Abstract
Background
Diagnosis of hair shaft abnormalities is based on light microscopic examination of more than 50 plucked hairs. The aim of this study was to verify whether hair shaft abnormalities may be visualized by trichoscopy (hair and scalp videodermoscopy) and to analyze trichoscopic features of common genetic hair shaft dysplasias.
Method
Patients with known genetic hair shaft disorders were included into the study. Trichoscopy was performed with the use of Fotofinder II videodermoscope. Images performed at 20-fold and 70-fold magnification were analysed. In selected cases 160-fold magnification was used for better visualization of hair shafts.
Results
Our results show that characteristic light microscopy features of Netherton syndrome, monilethrix, woolly hair syndrome, pili torti, pili annulati and trichothiodystrophy may be visualized by trichoscopy.
Conclusion
Genetic hair shaft abnormalities may be diagnosed by trichoscopy in a single diagnostic session without the need of plucking or cutting them for diagnostic purposes.
Keywords: alopecia, dermoscopy, hair, trichoscopy, videodermoscopy
Introduction
A number of autosomal dominant, autosomal recessive and X-linked single gene disorders are characterized by hair abnormalities. Hair changes may be a significant finding or even the initial presentation of a syndrome giving the clue to the diagnosis, in diseases such as trichothiodystrophy[1] or Netherton syndrome.[2]
Hair shaft abnormalities encompass a group of congenital or acquired alterations which involve the hair shaft. They usually lack macroscopic features, which would enable easy diagnosis in medical practice. Thus, the usual diagnostic method is light microscopy and every time about 50 hairs are plucked to decrease the risk of missing a hair with the characteristic abnormality under light microscopy.[3]
Dermoscopy is a method based on epiluminescence microscopy which is used to visualize living skin in magnification. It is known for its value in differential diagnosis of pigmented skin lesions and early detection of melanoma.[4] Videodermoscopy, known also as computerized dermoscopy, allows working with higher magnifications compared to a hand-held dermoscope. The usual working magnification is 20-160-fold in the case of videodermoscopy and 10-20-fold in regular dermoscopy.
Videodermoscopy of hair and scalp (trichoscopy) is a new method, which allows viewing hair shafts in vivo in many-fold magnification without the need of pulling hair for diagnostic purposes of examination.[5,6] Several reports indicate the usefulness of trichoscopy in diagnosing hair and scalp disorders, including androgenic alopecia, alopecia areata or lipedematous alopecia.[7,8,9,10]
The aim of this study was to verify whether hair shaft abnormalities may be visualized by trichoscopy and to analyze trichoscopic features of common genetic hair shaft abnormalities.
Methods
A total of 13 (4 with pili annulati, 3 with pili torti, 3 with trichithiodystrophy, 2 with woolly hair syndrome, 1 with monilethrix) patients with known hair shaft abnormalities were included into the study. In all patients, light microscopy evaluation of a sample of hairs preceded trichoscopy. Trichoscopic images were performed with the use of Fotofinder II videodermoscope. In every patient hairs were visualized at 20-fold and 70-fold magnifications. In selected cases a 160-fold magnification was used for better visualization of hair shaft abnormalities. When applicable, polarized light microscopy, scanning electron microscopy were performed.
Results
Netherton Syndome
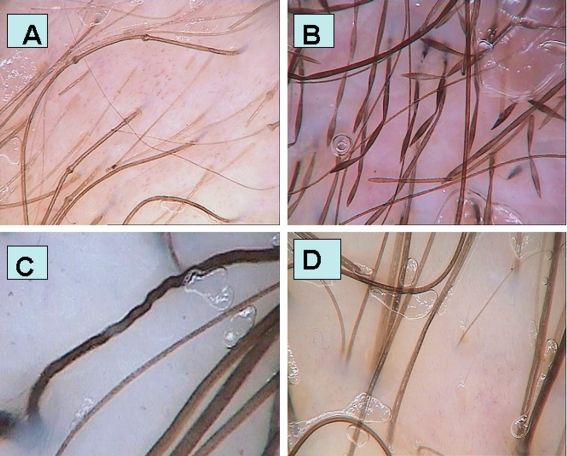
Trichorhexis invaginata and golf tee-like end were visualized by trichoscopy [Fig 1A]. Trichorhexis invaginata could be visualized at 20-fold magnification, whereas golf tee-like ends were best visualized at 70-fold and 160-fold magnification. These characteristic features could be also visualized within the eyebrows.
