Abstract
Although the Unified Huntington's Disease Rating Scale (UHDRS) is widely used in the assessment of Huntington disease (HD), the ability of individual items to discriminate individual differences in motor or behavioral manifestations has not been extensively studied in HD gene expansion carriers without a motor-defined clinical diagnosis (i.e., prodromal-HD or prHD). To elucidate the relationship between scores on individual motor and behavioral UHDRS items and total score for each subscale, a non-parametric item response analysis was performed on retrospective data from two multicentre, longitudinal studies. Motor and Behavioral assessments were supplied for 737 prHD individuals with data from 2114 visits (PREDICT-HD) and 686 HD individuals with data from 1482 visits (REGISTRY). Option characteristic curves were generated for UHDRS subscale items in relation to their subscale score. In prHD, overall severity of motor signs was low and participants had scores of 2 or above on very few items. In HD, motor items that assessed ocular pursuit, saccade initiation, finger tapping, tandem walking, and to a lesser extent saccade velocity, dysarthia, tongue protrusion, pronation/supination, Luria, bradykinesia, choreas, gait and balance on the retropulsion test were found to discriminate individual differences across a broad range of motor severity. In prHD, depressed mood, anxiety, and irritable behavior demonstrated good discriminative properties. In HD, depressed mood demonstrated a good relationship with the overall behavioral score. These data suggest that at least some UHDRS items appear to have utility across a broad range of severity, although many items demonstrate problematic features.
Keywords: UHDRS, Item Response Theory, Huntington disease
INTRODUCTION
Huntington disease (HD) is an autosomal dominant neurodegenerative disease associated with severe motor, psychiatric and cognitive impairment.1 Although the gene expansion responsible for HD is present at birth, the onset of clinical motor manifestations normally occurs in mid-life. Non-motor manifestations of HD, including mood and cognitive disturbances, are often present in carriers of the HD gene expansion many years before motor onset.2–6 Unfortunately, the relationship between these changes, their latency to onset and aetiology are poorly understood, resulting in challenges when developing new rating scales that measure efficacy of both pharmacological and non-pharmacological interventions.
The Unified Huntington's Disease Rating Scale (UHDRS) was developed for the clinical assessment of HD7, and is the current ‘gold-standard’ outcome measure used in clinical trials of HD. The UHDRS is divided into multiple subscales, assessing motor and cognitive function, behavioral (i.e., psychiatric) symptoms, and functional capacity. The scale was developed and tested in gene expansion carriers who had manifest motor symptoms of HD,7 and may not be sensitive to changes in the earlier stages of the disease.8–10
The Functional Rating Scale Taskforce for pre-Huntington Disease (FuRST-pHD) is a multinational, multidisciplinary initiative with the goal of developing a data-driven, comprehensive, psychometrically sound, rating scale for assessing symptoms and functional ability in HD gene expansion carriers who have not yet met the motor-defined diagnostic criteria for HD (i.e., prodromal HD). Such a scale is essential in order to develop early interventions that may alter the course of HD in individuals with the CAG expansion. FuRST-pHD has established a collaborative approach for identifying which relevant changes should be assessed, and for developing rating scale items best able to measure severity.11
PREDICT-HD is an ongoing NIH- and CHDI-funded multi-centre, longitudinal study in pre-HD gene expansion carriers in the United States, Canada, Europe and Australia.5 REGISTRY is funded by the CHDI Foundation and managed by the European Huntington’s Disease Network (EHDN) and is an ongoing, multi-centre, longitudinal observational study collecting data in HD and pre-HD gene expansion carriers in Europe.12 The current study used item response theory (IRT) analysis of UHDRS data collected from prodromal HD (prHD) and HD individuals (from PREDICT-HD and REGISTY, respectively) to explore the relationship between scores on individual UHDRS motor and behavioral items and the respective subscale total score. IRT analyses are useful in evaluating the performance of individual items on rating scales by assessing the relationship between scores assigned to an item in the target population and the underlying disease severity measure (e.g., severity of motor signs in HD).13 The fundamental premise of IRT is that the probability of a particular score for an item of a rating scale is a function of the underlying trait or condition that is being measured.13 In this respect, IRT has been used in the evaluation of various rating scales, including the Hamilton Depression14–17 and Anxiety Rating15 Scales, Montgomery-Asberg Depression Rating Scale,17 Beck Depression Inventory,18 Somatic Symptoms Inventory,19 and Sexual Interest and Desire Inventory.20
METHODS
Participants
Retrospective de-identified data from two ongoing multicentre, longitudinal observational research studies conducted in United States, Canada, Europe and Australia were used in the analyses. Demographic data obtained from the PREDICT-HD and REGISTRY studies included age and gender, as well as group means for CAG repeated length and disease burden scores (Table 1). The dataset used in the present analyses included UHDRS Motor and Behavioral assessments that were supplied for 737 prHD gene expansion carriers (CAG repeat length > 36; UHDRS Diagnostic Confidence Level ≤ 3) with data from 2114 visits (PREDICT-HD) and 686 HD gene expansion carriers (CAG repeat length ≥ 36; UHDRS Diagnostic Confidence Level = 4, possessing motor abnormalities that are unequivocal signs of HD)7 with data from 1482 visits (REGISTRY). All data collection sites had IRB/EC approval and all participants provided informed consent.
TABLE 1.
Demographics and clinical characteristics of prHD (PREDICT-HD) and HD (REGISTRY) participants.
| Characteristic | PrHD | HD |
|---|---|---|
| Male gender | 36.8%, N=271 | 47.7%, N=327 |
| Age | 42.50 ± 0.22 (18–79) | 52.07 ± 0.30 (19–87) |
| CAG repeat length | 42.51 ± 0.10 (38–61) | 44.78 ± 0.15 (37–75) |
| aDisease/genetic burden score | 277.54 ± 1.66 (90.75–813.70) | 442.25 ± 2.73 (91.5 – 1165.5) |
| bExpected yrs to onset | 13.71 ± 0.15 (3.44–42.46) | n/a |
| UHDRS Total Motor | 5.58 ± 0.13 (0–45) | 43.06 ± 0.55 (0–106) |
| UHDRS Total Behavioral-Frequency | 6.67 ± 0.13 (0–44) | 6.32 ± 0.18 (0–34) |
| UHDRS Total Behavioral-Severity | 4.81 ± 0.12 (0–44) | 5.97 ± 0.16 (0–31) |
Gender and CAG repeat length were based on first visit data, all remaining variables averaged across all visits. Mean ± SEM, range in parenthesis.
Disease Burden Score = (CAG repeat length – 35.5) × age; Penney JB Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. Ann Neurol. CAG repeat number governs the development rate of pathology in Huntington's disease. Ann Neurol. 1997;41(5):689–692.
Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR; International Huntington's Disease Collaborative Group. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin Genet. 2004 Apr;65(4):267–77.
Clinical Assessments
The UHDRS Motor Subscale has 15 items measuring eye movements (ocular pursuit, saccade initiation and velocity), speech (dysarthia), motor coordination (tongue protrusion, finger taps, pronate/supinate, Luria-manual sequencing), rigidity, bradykinesia, dystonia, chorea, and gait/balance. The UHDRS Behavioral Subscale includes 11 items that independently assess frequency and severity of psychiatric-related symptoms, including depressed mood, apathy, low self-esteem/guilt, suicidal thoughts, anxiety, irritable behavior, aggressive behavior, obsessional thinking, compulsive behavior, delusions, and hallucinations. Each UHDRS item is rated on a 5-point scale, with each item contributing 0–4 points to the total score. The UHDRS’99 Motor and Behavioral Subscales were administered to each patient at each visit by a qualified rater. A total score was separately calculated for the Motor and Behavioral Subscales by summing up all the individual motor and behavioral items (separately for frequency and severity), respectively, with higher scores representing more severe manifestations.7
Analyses
Non-parametric IRT analyses of the UHDRS Motor and Behavioral Subscales were performed to determine the relationship between scores on the individual UHDRS items and total score on the relevant UHDRS subscale (i.e., Motor and Behavioral subscales). IRT software (TESTGRAF)21,22 was used to generate Option Characteristic Curves (OCCs) that display the probability of a particular option score (i.e., a score of 0, 1, 2, 3, 4) on each UHDRS item as a function of overall level of severity (i.e., total Motor or Behavioral score). As such, OCCs provide a graphical representation of how informative a particular item (or symptom) is as a measure of illness. The item is effective if the options discriminate differences in severity; as there is an increase in severity of the underlying trait (increased total score on the scale) the probability of low scores on the individual item decrease and the probability of higher scores increase. Highly discriminating items are characterized by (1) OCCs that rise and fall quickly, indicating good discrimination across the range of severity, and (2) OCCs whose weights correspond to the ordinal position in which they are most likely to be endorsed. In the present study, we used retrospective data from PREDICT-HD and REGISTRY to generated OCCs from the UHDRS Motor and Behavioral Subscales to illustrate the relationship between scoring patterns for each item and the range of total UHDRS subscale score in prHD and HD. It should be noted that multiple scores were included from each participant, yet no corrections for repeated assessments were employed in the analysis. This is justified because Item Response Theory assumes local independence (i.e., independence among item responses at any given level of severity), and so the analyses are not invalidated by virtue of covariance among an individual’s item scores. In addition, given that multiple observations were obtained from individuals with scores throughout the continuum of severity, it is unlikely that any systematic biased would have occurred. A thorough description of the TESTGRAF software may be obtained at www.psych.mcgill.ca/faculty/ramsay/ramsay.html.
RESULTS
UHDRS Motor Subscale in prHD Participants
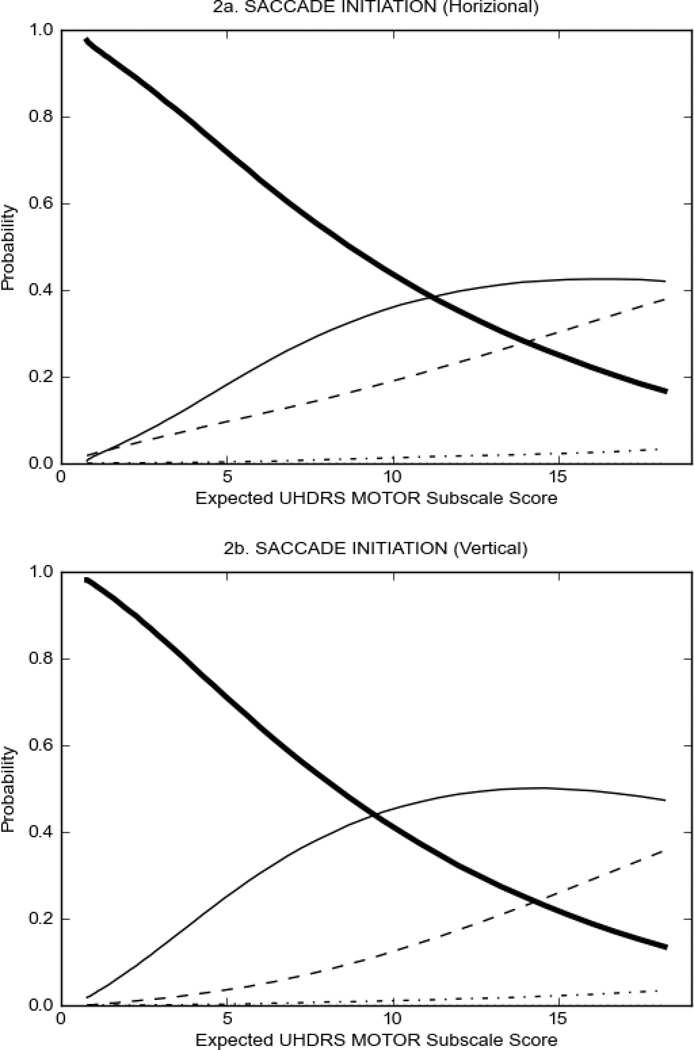
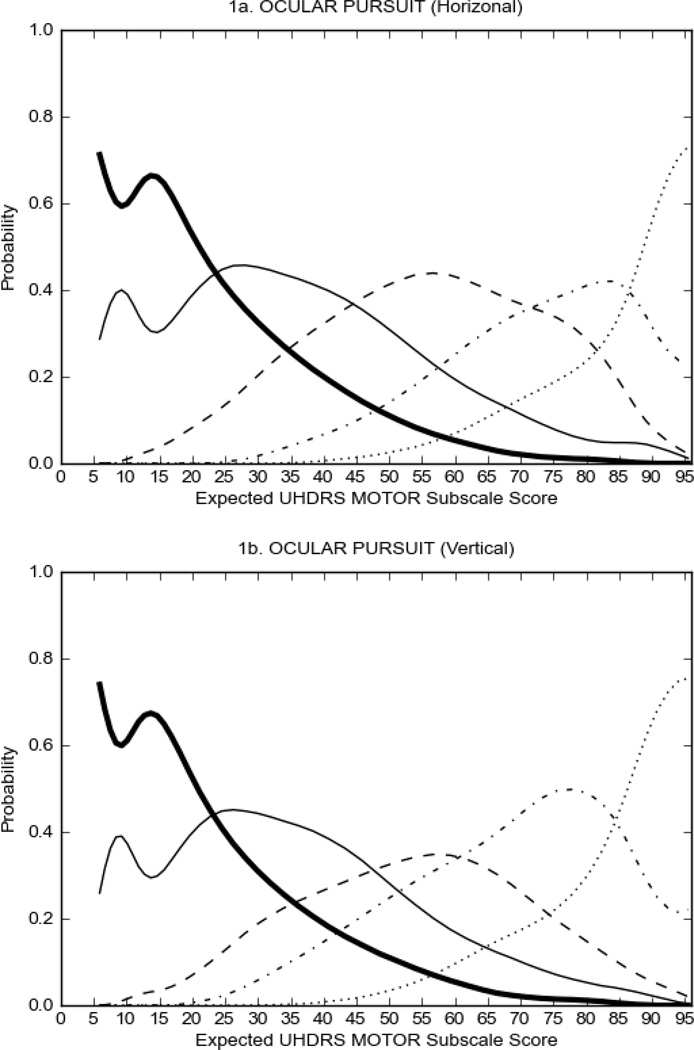
prHD participants had scores of 2 or more on very few items, and the overall severity of motor signs was significantly lower than that observed in HD participants (prHD = 5.58 ± 0.13; HD = 43.06 ± 0.55, p<0.01, Table 1). There were indications that some items performed better than others (Figure 1), including saccade initiation (Figure 1A) in which the option with the highest probability of being scored increased from 0 to 2 as motor symptom severity increased; however, neither Option 3 nor Option 4 had a high probability of being scored even at high levels of overall severity of motor signs. For the other items in the UHDRS motor subscale, scores of '0' (Luria, rigidity, dystonia, tongue protrusion, Figure 1B) or at the most scores of '1' (occular pursuit, dysarthria, saccade velocity, bradykinesia, chorea, finger taps, pronation/supination, Figure 1C,D) had the highest probability of being scored across the full range of severity. These items appear to have limited usefulness in assessing motor manifestations in the prHD population.
FIGURE 1.
OCCs for representative UHDRS motor items in relation to UHDRS Motor Subscale Score in prHD participants. The remaining OCCs are available as supplemental material online.
