Abstract
Background
Brooke-Spiegler syndrome is a rare condition with a predisposition to develop cutaneous adnexal neoplasms, especially cylindromas, trichoepitheliomas and spiradenomas. Malignant transformation of cylindromas is rare. In such cases usually cylindrocarcinomas develop within these lesions. We present an unusual case of basal cell carcinoma developing within a preexisting cylindroma.
Main observations
58-year-old woman with a 30-year history of multiple dermal cylindromas extensively involving her scalp was referred for dermatological treatment. The patient reported that one of the long-lasting lesions, 5.5 cm in size, ulcerated within the foregoing few weeks. Histopathology confirmed cylindromas and basal cell carcinoma within the ulcerating tumor. Surgical excision of largest cylindroma tumors led to cosmetic and functional improvement. Magnetic resonance and computed tomography showed tumor infiltration into the skull lamina externa. Metastases were excluded by chest radiography and abdominal ultrasound examination.
Conclusion
Patients with Brooke-Spiegler syndrome should be followed-up for malignant transformation of skin tumors to prevent deep penetration and possible metastases.
Keywords: basal cell carcinoma, cylindroma, trichoepithelioma, syringoma, computed tomography, magnetic resonance imaging, turban tumor
Background
Brooke-Spiegler syndrome is a rare disease with a predisposition to develop numerous cutaneous adnexal neoplasms such as cylindromas, trichoepitheliomas, spiradenomas, trichoblastomas, basal cell carcinomas, follicular cysts and organoid nevi.[1] Most commonly these lesions develop on the head and neck in middle-aged and elderly females. Development of conjuction tumors (i.e. spiradenoma and cylindroma), carcinosarcomas and cylindrocarcinomas as well as concomitant presence of basal cell carcinomas was reported. [2,3,4,5]
We present an unusual case of basal cell carcinoma developing within a preexisting cylindroma in a patient with Brooke-Spiegler syndrome and review current literature about genetics and histogenesis of this disease.
Case Report
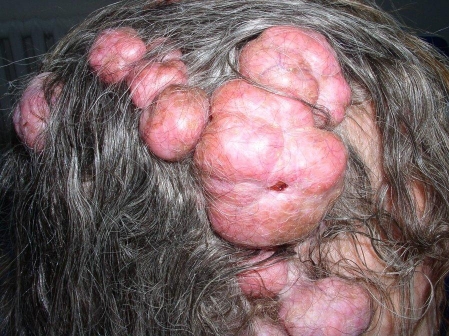
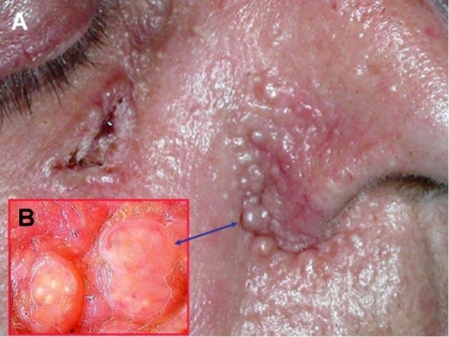
A 58-year-old woman with multiple tumors extensively involving her scalp was referred for dermatologic treatment. She reported that lesions have been appearing and continuously growing for the past 30 years. Clinical examination revealed numerous firm, rubbery pink tumors located on her scalp and neck and several nodular lesions on the right cheek and the upper back [Fig. 1–5]. The tumor of the left parietal area had been ulcerating for several weeks [Fig. 2]. No other abnormalities were found on clinical examination. The patient gave negative family history.
Figure 1.
Multiple cylindromas of the scalp forming a turban tumor.
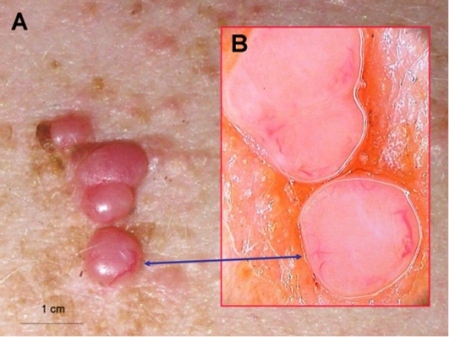
Figure 5.