Figure 1.
Trichoscopy images of (A) Netherton syndrome with trichorhexis invaginata and golf tee like hairs (50-fold magnification); (B) monilethrix - "the regularly bended ribbon sign" (70-fold magnification); (C) woolly hair syndrome - hair shafts resembling a "crawling snake", with ondulations placed very closely together in (70-fold magnification); (D) healthy person's with curly hair - slight variations in hair shaft thickness, when it is evaluated at different lengths (70-fold magnification).
Monilethrix
Trichoscopy performed in monilethrix patient demonstrated an unusual picture of hair shafts with beaded appearance, bended regularly at multiple locations with tendency to curve in different directions, giving it an appearance of a regularly bended ribbon [Fig 1B]. The relative number of beaded hairs to normally appearing hairs was considerably higher in the temporal and occipital area (90%), as compared to the vertex and parietal area of the scalp (40%).
The term "regularly bended ribbon sign" for describing this dermoscopic feature of monilethrix was recently suggested.[11]
Woolly hair syndrome (WHS)
Light microscopy revealed ovoid cross sections, 180-degree longitudinal twisting, trichorrhexis nodosa and pili annulati. By scanning microscopy examination, the hairs were flat, appearing as oval or irregular on transverse sections with longitudinal and transverse grooves. In the distal end of the cuticle, cells were either damaged or absent, and the cortex and medulla were vacuolated.
Trichoscopy allowed to distinguish between healthy curly hair and the WHS. In WHS hair shafts demonstrated a "crawling snake" appearance, with short wave cycles [Fig. 1C]. Apart from that, the examination demonstrated broken hair shafts.
For comparison, hairs of healthy persons with curly hair were investigated in trichoscopy. The hair shafts in healthy persons with curly hair do not differ significantly from hair shafts observed in persons with straight hair. However, one can observe slight differences in the hair shaft thickness, when it is evaluated at different lengths [Fig. 1D].
Pili annulati
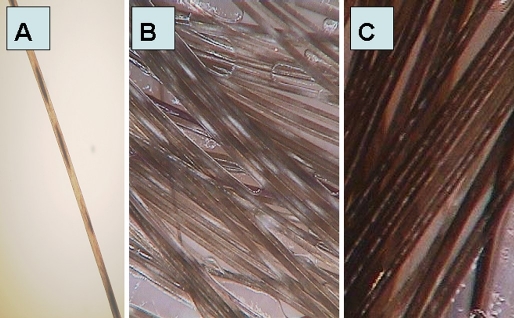
Microscopy of hairs from patients with pili annulati showed a random pattern of intermittent abnormal cavities [Fig. 2A]. Light bands observed by clinical examination appeared as dark bands when imaged by light microscopy, reflecting cortical spacers containing air in the light bands and fluid in the dark bands.[12]
Figure 2.
Pili annulati: (A) microscopic examination (x40); (B) trichoscopy image in pili annulati with regular, width, white bands with misty-like appearance (x70); (C) trichoscopy image in normal thick hair shaft with "intermittent medulla symptom", easily mistaken with pili annulati.
Trichoscopy in patients with pili annulati demonstrated hair shafts with regular light bands, which corresponded to dark cavities visible in microscopical examination [Fig. 2B]. These features were visible in patients with dark and light hairs. "Intermittent medulla", observed with thick hair shafts, can be wrongly diagnosed as pili annulati. In these cases, the medulla visible as light-colored constitutes less than 50% of the hair shaft width. Typical intermittent medulla in a healthy person is shown in Fig. 2C for comparison.
