU v. non-IDU | AIDS v. no AIDS | CD4 per 100 cell/µL decrease |
log HIV-1 RNA ≥5 v. <5 log copies |
| All cause mortality | 0.84 (0.74–0.94) | 1.47 (1.41–1.54) | 2.38 (2.13–2.67) | 1.98 (1.78–2.20) | 1.22 (1.18–1.26) | 1.25 (1.13–1.38) |
| AIDS | 0.90 (0.75–1.08) | 1.19 (1.11–1.28) | 1.58 (1.31–1.91) | 3.20 (2.71–3.78) | 1.43 (1.34–1.53) | 1.31 (1.12–1.53) |
| Non-AIDS related | 0.81 (0.67–0.98) | 1.75 (1.64–1.87) | 3.27 (2.77–3.87) | 1.35 (1.14–1.59) | 1.08 (1.03–1.14) | 1.24 (1.07–1.44) |
| Unknown | 0.72 (0.52–1.00) | 1.58 (1.41–1.77) | 2.91 (2.17–3.89) | 1.46 (1.11–1.93) | 1.24 (1.12–1.36) | 1.12 (0.87–1.45) |
| AIDS | 0.90 (0.75–1.08) | 1.19 (1.11–1.28) | 1.58 (1.31–1.91) | 3.20 (2.71–3.78) | 1.43 (1.34–1.53) | 1.31 (1.12–1.53) |
| Non-specified AIDS | 1.29 (0.92–1.80) | 1.36 (1.18–1.56) | 1.58 (1.09–2.31) | 2.47 (1.77–3.46) | 1.65 (1.43–1.90) | 1.15 (0.84–1.59) |
| AIDS infection | 0.96 (0.74–1.24) | 1.07 (0.95–1.19) | 1.88 (1.44–2.46) | 2.29 (1.80–2.91) | 1.69 (1.52–1.88) | 1.41 (1.11–1.79) |
| AIDS malignancy | 0.58 (0.40–0.84) | 1.24 (1.09–1.40) | 1.18 (0.80–1.74) | 6.57 (4.81–8.97) | 1.05 (0.95–1.17) | 1.25 (0.94–1.65) |
| Non AIDS malignancy | 0.50 (0.31–0.79) | 2.32 (2.04–2.63) | 2.41 (1.62–3.58) | 1.26 (0.89–1.78) | 1.14 (1.02–1.27) | 0.84 (0.62–1.13) |
| Non-AIDS infection | 0.82 (0.52–1.28) | 1.51 (1.28–1.78) | 3.56 (2.35–5.39) | 1.84 (1.23–2.74) | 1.12 (0.97–1.28) | 1.85 (1.25–2.73) |
| CVD1 | 0.89 (0.56–1.41) | 2.05 (1.76–2.39) | 2.50 (1.58–3.94) | 0.97 (0.63–1.50) | 1.08 (0.95–1.23) | 1.54 (1.05–2.27) |
| MI/IHD2 | 0.47 (0.18–1.18) | 2.42 (1.92–3.06) | 1.80 (0.80–4.05) | 1.37 (0.71–2.64) | 1.05 (0.85–1.29) | 1.68 (0.91–3.10) |
| Stroke | 1.33 (0.51–3.48) | 1.80 (1.26–2.57) | 0.77 (0.17–3.44) | 0.49 (0.15–1.63) | 1.14 (0.84–1.54) | 1.07 (0.45–2.57) |
| Other heart disease | 1.14 (0.59–2.20) | 1.84 (1.42–2.38) | 4.29 (2.28–8.07) | 0.88 (0.44–1.74) | 1.09 (0.89–1.34) | 1.66 (0.91–3.04) |
| Violent deaths | 0.68 (0.41–1.11) | 1.23 (1.01–1.49) | 3.68 (2.47–5.49) | 1.39 (0.89–2.18) | 0.84 (0.74–0.95) | 0.90 (0.61–1.31) |