Multiple cylindromas on patient's back (A). Dermoscopy shows homogenous pink nodules with enlarged irregular vessels at the periphery. (B)
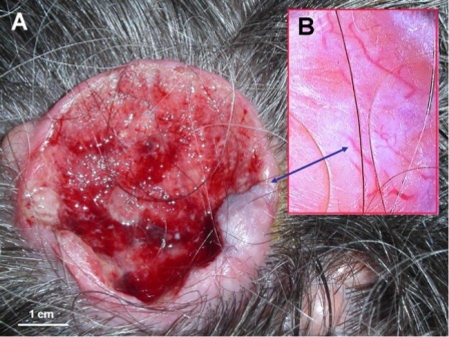
Figure 2.
Basal cell carcinoma within a cylindroma (A). Dermoscopy of ulcer's edge shows arborizing vessels, which are characteristic for both, basal cell carcinoma and cylindroma (B).
Figure 3.
Cylindromas of the neck area (A). Dermoscopy shows prominent arborizing vessels on pink background and numerous whitish ovoid areas (B).
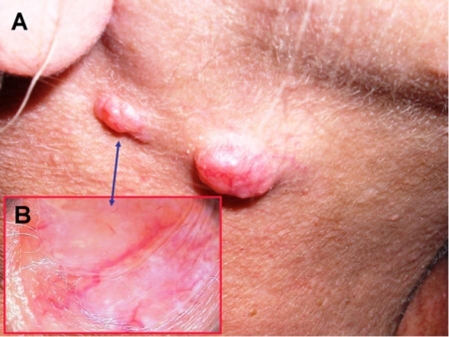
Figure 4.
Basal cell carcinoma of the right cheek area and multiple trichoepitheliomas in the nasolabial area (A). Dermoscopic view of two trichoepitheliomas (B).
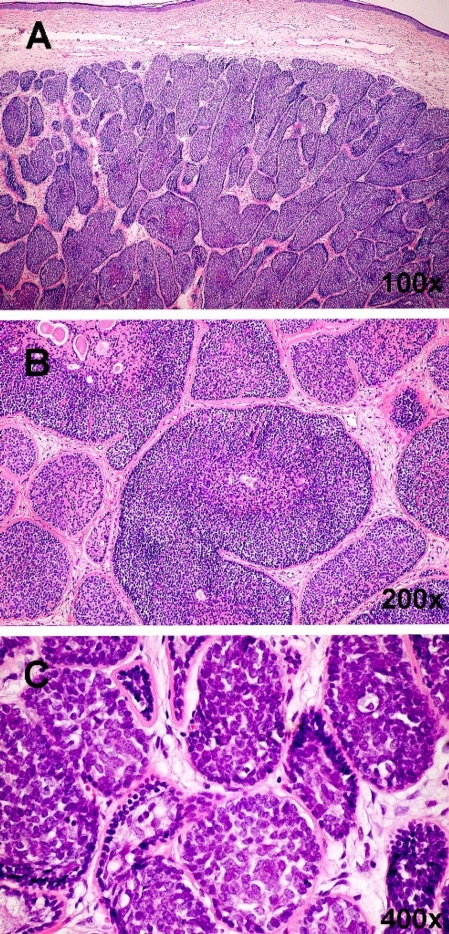
Results of basic laboratory tests (blood cell count, erythrocyte sedimentation rate, routine biochemistry tests including alkaline phosphatase, C-reactive protein and urine analysis) were within normal range. Histopathologic examination revealed basal cell carcinoma within cylindroma in the ulcerated lesion [Fig. 6,7], cylindromas of the scalp, basal cell carcinoma of the cheek and trichoepithelioma in the nasolabial area.