Pili torti
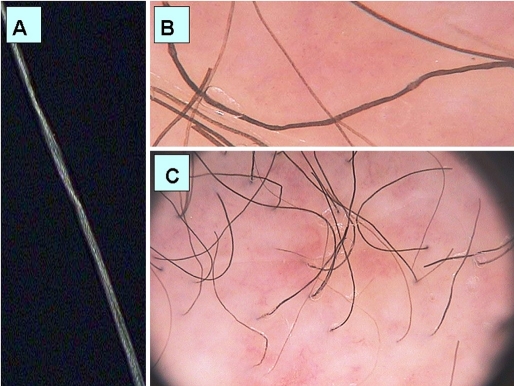
On microscopy, groups of three or four regularly spaced twists were seen at irregular intervals along the shaft in some of the hairs of the sample. Only part of the hair length was affected [Fig. 3A]. Trichoscopy of pili torti showed at 70-fold magnification regular twists of the hair shaft along the long axis [Fig. 3B]. Images taken at a 20-fold magnification demonstrate the hair shafts acutely bent at different angles at irregular intervals [Fig. 3C].
Figure 3.
Pili torti: (A) microscopic image of hair shaft in pili torti with group of regularly spaced twists; (B) trichoscopy image in pili torti with regular twists of the hair shaft along the long axis (70-fold magnification); (C) trichoscopy image in pili torti with hair shafts acutely bended at different angles at irregular intervals (20-fold magnification).
Trichothiodystrophy
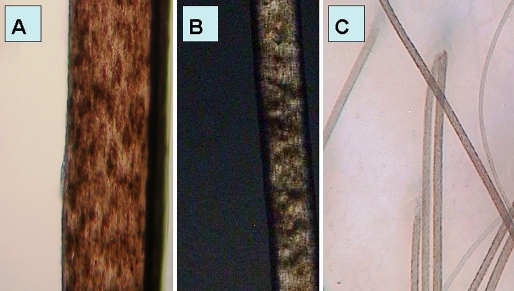
Light microscopy showed hair shafts with an irregular, undulating contour [Fig. 4A] and transverse fractures through the hair shaft (trichoschisis). Polarized light microscopy showed bright and dark bands [Fig. 4B]. Hair shafts were notably flattened, folded most probably during the hair mounting process.
Figure 4.
Trichothiodystrophy: (A) Light microscopy examination (100-fold magnification); (B) trichothiodystrophy in polarizing microscope with tiger tail banding (40-fold magnification); (C) trichoscopy image in trichothiodystrophy - hair shafts with non-homogenous structure of grains of sand and with slightly wavy contour.
Trichoscopy in trichotiodystrophy patients was non-specific. At a higher magnification hairs showed a non-homogenous structure reassembling grains of sand within the hair shaft and a slightly wavy contour [Fig. 4C].
Discussion
The results of this study show that characteristic light microscopy features of most genetic hair shaft abnormalities may be visualized by trichoscopy without the need of plucking hairs for diagnostic purposes. This is the case in Netherton syndrome, monilethrix, woolly hair syndrome, pili torti, pili annulati and trichothiodystrophy.
The Netherton syndrome is characterized by three major clinical features: ichthyosiform dermatosis (non-bullous ichthyosiform erythroderma or as ichthyosis linearis circumflexa), atopy and a characteristic hair abnormality. Clinically hair appears sparse, dull, brittle and short. The hair shaft abnormality, called bamboo hair or trichorhexis invaginata is microscopically characterized by an invagination of the distal portion of the hair shaft into its proximal portion forming a "ball in cup" appearance and is considered pathognomonic for Netherton syndrome. Occasionally ragged, cupped proximal hair end may be seen, where the fragile node has fractured. This abnormality is often referred to as "golf-tee hairs".[13,14] The diagnosis of Netherton syndrome can be difficult. Often, there is no family history and concomitant atopy may lead to misdiagnosis of atopic dermatitis or severe eczema. Differential diagnosis has to include Omenn's syndrome, generalized seborrhoeic dermatitis, erythrodermic psoriasis, staphylococcal scaled skin syndrome, non-bullous ichtiosiform erythroderma and atopic dermatitis.[15] The basis for diagnosis is microscopically confirmed trichorrhexis invaginata. Although a single hair with characteristic invagination is sufficient to establish the diagnosis of Netherton syndrome[16], usually the diagnosis can be confirmed at the age of several months or even several years.[17] This results from the frequent difficulty to spot hairs with pathognomonic features of Netherton syndrome. Often samples of hundreds of hairs are not sufficient to find characteristic features of trichorrhexis invaginata. Plucking hairs is often not helpful as the most abnormal and diagnostically useful hairs are short and forceps miss them.[18] Our study shows that these specific features (trichorhexis invaginata and golf tee-like ends) may be searched for and identificated by trichoscopy [Fig. 1A]. This method is especially beneficial in patients with a low proportion of hairs with characteristic Netherton syndrome abnormalities. In such cases trichoscopy should be considered a method of choice, as it allows to exam practically all hairs during one diagnostic session. It also allows investigating eyebrows, which often carry characteristic trichorhexis invaginata features.[19]