| Suicide | 0.56 (0.25–1.28) | 1.32 (1.00–1.76) | 2.09 (1.03–4.23) | 1.32 (0.61–2.83) | 0.81 (0.67–0.99) | 0.80 (0.43–1.48) |
| Substance abuse | 0.80 (0.36–1.78) | 1.07 (0.74–1.56) | 6.88 (3.46–13.68) | 1.28 (0.60–2.77) | 0.74 (0.60–0.92) | 0.92 (0.48–1.75) |
| Other violent death | 0.64 (0.24–1.73) | 1.26 (0.88–1.81) | 3.14 (1.33–7.42) | 1.52 (0.67–3.46) | 1.03 (0.80–1.32) | 1.04 (0.50–2.17) |
| Liver-related | 1.33 (0.87–2.06) | 1.63 (1.34–1.98) | 6.05 (4.03–9.09) | 1.46 (0.94–2.28) | 1.05 (0.91–1.20) | 1.20 (0.80–1.80) |
| Hepatitis-related | 1.28 (0.71–2.33) | 1.51 (1.15–1.99) | 7.90 (4.55–13.73) | 1.14 (0.62–2.08) | 1.08 (0.90–1.30) | 1.34 (0.78–2.30) |
| Other liver-related | 1.41 (0.75–2.66) | 1.74 (1.32–2.30) | 4.53 (2.41–8.51) | 1.95 (1.00–3.79) | 1.00 (0.82–1.23) | 1.07 (0.58–1.96) |
| Respiratory disease | 1.41 (0.57–3.47) | 1.55 (1.06–2.25) | 4.94 (1.96–12.45) | 2.26 (0.94–5.45) | 1.13 (0.82–1.55) | 3.62 (1.30–10.09) |
| Renal failure | 0.33 (0.08–1.46) | 1.59 (1.11–2.28) | 1.26 (0.35–4.47) | 0.91 (0.36–2.30) | 1.73 (1.18–2.55) | 1.65 (0.66–4.14) |
| Other causes with N<20 | 0.96 (0.54–1.72) | 1.47 (1.18–1.83) | 2.27 (1.27–4.04) | 1.22 (0.71–2.09) | 1.37 (1.13–1.66) | 1.44 (0.86–2.42) |
Models were mutually adjusted for age, sex, IDU, CD4, viral load, prior AIDS diagnosis, cohort and year of starting ART
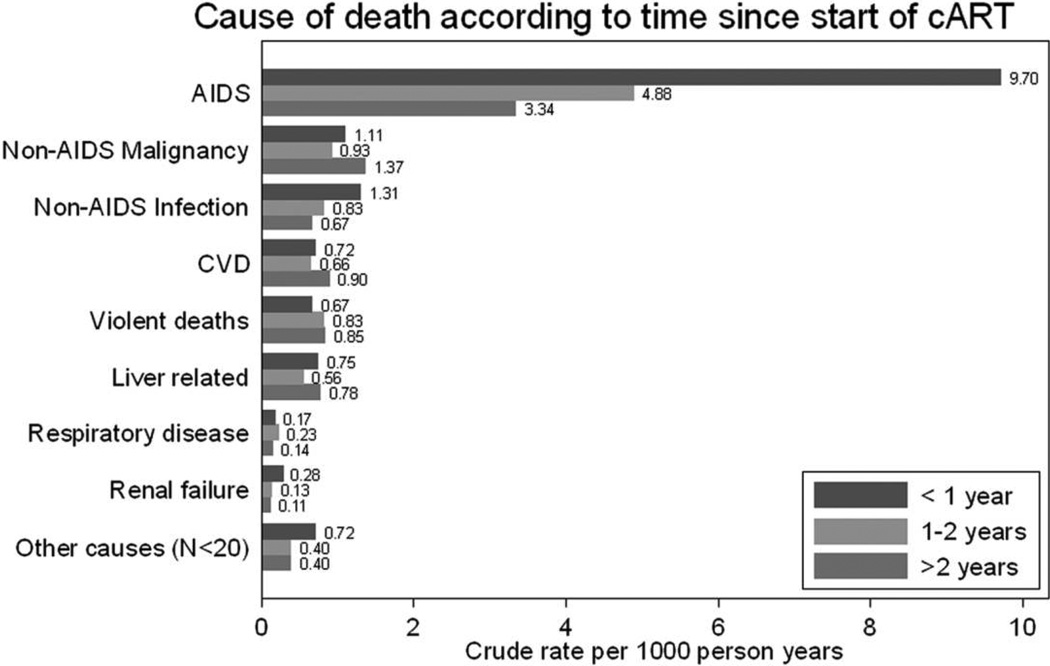
Figure 2 displays cause-specific mortality rates according to time since starting ART. Rates of death due to AIDS, non-AIDS infection, and renal failure decreased markedly with increasing time receiving ART. Table 6 shows cause-specific mortality incidence rate ratios according to duration of ART and calendar year of starting ART, adjusted for prognostic factors. Rates of AIDS-related mortality decreased with both time on ART (P < .001, by test for trend) and with year of starting ART (P < .001). Rates of death due to non-AIDS infection and renal failure also decreased with time receiving ART.