Figure 6.
Cylindroma (H&E). The pattern of cylindroma consists of dermal nests, lobules or cords of basaloid tumor cells (A). Dilated thin-wall blood vessels corresponding to telangiectasia clinically are present between the tumor mass and epidermis. Lobules are surrounded by a dense, intensely eosinophilic hyaline membrane (B). Similar eosinophilic material is present inside the nests admixed with tumor cells. The pattern formed by these almost interlocking nests has been compared to a jigsaw puzzle. Within the nests are two cell types: palisaded small cells with oval dark nuclei situated peripherally and large cells with prominent vesicular nuclei located intralobularly (C).
Figure 7.
BCC (H&E, 200x) Ulcerated tumor with the presence of basal cell carcinoma features underneath the ulceration with accompanying inflammatory cells.
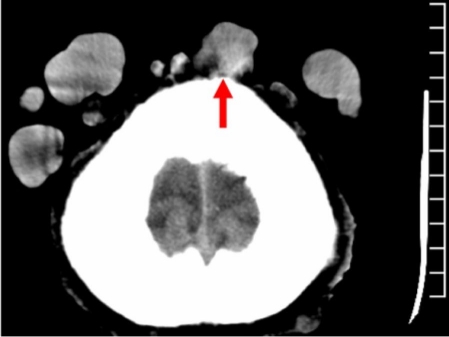
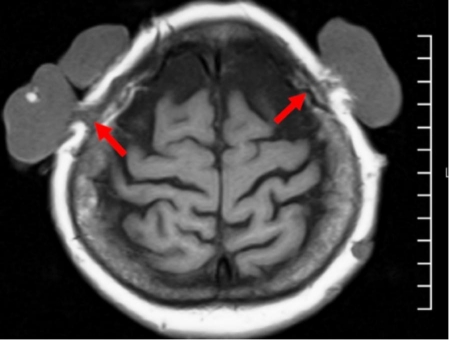
Due to the reported long period of tumor ulceration, imaging diagnostic procedures (skull radiography, magnetic resonance and computed tomography) were performed in order to assess the integrity of the skull bones and brain structures. Computed tomography revealed deformation of lamina externa of both frontal parietal bones [Fig. 8]. Magnetic resonance imaging revealed no brain or meningeal lesions. However, the lesions in the vertex area were attached to the lamina externa of the skull causing its blurring on magnetic resonance image [Fig. 9]. The largest tumor located in the right patrietal area was found to be connected by pedicle with epicranial aponeurosis and lamina externa of the right parietal bone. Many other tumors varied in size were found to be connected with lamina externa of the skull [Fig. 8–9]. Chest radiography and abdominal ultrasound examination excluded malignancy or matastases.
Figure 8.
Computer tomography scan of the scalp. Deformation of lamina externa of right frontal bone.
Figure 9.
Magnetic resonance imaging of the scalp. Blurring of lamina externa of both parietal bones.
The patient gave no consent for a major surgical intervention. Largest nodular lesions of the scalp were progressively surgically removed in local anesthesia. Sutured wounds healed uneventfully, however surgical excision of largest cylindroma tumors led to cosmetic and functional improvement.