Monilethrix is an autosomal dominant hair disorder characterized by periodic thinning of hair shafts and a tendency to fracture at constricted points. This abnormality results clinically in hair fragility and patchy dystrophic alopecia.[20] The hairs are seldom longer than 5 to 8 centimeters. The effect of disease on hair is variable and may range even within families from close to normal or mild occipital hair loss to near total alopecia. Other hairy areas, such as the eyebrows, eyelashes, axillary hair, pubic hair and body hair may also be involved. Follicular abnormalities seen in monilethrix range from subtle perifollicular erythema and hyperkeratosis to horny follicular papule formation.[21] Mutations in hair specific keratins, especially hHb1 and hHb6, which are primarily expressed in cortical trichocytes, cause predominantly structure abnormalities of hair cortex. These are in particular multiple constrictions of hair shaft, which alternate with elliptical nodosities.[22] Despite characteristic microscopic findings, the disease is not simple to diagnose in dermatological practice, especially in mild cases of the disease. Recently, a case of a patient with monilethtix has been described, in which the diagnosis was first established at the age of 44.[11] Trichoscopy imaging of the scalp and hair in monilethrix demonstrated an unusual picture of hair shafts with beaded appearance, bended regularly at multiple locations with tendency to curve in different directions, giving it an appearance of a regularly bended ribbon.
The term "regularly bended ribbon sign" for describing this dermoscopic feature of monilethrix was recently suggested.[11]
The relative number of beaded hairs to normally appearing hairs was considerably higher in the temporal and occipital area as compared to the rest of the scalp. The existence of pseudomonilethrix is an issue of controversy. According to some authors pseudomonilethrix is a hereditary hair weakness, in which hair shaft exhibit irregular artifactual thickenings or flattenings.[23] Other authors suggest that true pseudomonilethrix does not exist and that sporadic occurrence of irregular hair shaft thickenings is an artifact produced by either procedure of preparing hairs for microscopic examination[24] or by excessive use of cosmetic hair care products.[25] The differential diagnosis between monilethrix and pseudomonilethrix can be made easily by trichoscopy.
Pili annulati is a rare hair shaft disorder characterized by discrete banding of hairs. The etiopathogenesis of this disorder remains unknown but it is likely to be a single gene defect.[26] It is often an incidental finding with alternating light and dark bands causing a slightly spangled appearance of the hair. The phenotype is variable, with not every hair affected, and with variability along a single hair shaft.[27] There is no association with hair fragility, however Gunter et al observed that with onset of hair thinning due to androgenic alopecia, progressive reduction of hair shaft diameter may cause increased fragility in pili annulati.[28] Light microscopy of pili annulati shows a random pattern of intermittent abnormal cavities. Light bands observed by clinical examination appear as dark bands when imaged by light microscopy. The spangled appearance is due to cortical spacer containing air in the light bands and fluid in the dark bands.[29] Trichoscopy in patients with pili annulati demonstrated hair shafts with regular light bands, which correspond to dark cavities visible in microscopical examination. These features are visible in patients with dark and light hairs. This has to be distinguished from a symptom called "intermittent medulla", observed in normal thick hair shafts, which may be wrongly diagnosed as pili annulati. In these cases, the medulla visible as light-colored constitutes less than 50% of the hair shaft width, the light bands are short and the intervals between them are usually shorter than the bands' own length. Our study shows that this feature can be distinguished by trichoscopy from the light bands in pili annulati, which take up more than a half of hair shaft width. The boarders of bands are not clear-cut and the intervals between these bands are usually longer than the bands' own length.