UHDRS Motor Subscale in HD Participants
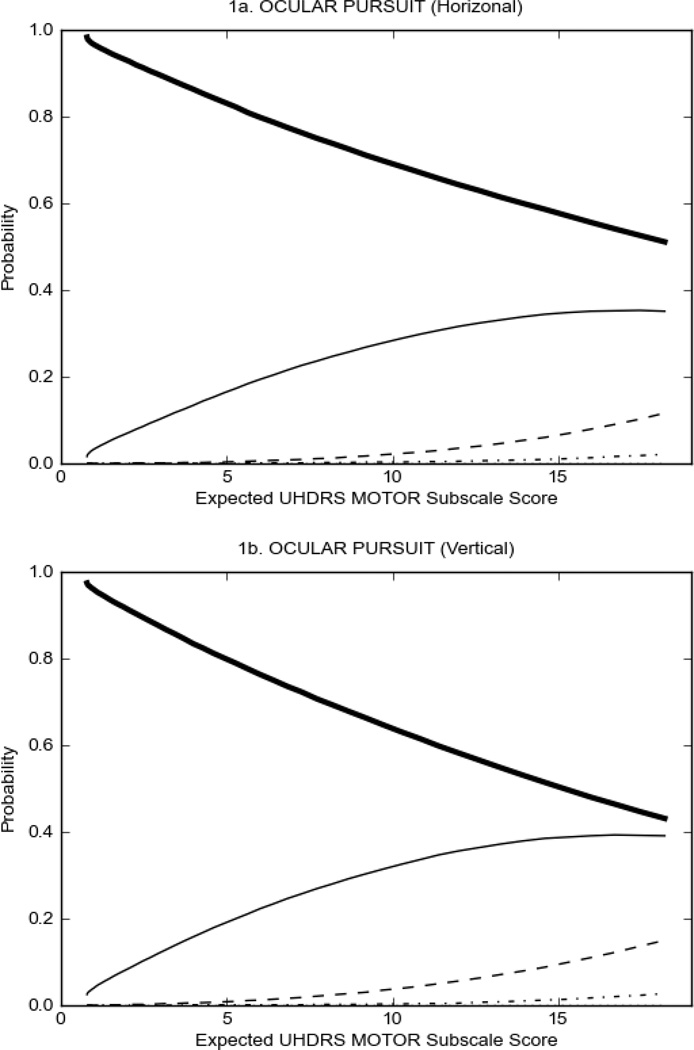
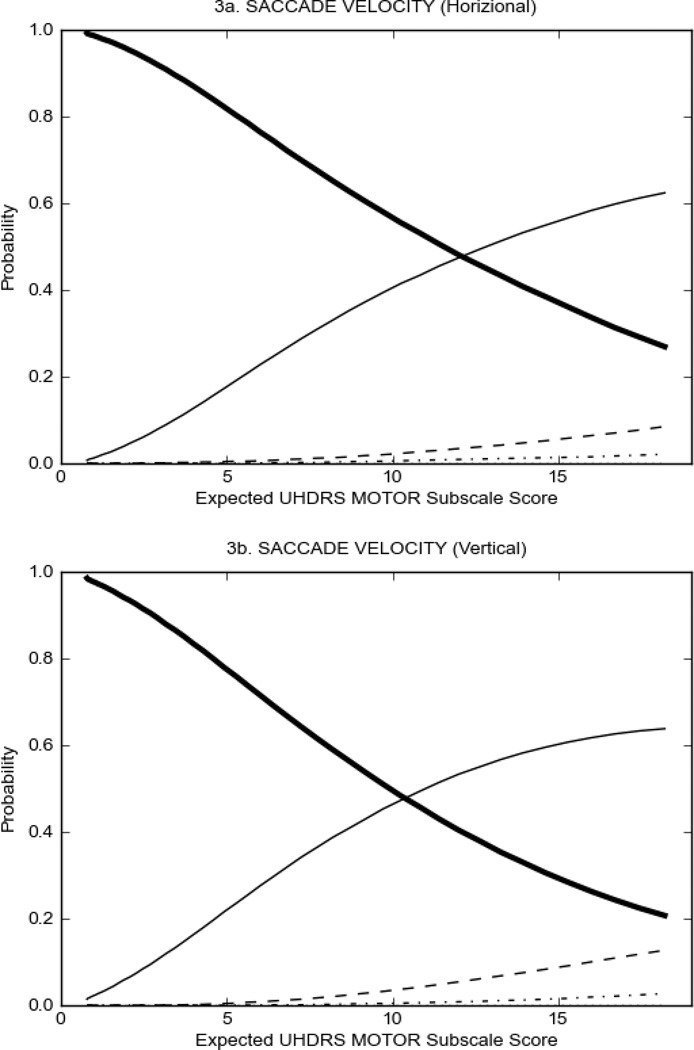
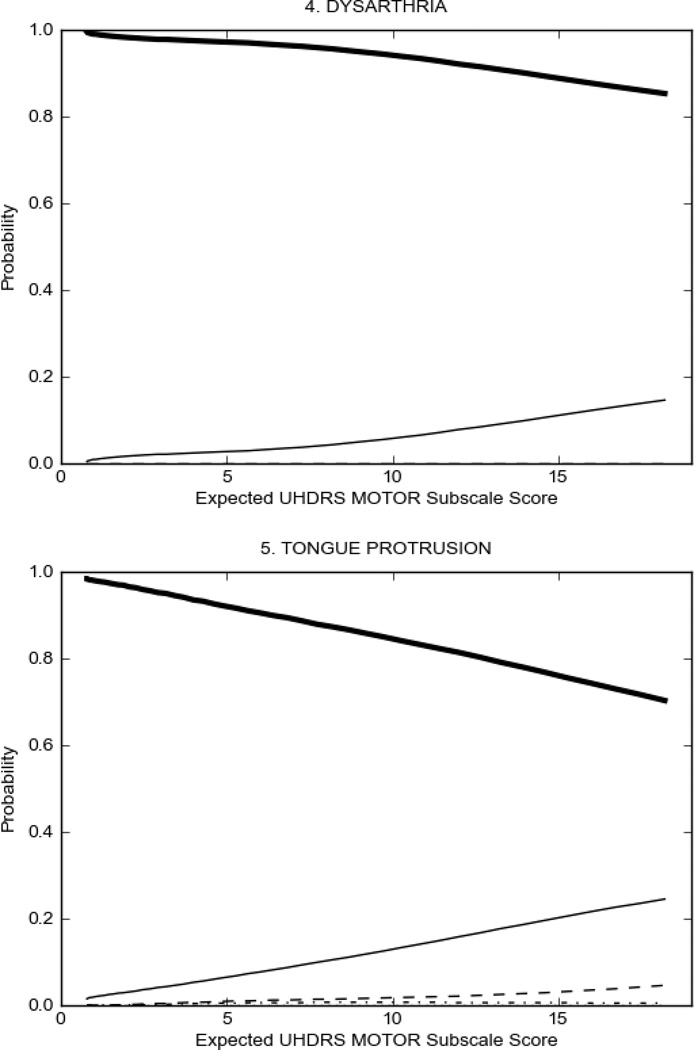
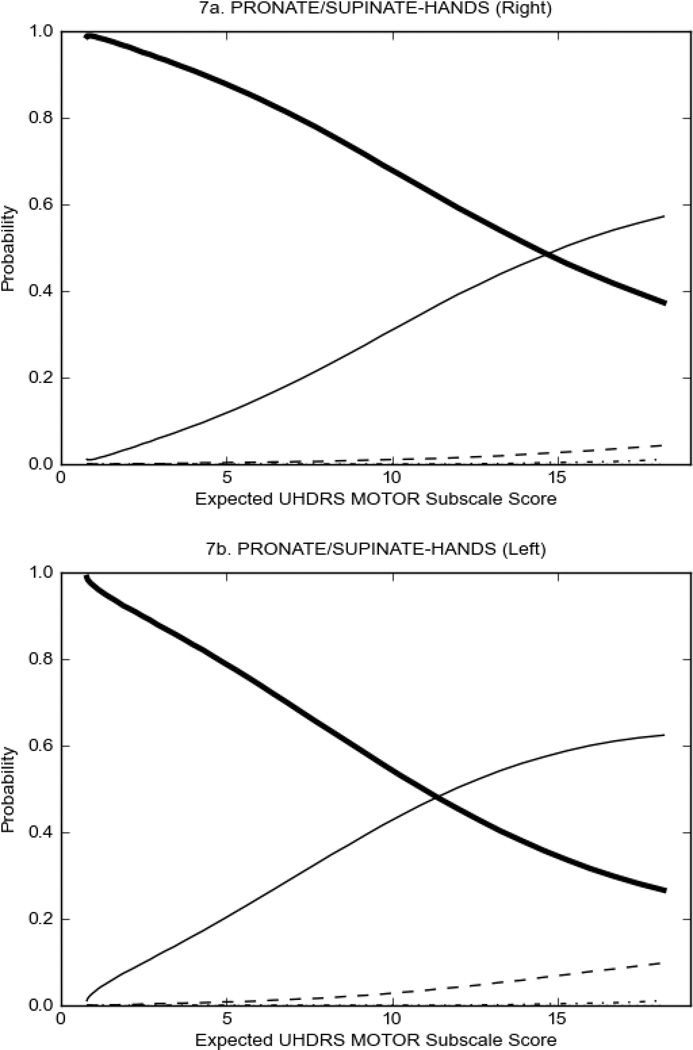
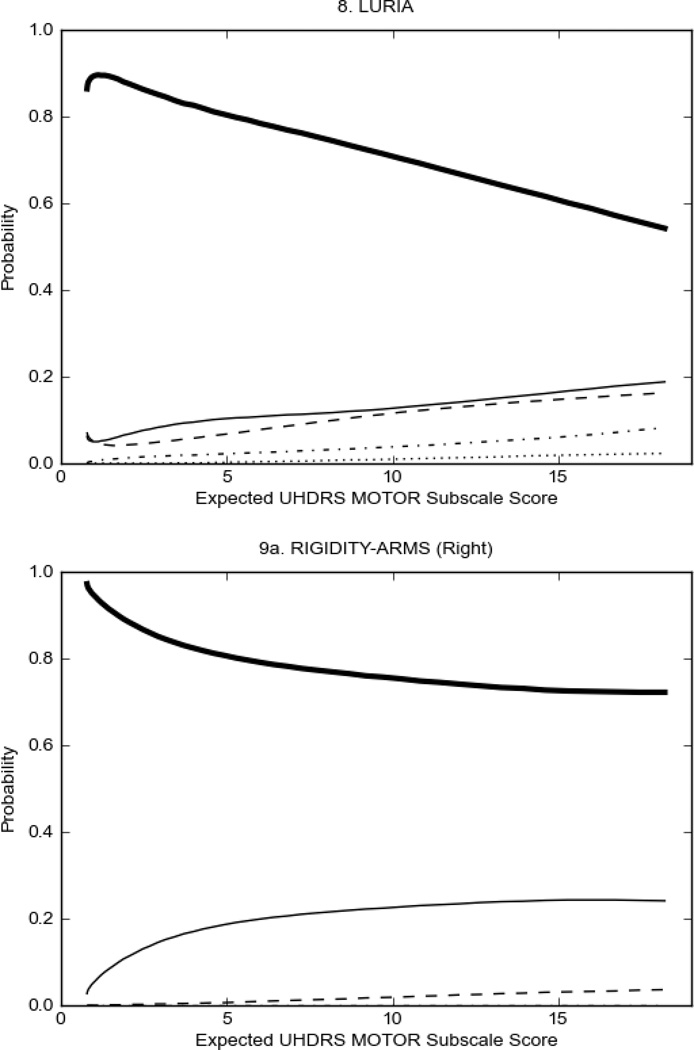
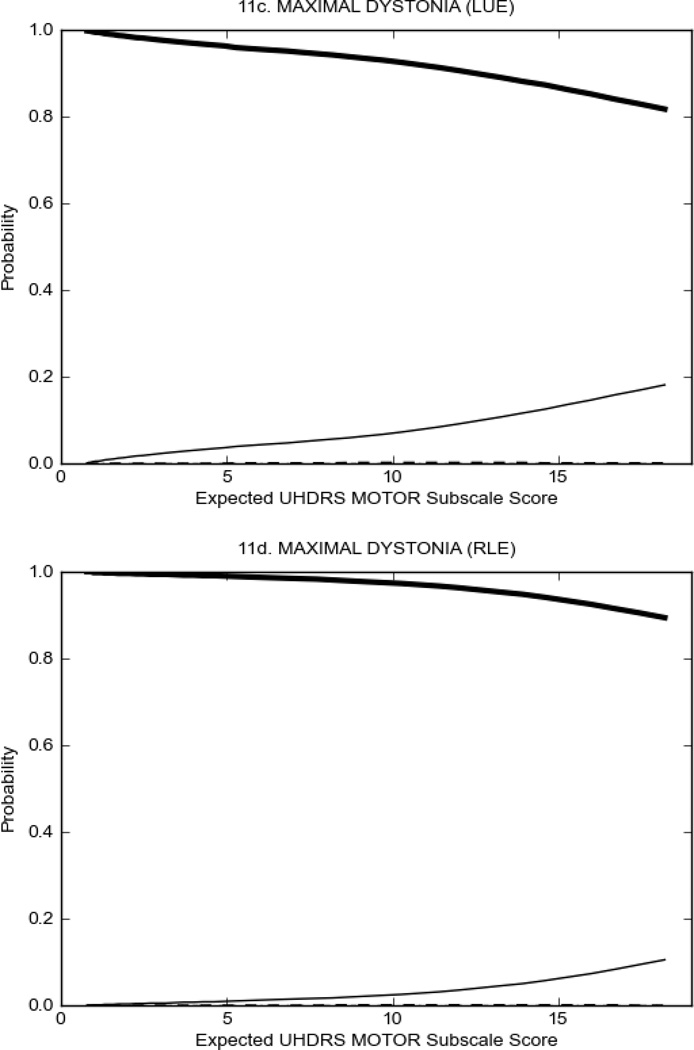
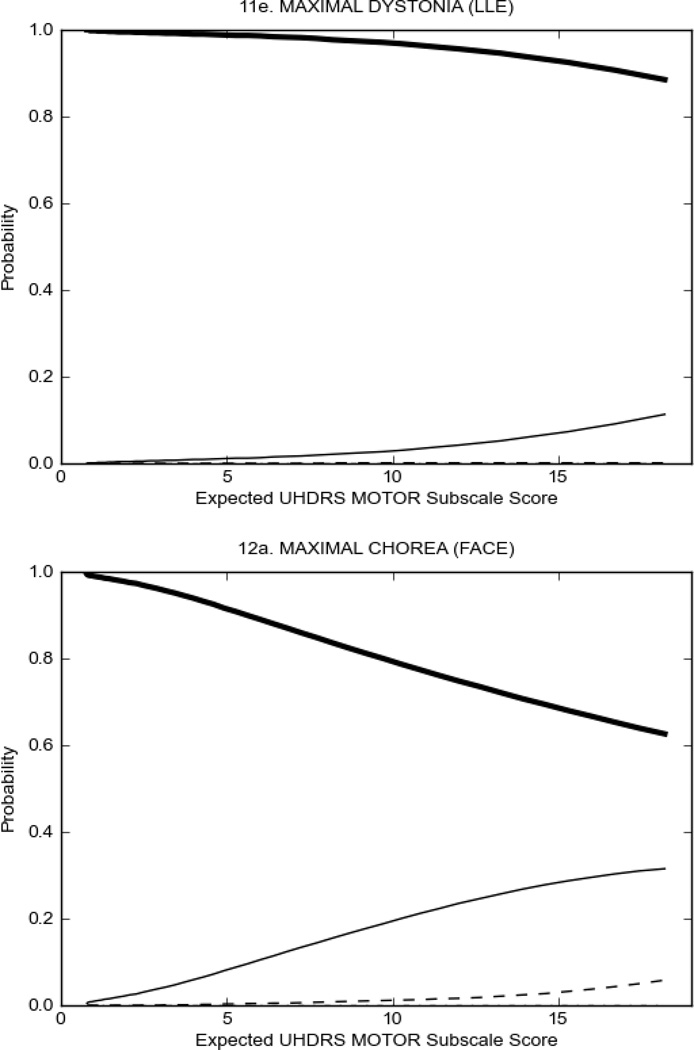
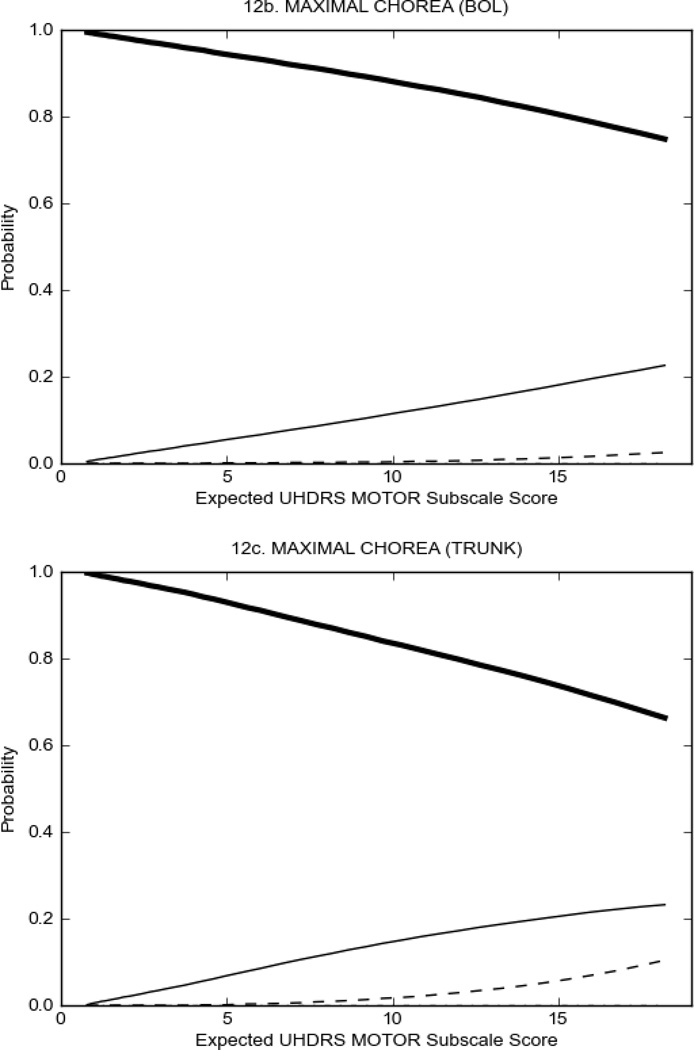
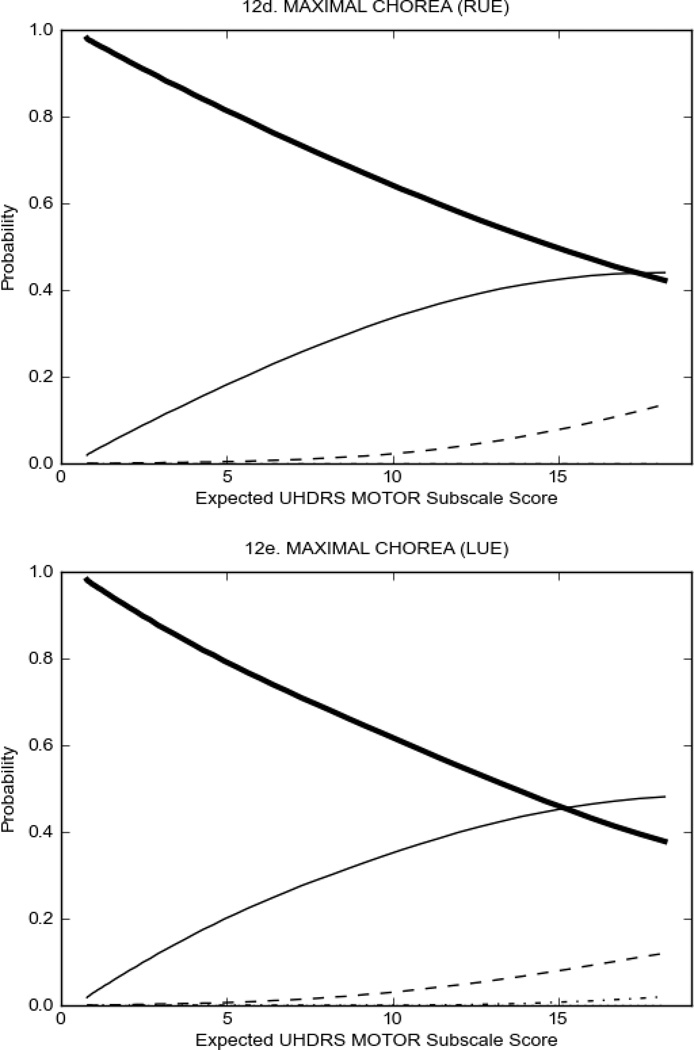
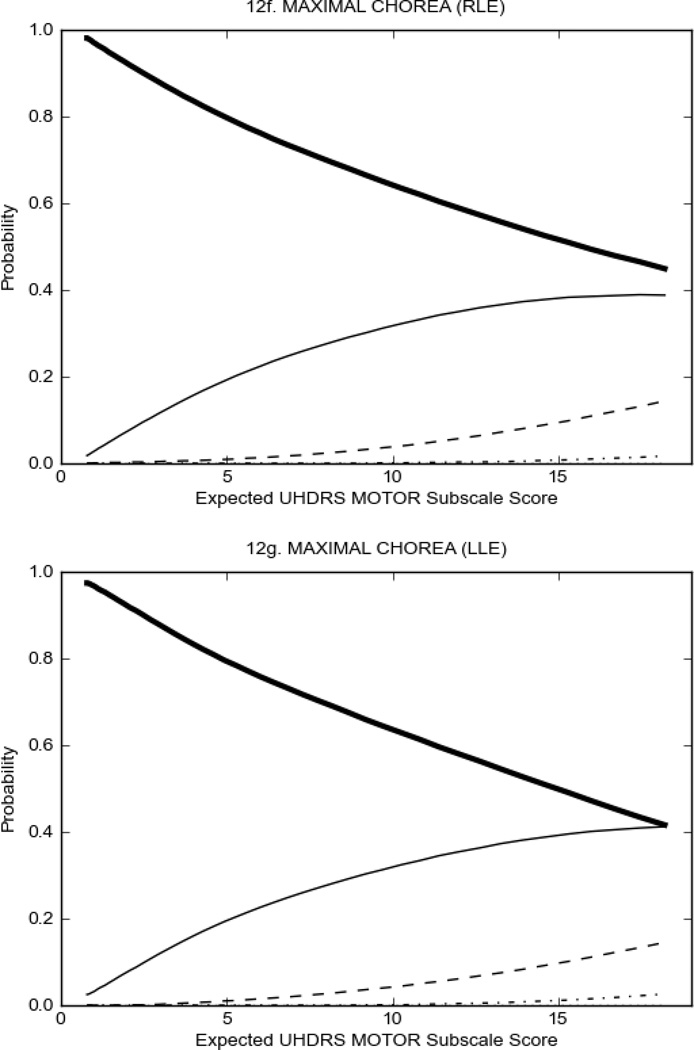
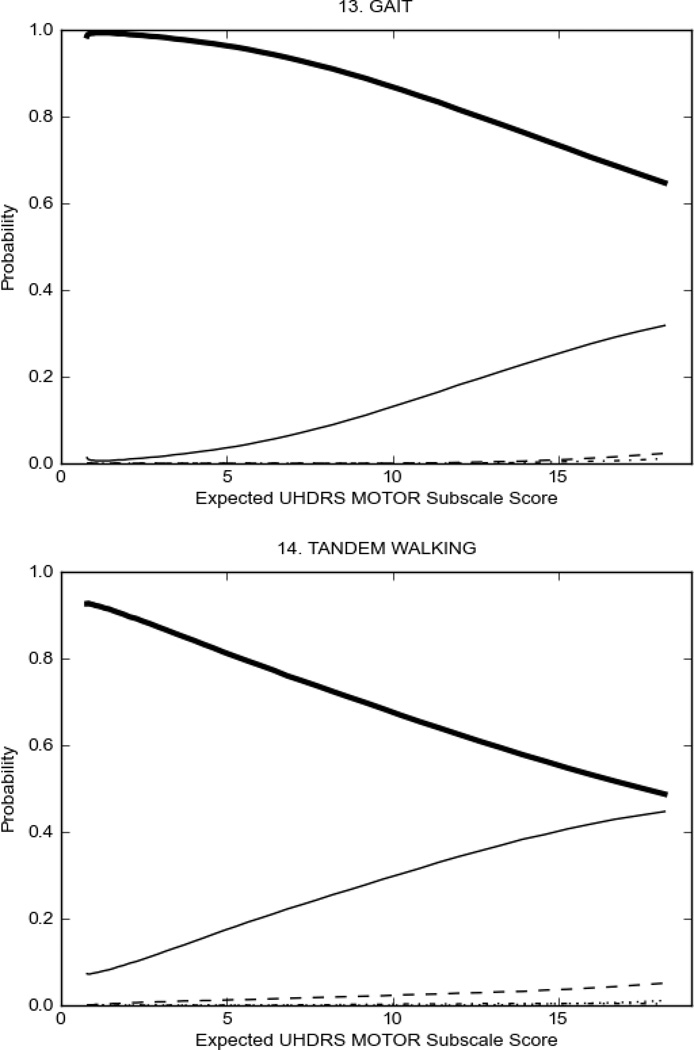
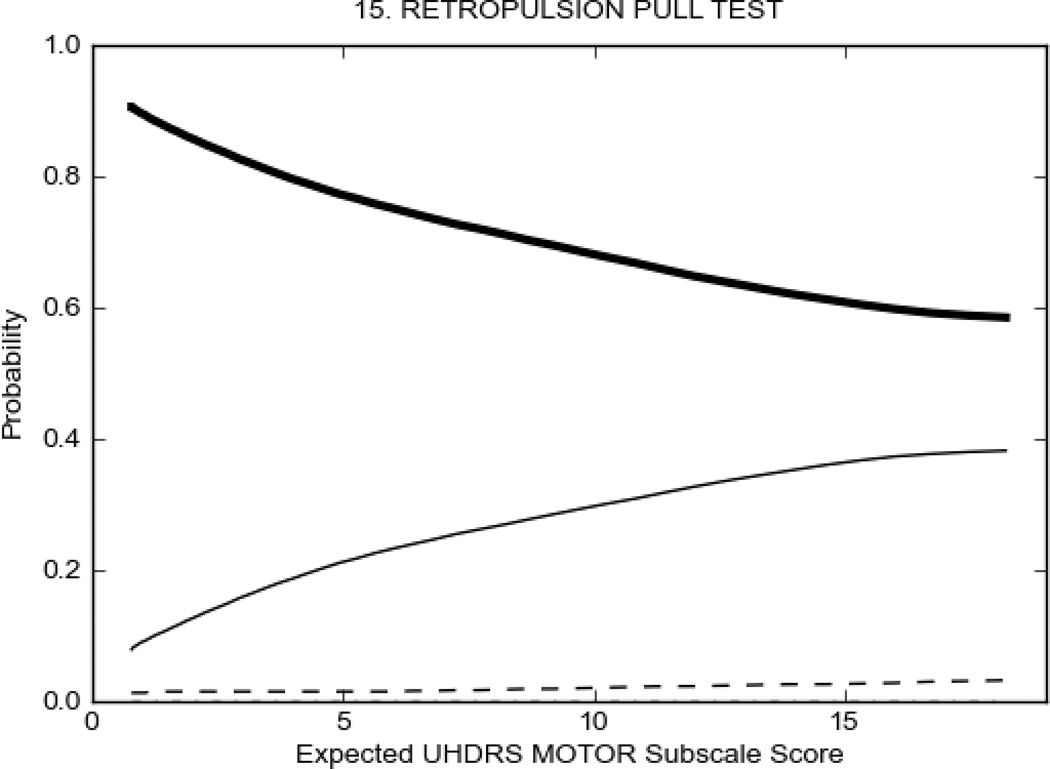
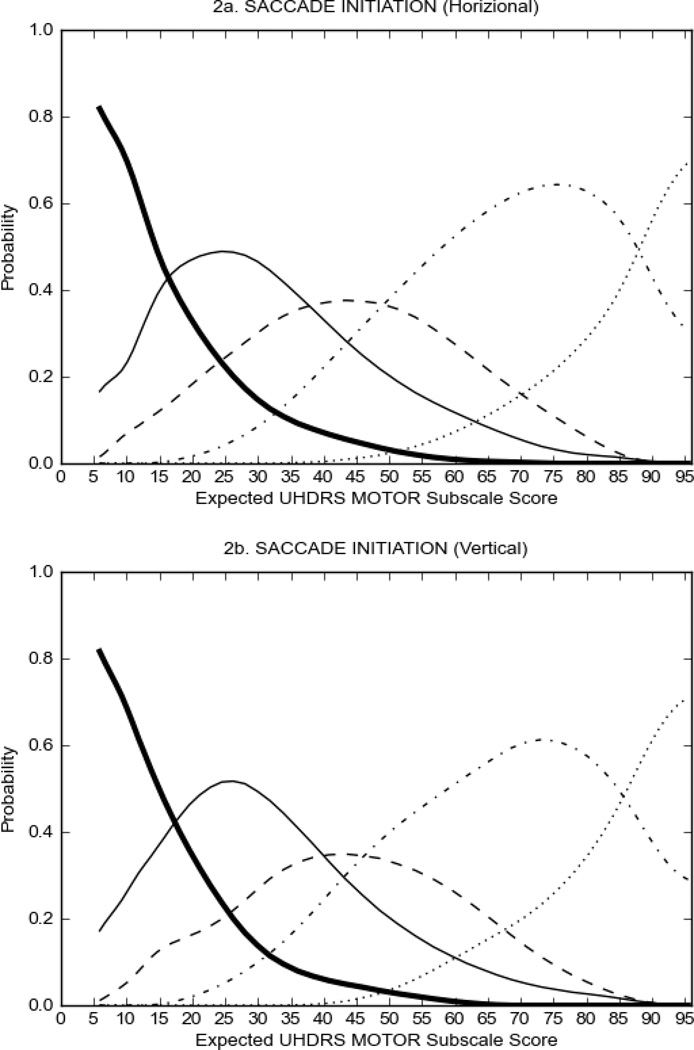
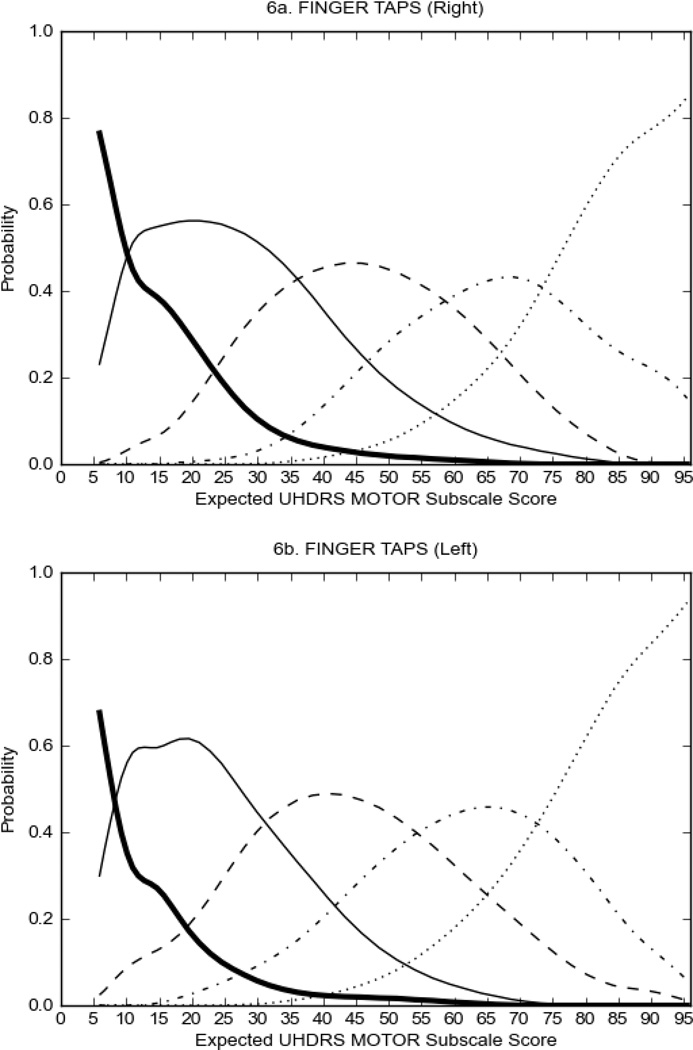
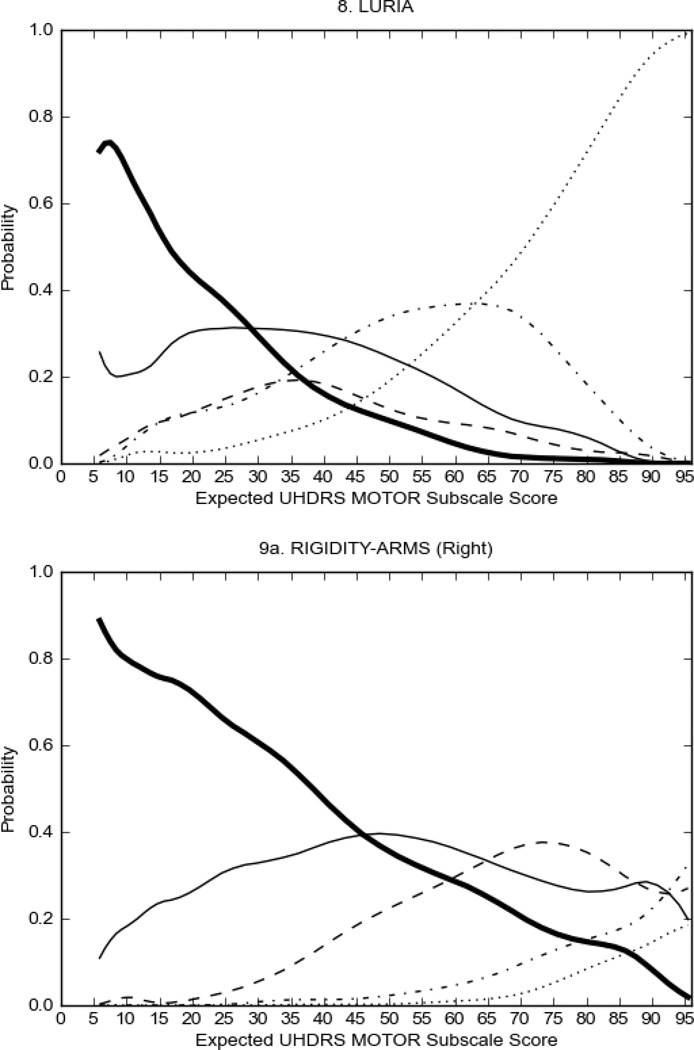
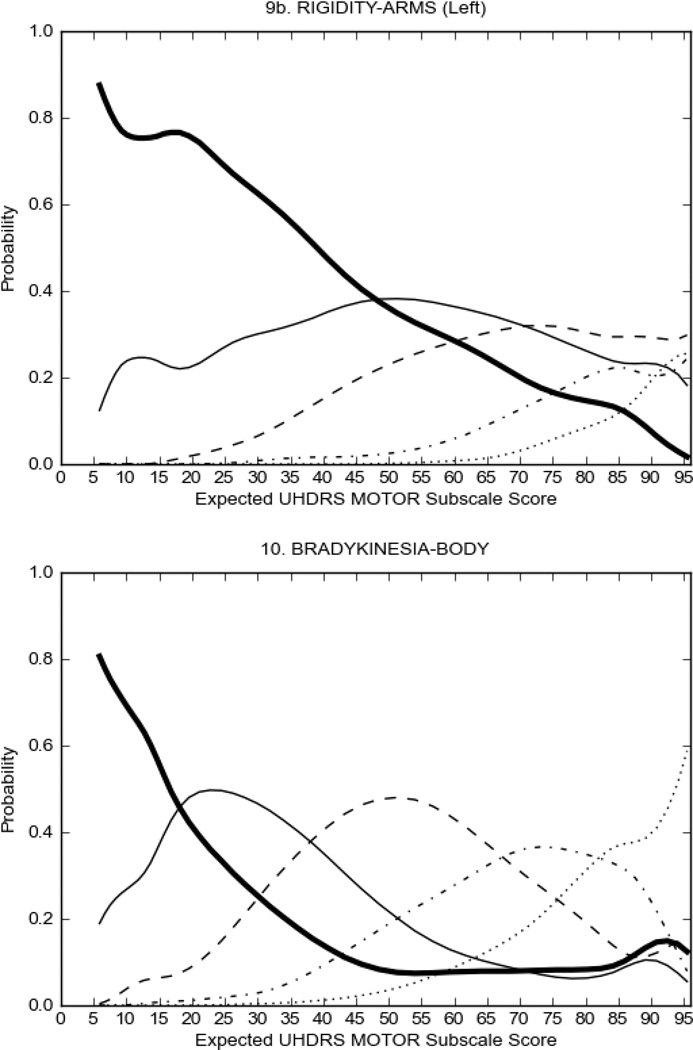
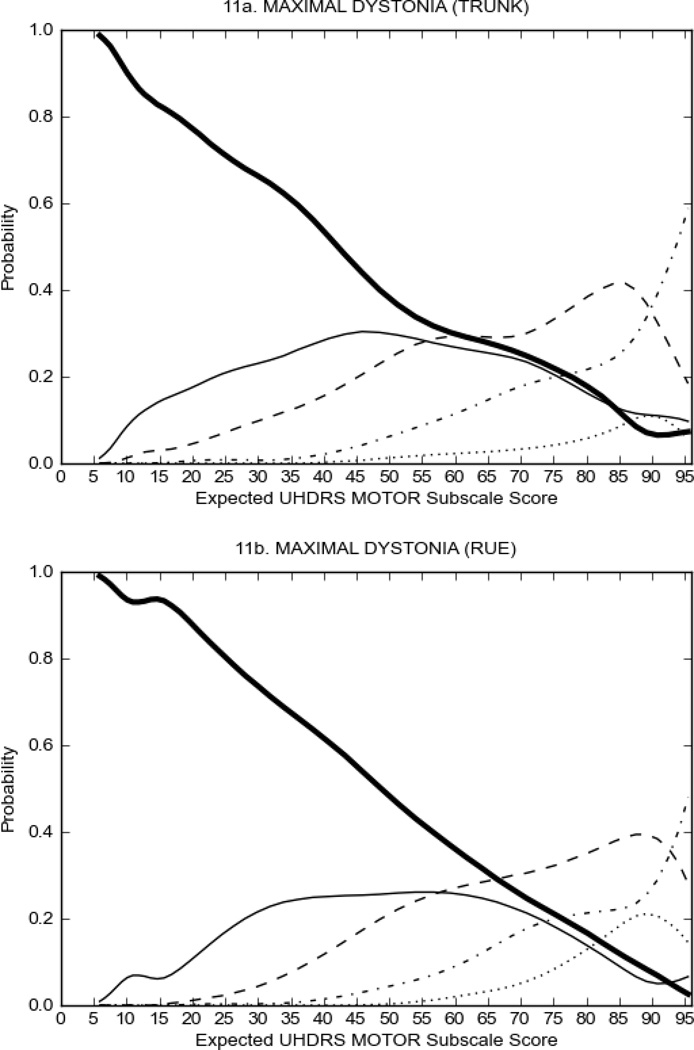
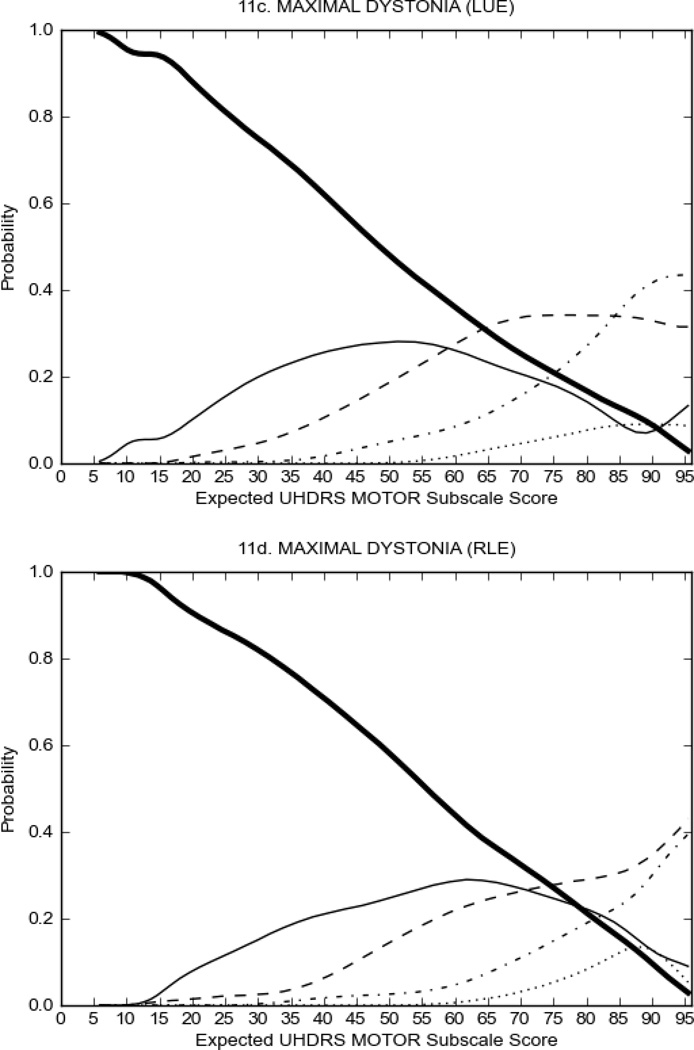
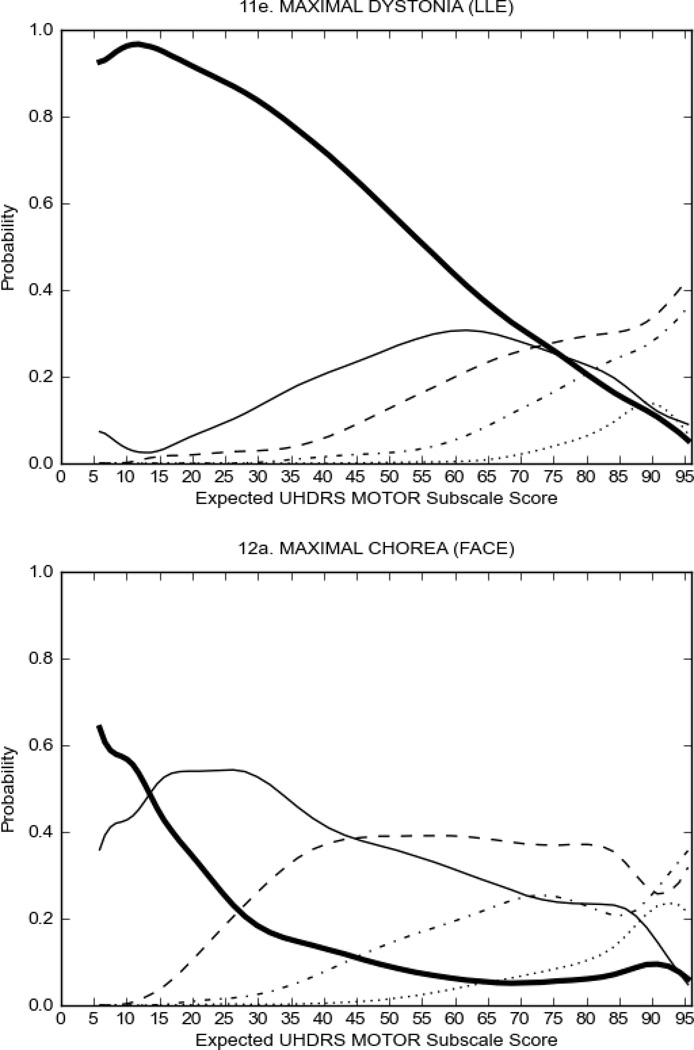
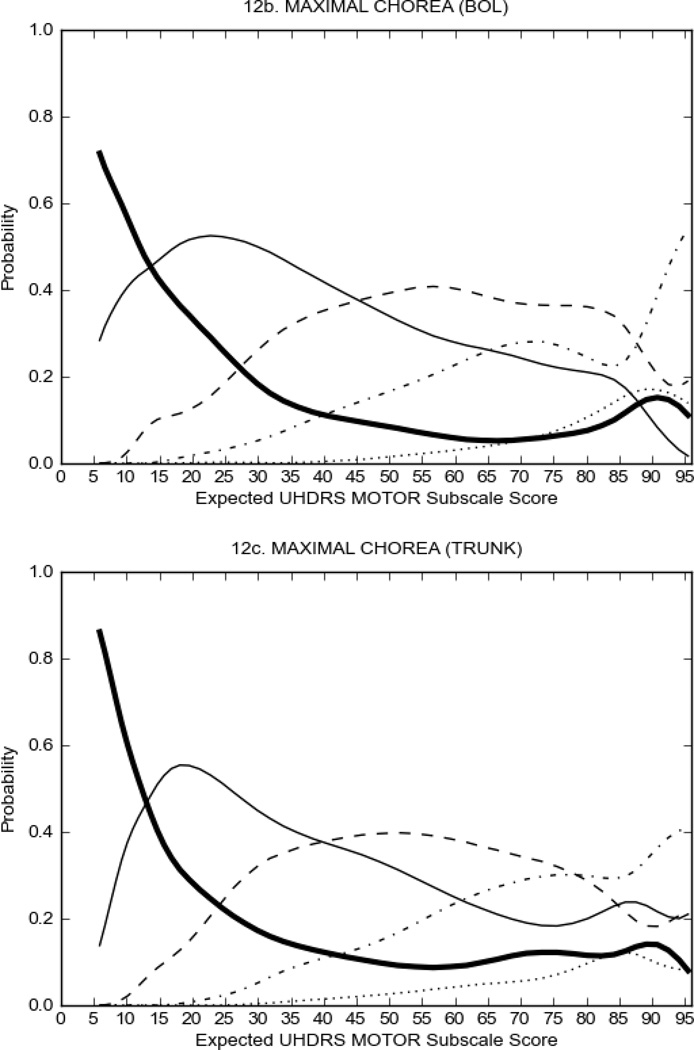
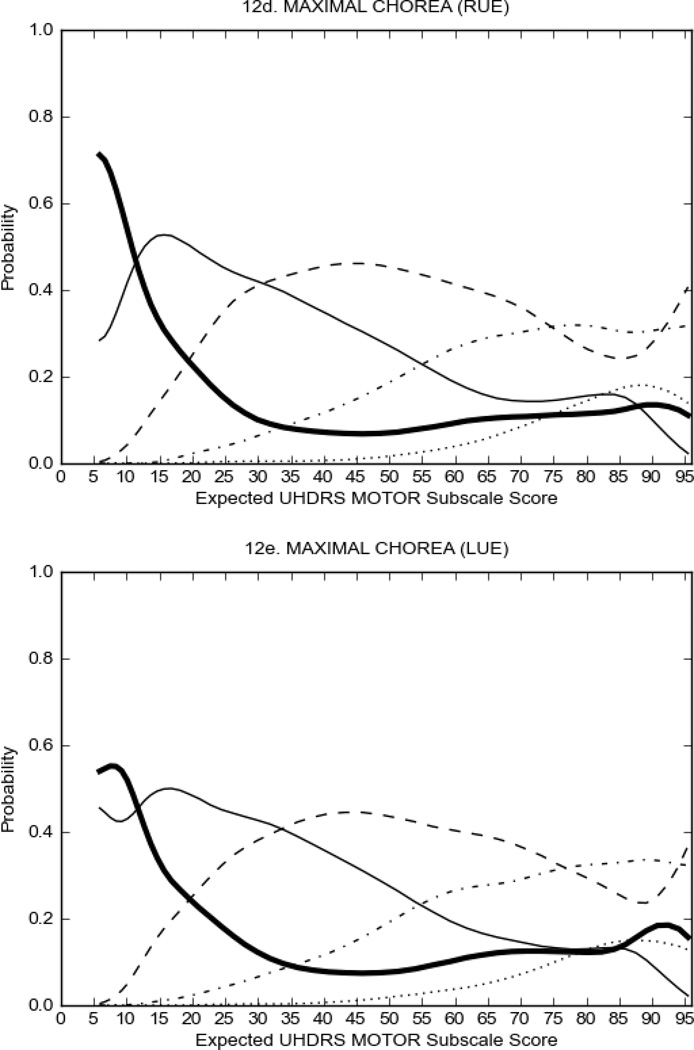
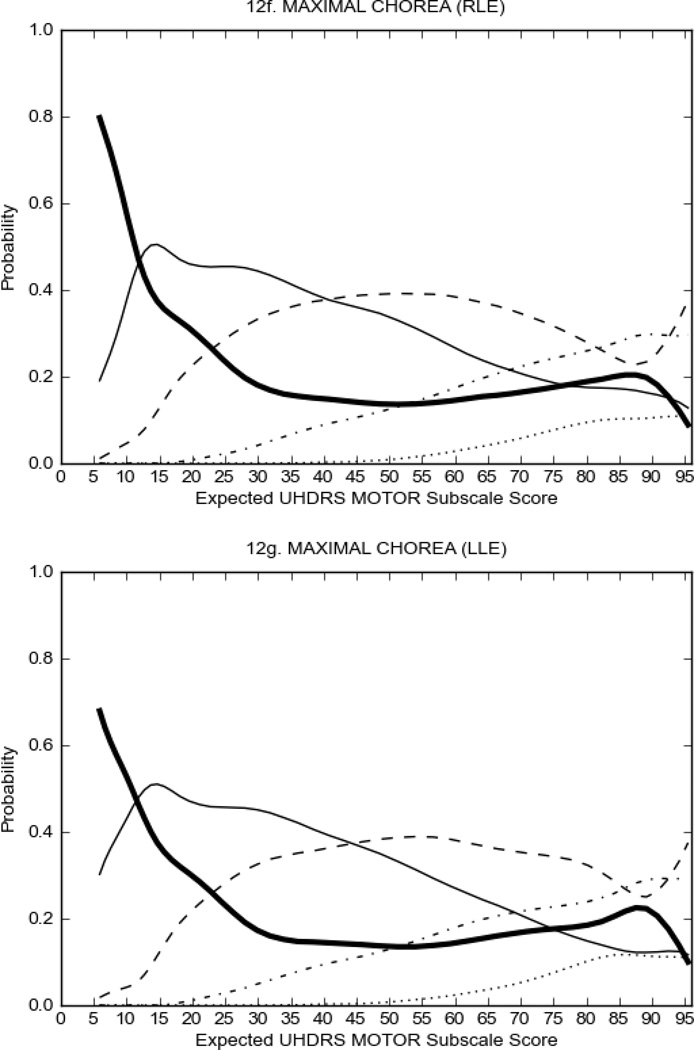
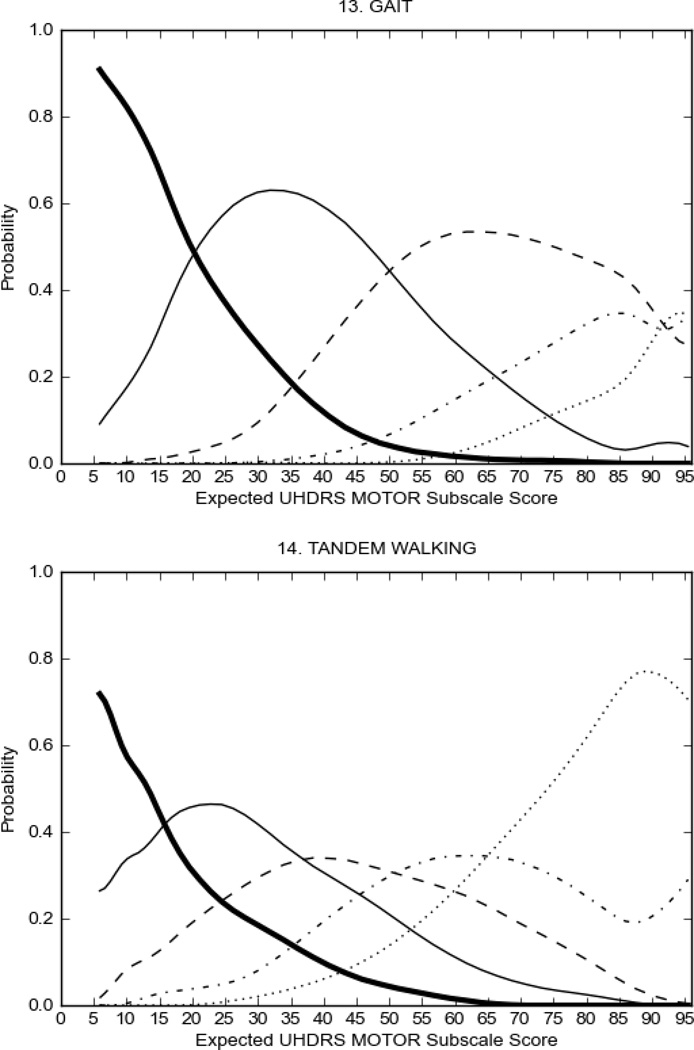
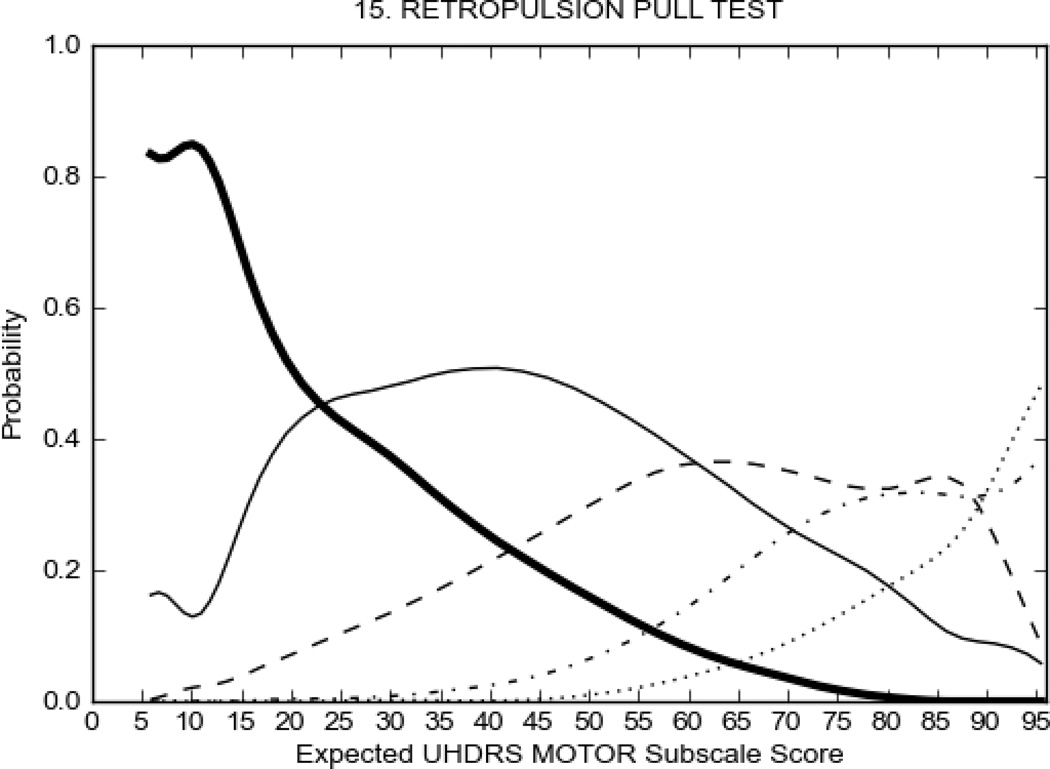