Figure 2.
Bar graph showing crude cause-specific mortality rate according to length of time since start of combination antiretroviral therapy (cART). CVD, cardiovascular disease.
Table 6.
Adjusted Cause-Specific Incidence Rate Ratios by Time Receiving Antiretroviral Therapy (ART) and Calendar Year of Starting ART, from Poisson Models
| Model | Cause of death | |||||||
|---|---|---|---|---|---|---|---|---|
| AIDS | Non-AIDS malignancy | Non-AIDS infection | Cardiovascular disease | Violence | Liver related | Respiratory | Renal failure | |
| Time receiving ARTa | ||||||||
| <1 year | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1–1.99 years | 0.50 (0.41–0.61) | 0.85 (0.52–1.38) | 0.68 (0.42–1.10) | 0.92 (0.51–1.65) | 1.24 (0.71–2.18) | 0.72 (0.39–1.33) | 1.53 (0.51–4.60) | 0.48 (0.15–1.53) |
| ≥2 years | 0.33 (0.28–0.39) | 1.24 (0.84–1.82) | 0.63 (0.42–0.96) | 1.22 (0.76–1.96) | 1.17 (0.72–1.89) | 0.90 (0.57–1.43) | 1.07 (0.37–3.13) | 0.38 (0.15–0.95) |
| P, for trend | <.001 | .18 | .036 | .32 | .61 | .80 | .96 | .042 |
| Year of starting ARTb | ||||||||
| 1996–1997 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1998–1999 | 0.82 (0.69–0.99) | 0.77 (0.54–1.09) | 0.66 (0.40–1.09) | 0.85 (0.55–1.32) | 1.26 (0.83–1.93) | 1.01 (0.65–1.58) | 1.88 (0.59–6.00) | 0.62 (0.22–1.71) |
| 2000–2002 | 0.70 (0.58–0.85) | 0.66 (0.45–0.98) | 1.09 (0.68–1.73) | 0.78 (0.49–1.26) | 0.66 (0.38–1.14) | 0.72 (0.43–1.21) | 1.17 (0.32–4.25) | 0.52 (0.17–1.52) |
| 2003–2006 | 0.72 (0.56–0.92) | 0.83 (0.47–1.46) | 1.63 (0.92–2.92) | 0.74 (0.35–1.56) | 1.02 (0.48–2.18) | 0.42 (0.16–1.13) | 3.09 (0.74–12.95) | 0.56 (0.14–2.30) |
| P, for trend | <.001 | .11 | .074 | .28 | .33 | .059 | .32 | .28 |
NOTE. Data are hazard ratio (95% confidence interval), unless otherwise indicated.
Adjusted for age, sex, injection drug use, CD4, log viral load, AIDS at baseline, and year of starting ART.
Adjusted for age, sex, injection drug use, CD4, log viral load, AIDS at baseline, and time receiving ART.