Pili torti is a rare hair shaft dysplasia due to a structural defect in which the hair shafts are flattened or ridged and twisted in their own axis, resulting in the clinical appearance of "corkscrew hairs" and in spontaneous breakage. Pili torti may be associated with many syndromes (Menkes, Bazex or Crandall syndrome) or may occur as an inherited, isolated phenomenon with the onset at birth or in the early months of life.[30] Acquired pili torti have been reported after treatment with oral retinoids.[31] A localized form has been reported after trauma and in cicatrical alopecia.[30] Hair is often sparse, coarse, dry and fragile and affected persons frequently report that they have never required a haircut. Affected hairs are commonly found on the nape of the neck, above the ears and in the eyebrows. The hairs are dry and brittle and break at different lengths.[30] On microscopy, groups of three or four (up to 20) regularly spaced twists, each 0.4-0.9 mm in width, were seen at irregular intervals along the shaft in some hairs within a sample and only part of the hair length was affected.[32] Trichoscopy in pili torti showed typical, regular twists of the hair shaft along the long axis. This was best visible at 70-fold and higher magnifications. The 20-fold magnification demonstrated hair shafts with sharp bending at irregular intervals.
In woolly hair syndrome the term "woolly hair" refers to an abnormal variant of fine, tightly curled hair that often exhibits decreased pigmentation. In 1974, Hutchinson et al[33] classified woolly hair into 3 variants: woolly hair nevus, autosomal dominant hereditary woolly hair and autosomal recessive familial woolly hair. Since then, woolly hair has also been observed in association with several genetic conditions, especially the Noonan syndrome, which is characterized by short stature, typical facial dysmorphology and heart defects[34] and in the phenotypically overlapping cardiofaciocutaneous syndrome, which is characterized by congenital heart defect, developmental delay, peculiar facial appearance with bitemporal constriction, prominent forehead, downslanting palpebral fissures, abnormalities of the skin and curly sparse hair.[35] Distinguishing woolly hair from curly hair may require evaluation of hair shafts by either light or electron microscopy.[36] Light microscopy revealed ovoid cross sections, 180-degree longitudinal twisting, trichorrhexis nodosa and pili annulati. Scanning electron microscopy revealed hairs which were flat, appearing as oval or irregular on transverse sections with longitudinal and transverse grooves. In the distal end of the cuticle cells were either damaged or absent and the cortex and medulla were vacuolated.
Our study shows that trichoscopy allows to distinguish between healthy curly hair and the woolly hair syndrome. In woolly hair syndrome trichoscopy showed the image of hair shafts resembling a "crawling snake", with short wave cycles. Apart from that, trichoscopy also demonstrates broken hair shafts which correspond to increased fragility of hair shafts due to longitudinal twisting. In healthy persons with curly hair trichoscopy does not differ significantly from hair shafts observed in persons with straight hair. However, one can observe slight differences in the hair shaft thickness, when it is evaluated at different lengths.
Trichothiodystrophy, or sulfur-deficient brittle hair, identifies a group of rare and complex neuroectodermal disorders with remarkable clinical heterogeneity.[37]
Clinical symptoms of trichothiodystrophy vary widely in nature and severity, and the single common feature in all patients is fragile hair.[3] In addition, hair loss may occur with periodic cyclicity and during infections intermittent hair loss was observed.[38] Scalp hairs, eyebrows and eyelashes are brittle, unruly, of variable lengths. With polarizing microscopy, using crossed polarizers, hair shafts show a distinctive hair feature: striking alternating bright and dark bands, often referred to as "tiger tail" banding.[37]
The structural abnormality that causes the interrupted transverse bright lines along the hair shafts is not completely understood. An X-ray microanalysis revealed alternating content of sulfur along the long axis of the hair.[32] The X-ray analysis results also showed that calcium was absent in tracts corresponding to dark bands, whereas it was normally present in light bands.[39] Amino-acid analysis of hair in trichothiodystrophy shows notably low cysteine content (less then half of normal content), that parallels the low total sulfur content.[38] Light microscopy showed hair shafts with an irregular, undulating contour and clean transverse fractures through the hair shaft (trichoschisis). The basis of this undulation is not understood, although it may reflect a weak and unstable internal cortical structure, a consequence of the major reduction in disulfide crosslinking, reflected by the low total sulfur content.[40] Cross-sectional examination of the cuticle in hair shows lack of the exocuticle and A layer.[41]
The bright and dark bands seen with polarized light correspond to the undulating orientation of the cortical fibers in trichotiodystrophy. Hair shafts are notably flattened and may fold over like ribbon during the hair mounting process.[40]
The diagnosis of trichotiodystrophy cannot be made on the basis of seeing a few hairs that appear to have alternating bright and dark bands; rather all hair should show the tiger tail pattern and all should have an undulating irregular contour.[37]
It is not possible to demonstrate with a videodermoscope the phenomena observed under a polarized light microscope. Trichoscopy can only suggest the necessity for further diagnosis of trichotiodystrophy, when hair shafts assessed at a high magnification have a non-homogenous structure resembling grains of sand and their contour is very slightly wavy.