In HD participants (Figure 2), UHDRS motor items assessing ocular pursuit, saccade initiation, tandem walking and finger taps (Figure 2A) showed reasonable approximation of the “ideal” OCC, where the trait level is represented by the total UHDRS Motor Subscale Score. That is, as total Motor Subscale scores increase the probability of low scores on the individual item decrease and the probability of higher scores increase. From an IRT perspective, these items demonstrate desirable psychometric features including a clear identification of the range of severity scores over which each option is most likely scored, rapid changes in the curves which correspond to changes in severity, and an orderly relation between the weight assigned to the option and the region of severity over which an item had the highest probability of being scored.13
FIGURE 2.
OCCs for representative UHDRS motor items in relation to UHDRS Motor Subscale Score in HD participants. The remaining OCCs are available as supplemental material online.
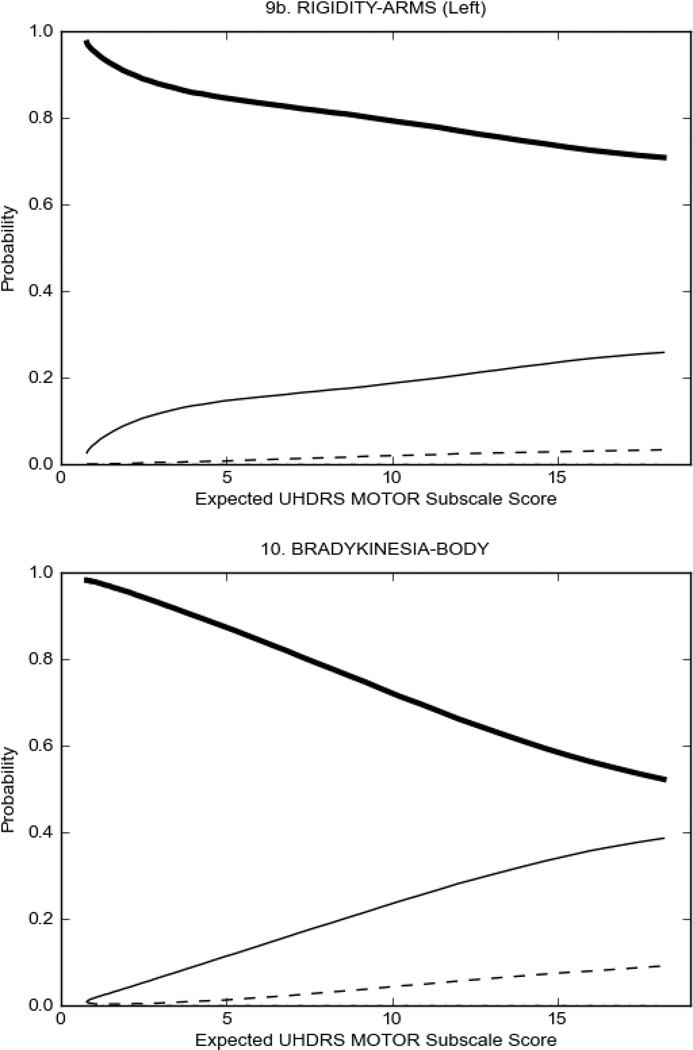
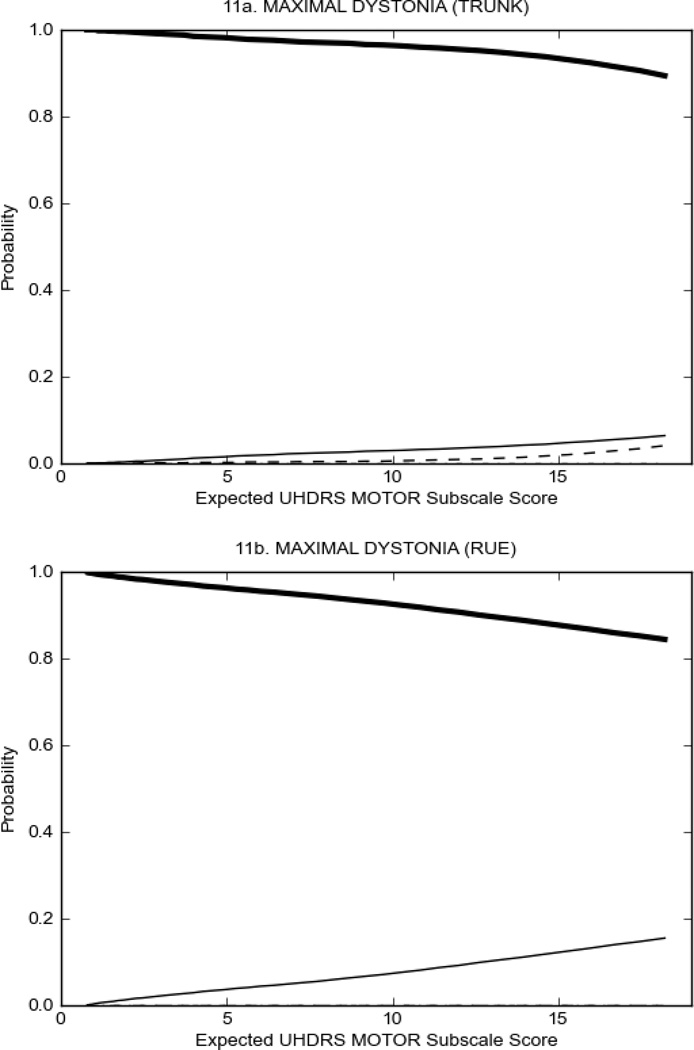
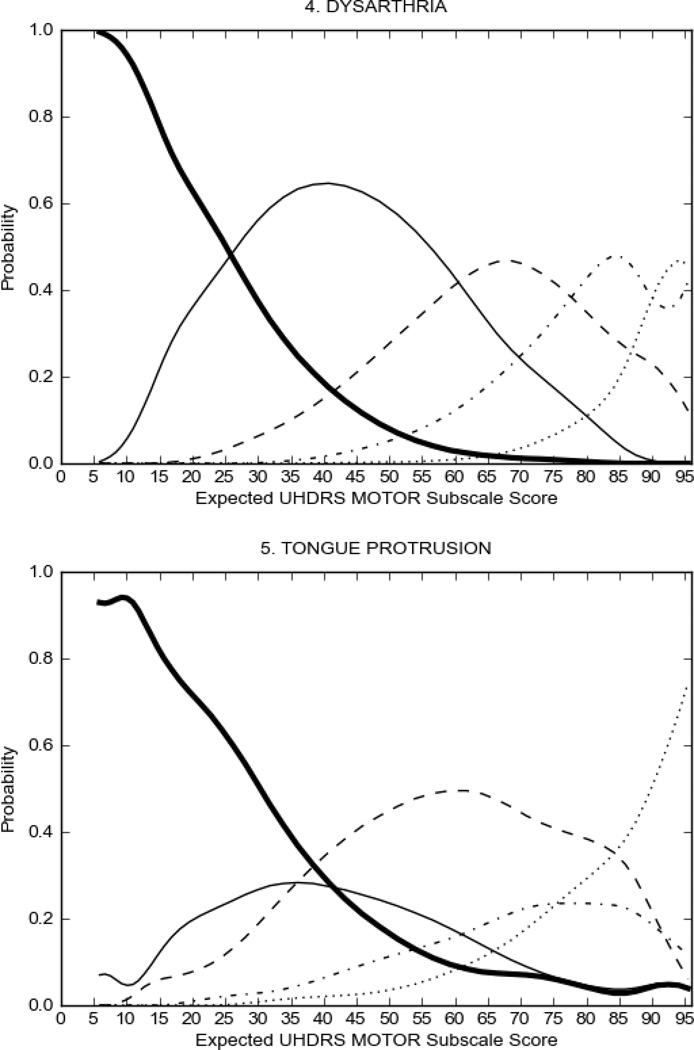