Discussion
Cylindromas are benign skin appendage tumors. They are most frequently found in scalp and neck skin, with a strong predilection for middleaged and elderly females. Solitary lesions are firm, rubbery nodules with pink, red, or sometimes blue coloring that range in size from a few millimeters to about 6 centimeters. The average diameter of these tumors is about 1 cm. The solitary form usually begins in middle age or later as a slow-growing nodule exhibiting no symptoms. Cylindromas are histopathologically characterized mainly by irregularly shaped islands of basaloid cells arranged in a "jigsaw puzzle" pattern, surrounded by an eosinophilic hyaline sheath. In the tumor islands, a peripheral palisade of relatively undifferentiated cells with small, dark nuclei can be distinguished from more differentiated cells with large, pale nuclei, which resemble ductal or secretory cells.[6,7]
Conventional wisdom classified cylindroma as a neoplasm of apocrine differentiation.[8,9] Evidence has also been presented in favor of an eccrine origin of cylindroma.[10] However, the fact that cylindromas arise in hair follicle-bearing regions, but not in palmoplantar skin, rich in eccrine glands, yet devoid of pilosebaceous units and apocrine glands argues against an eccrine origin. Thus, histogenesis of cylindromas has remained a subject of intense and controversial debate. Massoumi et al[7] suggested that cylindroma originates from epithelial hair follicle cells, whose exact differentiation pathway (follicular versus apocrine versus sebaceous, and possibly even eccrine) may be dictated by the nature of a given patient and a given skin location as in every normal hair follicle.[11,12]
Solitary cylindromas occur sporadically and typically are not inherited. Multiple tumors are inherited in an autosomal dominant manner. The multiple form most commonly occurs on the head and neck but can also be seen on the trunk and the extremities. Lesions grow slowly, and additional lesions develop over time. At an advanced stage cylindromas form masses of numerous pink, red, or occasionally bluish nodules. When they coalesce on the scalp, they reassemble a turban, and are being referred to as a "turban tumor". Such a mass of nodules is sometimes called also a "tomato tumor".[6,13]
Multiple cylindromas characteristically develop in Brooke-Spiegler syndrome (OMIM number 605041). This is a rare autosomal dominant disorder described first by Spiegler and Brooke.[14,15] The disease is characterized by the development of adnexal tumors, mostly cylindromas, but also trichoepitheliomas and spiradenomas.[16,17] Typically cylindromas occur on the scalp while trichoepitheliomas develop on the face, particulary around the nose.[18] Lesions usually appear at puberty or early adulthood and continue to arise throughout life. The diseases affects predominantly women. The reported male:female ratio is 1:6-9.6.[9,19]
Some patients may present only multiple trichoepitheliomas or cylindromas as unique clinical manifestation. Recent genetic studies show that two autosomal dominantly inherited genodermatoses, multiple familial trichoepithelioma (OMIM number 601606) and familial cylindromatosis (OMIM number 132700), which have been originally described as distinct entities have the same genetic origin.[20] Familial cylindromatosis is clinically characterized by presence of multiple cylindromas, whereas in multiple familial trichoepithelioma development of trichoepitheliomas is observed.
Other lesions reported in Brooke-Spiegler syndrome include parotid basal cell adenomas, organoid nevi, syringomas, and basal cell carcinomas.
Brooke-Spiegler syndrome as well as sporadic cylindromas have been shown to result from mutations leading to loss of both alleles of the cylindromatosis gene (CYLD1). CYLD1 is a tumor suppressor gene which has been shown to inhibit tumor cell proliferation by blocking Bcl-3 dependent NF-κB signaling.[21,22] Loss of CYLD1 function increases resistance to apoptosis. In 2007, Stegmeier et al[23] noted that the CYLD1 gene encodes a deubiquitinating enzyme. The enzyme removes Lys-63-linked ubiquitin chains from I-κB kinase signaling components. By this mechanism, the enzyme inhibits NF-κB pathway activation and stimulates apoptosis. Thus, inactivation of CYLD1 enhances the action of NF-κB and leads to increased resistance to apoptosis and carcinogenesis.