In general, it has to be pointed out that for investigating a possible hair shaft abnormality ex vivo, an appropriate sample of hairs must be collected. Hairs must be in sufficient amount to provide a reasonable chance of finding relevant positive clues. A sample of 50 hairs is usually adequate to allow detection of most significant abnormalities. There are exceptions, and Netherton's syndrome is one, where repeated samples of several hundred hairs may be needed to confirm the diagnosis. It is precisely in such cases that trichoscopy proves to be particularly useful, as it allows a rapid, simultaneous examination of almost all hairs to find the one with the specific feature.
In the diagnosis of monilethrix, pili torti, pili annulati and woolly hair, trichoscopy gives comparable results to a microscopic examination, but it has the benefit of being also quick, easy and patient-friendly. From all evaluated hair shaft diseases, only in the case of trichotiodystrophy is trichoscopy insufficient for making the diagnosis.
In conclusion, our study shows that in all hair shaft abnormalities with the exception of trichotiodystrophy, the diagnosis may be established based solely on trichoscopy directly at the dermatologists' office without the need of plucking or cutting hairs for diagnostic procedures.
References
- Silengo M, Valenzise M, Sorasio L, Ferrero GB. Hair as a diagnostic tool in dysmorphology. Clin Genet. 2002;62:270–272. doi: 10.1034/j.1399-0004.2002.620403.x. [DOI] [PubMed] [Google Scholar]
- Furdon SA, Clark DA. Scalp hair characteristics in the newborn infant. Adv Neonatal Care. 2003;3:286–296. doi: 10.1016/j.adnc.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Itin PH, Fistarol SK. Hair shaft abnormalities - clues to diagnosis and treatment. Dermatology. 2005;211:63–71. doi: 10.1159/000085582. [DOI] [PubMed] [Google Scholar]
- Zalaudek I, Argenziano G, Di Stefani A, Ferrara G, Marghoob AA, Hofmann-Wellenhof R, Soyer HP, Braun R, Kerl H. Dermoscopy in general dermatology. Dermatology. 2006;212:7–18. doi: 10.1159/000089015. [DOI] [PubMed] [Google Scholar]
- Olszewska M, Rudnicka L, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy. Arch Dermatol. 2008;144:1007. doi: 10.1001/archderm.144.8.1007. [DOI] [PubMed] [Google Scholar]
- Rudnicka L, Olszewska M, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651–654. [PubMed] [Google Scholar]
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637–640. [PubMed] [Google Scholar]
- Olszewska M, Rudnicka L. A Novel Method for Diagnosing and Monitoring Androgenic Alopecia. Dermatology. 2006;212:290–291. [Google Scholar]
- Olszewska M, Rudnicka L. Videodermoscopy as tool for differential diagnosis and monitoring of hair loss. Exp Dermatol. 2006;15:5. [Google Scholar]
- Piraccini BM, Voudouris S, Pazzaglia M, Rech G, Vicenzi C, Tosti A. Lipedematous alopecia of the scalp. Dermatol Online J. 2006;12:6. [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Czuwara J, Olszewska M, Rudnicka L. Dermoscopy as a tool for rapid diagnosis of monilethrix. J Drugs Dermatol. 2007;6:222–224. [PubMed] [Google Scholar]
- Giehl KA, Ferguson DJ, Dean D, Chuang YH, Allen J, Berker DA, Tosti A, Dawber RP, Wojnarowska F. Alterations in the basement membrane zone in pili annulati hair follicles ad demonstrated by electron microscopy and immunohistochemistry. Br J Dermatol. 2004;150:722–727. doi: 10.1111/j.0007-0963.2004.05837.x. [DOI] [PubMed] [Google Scholar]