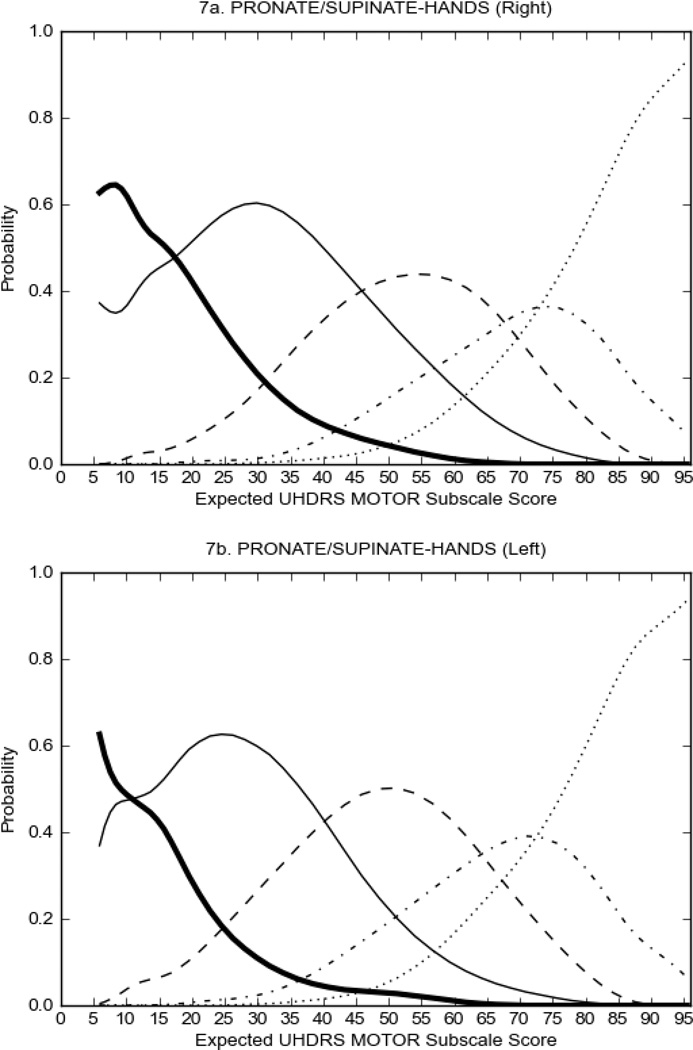
Figure 2 also shows items that display some discrimination across motor severity, but were less successful in their ability to discriminate over the full range of severity. For example, in items assessing saccade velocity, tongue protrusion and Luria (Figure 2B), the middle options had a lower probability of being scored as compared to options '0' or '4' across the severity range. For items assessing chorea, gait, and rigidity (Figure 2C), the probability of Options 3 and 4 (i.e., the most severe level of these signs) being scored was very low. As a result, at the higher severity range, the sensitivity of these items to detect changes in motor severity is reduced. It is possible that not enough participants in the population exhibited these levels of manifestation, and that these options may be endorsed with a greater frequency in a more affected population. Conversely, dystonia (Figure 2D) showed poor discriminative properties, which were manifested primarily at the highest levels of motor severity and did not discriminate well at lower motor scores.