Independently, it was shown that CYLD1 is also required for the cell's timely entry into mitosis. This indicates that CYLD1 also may exhibit tumor-promoting activities (enhancer of mitotic entry), what may provide an explanation for the benign nature of most cylindroma lesions.[23]
In recent years several mutations in CYLD gene were identified not only in patients with Brooke-Spiegler syndrome, but also in familial cylindromatosis and multiple familial trichoepithelioma.[24,25,26] These findings, taken together with the observations that features of Brooke-Spiegler syndrome, familial cylindromatosis and multiple familial trichoepithelioma can occur in the same patient or in different patients within a single family, and that a single CYLD1 mutation can be associated with both cylindromas or trichoepitheliomas[20] may suggest that these syndromes not only share a common genetic basis but may represent phenotypic variation of the same disease.[27] The exact role of CYLD1 in skin tumors, however needs to be established since it has also been shown that CYLD1 expression is lowered or absent in basal cell carcinomas and squamous cell carcinomas.[28,29]
Treatment options in Brooke-Spiegler syndrome are limited. For solitary tumors, surgical excision is the treatment of choice. Other forms of therapy include electrodesiccation, curettage, cryosurgery and excision with the use of carbon dioxide or Nd:YAG laser.[30,31,32,33] Multiple cylindromas usually require numerous tumor excisions or extensive plastic surgery with coverage by spitthickness graft. In case of malignant transformation, surgical treatment may include local bone decortication followed by postoperative radiation. In order to plan the extent of scalp surgery in such cases computed tomography and/or magnetic resonance imaging for evaluation of skull integrity is necessary.
Among non-invasive treatment methods, patients may benefit from intralesional or oral 5-fluorouracil, oral adriamicin, cisplatin or cyclophosphamide, which may be applied in extremely affected patients.[34,35]
Recently it was suggested that targeting NF-κB in cylindroma therapy may be of therapeutic potential in patients with multiple tumors in the course of Brooke-Spiegler syndrome. In one case of familial trichoepitheliomas significant improvement was achieved with a combination of adalimumab and aspirin. This combination was chosen in order to block TNFα induced activation of NF-κB at two different levels of the signaling pathway.[36] Several therapeutic agents, including sodium salicylate, COX2 inhibitors (i.e. celecoxibe), corticoids and prostaglandins (i.e. 15d-PGJ2), as well as phytomedical products inhibit NF-κB activity and thus may be of benefit in Brooke-Spiegler syndrome[37,38] However their possible clinical efficacy remains to be elucidated. In one study some improvement was observed after topical application of salicylic acid.[39]
Malignant transformation of cylindromas is very rare.[40,41] In such cases usually cylindrocarcinomas develop within these lesions.[42,43,44] Less frequently malignant spiradenomas were observed.[43] Our patient developed basal cell carcinoma within a cylindroma in the course of Brooke-Spiegler syndrome. This is an unusual case and it favors of the theory of Massoumi et al[7] that cylindromas originate from epithelial hair follicle cells. Clinically this case provides further evidence that in patients with Brooke-Spiegler syndrome lifelong follow-up care is strictly recommended due to the tendency to develop new skin tumors and risk of malignant transformation with skull erosions. These may lead to dura mater infiltration, hemorrhage and meningitis.[45]
Table 1. Synonyms for (tumors in) Brooke-Spiegler syndrome.[46].
|
Ancell's syndrome Ancell-Spiegler cylindroma Brooke's epithelioma Brooke's disease Brooke's syndrome Brooke's tumour Brooke-Fordyce disease Brooke-Fordyce trichoepithelioma Brooke-Spiegler phakomatosis Spiegler's syndrome Spiegler's tumour |
Conclusion
Patients with Brooke-Spiegler syndrome should be thoroughly examined for both, malignant neoplasia and malignant transformation of benign tumors. In patients with malignant transformation within dermal cylindromas, imaging techniques such as computed tomography and magnetic resonance imaging should be performed to exclude bone damage and choose optimal form of therapy.