- Sun JD, Linden KG. Netherton syndrome: a case report and review of the literature. Int J Dermatol. 2006;45:693–697. doi: 10.1111/j.1365-4632.2005.02637.x. [DOI] [PubMed] [Google Scholar]
- de Berker DA, Paige DG, Ferguson JD, Dawber RP. Golf tee hairs in Netherton disease. Pediatr Dermatol. 1995;12:7–11. doi: 10.1111/j.1525-1470.1995.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Ong C, O'Toole EA, Ghali L, Malone M, Smith VV, Callard R, Harper JI. LEKTI demonstrable by immunohistochemistry of the skin: a potential diagnostic skin test for Netherton syndrome. Br J Dermatol. 2004;151:1253–1257. doi: 10.1111/j.1365-2133.2004.06180.x. [DOI] [PubMed] [Google Scholar]
- de Berker D. Clinical relevance of hair microscopy in alopecia. Clin Exp Dermatol. 2002;27:366–372. doi: 10.1046/j.1365-2230.2002.01078.x. [DOI] [PubMed] [Google Scholar]
- Chavanas S, Garner C, Bodemer C, Ali M, Teillac DH, Wilkinson J, Bonafé JL, Paradisi M, Kelsell DP, Ansai S, Mitsuhashi Y, Larrègue M, Leigh IM, Harper JI, Taïeb A, Prost Y, Cardon LR, Hovnanian A. Localization of the Netherton syndrome gene to chromosome 5q32, by linkage analysis and homozygosity mapping. Am J Hum Genet. 2000;66:914–921. doi: 10.1086/302824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sárdy M, Fáy A, Kárpáti S, Horváth A. Comèl-Netherton syndrome and peeling skin syndrome type B: overlapping syndromes or one entity? Int J Dermatol. 2002;41:264–268. doi: 10.1046/j.1365-4362.2002.01418.x. [DOI] [PubMed] [Google Scholar]
- Rakowska A, Kowalska-Oledzka E, Slowinska M, Rosinska D, Rudnicka L. Hair shaft videodermoscopy in netherton syndrome. Pediatr Dermatol. 2009;26:320–322. doi: 10.1111/j.1525-1470.2008.00778.x. [DOI] [PubMed] [Google Scholar]
- Gummer CL, Dawber RP, Swift JA. Monilethrix: an electron microscopic and electron histochemical study. Br J Dermatol. 1981;105:529–541. doi: 10.1111/j.1365-2133.1981.tb00796.x. [DOI] [PubMed] [Google Scholar]
- Brzezińska-Wcisło L, Bogdanowski T, Szeremeta-Bazylewicz G, Pierzchała E. Monilethrix - rare syndrome of structural hair abnormalities. Pol Merkur Lekarski. 1999;7:226–228. [PubMed] [Google Scholar]
- Djabali K, Panteleyev AA, Lalin T, Garzon MC, Longley BJ, Bickers DR, Zlotogorski A, Christiano AM. Recurrent missense mutations in the hair keratin gene hHb6 in monilethrix. Clin Exp Dermatol. 2003;28:206–210. doi: 10.1046/j.1365-2230.2003.01196.x. [DOI] [PubMed] [Google Scholar]
- Camacho F, Ferrando J, Rodriguez-Pichardo A. Acquired pseudomonilethrix in a family with monilethrix. Eur J Dermatol. 1993;3:651–655. [Google Scholar]
- Zitelli JA. Pseudomonilethrix. An artifact. Arch Dermatol. 1986;122:688–690. doi: 10.1001/archderm.122.6.688. [DOI] [PubMed] [Google Scholar]
- Itin PH, Schiller P, Mathys D, Guggenheim R. Cosmetically induced hair beads. J Am Acad Dermatol. 1997;36:260–261. doi: 10.1016/s0190-9622(97)70293-7. [DOI] [PubMed] [Google Scholar]
- Green J, Fitzpatrick E, de Berker D, Forrest SM, Sinclair RD. A gene for pili annulati maps to the telomeric region of chromosome 12q. J Invest Dermatol. 2004;123:1070–1072. doi: 10.1111/j.0022-202X.2004.23500.x. [DOI] [PubMed] [Google Scholar]