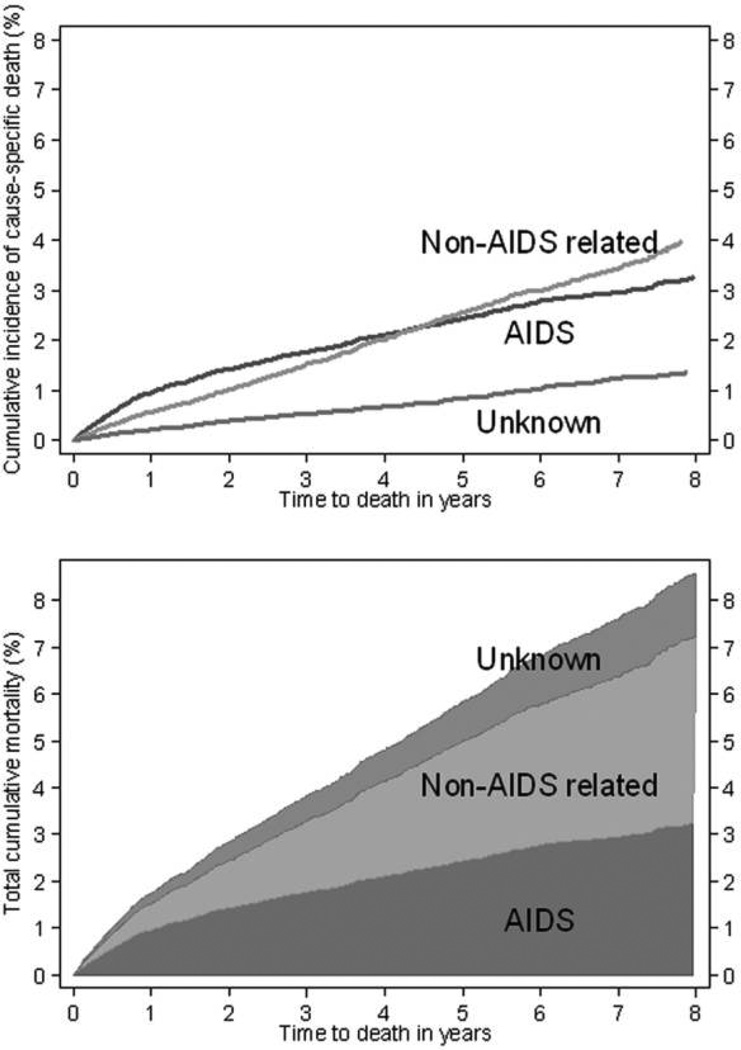
Figure 3 (upper panel) shows cumulative incidence for AIDS-related, non-AIDS-related, and unknown deaths. The majority of deaths during the first year of receiving ART were AIDS related, but because rates of AIDS-related death decrease with time receiving ART, the cumulative incidence of non–AIDS-related deaths exceeded that of AIDS-related deaths after ~4 years of ART. Figure 3 (lower panel) shows that the total cumulative mortality at 8 years was ~9%, with <4% of deaths classified as AIDS related.
Figure 3.
Cumulative incidence of AIDS-related, non–AIDS-related, and unknown deaths (upper panel) and total cumulative mortality partitioned by cause of death (lower panel).
DISCUSSION
We retrospectively assigned a cause of death for 85% of the 1876 deaths among nearly 40,000 individuals who started ART during 1996–2006 in Europe and North America. Rates of AIDS, the leading cause of death, decreased with time receiving ART, although because time receiving ART is equivalent to follow-up time, no causal effect of time receiving ART can be inferred. However, there was little variation in rates of non-AIDS causes of deaths with year of starting ART and time receiving ART. Immunodeficiency was associated with death due to AIDS, non-AIDS malignancy, and renal failure. Rates of death due to AIDS, non-AIDS infection, cardiovascular disease, and respiratory disease were higher in patients with baseline viral loads >5 log copies/mL. Those infected through injection drug use had higher rates of all specific causes of death, with particularly high rates of liver-related and violent death.
Associations with risk factors could only be analyzed for frequent causes of death, reflecting the success of ART in reducing overall mortality. We used the CoDe system, which is specifically designed for classifying causes of death in HIV-1–infected persons [12]. However, deaths were classified retrospectively without complete clinical histories. Classifications may differ from those that would be assigned if full documentation was available. We may have grouped diseases with different etiologies; for example, deaths from myocardial infarction/ischemic heart disease, stroke, and the more-general unspecified heart-related deaths. It is likely that some deaths coded as unspecified heart-related were due to dysrhythmias, possibly attributable to drug overdose, which would be better classified as violent death. We may have inappropriately assigned some causes of death to AIDS in patients with extremely low CD4 counts and multiple AIDS conditions but a nonspecific cause of death, such as respiratory failure or coma. Nonetheless, the majority of deaths are likely to have been classified accurately, and misclassifications are unlikely to be associated with risk factors such as age or time since initiation of ART. There was some evidence of between-cohort heterogeneity in death rates, particularly for AIDS-related deaths. Such heterogeneity could be attributable to differences in patient characteristics beyond those included in existing models (eg, socioeconomic status) or to underascertainment of deaths in some cohorts.