References
- Uede K, Yamamoto Y, Furukawa F. Brooke-Spiegler syndrome associated with cylindroma, trichoepithelioma, spiradenoma, and syringoma. J Dermatol. 2004;31:32–38. doi: 10.1111/j.1346-8138.2004.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Michal M, Lamovec J, Mukensnabl P, Pizinger K. Spiradenocylindromas of the skin: tumors with morphological features of spiradenoma and cylindroma in the same lesion: report of 12 cases. Pathol Int. 1999;49:419–425. doi: 10.1046/j.1440-1827.1999.00890.x. [DOI] [PubMed] [Google Scholar]
- Kazakov DV, Soukup R, Mukensnabl P, Boudova L, Michal M. Brooke-Spiegler syndrome: report of a case with combined lesions containing cylindromatous, spiradenomatous, trichoblastomatous, and sebaceous differentiation. Am J Dermatopathol. 2005;27:27–33. doi: 10.1097/01.dad.0000138049.86662.3e. [DOI] [PubMed] [Google Scholar]
- Garat H, Loche F, Gorguet B, Rumeau H, Lamant L, Bazex J. Brooke-Spiegler syndrome. Ann Dermatol Venereol. 1999;126:513–517. [PubMed] [Google Scholar]
- De Francesco V, Frattasio A, Pillon B, Stinco G, Scott CA, Trotter D, Patrone P. Carcinosarcoma arising in a patient with multiple cylindromas. Am J Dermatopathol. 2005;27:21–26. doi: 10.1097/01.dad.0000141548.69423.c7. [DOI] [PubMed] [Google Scholar]
- Scheinfeld NS, Mokashi A, Celebi JT, Oppenheim AR Cylindroma. http://www.emedicine.com/derm/topic94.htm (3.08.2007).
- Massoumi R, Podda M, Fassler R, Paus R. Cylindroma as tumor of hair follicle origin. J Invest Dermatol. 2006;126:1182–1184. doi: 10.1038/sj.jid.5700218. [DOI] [PubMed] [Google Scholar]
- Weedon D. Skin pathology, 2nd ed. London: Churchill-Livingstone; 2002. pp. 890–891. [Google Scholar]
- Klein W, Chan E, Seykora JT. Tumors of the epidermal appendages. Chap. 30. In: Lever's histopathology of the skin (Elder DE et al., eds), 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 897–898. [Google Scholar]
- Ishihara M, Mehregan DR, Hashimoto K, Yotsumoto S, Toi Y, Pietruk T, Mehregan AH, Mehregan DA. Staining of eccrine and apocrine neoplasms and metastatic adenocarcinoma with IKH-4, a monoclonal antibody specific for the eccrine gland. J Cutan Pathol. 1998;25:100–105. doi: 10.1111/j.1600-0560.1998.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niches. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Wu P, Hou L, Plikus M, Hughes M, Schehnet J, Suksaweang S, Widelitz R, Jiang TX, Chuong CM. Evo-devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:249–270. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas AA, Norton SA, Fitzpatrick JE. Solitary violaceous nodule on the face. Dermal cylindroma (also known as cylindroma, dermal eccrine cylindroma, Spiegler's tumor, turban tumor, and tomato tumor) Arch Dermatol. 1993;129:498–499. doi: 10.1001/archderm.129.4.498. [DOI] [PubMed] [Google Scholar]
- Spiegler H. Uber endothelioma der haut. Arch Derm Syph. 1899;50:163–176. [Google Scholar]
- Brooke H. Epithelioma adenoides cysticum. Br J Dermatol. 1892;4:269. [Google Scholar]
- Uede K, Yamamoto Y, Furukawa F. Brooke-Spiegler syndrome associated with cylindroma, trichoepithelioma, spiradenoma, and syringoma. J Dermatol. 2004;31:32–38. doi: 10.1111/j.1346-8138.2004.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Kim C, Kovich OI, Dosik J. Brooke-Spiegler syndrome. Dermatol Online J. 2007;13:10. [PubMed] [Google Scholar]