UHDRS Behavioral Subscale in prHD Participants
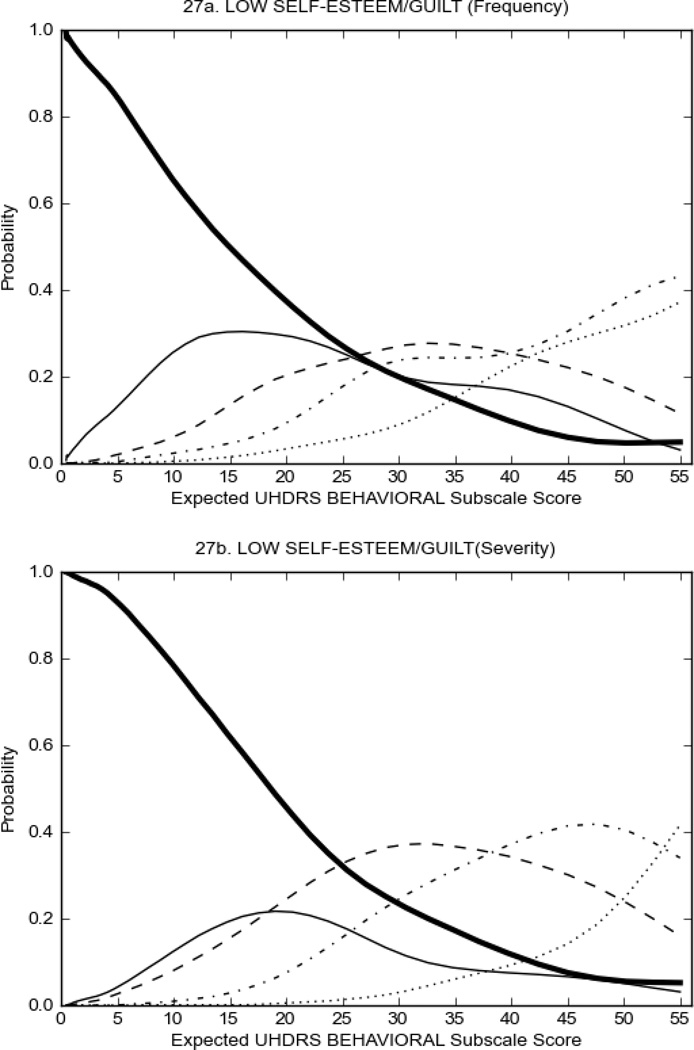
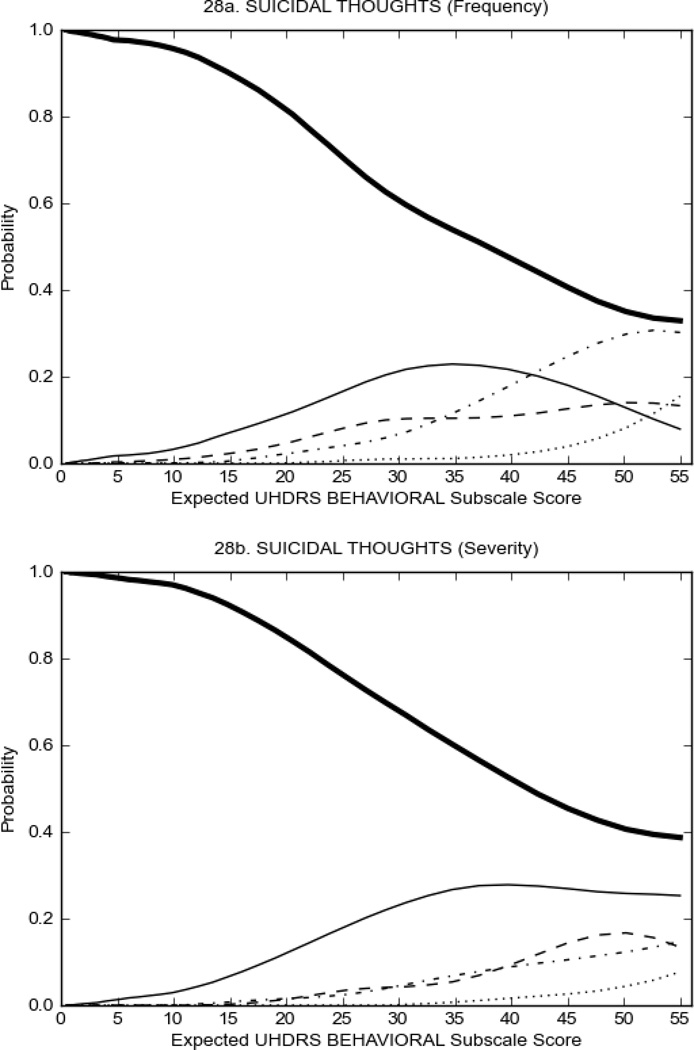
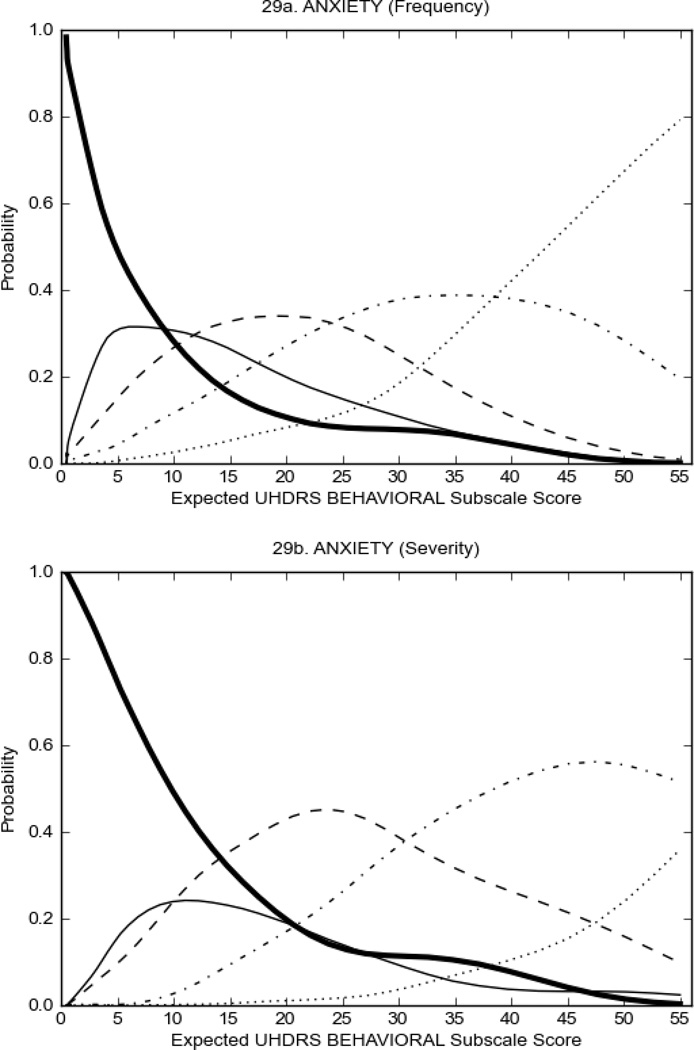
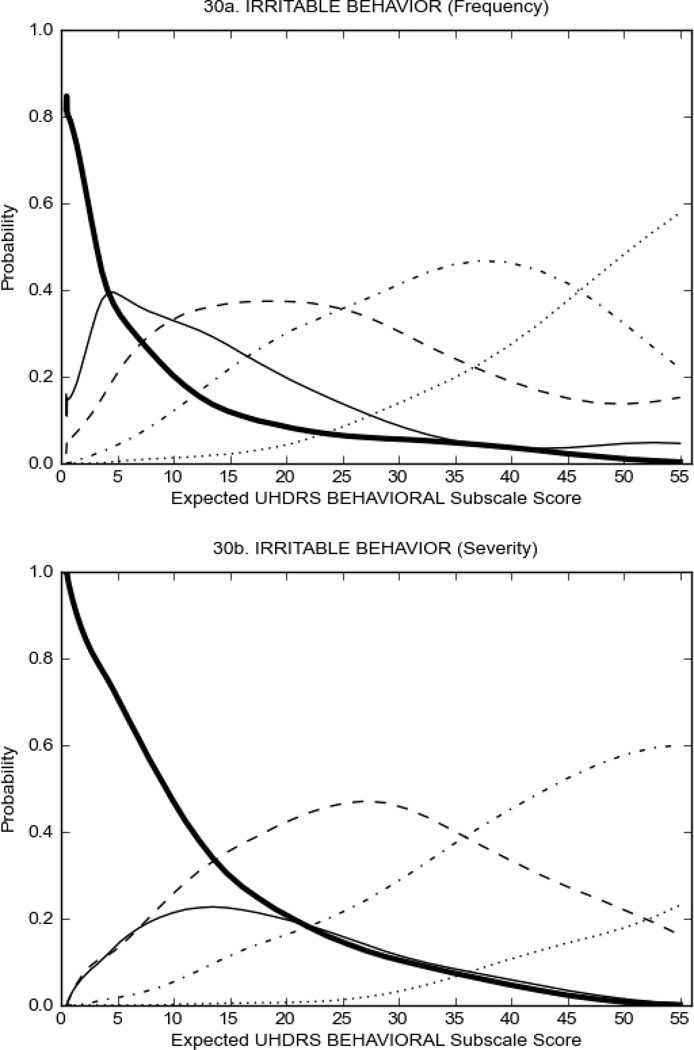
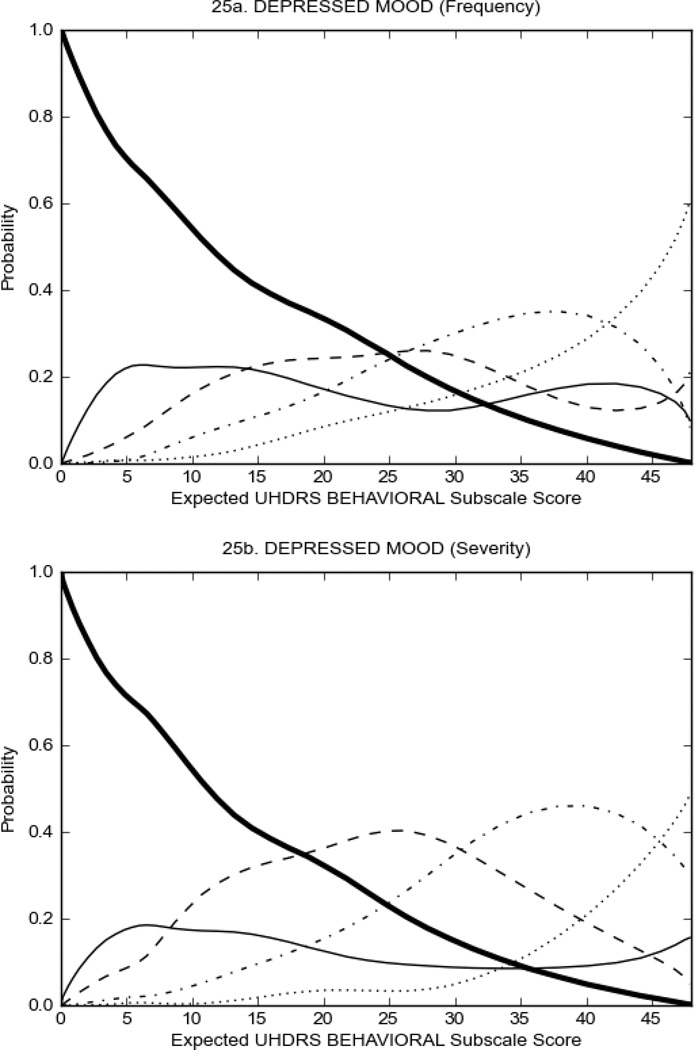
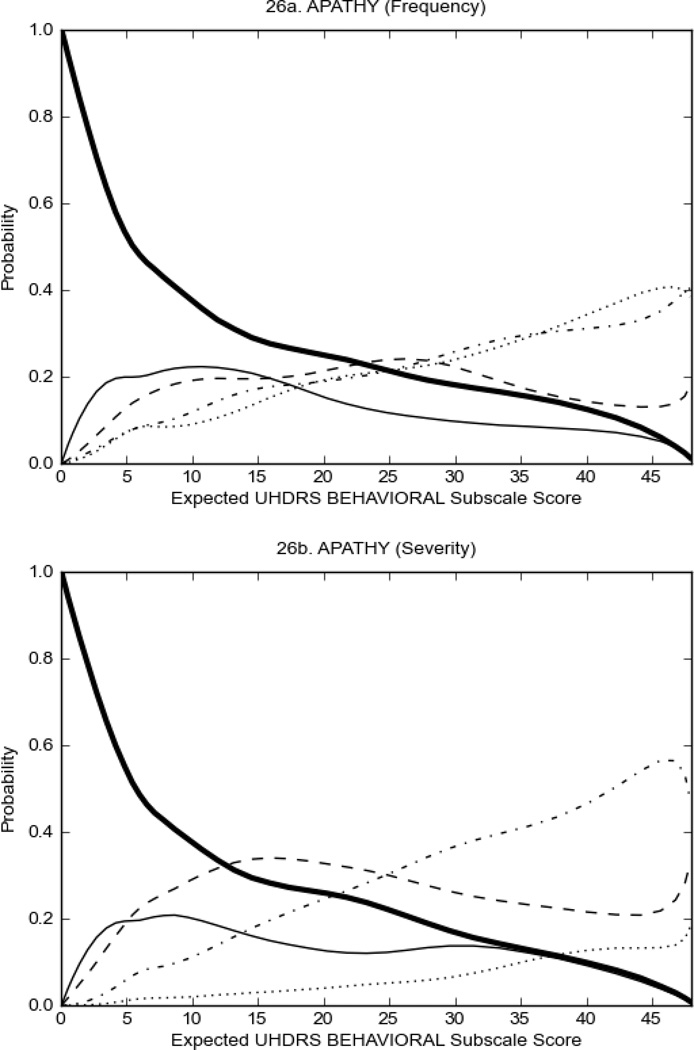
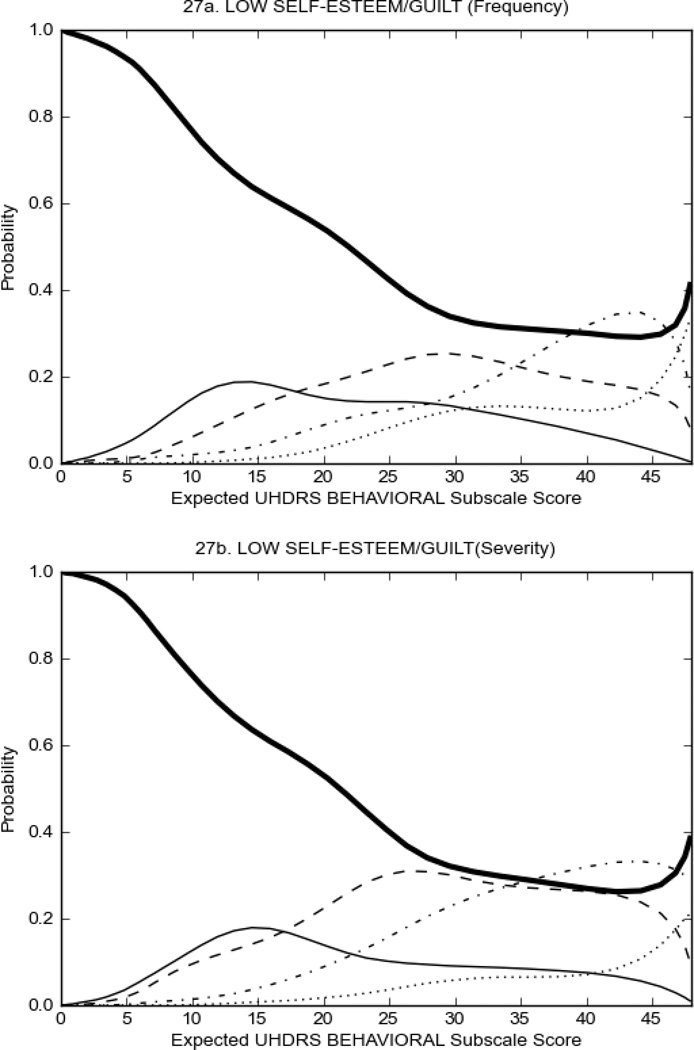
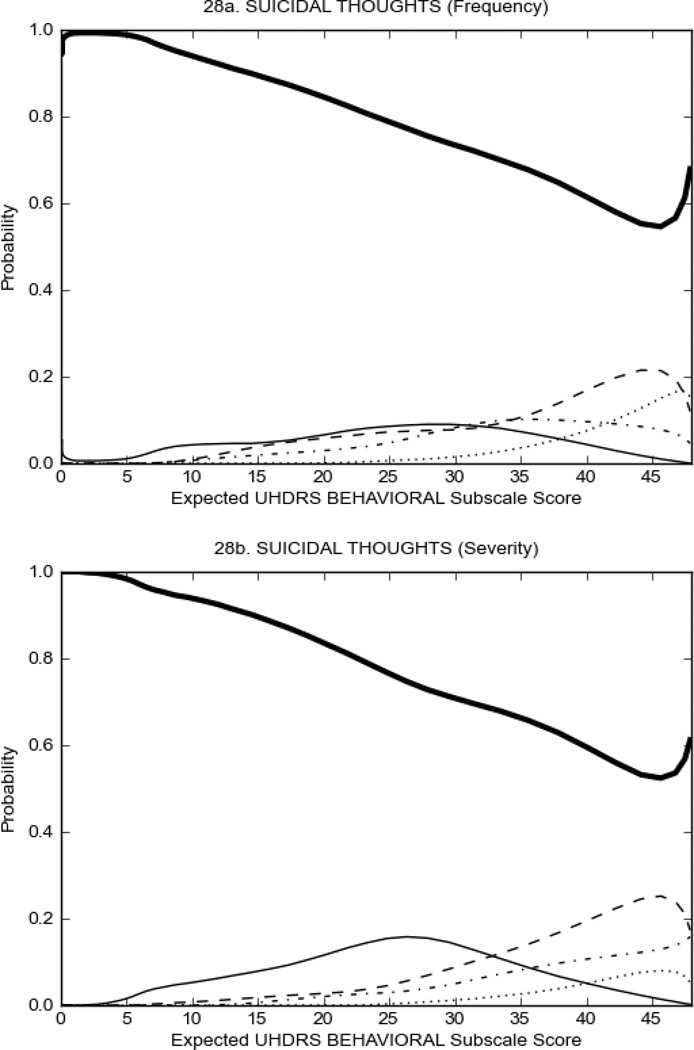
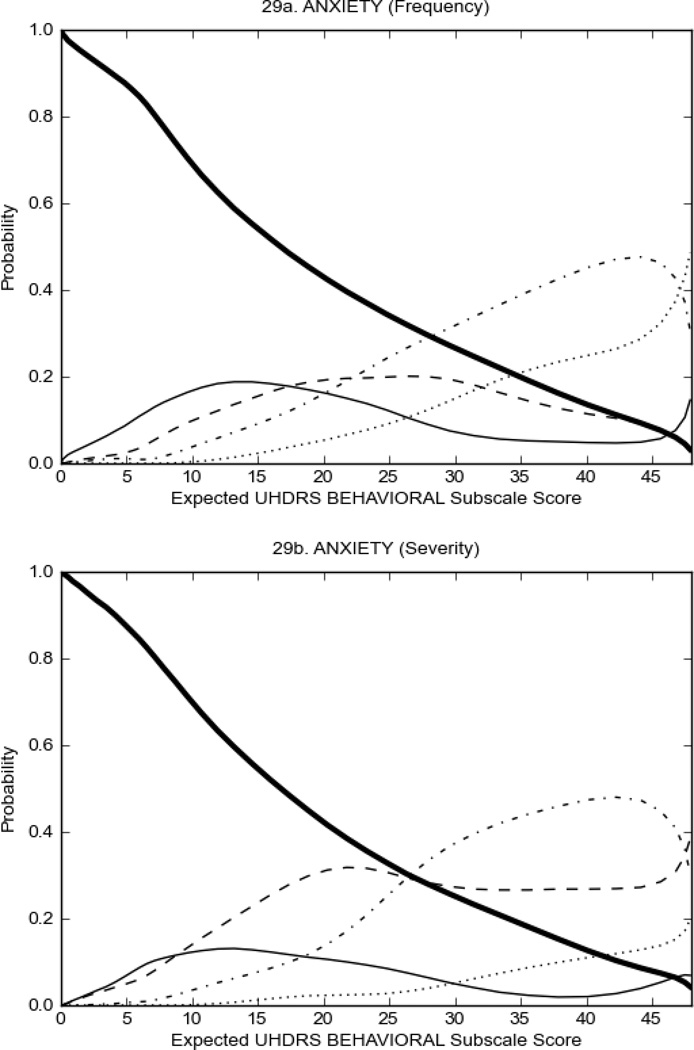
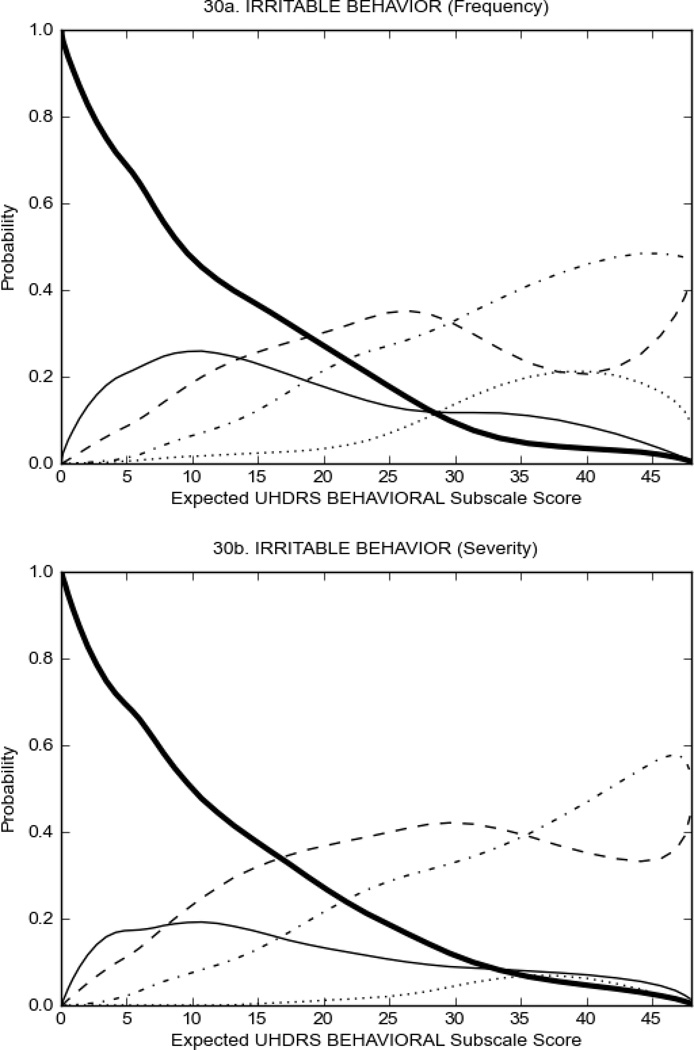
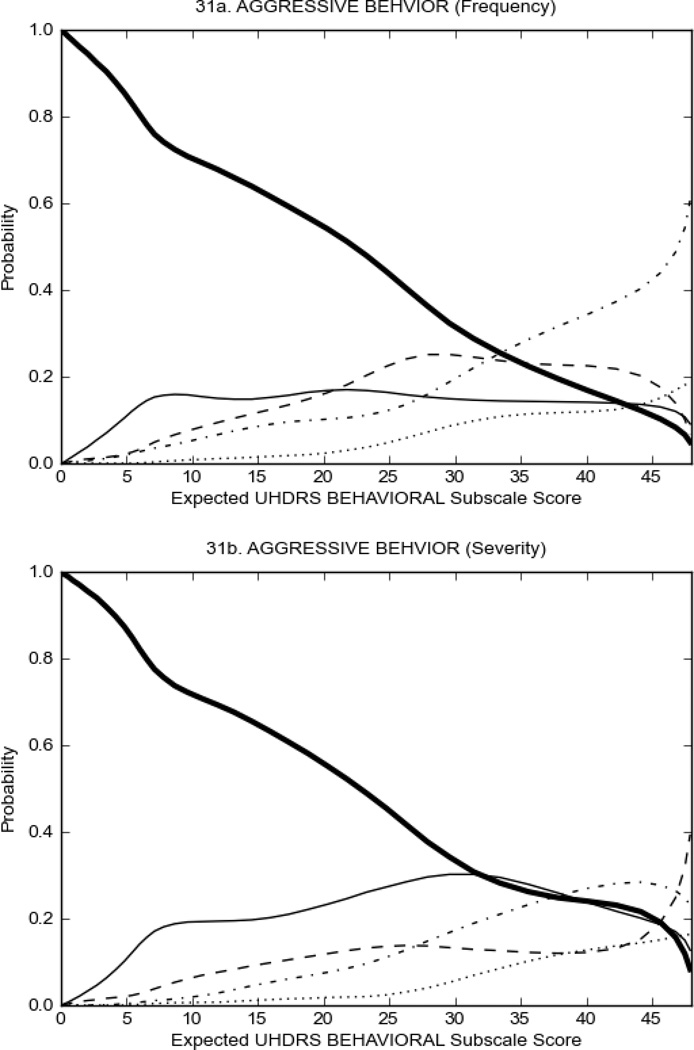
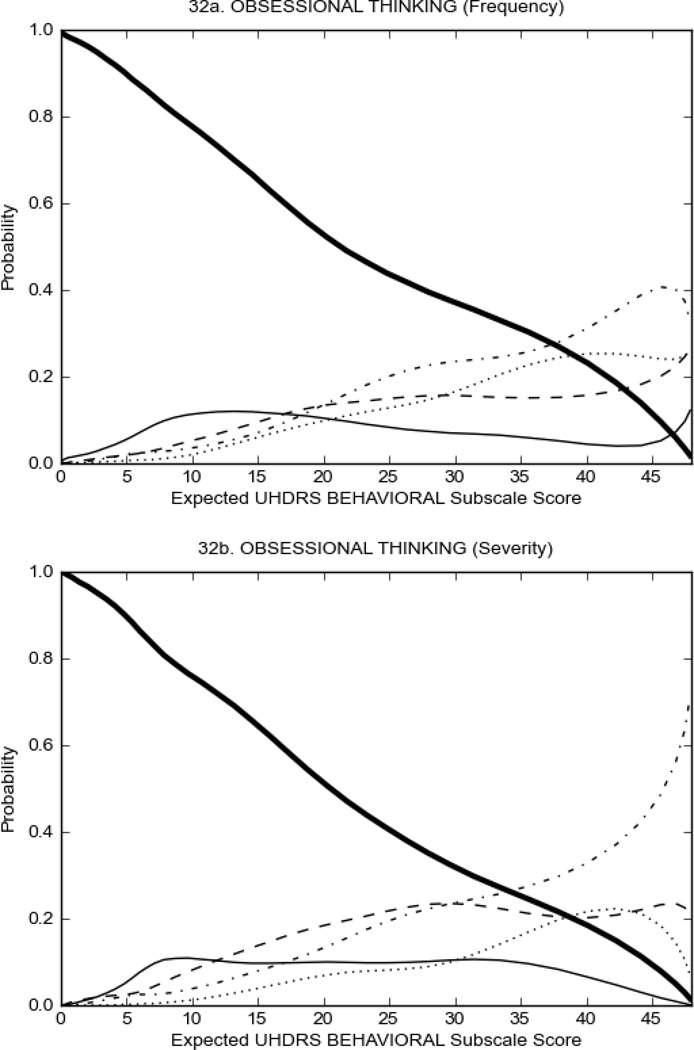
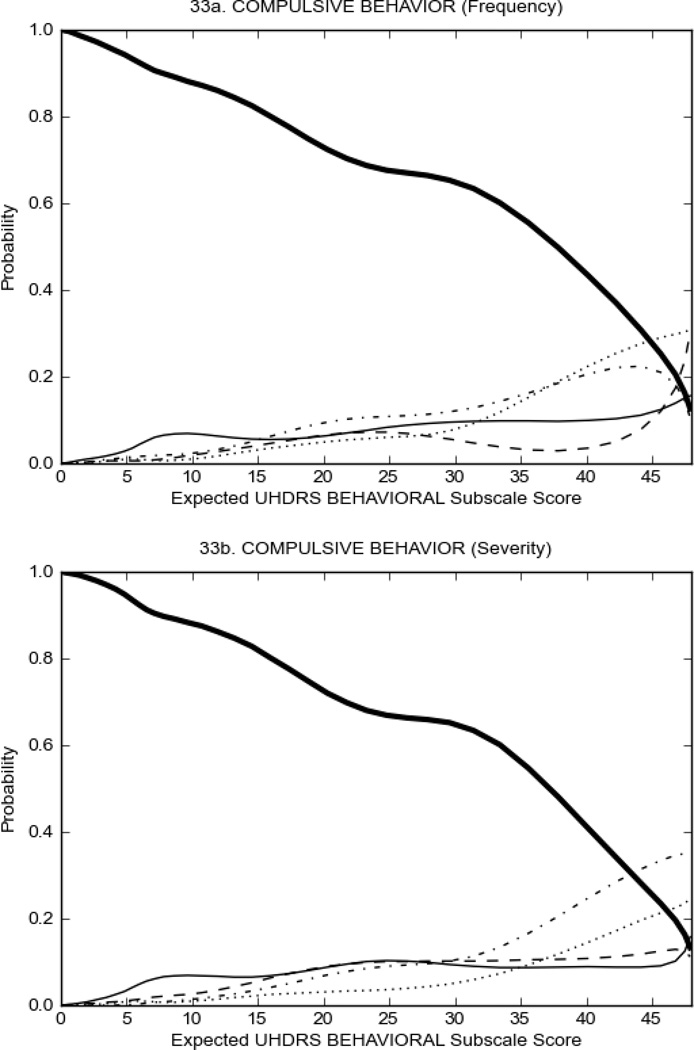
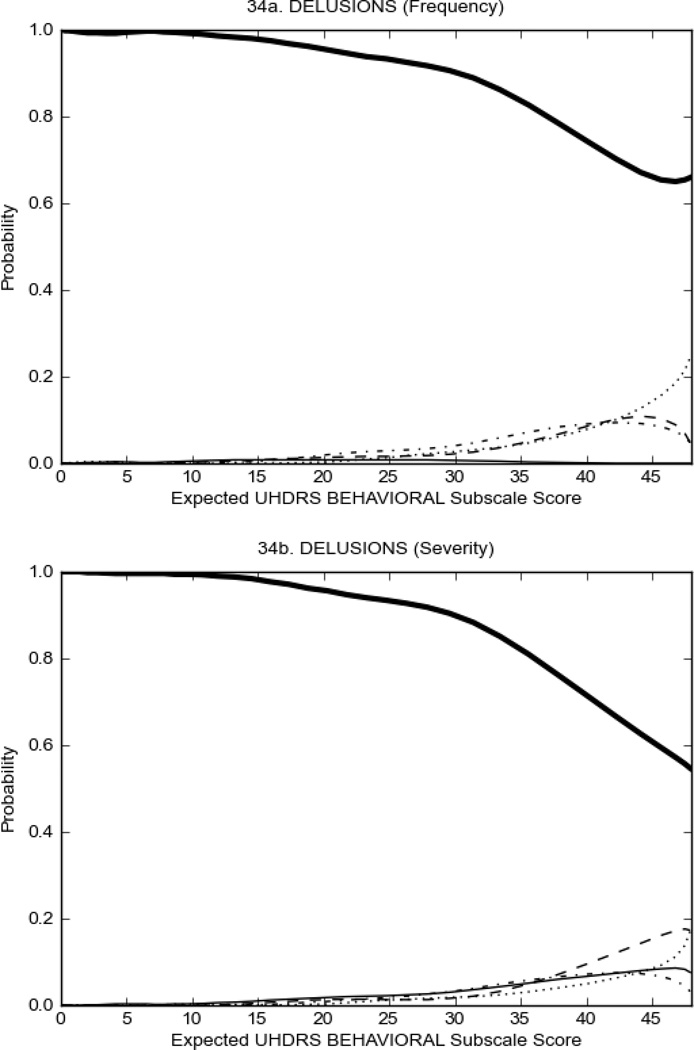
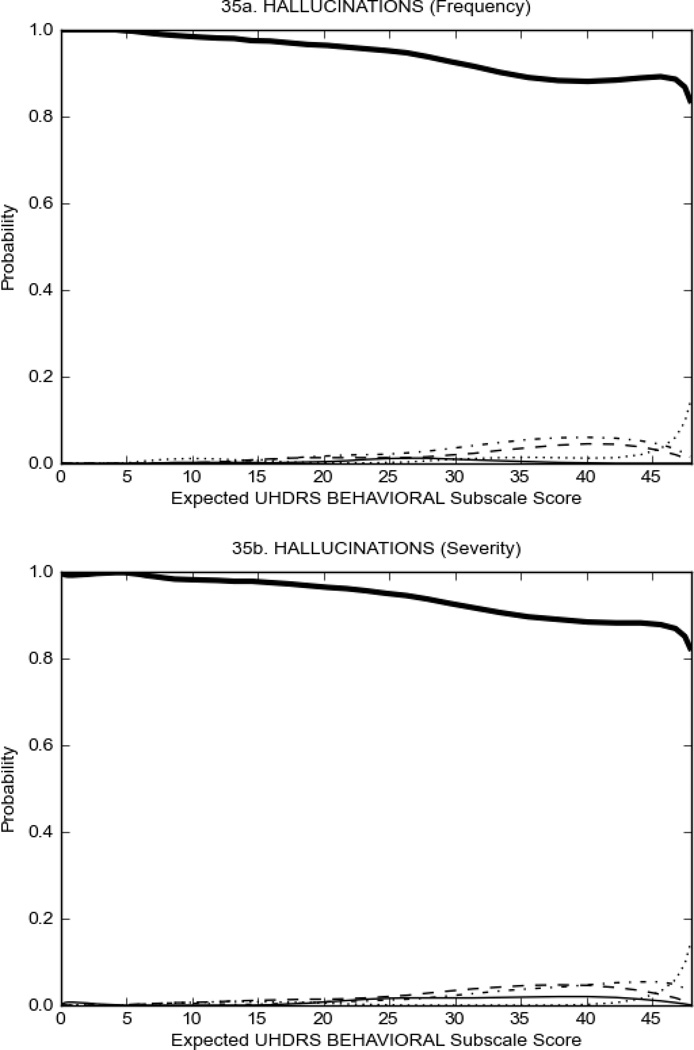
Figure 3 shows the performance of UHDRS behavioral items in relation to total Behavioral Subscale score in prHD participants. Depressed mood, anxiety, irritable behavior (Figure 3A), and to a lesser extent guilt and apathy (Figure 3B) demonstrated good discriminative properties across a broad range of severity. By contrast, aggressive behavior, compulsive behavior and obsessional thinking (Figure 3C) did not discriminate well at lower levels of severity and scores greater than '0' were only observed at higher levels of severity, indicating that these symptoms are of relevance in patients that exhibit higher levels of psychiatric disturbances. For the remaining items (suicidal thoughts, delusions, hallucinations; Figure 3D), the '0' option had the highest probability of being scored across the entire severity range, indicating that that these may not manifest in a prHD population.
FIGURE 3.
OCCs for representative UHDRS behavioral items in relation to UHDRS Behavioral Subscale Score in prHD participants. The remaining OCCs are available as supplemental material online.
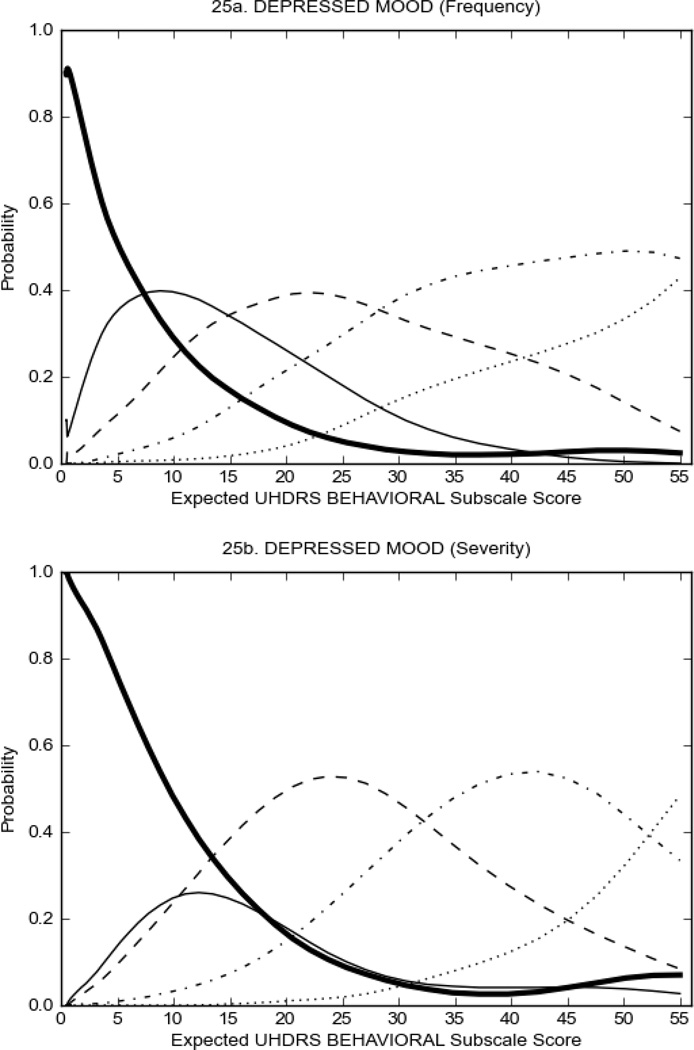
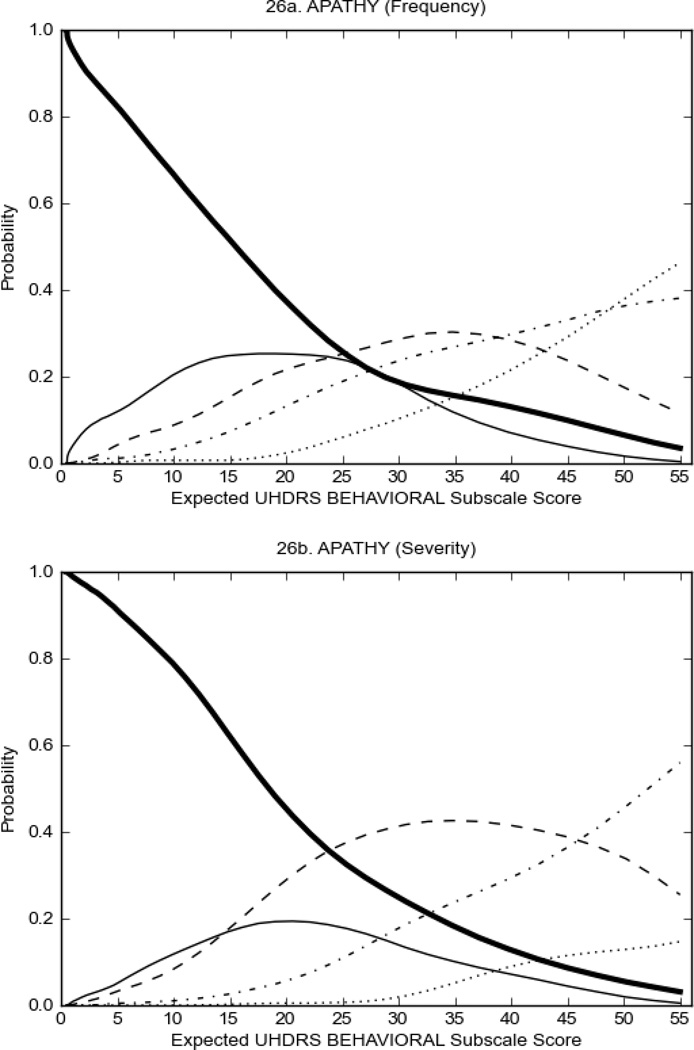
UHDRS Behavioral Subscale in HD Participants
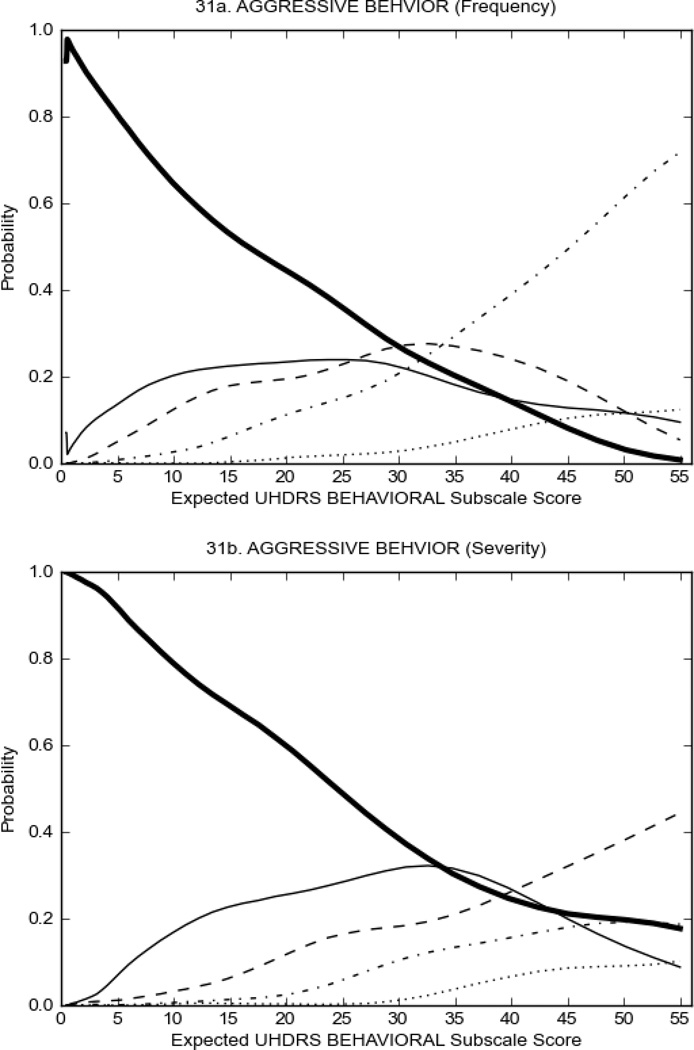
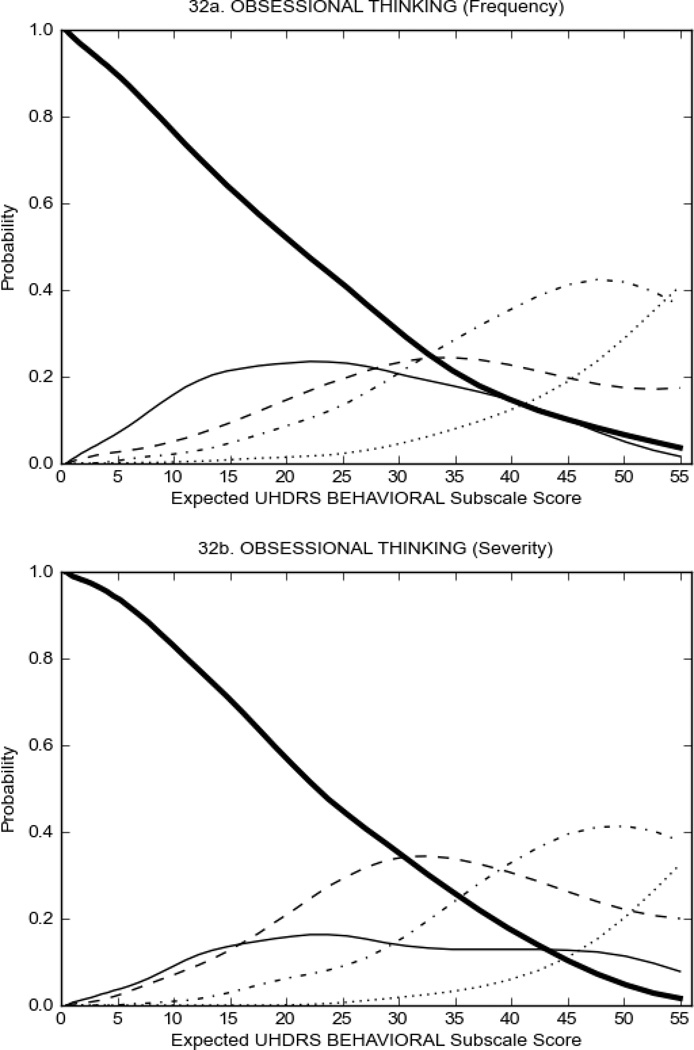
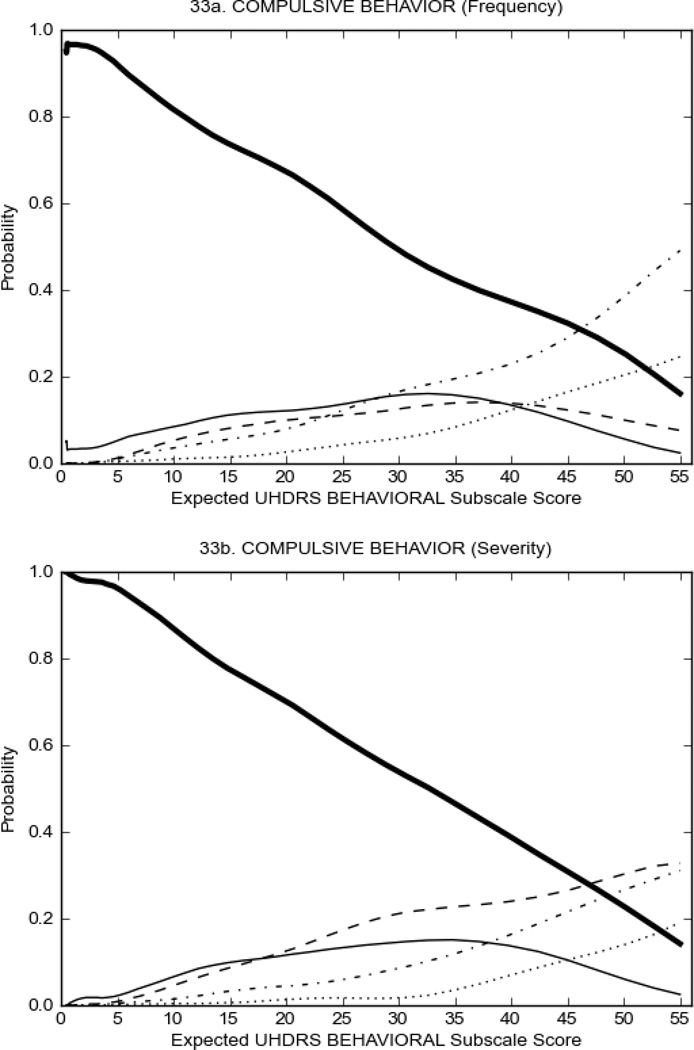
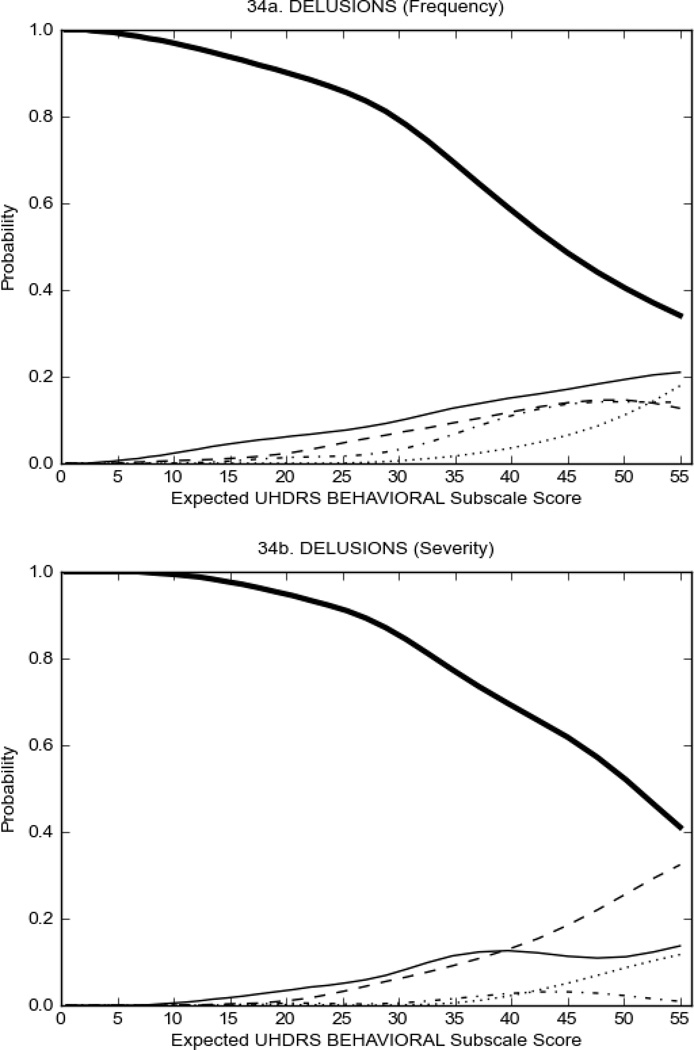
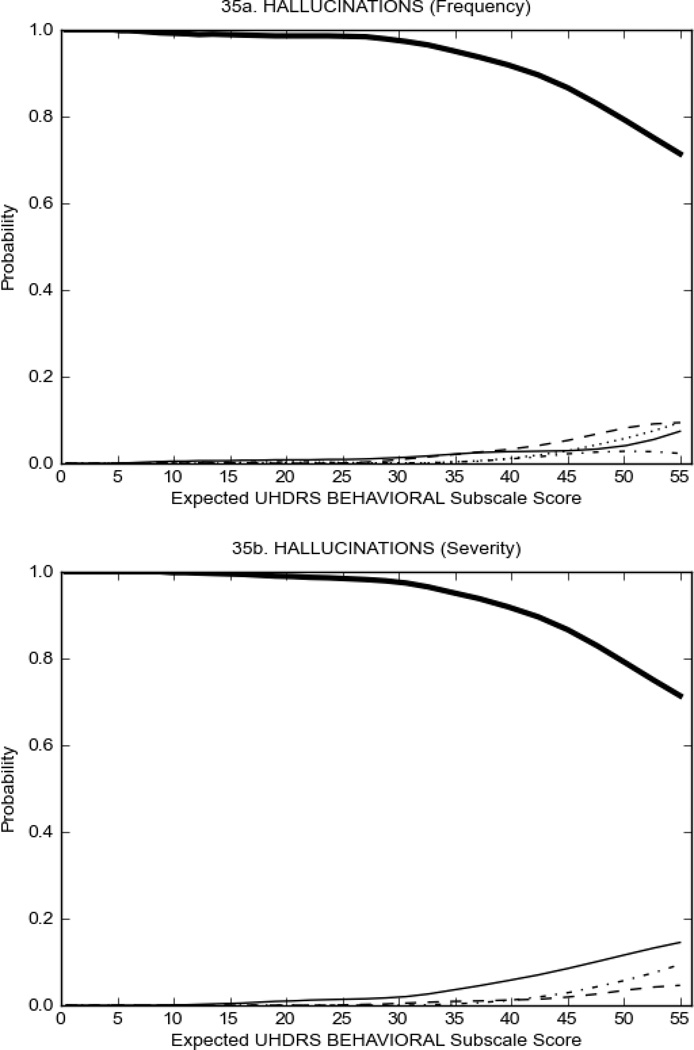
Figure 4 shows the performance of UHDRS behavioral items in relation to total Behavioral Subscale scores in HD participants. In line with previous studies, psychiatric symptoms were present in HD.2–6 There are clear differences in items of the UHDRS Behavioral Subscale with respect to their performance in discriminating different levels of severity, with depressed mood (Figure 4A) showing the best discriminative properties. The remaining items did not discriminate across levels of severity, including: items that had scoring above '0' only at the highest levels of severity (aggressive behavior, apathy; Figure 4B); items that poorly discriminated in the mid-range of scoring (guilt, anxiety, irritable behavior; Figure 4C); and items that had no scoring across most of the severity range (suicidal thought, obsessional thinking, compulsive behavior, delusions; Figure 4D).
FIGURE 4.
OCCs for representative UHDRS behavioral items in relation to UHDRS Behavioral Subscale Score in HD participants. The remaining OCCs are available as supplemental material online.
DISCUSSION
The present study assessed how well items on the UHDRS Motor and Behavioral Subscales captured scoring across disease severity in two populations: HD gene expansion carriers with and without a motor diagnosis. The total score on the Motor Subscale was significantly lower in the prHD population as compared to the HD population. This is not surprising as the classification of “prHD” is defined as the absence of sufficient motor signs to meet conventional diagnostic criteria for manifest disease.23 Examination of the UHDRS Motor Subscale in the prHD population (Figure 1) showed modest scoring on the majority items; that is, scores above 2 were rare, with a score of ‘0’ often being the one with the highest probability across the full range of motor severity. An obvious interpretation of these results is that the prHD population exhibit less severe motor manifestations than the HD population. Since the UHDRS was designed to measure motor signs in a manifest HD population, it is sub-optimal for the assessment of milder motor signs prevalent in the prHD population. It may be necessary to design new scales or develop quantitative motor devices that are more sensitive to detecting subtle changes in a prHD population that exhibit mild motor signs.24,25,26 It is important to note that although prHD and HD stratifications in the present analyses were based on UHDRS Diagnostic Confidence Level scores, the groups may also have differed with respect to CAG repeat length and disease burden scores (see Table 1). However, because only group means were provided for CAG repeat length, the contribution of CAG repeat length to differences between prHD and HD could not be determined.