Non–HIV-1 markers of disease risk, such as hypertension, smoking, and alcohol misuse, may be strongly associated with non-AIDS morbidity and mortality but were not available to us. We did not analyze associations of ART regimen with mortality because of the likelihood of unmeasured confounding by indication. Associations of baseline CD4 count with cause-specific mortality should be interpreted with caution because CD4 count prior to death was used in assigning the cause of death. Six deaths in injection drug users without direct evidence of hepatitis C infection were classified as hepatitis related, which will have increased the estimated association of injection drug use with hepatitis-related liver disease. However, rates of liver-related death are 6 times higher in injection drug users, possibly because of drug dependency, alcohol abuse, or coinfection with hepatitis.
AIDS opportunistic infections have been reported to be the most frequent cause of death in the early ART era [17]. Reported proportions of AIDS-related deaths in the ART era range from 31% (D:A:D collaboration) [18], 40% in Spain [19] and Australia [20], 46% in the United Kingdom [21], to 50% in the United States [22]. Because patients with nonassigned causes of death had less advanced HIV-1 disease, the proportion of AIDS-defining deaths is somewhat overestimated in our study. Among HIV-1–infected adults in France, the proportion of AIDS-related deaths decreased from 47% to 36% between 2000 and 2005, and the proportion of deaths due to cancer not related to AIDS or hepatitis increased from 11% to 17% [13, 23]. Compared with our study, the higher proportion of liver-related disease in France (13%–15%) [13] and the D:A:D collaboration (15%) [18] may be attributable to a higher proportion of injection drug users.
Acute renal failure remains prevalent among hospitalized patients with HIV-1 and is associated with increased mortality in the ART era [24]. Patterns of death due to renal failure were similar to those due to AIDS, with a strong inverse association with CD4 count and a decrease associated with time receiving ART. The same pattern has been described for HIV-1–associated nephropathy, which is caused by viral replication within the kidney and associated with end-stage renal disease [25]. Alternatively, renal failure can be linked to HIV-1 through shared risk factors such as hepatitis C [26], antiretroviral drugs (particularly indinavir and tenofovir), or nephrotoxic drugs used to treat opportunistic infections.
Ongoing HIV-1 replication is associated with an excess risk of opportunistic disease and death, even after allowing for CD4 count [27]. In our study, death rates due to infections, as well as respiratory tract and cardiovascular disease, were associated with baseline viral loads >5 log copies/mL but not with lower CD4 counts. Systemic immune activation secondary to high viral replication, rather than or additional to immunodeficiency, may promote deaths due to infectious and cardiovascular causes. Several infections, including herpes simplex [28], hepatitis C [29], and tuberculosis [30], have been shown to induce HIV-1 viremia and worsen outcomes.
ART continues to dramatically reduce rates of mortality attributable to HIV-1 infection in high-income countries. Lower rates of deaths due to AIDS after the year 2000 may result from newer ART regimens having better potency and tolerability. However, AIDS remains the most common cause of death, particularly early after initiation of treatment. The strong inverse association of rates of death due to AIDS with CD4 counts at the time of starting ART supports arguments for earlier initiation of ART [31–33]. Conditions associated with social and lifestyle factors contribute the next most frequent causes of death, with violence and liver-related diseases (mainly due to hepatitis) contributing 15% of all deaths. The importance of lifestyle is reinforced by the observation that the most common non-AIDS malignancy reported was lung cancer, likely associated with smoking. Many endocarditis cases occurred in patients infected with HIV-1 via injection drug use, who likely acquired endocarditis through injection drug use. Increases in rates of deaths due to causes associated with aging, such as non-AIDS malignancies and cardiovascular disease, imply that the process of aging will become a dominant factor in HIV-1 mortality in the next decade. Interventions to address risk factors for lifestyle-related causes of death, as well as monitoring for and care of diseases associated with old age, will be necessary if the full benefit of ART in decreasing mortality is to continue in the second decade of ART.