- Saunders H, Tucker P, Saurine T, Watkins F. Pedigree of multiple benign adnexal tumours of Brooke-Spiegler type. Australas J Dermatol. 2003;44:144–148. doi: 10.1046/j.1440-0960.2003.00663.x. [DOI] [PubMed] [Google Scholar]
- Szepietowski JC, Wasik F, Szybejko-Machaj G, Bieniek A, Schwartz RA. Brooke-Spiegler syndrome. J Eur Acad Dermatol Venereol. 2001;15:346–349. [PubMed] [Google Scholar]
- Poblete Gutiérrez P, Eggermann T, Höller D, Jugert FK, Beermann T, Grussendorf-Conen EI, Zerres K, Merk HF, Frank J. Phenotype diversity in familial cylindromatosis: a frameshift mutation in the tumor suppressor gene CYLD underlies different tumors of skin appendages. J Invest Dermatol. 2002;119:527–531. doi: 10.1046/j.1523-1747.2002.01839.x. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fässler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Stegmeier F, Sowa ME, Nalepa G, Gygi SP, Harper JW, Elledge SJ. The tumor suppressor CYLD regulates entry into mitosis. Proc Natl Acad Sci U S A. 2007;104:8869–8874. doi: 10.1073/pnas.0703268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AL, Kellermayer R, Szigeti R, Tészás A, Azmi S, Celebi JT. CYLD mutations underlie Brooke-Spiegler, familial cylindromatosis, and multiple familial trichoepithelioma syndromes. Clin Genet. 2006;70:246–249. doi: 10.1111/j.1399-0004.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- Liang YH, Gao M, Sun LD, Liu LJ, Cui Y, Yang S, Fan X, Wang J, Xiao FL, Zhang XJ. Two novel CYLD gene mutations in Chinese families with trichoepithelioma and a literature review of 16 families with trichoepithelioma reported in China. Br J Dermatol. 2005;153:1213–1215. doi: 10.1111/j.1365-2133.2005.06960.x. [DOI] [PubMed] [Google Scholar]
- España A, García-Amigot F, Aguado L, García-Foncillas J. A novel missense mutation in the CYLD gene in a Spanish family with multiple familial trichoepithelioma. Arch Dermatol. 2007;143:1209–1210. doi: 10.1001/archderm.143.9.1209. [DOI] [PubMed] [Google Scholar]
- Zhang G, Huang Y, Yan K, Li W, Fan X, Liang Y, Sun L, Li H, Zhang S, Gao M, Du W, Yang S, Liu J, Zhang X. Diverse phenotype of Brooke-Spiegler syndrome associated with a nonsense mutation in the CYLD tumor suppressor gene. Exp Dermatol. 2006;15:966–970. doi: 10.1111/j.1600-0625.2006.00501.x. [DOI] [PubMed] [Google Scholar]
- Seton-Rogers S. A new pathway for CYLD. Nature Rev Cancer. 2006;6:485–485. [Google Scholar]
- Bowen S, Gill M, Lee DA, Fisher G, Geronemus RG, Vazquez ME, Celebi JT. Mutations in the CYLD gene in Brooke-Spiegler syndrome, familial cylindromatosis, and multiple familial trichoepithelioma: lack of genotype-phenotype correlation. J Invest Dermatol. 2005;124:919–920. doi: 10.1111/j.0022-202X.2005.23688.x. [DOI] [PubMed] [Google Scholar]
- Martins C, Bártolo E. Brooke-Spiegler syndrome: treatment of cylindromas with CO2 laser. Dermatol Surg. 2000;26:877–881. doi: 10.1046/j.1524-4725.2000.00034.x. [DOI] [PubMed] [Google Scholar]
- Retamar RA, Stengel F, Saadi ME, Kien MC, Della Giovana P, Cabrera H, Chouela EN. Brooke-Spiegler syndrome - report of four families: treatment with CO2 laser. Int J Dermatol. 2007;46:583–586. doi: 10.1111/j.1365-4632.2007.01689.x. [DOI] [PubMed] [Google Scholar]