- Streck AP, Moncores M, Sarmento DF, Barbosa HS, Weissmüller G, Baetas-Da-Cruz W. Study of nanomechanical properties of human hair shaft in a case of pili annulati by atomic force microscopy. J Eur Acad Dermatol Venereol. 2007;21:1109–1110. doi: 10.1111/j.1468-3083.2006.02096.x. [DOI] [PubMed] [Google Scholar]
- Hofbauer GF, Tsambaos D, Spycher MA, Trüeb RM. Acquired hair fragility in pili anulati: causal relationship with androgenetic alopecia. Dermatology. 2001;203:60–62. doi: 10.1159/000051706. [DOI] [PubMed] [Google Scholar]
- Giehl KA, Ferguson DJ, Dean D, Chuang YH, Allen J, Berker DA, Tosti A, Dawber RP, Wojnarowska F. Alterations in the basement membrane zone in pili annulati hair follicles as demonstrated by electron microscopy and immunohistochemistry. Br J Dermatol. 2004;150:722–727. doi: 10.1111/j.0007-0963.2004.05837.x. [DOI] [PubMed] [Google Scholar]
- Richards KA, Mancini AJ. Three members of a family with pili torti and sensorineural hearing loss: The Bjornstad syndrome. J Am Acad Dermatol. 2002;46:301–303. doi: 10.1067/mjd.2002.107969. [DOI] [PubMed] [Google Scholar]
- Strumia R, Borghi A, Colombo E, Manzato E, Gualandi M. Low prevalence of twisted hair in anorexia nervosa. Clin Exp Dermatol. 2005;30:349–350. doi: 10.1111/j.1365-2230.2005.01745.x. [DOI] [PubMed] [Google Scholar]
- de Berker D, Sinclair RD. The hair shaft: normality, abnormality, and genetics. Clin Dermatol. 2001;19:129–134. doi: 10.1016/s0738-081x(00)00123-1. [DOI] [PubMed] [Google Scholar]
- Hutchinson PE, Cairns RJ, Wells RS. Woolly hair. Clinical and general aspects. Trans St Johns Hosp Dermatol Soc. 1974;60:160–177. [PubMed] [Google Scholar]
- Noordam K. Expanding the genetic spectrum of Noonan syndrome. Horm Res. 2007;68 Suppl 5:24–27. doi: 10.1159/000110468. [DOI] [PubMed] [Google Scholar]
- Bazzi H, Christiano AM. Broken hearts, woolly hair, and tattered skin: when desmosomal adhesion goes awry. Curr Opin Cell Biol. 2007;19:515–520. doi: 10.1016/j.ceb.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Valentine MC, Sybert VP. Hereditary woolly hair and keratosis pilaris. J Am Acad Dermatol. 2006;54:S35–S39. doi: 10.1016/j.jaad.2005.01.092. [DOI] [PubMed] [Google Scholar]
- Itin PH, Sarasin A, Pittelkow MR. Trichothiodystrophy: update on the sulfur-deficient brittle hair syndromes. J Am Acad Dermatol. 2001;44:891–920. doi: 10.1067/mjd.2001.114294. [DOI] [PubMed] [Google Scholar]
- Liang C, Kraemer KH, Morris A, Schiffmann R, Price VH, Menefee E, DiGiovanna JJ. Characterization of tiger-tail banding and hair shaft abnormalities in trichothiodystrophy. J Am Acad Dermatol. 2005;52:224–232. doi: 10.1016/j.jaad.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Richetta A, Giustini S, Rossi A, Calvieri S. What's new in trichothiodystrophy. J Eur Acad Dermatol Venereol. 2001;15:1–4. doi: 10.1046/j.1468-3083.2001.00218.x. [DOI] [PubMed] [Google Scholar]
- Sperling LC, DiGiovanna JJ. "Curly" wood and tiger tails: an explanation for light and dark banding with polarization in trichothiodystrophy. Arch Dermatol. 2003;139:1189–1192. doi: 10.1001/archderm.139.9.1189. [DOI] [PubMed] [Google Scholar]
- Forslind B, Andersson MK, Alsterborg E. Hereditary hair changes revealed by analysis of single hair fibres by scanning electron microscopy. Scanning Microsc. 1991;5:867–875. [PubMed] [Google Scholar]