Acknowledgments
We are grateful to all patients, doctors, nurses and other persons who were involved with the participating cohort studies
Financial support. The ART Cohort Collaboration is supported by the UK Medical Research Council grant G0600599. Sources of funding of individual cohorts include the Agence Nationale de Recherche contre le SIDA (ANRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), the French, Italian and Swiss Ministries of Health, the Stichting HIV Monitoring, the European Commission, the British Columbia and Alberta Governments, the Michael Smith Foundation for Health Research, the Canadian Institutes of Health Research and unrestricted grants from GlaxoSmithKline, Roche and Boehringer-Ingelheim.
STUDY GROUPS
The Antiretroviral Therapy Cohort Collaboration Study Group
Writing committee
John Gill, Division of Infectious Diseases, University of Calgary, Calgary, Canada; Margaret May, Department of Social Medicine, University of Bristol, Bristol, United Kingdom; Charlotte Lewden, INSERM, U897, and Université de Bordeaux, ISPED, Bordeaux, France; Mike Saag, Division of Infectious Disease, Department of Medicine, University of Alabama, Birmingham; Michael Mugavero, Division of Infectious Disease, Department of Medicine, University of Alabama, Birmingham; Peter Reiss, Academisch Medisch Centrum bij de Universiteit van Amsterdam, Amsterdam, The Netherlands; Bruno Ledergerber, Zurich University Hospital, Zurich, Switzerland; Amanda Mocroft, University College London Medical School, Royal Free Campus, United Kingdom; Ross Harris, Department of Social Medicine, University of Bristol, Bristol, United Kingdom; Christoph A. Fux, University Clinic for Infectious Diseases and University of Berne, Switzerland; Amy Justice, Yale University School of Medicine, New Haven, and VA Connecticut Healthcare System, West Haven, Connecticut; Dominique Costagliola, INSERM, U943, Université Pierre et Marie Curie, Paris, France; Jordi Casabona, CEEISCAT, ICO/Departament de Salut, Universitat Autònoma de Barcelona and CIBER Epidemiology and Public Health (CIBERESP), Badalona, Spain; Robert S Hogg, British Columbia Columbia Centre for Excellence in HIV/AIDS and Simon Fraser University, Vancouver, Canada; Pavel Khaykin, Zentrum der Inneren Medizin, J.W. Goethe Universität, Frankfurt, Germany; Fiona Lampe, Division of Population Health, University College, London, United Kingdom; Janne Vehreschild, Department of Internal Medicine, University of Cologne, Germany; Jonathan A C Sterne, Department of Social Medicine, University of Bristol, Bristol, United Kingdom.
Cause of Death (CoDe) committee
John Gill, Charlotte Lewden, Mike Saag, Matthias Egger, Michael Mugavero, Peter Reiss, Bruno Ledergerber, Amanda Mocroft, Ross Harris, Margaret May, Jonathan A C Sterne.
Steering committee
Hans-Reinhard Brodt (Frankfurt), Jordi Casabona (PISCIS), Geneviève Chêne (Aquitaine), Dominique Costagliola (FHDH), François Dabis (Aquitaine), Antonella D’Arminio Monforte (ICONA), Frank de Wolf (ATHENA), Matthias Egger (SHCS), Gerd Fätkenheuer (Köln/Bonn), John Gill (South Alberta Clinic), Jodie Guest (HAVACS), Robert S Hogg (BCCfE-HIV), Amy Justice (VACS), Ole Kirk (Euro-SIDA), Mari Kitahata (Washington), Fiona Lampe (Royal Free), Bruno Ledergerber (SHCS), Peter Reiss (ATHENA), Michael Saag (Alabama), Tim Sterling (CHORUS/Vanderbilt).
Coordinating team
Margaret May, Ross Harris, Jonathan Sterne (Principal Investigator).
Footnotes
Potential conflicts of interest. All authors: no conflicts.
Presented in part: 16th Conference on Retroviruses and Opportunistic Infections, Montreal, Canada, February 2009 (abstract 708).