Examination of the UHDRS Motor Subscale in the HD population (Figure 2) revealed that a number of items (e.g., ocular pursuit, saccade initiation, finger taps, tandem walking and to a lesser extent saccade velocity, dysarthria, tongue protrusion, pronation/supination, Luria, bradykinesia, chorea, gait, and retropulsion) showed favorable item response characteristics, thus supporting their utility in assessing motor signs in HD. Other items (e.g., dystonias and rigidity of the arms), however, did not discriminate individual differences over the full range of severity and contain options that do not track with changes in overall motor severity. Although the UHDRS appears to provide useful information about the status of a given HD patient, the scale could nevertheless be improved to address these issues. It may be possible to alter the wording and scoring options of these items to improve item performance. There were, however, some items (e.g., the dystonia items) where the '0' option had the highest probability of being scored across almost the full range of severity, indicating that these items are not sensitive to changes in disease and appear to be less relevant for motor assessment in HD. This latter issue suggests that the UHDRS itself could be refined by removing irrelevant or redundant items resulting in increased sensitivity and reduced noise.26,27 It is also important to note that the motor phenotype in HD is not homogenous and, although the phenotype that is characterized by prominent dystonia may be present in only a subset of participants,28 it is none-the-less an important feature to assess when this phenotype needs to be captured.
It is well-established that in addition to motor manifestations, mood and other psychiatric disturbances are also present in HD2–6 and reported to be functionally debilitating.29,30 In the present study, the total Behavioral Frequency Subscale score did not vary substantially between the prHD and HD population (prHD = 6.67 ± 0.13; HD = 6.32 ± 0.18; n.s.), whereas the total Behavioral Severity Subscale score was higher in HD than prHD (prHD = 4.81 ± 0.12; HD = 5.97 ± 0.16; p<0.01) (Table 1). It is possible, therefore, that while motor signs progress from prHD to HD, the frequency of behavioral manifestations do not progress or may in fact be under-reported due to decreased self-awareness of behavioral manifestations.31 On the other hand, the severity of the behavioral manifestations are greater in HD than prHD; indications that the functional impact of these manifestations are more pronounced in HD than in prHD. Interestingly, when comparing prHD and HD participants, some items on the UHDRS Behavioral Subscale were generally less problematic in prHD in discriminating across ranges of severity. The reason for these discrepancies are not clear but may be related to the presence of motor manifestations in HD which could overshadow the ability to assess behavioral symptoms and thus does not adequately capture the mood and psychiatric disturbances that may occur in HD.
It is important to note that even though some items may not perform well from an IRT perspective, their assessment from a clinical perspective is none-the-less important and may provide critical clinical information. For example, although a high proportion of participants did not endorse the UHDRS Suicidal Thoughts Item and the '0' option had the highest probability of being scored, 8.3% of prHD and 9.4% of HD participants reported frequency of Suicidal Thoughts with scores of 2 or more (i.e., thoughts about suicide at least once a month or more often). Indeed, elevated rates of suicidal behavior in HD is a significant concern32,33 and thus it is important to be aware of those that may be at an increased risk; including regulatory requirements that suicidality be closely monitored during clinical trials.34
To examine motor and behavioral manifestations in gene expansion carriers, we used an IRT approach to assess the discriminative properties of individual items on the UHDRS Motor and Behavioral Subscales in prHD and HD participants using retrospective data from two ongoing multicentre, longitudinal observational research studies. Although there is the possibility that some (albeit relatively few) of the participants may have participated in both PREDICT-HD and REGISTRY or where from the same families that may have similar behavioral and motoric profiles, there was no way of identifying those subjects based on the de-indentified data that was provided. However, given the large sample sizes used in the analyses it is unlikely that these individuals would have systematically biased the results. Further, it is important to note that comparisons were made between scores generated and stratified based on UHDRS Diagnostic Confidence Level scores. As such, none of the scores that contributed to the data were duplicates and were treated as independent data points.
The cohorts used these analyses are out-patient ambulatory participants and thus limiting conclusions with respect to more advanced disease and/or higher severity of motor signs. These participants, however, are likely representative of those that would participate in clinical trials. Also worth noting is that that some pharmacological treatments are effective in treatment of psychiatric and motor problems in HD35 and thus may mask the prevalence of these manifestations in the PREDICT and REGISTRY participants. Nevertheless, the present results suggest that the UHDRS will likely perform sub-optimally in the measurement of treatment-induced changes in this population and provide useful information about the performance of specific UHDRS items in prHD and how their assessment might be improved. The results suggest that some UHDRS items appear to have utility across a broad range of disease severity; although many items demonstrate problematic features and some issues remain with regard to the use of individual UHDRS items as measures of severity in these populations. In addition, these analyses highlight the advantages of using existing data bases to improve assessment and measurement. As part of the FuRST-pHD, we are using this approach to help identify and develop items to better assess changes experienced in HD.