- Rallan D, Harland CC. Brooke-Spiegler syndrome: treatment with laser ablation. Clin Exp Dermatol. 2005;30:355–357. doi: 10.1111/j.1365-2230.2005.01759.x. [DOI] [PubMed] [Google Scholar]
- Tarstedt M, Molin L. Nd:YAG laser for effective treatment of multiple cylindroma of the scalp. J Cosmet Laser Ther. 2004;6:41–43. doi: 10.1080/14764170410030804. [DOI] [PubMed] [Google Scholar]
- Farhat F, Kattan J, Culine S, Bekradda M, Droz JP. Efficacy of the combination of 5 fluorouracil, adriamycin and cisplatin (FAP protocol) in the treatment of metastatic cylindroma. Bull Cancer. 1994;81:47–50. [PubMed] [Google Scholar]
- Triozzi PL, Brantley A, Fisher S, Cole TB, Crocker I, Huang AT. 5-Fluorouracil, cyclophosphamide, and vincristine for adenoid cystic carcinoma of the head and neck. Cancer. 1987;59:887–890. doi: 10.1002/1097-0142(19870301)59:5<887::aid-cncr2820590505>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Fisher GH, Geronemus RG. Treatment of multiple familial trichoepitheliomas with a combination of aspirin and a neutralizing antibody to tumor necrosis factor alpha: A case report and hypothesis of mechanism. Arch Dermatol. 2006;142:782–783. doi: 10.1001/archderm.142.6.782. [DOI] [PubMed] [Google Scholar]
- Umezawa K. Inhibition of tumor growth by NF-kappaB inhibitors. Cancer Sci. 2006;97:990–995. doi: 10.1111/j.1349-7006.2006.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Jeon YT, Kim SH, Song YS. NF-kappaB as a potential molecular target for cancer therapy. Biofactors. 2007;29:19–35. doi: 10.1002/biof.5520290103. [DOI] [PubMed] [Google Scholar]
- Oosterkamp HM, Neering H, Nijman SM, Dirac AM, Mooi WJ, Bernards R, Brummelkamp TR. An evaluation of the efficacy of topical application of salicylic acid for the treatment of familial cylindromatosis. Br J Dermatol. 2006;155:182–185. doi: 10.1111/j.1365-2133.2006.07224.x. [DOI] [PubMed] [Google Scholar]
- Iyer PV, Leong AS. Malignant dermal cylindromas. Do they exist? A morphological and immunohistochemical study and review of the literature. Pathology. 1989;21:269–274. doi: 10.3109/00313028909061072. [DOI] [PubMed] [Google Scholar]
- Pizinger K, Michal M. Malignant cylindroma in Brooke-Spiegler syndrome. Dermatology. 2000;201:255–257. doi: 10.1159/000018499. [DOI] [PubMed] [Google Scholar]
- Völter C, Baier G, Schwager K, Müller JG, Rose C. Cylindrocarcinoma in a patient with Brooke-Spiegler syndrome. Laryngorhinootologie. 2002;81:243–246. doi: 10.1055/s-2002-25034. [DOI] [PubMed] [Google Scholar]
- Köstler E, Schönlebe J, Mentzel T, Haroske G, Wollina U. Psoriasis and Brooke-Spiegler syndrome with multiple malignancies. J Eur Acad Dermatol Venereol. 2005;19:380–381. doi: 10.1111/j.1468-3083.2005.01037.x. [DOI] [PubMed] [Google Scholar]
- Durani BK, Kurzen H, Jaeckel A, Kuner N, Naeher H, Hartschuh W. Malignant transformation of multiple dermal cylindromas. Br J Dermatol. 2001;145:653–656. doi: 10.1046/j.1365-2133.2001.04460.x. [DOI] [PubMed] [Google Scholar]
- Wyld L, Bullen S, Browning FS. Transcranial erosion of a benign dermal cylindroma. Ann Plast Surg. 1996;36:194–196. doi: 10.1097/00000637-199602000-00017. [DOI] [PubMed] [Google Scholar]
- Dictionary of Medical Eponyms: http://www.whonamedit.com/synd.cfm/1742.html (14.10.2007).