References
- 1.ART-CC. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mocroft A, Brettle R, Kirk O, et al. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS. 2002;16:1663–1671. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 4.Lau B, Sharrett AR, Kingsley LA, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166:64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 5.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. ICD-9, International Classification of Diseases and Related Health Problems, 9th revision. Geneva: World Health Organization; 1975. [Google Scholar]
- 8.World Health Organization. International Statistical Classification of Diseases and Related health Problems, 10th revision. Geneva: World Health Organization; 2007. [Google Scholar]
- 9.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 10.May M, Royston P, Egger M, Justice AC, Sterne JA. Development and validation of a prognostic model for survival time data: application to prognosis of HIV positive patients treated with antiretroviral therapy. Stat Med. 2004;23:2375–2398. doi: 10.1002/sim.1825. [DOI] [PubMed] [Google Scholar]
- 11.May M, Sterne JA, Sabin C, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21:1185–1197. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewden C, Salmon D, Morlat P, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–130. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 13.Lewden C, May T, Rosenthal E, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: the “Mortalite 2000 and 2005” surveys (ANRS EN19 and Mortavic) J Acquir Immune Defic Syndr. 2008;48:590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 14.Clifford GM, Rickenbach M, Polesel J, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135–2141. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 15.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 16.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 17.Smit C, Geskus R, Walker S, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 18.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 19.Martinez E, Milinkovic A, Buira E, et al. Incidence and causes of death in HIV-infected persons receiving highly active antiretroviral therapy compared with estimates for the general population of similar age and from the same geographical area. HIV Med. 2007;8:251–258. doi: 10.1111/j.1468-1293.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 20.Petoumenos K, Law MG. Risk factors and causes of death in the Australian HIV Observational Database. Sex Health. 2006;3:103–112. doi: 10.1071/sh05045. [DOI] [PubMed] [Google Scholar]
- 21.Sabin CA, Smith CJ, Youle M, et al. Deaths in the era of HAART: contribution of late presentation, treatment exposure, resistance and abnormal laboratory markers. AIDS. 2006;20:67–71. doi: 10.1097/01.aids.0000196178.73174.24. [DOI] [PubMed] [Google Scholar]
- 22.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 23.Bonnet F, Burty C, Lewden C, et al. Changes in cancer mortality among HIV-infected patients: the Mortalite 2005 Survey. Clin Infect Dis. 2009;48:633–639. doi: 10.1086/596766. [DOI] [PubMed] [Google Scholar]
- 24.Wyatt CM, Arons RR, Klotman PE, Klotman ME. Acute renal failure in hospitalized patients with HIV: risk factors and impact on in-hospital mortality. AIDS. 2006;20:561–565. doi: 10.1097/01.aids.0000210610.52836.07. [DOI] [PubMed] [Google Scholar]
- 25.Post FA, Campbell LJ, Hamzah L, et al. Predictors of renal outcome in HIV-associated nephropathy. Clin Infect Dis. 2008;46:1282–1289. doi: 10.1086/529385. [DOI] [PubMed] [Google Scholar]
- 26.Wyatt CM, Malvestutto C, Coca SG, Klotman PE, Parikh CR. The impact of hepatitis C virus coinfection on HIV-related kidney disease: a systematic review and meta-analysis. AIDS. 2008;22:1799–1807. doi: 10.1097/QAD.0b013e32830e0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundgren JD, Babiker A, El-Sadr W, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–1155. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 28.Nagot N, Ouedraogo A, Konate I, et al. Roles of clinical and subclinical reactivated herpes simplex virus type 2 infection and human immunodeficiency virus type 1 (HIV-1)-induced immunosuppression on genital and plasma HIV-1 levels. J Infect Dis. 2008;198:241–249. doi: 10.1086/589621. [DOI] [PubMed] [Google Scholar]
- 29.Daar ES, Lynn H, Donfield S, et al. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J Infect Dis. 2001;183:589–595. doi: 10.1086/318539. [DOI] [PubMed] [Google Scholar]
- 30.DeSimone JA, Pomerantz RJ, Babinchak TJ. Inflammatory reactions in HIV-1-infected persons after initiation of highly active antiretroviral therapy. Ann Intern Med. 2000;133:447–454. doi: 10.7326/0003-4819-133-6-200009190-00013. [DOI] [PubMed] [Google Scholar]
- 31.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.When To Start Consortium. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marin B, Thiebaut R, Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23:1743–1753. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]