Acknowledgments
CHDI Foundation, Inc. – a not-for-profit research organisation whose mission is to rapidly and collaboratively discover and develop therapies that slow the progression of Huntington’s disease – initiated and sponsored the development of the FuRST-pHD.
APPENDIX
Investigators and Coordinators
PREDICT-HD: Peg Nopoulos, MD, Robert Rodnitzky, MD, Ergun Uc, MD, BA, Leigh J. Beglinger, PhD, Vincent A. Magnotta, PhD, Stephen Cross, BA, Nicholas Doucette, BA, Andrew Juhl, BS, Jessica Schumacher, BA, Mycah Kimble, BA, Pat Ryan, MS, MA, Jessica Wood, MD, PhD, Eric Epping, MD, PhD, Thomas Wassink, MD, and Teri Thomsen, MD (University of Iowa Hospitals and Clinics, Iowa City, Iowa, USA); David Ames, MD, Edmond Chiu, MD, Phyllis Chua, MD, Olga Yastrubetskaya, PhD, Joy Preston, Anita Goh, D.Psych, and Angela Komiti, BS, MA (The University of Melbourne, Kew, Victoria, Australia); Lynn Raymond, MD, PhD, Rachelle Dar Santos, BSc, Joji Decolongon, MSC, and David Weir, BSc (University of British Columbia, Vancouver, British Columbia, Canada); Adam Rosenblatt, MD, Christopher A. Ross, MD, PhD, Barnett Shpritz, BS, MA, OD, and Claire Welsh (Johns Hopkins University, Baltimore, Maryland, USA); William M. Mallonee, MD and Greg Suter, BA (Hereditary Neurological Disease Centre, Wichita, Kansas, USA); Ali Samii, MD, Hillary Lipe, ARNP, and Kurt Weaver, PhD (University of Washington and VA Puget Sound Health Care System, Seattle, Washington, USA); Randi Jones, PhD, Cathy Wood-Siverio, MS, Stewart A. Factor, DO, and Claudia Testa, MD, PhD (Emory University School of Medicine, Atlanta, Georgia, USA); Roger A. Barker, BA, MBBS, MRCP, Sarah Mason, BSC, Anna Goodman, PhD, and Anna DiPietro (Cambridge Centre for Brain Repair, Cambridge, UK); Elizabeth McCusker, MD, Jane Griffith, RN, and Kylie Richardson, PhD (Westmead Hospital, Sydney, Australia); Bernhard G. Landwehrmeyer, MD, Daniel Ecker, MD, Patrick Weydt, MD, Michael Orth MD, PhD, Sigurd Süβmuth, MD, RN, Katrin Barth, RN, and Sonja Trautmann, RN (University of Ulm, Ulm, Germany); Kimberly Quaid, PhD, Melissa Wesson, MS, and Joanne Wojcieszek, MD (Indiana University School of Medicine, Indianapolis, IN); Mark Guttman, MD, Alanna Sheinberg, BA, Adam Singer, and Janice Stober, BA, BSW (Centre for Addiction and Mental Health, University of Toronto, Markham, Ontario, Canada); Susan Perlman, MD and Arik Johnson, PsyD (University of California, Los Angeles Medical Center, Los Angeles, California, USA); Michael D. Geschwind, MD, PhD and Jon Gooblar, BA (University of California San Francisco, California, USA); Tom Warner, MD, PhD, Stefan Klöppel, MD, Maggie Burrows, RN, BA, Marianne Novak, MD, Thomasin Andrews, MD, BSC, MRCP, Elisabeth Rosser, MBBS, FRCP, and Sarah Tabrizi, BSC, PhD (National Hospital for Neurology and Neurosurgery, London, UK); Anne Rosser, MD, PhD, MRCP and Kathy Price, RN (Cardiff University, Cardiff, Wales, UK); Amy Chesire, LCSW-R, MSG, Frederick Marshall, MD, and Mary Wodarski, BA (University of Rochester, Rochester, New York, USA); Oksana Suchowersky, MD, FRCPC, Sarah Furtado, MD, PhD, FRCPC, and Mary Lou Klimek, RN, BN, MA (University of Calgary, Calgary, Alberta, Canada); Peter Panegyres, MB, BS, PhD, Carmela Connor, BP, MP, DP, and Elizabeth Vuletich, BSC (Neurosciences Unit, Graylands, Selby-Lemnos & Special Care Health Services, Perth, Australia); Joel Perlmutter, MD and Stacey Barton, MSW, LCSW (Washington University, St. Louis, Missouri, USA); Sheila A. Simpson, MD and Daniela Rae, RN (Clinical Genetics Centre, Aberdeen, Scotland, UK); David Craufurd, MD, Ruth Fullam, BSC, and Elizabeth Howard, MD (University of Manchester, Manchester, UK); Pietro Mazzoni, MD, PhD, Karen Marder, MD, MPH, Carol Moskowitz, MS, and Paula Wasserman, MA (Columbia University Medical Center, New York, New York, USA); Diane Erickson, RN, Dawn Miracle, BS, MS, and Rajeev Kumar, MD (Colorado Neurological Institute, Englewood, Colorado, USA); Vicki Wheelock, MD, Terry Tempkin, RNC, MSN, Nicole Mans, BA, MS, and Kathleen Baynes, PhD (University of California Davis, Sacramento, California, USA); Joseph Jankovic, MD, Christine Hunter, RN, CCRC, and William Ondo, MD (Baylor College of Medicine, Houston, Texas, USA); Justo Garcia de Yebenes, MD, Monica Bascunana Garde, Marta Fatas, BA, and Jose Luis Lópenz Sendon, MD (Hospital Ramón y Cajal, Madrid, Spain); Martha Nance, MD, Dawn Radtke, RN, and David Tupper, PhD (Hennepin County Medical Center, Minneapolis, Minnesota, USA); Wayne Martin, MD, Pamela King, BScN, RN, and Satwinder Sran, BSC (University of Alberta, Edmonton, Alberta, Canada); Anwar Ahmed, PhD, Stephen Rao, PhD, Christine Reece, BS, Janice Zimbelman, PhD, PT, Alexandra Bea, BA, and Emily Newman, BA (Cleveland Clinic Foundation, Cleveland, Ohio, USA).
REGISTRY: Raphael M. Bonelli, Brigitte Herranhof; Anna Hödl; Michael Koppitz, Markus Magnet; Daniela Otti; Annamaria Painold; Karin Reisinger (LKH Graz, Abteilung für Psychiatrie, Graz, Austria); Anja Flamez, Vera Morez, Sylvie de Raedt (VUB, Neurology, Brussels, Belgium); Pascale Ribaï, Christine Verellen-Dumoulin (Institut de Pathologie et de Génétique (IPG), Charleroi, Belgium); Heli Hiivola; Kirsti Martikainen; Katri Tuuha (Rehabilitation Centre Suvituuli, Turku-Suvituuli, Finland); Christoph Michael Kosinski; Daniela Probst; Christian Sass; Johannes Schiefer; Christiane Schlangen; Cornelius J. Werner (Universitätsklinikum Aachen, Neurologische Klinik, Aachen, Germany); Josef Priller; Harald Prüß(Klinik und Poliklinik für Neurologie - Charité - Universitätsmedizin Berlin, Berlin, Germany); Jürgen Andrich; Rainer Hoffmann; Christian Prehn, Carsten Saft; Stephan Salmen; Katrin Straßburger (Huntington-Zentrum (NRW) Bochum im St. Josef-Hospital, Bochum, Germany); Herwig Lange (Reha Zentrum in Dinslaken im Gesundheitszentrums Lang, Dinslaken, Germany); Ulrike Hunger; Matthias Löhle; Simone Schmidt; Alexander Storch; Anett Wolz; Martin Wolz (Universitätsklinikum Carl Gustav Carus an der Technischen Universität Dresden, Klinik und Poliklinik für Neurologie, Dresden, Germany); Johann Lammbeck, Birgit Zucker (Universitätsklinik Freiburg, Neurologie, Freiburg, Germany); Alexander Münchau; Lars Stubbe; Simone Zittel (Universitätsklinikum Hamburg-Eppendorf, Klinik und Poliklinik für Neurologie, Hamburg, Germany); Walburgis Heinicke (Psychatrium Heiligenhafen, Heiligenhafen, Germany); Bernhard Longinus (Klinik für Psychiatrie und Psychotherapie Marburg-Süd, Marburg KPP, Germany); Jens Carsten Möller; Ida Rissling (Klinik für Neurologie, Philipps-Universität Marburg, Marburg Uni, Germany); Alexander Peinemann; Michael Städtler; Adolf Weindl (Huntington-Ambulanz im Neuro-Kopfzentrum - Klinikum rechts der Isar der Neurologischen Klinik und Poliklinik der Technischen Universität München, München, Germany); Stefan Bohlen; Herwig Lange; Ralf Reilmann (Universitätsklinikum Münster, Klinik und Poliklinik für Neurologie, Münster, Germany); Antonie Beister; Matthias Dose; Kathrin Hammer, Gabriele Leythaeuser; Ralf Marquard; Caroline Schrenk; Michele Schuierer; Alexandra Wiedemann (Isar-Amper-Klinikum - Klinik Taufkirchen (Vils), Taufkirchen, Germany); Daniel Ecker; Carolin Eschenbach, Bernhard Landwehrmeyer; Franziska Lezius; Michael Orth, Sonja Trautmann, Patrick Weydt (Universitätsklinikum Ulm, Neurologie, Ulm, Germany); Elisabetta Bertini; Claudia Mechi; Marco Paganini; Sivia Piacentini; Maria Romoli; Sandro Sorbi (Neurologia I- Unita' di Neurogenetica Departimento di Neurologia e Psichiatria, Universita' di Firenze, Florence, Italy); Tiziana Martino; Sara Orobello, Ferdinando Squitieri (Neurogenetics Unit, IRCCS Neuromedmed, Pozzilli, Italy); Arvid Heiberg; Marleen R van Walsem (Rikshospitalet, Dept. of Medical Genetics, Oslo-RH, Norway); Kathrine Bjørgo; Madelein Fannemel, Per Gørvell, Lars Retterstøl (Oslo-Ulleval, Norway); Inga Bjørnevoll; Sigrid Botne Sando (St. Olavs Hospital, Trondheim, Norway); Emilia Jadwiga Sitek; Jaroslaw Slawek, Witold Soltan (St Adalbert Specialistic Hospital, Gdansk, Gdansk, Poland); Magdalena Boczarska – Jedynak; Barbara Jasińska – Myga; Grzegorz Opala (Silesian Medical University Katowice, Department of Neurology of Old Age, Katowice, Poland); Krzysztof Banaszkiewicz Malgorzata Dec; Malgorzata Krawczyk; Monika Rudzińska, Andrzej Szczudlik, Magdalena Wójcik (Krakowska Akademia Neurologii, Krakow, Poland); Anna Bryl; Anna Ciesielska, Aneta Klimberg, Wojciech Kozubski, Jerzy Marcinkowski; Pani Justyna Sempołowicz, Daniel Zielonka (Poznan University of Poznań, Department of Social Medicine, Poland); Piotr Janik; Anna Kalbarczyk; Hubert Kwiecinski, Zygmunt Jamrozik (Medical University of Warsaw, Neurology; Warsaw-MU, Poland); Jakub Antczak; Katarzyna Jachinska, Maryla Rakowicz, Przemyslaw Richter; Danuta Ryglewicz; Gregorz Witkowski, Jacek Zaremba; Elzbieta Zdzienicka (Institute of Psychiatry and Neurology Dep. of Genetics, Dep. of Neurology, Dep. of Clinical Neurophysiology, Warsaw-IpiN, Poland); Cristina Costa, Ângela Timóteo (Hospital Fernando Fonseca, E.P.E. Serviço de Neurologia, Lisbon-Fernando Fonseca, Portugal); Miguel Coelho; Joaquim J Ferreira; Tiago Mestre; Mário M Rosa; Anabela Valadas (Neurological Clinical Research Unit, Institute of Molecular Medicine, Lisbon-Santa Maria, Portugal); Miguel Gago; Carolina Garrett; Maria Rosalia Guerra (Hospital São João E.P.E., Porto-São João, Portugal); Jordi Bas; Matilde Calopa, Nuria Busquets (Hospital Universitari de Bellvitge, Barcelona-Bellvitge, Spain); Esther Cubo; Natividad Mariscal; Jesús Sánchez (Servicio de Neurología Hospital General Yagüe, Burgos, Spain); Francisco J Barrero, Blas Morales (Hospital Universitario San Cecilio, Neurología, Granada, Spain); Rocío García-Ramos García; Clara Villanueva; Purificacion Pin Quiroga (Hospital Clínico Universitario San Carlos Madrid-Clinico, Spain); María José Saiz Artiga; Asunción Martínez ; Pedro-José García Ruíz-Espiga, Vicenta Sánchez (Madrid-Fundación Jiménez Díaz, Madrid FJD, Spain); Mónica Bascuñana; Patricia Trigo Cubillo; Marta Fatas; José Luis López-Sendon Moreno; Guillermo García Ribas; Christine Schwarz, Justo García de Yébenes (Hospital Ramón y Cajal, Neurología, Madrid RYC , Spain); Joakim Tedroff; Elisabeth Winnberg (NeuroHealth Consulting Sweden HB, Karolinska Institute, Stockholm-Ersta, Sweden); Jean-Marc Burgunder (Neurologische Klinik des Inselspitals, Bern, Switzerland); Irene Romero, Michael Schüpbach, Sabine Weber Zaugg (Zentrum für Bewegungsstörungen, Neurologische Klinik und Poliklinik, Bern, Switzerland); Monique S.E. van Hout; Jeroen P.P. van Vugt; A. Marit de Weert (Medisch Spectrum Twente, Enschede, The Netherlands); J.J.W. Bolwijn; M. Dekker; K.L. Leenders, J.C.H. van Oostrom (Polikliniek Neurologie, Groningen, The Netherlands); Berry Kremer; C.C.P. Verstappen (Universitair Medisch Centrum St. Radboud, Neurology, Nijmegen, The Netherlands); Kirsty Matheson; Daniela Rae; Sheila A Simpson; Fiona Summers; Alexandra Ure, Gwen Keenan (NHS Grampian, Clinical Genetics Centre, Aberdeen, U.K.); Johnathan Bisson, Monica Busse; Lynda Ellison-Rose; Olivia Handley, Sarah Hunt, Jenny Naji; Kathleen Price; Anne Rosser; (The Institute of Medical Genetics, University Hospital of Wales, Cardiff, U.K.); Maureen Edwards;Paul A. De Sousa; Teresa Hughes (Molecular Medicine Centre, Western General Hospital, Department of Clinical Genetics, Edinburgh, U.K.); Marie McGill; Pauline Pearson; Mary Porteous; Paul Smith (Scottish Huntington´s Association, Edinburgh, U.K.); Adam Zeman (Scottish Huntington´s Association, Edinburgh, U.K.); Peter Brockie; Jillian Foster; Nicola Johns; Jean Rothery, Gareth Thomas, Shona Yates (Scottish Huntington's Association Whyteman's Brae Hospital, Fife, U.K.); Alison Gordon; Rekha Hedge; Pauline Marshall; Stuart Ritchie (Scottish Huntingtons Association, Abercromby Centre, Glasgow, U.K.); Carol Chu; Emma Hobson; Stuart Jamieson; Jean Toscano; Sue Wild; Pam Yardumian (Chapel Allerton Hospital, Department of Clinical Genetics, Leeds, U.K.); Colin Bourne; Carole Clayton; Heather Dipple; Janet Grant; Diana Gross; Caroline Hallam; Julia Middleton; Ann Murch (Leicestershire Partnership Trust, Mill Lodge, Leicester, U.K.); Michael Patton, Maria Patterson (London-St. Georges-Hospital, U.K.); Natalie Arran; David Craufurd; Ruth Fullam; Liz Howard; Susan Huson; Lucy Partington-Jones; Nichola Ritchie; Julie Snowden; Annie Solom; Cheryl Stopford; Jennifer Thompson; Leann Westmoreland(Genetic Medicine, University of Manchester, Manchester Academic Health Sciences Centre and Central Manchester University Hospitals NHS Foundation Trust, Manchester, U.K.); Oliver Bandmann; Alison Bradbury, Kay Fillingham, Oliver Quarrell (The Royal Hallamshire Hospital, Sheffield, U.K.)
Footnotes
Potential conflict of interest: Nothing to Report
Authors Roles: Anthony L. Vaccarino: Research project: conception, organization and execution; Statistical analyses: design and execution; Manuscript: writing of first draft. Karen Anderson: Manuscript: review and critique. Beth Borowsky: Manuscript: review and critique. Kevin Duff: Manuscript: review and critique. Joseph Giuliano: Manuscript: review and critique. Mark Guttman: Manuscript: review and critique. Aileen K. Ho: Manuscript: review and critique. Michael Orth: Research project: Acquisition of data sets; Manuscript: review and critique. Jane S. Paulsen: Research project: Acquisition of data sets; Manuscript: review and critique. Terrence Sills: Research project: conception and organization; Manuscript: review and critique. Daniel P. van Kammen: Manuscript: review and critique. Kenneth R. Evans: Research project: conception and organization; Manuscript: review and critique. PREDICT-HD and REGISTRY Investigators and Coordinators: Manuscript: review and critique.
Financial Disclosure: None reported.
REFERENCES
- 1.Bates GP, Murphy KP. In: Huntington’s disease. Bates GP, Harper PS, Jones AL, editors. Oxford: Oxford University Press; 2002. pp. 387–426. [Google Scholar]
- 2.Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC Predict-HD Investigators of the Huntington Study Group. Psychiatric symptoms in Huntington's disease before diagnosis: the Predict-HD study. Biol Psychiatry. 2007;62(12):1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Julien C, Thompson JC, Wild S, et al. Psychiatric disorders in preclinical Huntington’s Disease. J Neurol, Neurosurg & Psychiatry. 2007;78:939–943. doi: 10.1136/jnnp.2006.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulsen JS, Zhao H, Stout JC, et al. Clinical markers of early disease in persons near onset of Huntington's disease. Neurology. 2001;57(4):658–662. doi: 10.1212/wnl.57.4.658. [DOI] [PubMed] [Google Scholar]
- 5.Paulsen JS, Hayden M, Stout JC, et al. Preparing for Preventive Clinical Trials: The PREDICT-HD Study. Arch Neurol. 2006;63:883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- 6.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington's disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiat. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huntington Study Group. Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 8.Biglan KM, Ross CA, Langbehn DR, et al. Motor abnormalities in premanifest persons with Huntington's disease: the PREDICT-HD study. Mov Disord. 2009;24(12):1763–1772. doi: 10.1002/mds.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurgens CK, van de Wiel L, van Es AC, et al. Basal ganglia volume and clinical correlates in 'preclinical' Huntington's disease. J Neurol. 2008;255(11):1785–1791. doi: 10.1007/s00415-008-0050-4. [DOI] [PubMed] [Google Scholar]
- 10.Rao AK, Louis ED, Marder KS. Clinical assessment of mobility and balance impairments in pre-symptomatic Huntington's disease. Gait Posture. 2009;30(3):391–393. doi: 10.1016/j.gaitpost.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans K, Anderson K, Borowsky B, et al. The Functional Rating Scale Taskforce for Pre-Huntington’s Disease: An Empirically-Driven Initiative for New Scale Development. Neurotherapeutics. 2009;6:205. [Google Scholar]
- 12. http://www.euro-hd.net/html/registry.
- 13.Santor DA, Ramsay JO. Progress in the technology of measurement: Applications of Item Response models. Psychol Assess. 1998;10:345–359. [Google Scholar]
- 14.Evans KR, Sills TL, DeBrota DJ, Gelwicks S, Engelhardt N, Santor D. An Item Response analysis of the Hamilton Depression Rating Scale using shared data from two pharmaceutical companies. J. Psychiatr Res. 2004;38:275–284. doi: 10.1016/j.jpsychires.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Santor DA, Coyne JC. Examining symptom expression as a function of symptom severity: Item performance on the Hamilton Rating Scale for Depression. Psychol Assess. 2001;13:127–139. [PubMed] [Google Scholar]
- 16.Vaccarino AL, Evans KR, Sills TL, Kalali AH. Symptoms of anxiety in depression: assessment of item performance of the Hamilton Anxiety Rating Scale in patients with depression. Depression Anxiety. 2008;25:1006–1013. doi: 10.1002/da.20435. [DOI] [PubMed] [Google Scholar]
- 17.Carmody TJ, Rush AJ, Bernstein I, et al. The Montgomery Asberg and the Hamilton ratings of depression: a comparison of measures. Eur Neuropsychopharmacol. 2006;16(8):601–611. doi: 10.1016/j.euroneuro.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y, Pilkonis PA, Frank E, Thase ME, Reynolds CF. Differential functioning of the Beck depression inventory in late-life patients: use of item response theory. Psychol Aging. 2002;17(3):379–391. doi: 10.1037//0882-7974.17.3.379. [DOI] [PubMed] [Google Scholar]
- 19.Vaccarino AL, Evans KR, Sills TL, Kalali AH. Prevalence and association of somatic symptoms in patients with major depressive disorder. J Affect Dis. 2008;110:270–276. doi: 10.1016/j.jad.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Sills T, Wunderlich G, Pyke R, et al. The Sexual Interest and Desire Inventory-Female (SIDI-F): item response analyses of data from women diagnosed with hypoactive sexual desire disorder. J Sex Med. 2005;2:801–818. doi: 10.1111/j.1743-6109.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay JO. TestGraf: a program for the graphical analysis of multiple choice test and questionnaire data. Montreal: McGill University; 2000. [Available online from http://ego.psych.mcgill.ca/pub/ramsay/testgraf] [Google Scholar]
- 22.Ramsay JO. Kernel smoothing approaches to nonparametric item characteristic curve estimation. Psychometrika. 1991;56:611–630. [Google Scholar]
- 23.Paulsen JS. Early Detection of Huntington's Disease. Future Neurol. 2010;5(1):85–104. doi: 10.2217/fnl.09.78. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bechtel N, Jauffret C, Say M, et al. Force-transducer-based finger tapping as a potential biomarker in premanifest and symptomatic HD- Cross-sectional results from the TRACK-HD Study. Clin Gen. 2009;76(s1):42. [Google Scholar]
- 25.Tabrizi SJ, Langbehn DR, Leavitt BR, et al. Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer C, Ludolph AC, Landwehrmeyer B, Schwenke C, Doble A, Orth M. Modification of the Unified Huntington’s Disease Rating Scale: Selection of items on the basis of the EHDI data. Clin Gen. 2009;76(s1):60. [Google Scholar]
- 27.Siesling S, Zwinderman AH, van Vugt JP, Kieburtz K, Roos RA. A shortened version of the motor section of the Unified Huntington's Disease Rating Scale. Mov Disord. 1997;12(2):229–234. doi: 10.1002/mds.870120214. [DOI] [PubMed] [Google Scholar]
- 28.Louis ED, Anderson KE, Moskowitz C, Thorne DZ, Marder K. Dystonia-predominant adult-onset Huntington disease: association between motor phenotype and age of onset in adults. Arch Neurol. 2000;57(9):1326–1330. doi: 10.1001/archneur.57.9.1326. [DOI] [PubMed] [Google Scholar]
- 29.Dewhurst K, Oliver JE, McKnight AL. Socio-psychiatric consequences of Huntington's disease. Br J Psychiatry. 1970;116(532):255–258. doi: 10.1192/bjp.116.532.255. [DOI] [PubMed] [Google Scholar]
- 30.Nehl C, Paulsen JS. Cognitive and psychiatric aspects of Huntington disease contribute to functional capacity. J Nerv Ment Dis. 2004;192(1):72–74. doi: 10.1097/01.nmd.0000106004.67587.57. [DOI] [PubMed] [Google Scholar]
- 31.Hoth KF, Paulsen JS, Moser DJ, et al. Patients with Huntington's disease have impaired awareness of cognitive, emotional, and functional abilities. J Clin Exp Neuropsychol. 2007;29(4):365–376. doi: 10.1080/13803390600718958. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen JS, Hoth KF, Nehl C, Stierman L. Critical periods of suicide risk in Huntington's disease. Am J Psychiatry. 2005;162(4):725–731. doi: 10.1176/appi.ajp.162.4.725. [DOI] [PubMed] [Google Scholar]
- 33.Craufurd D, Thompson JC, Snowden JS. Behavioral changes in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:219–226. [PubMed] [Google Scholar]
- 34.Hanson S, Davis M, Altevogt B. CNS Clinical Trials: Suicidality and Data Collection: Workshop Summary. Washington, D.C.: National Academies Press; 2010. [PubMed] [Google Scholar]
- 35.Bonelli RM, Hofmann P. A systematic review of the treatment studies in Huntington's disease since 1990. Expert Opin Pharmacother. 2007;8(2):141–153. doi: 10.1517/14656566.8.2.141. [DOI] [PubMed] [Google Scholar]